Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds
Abstract
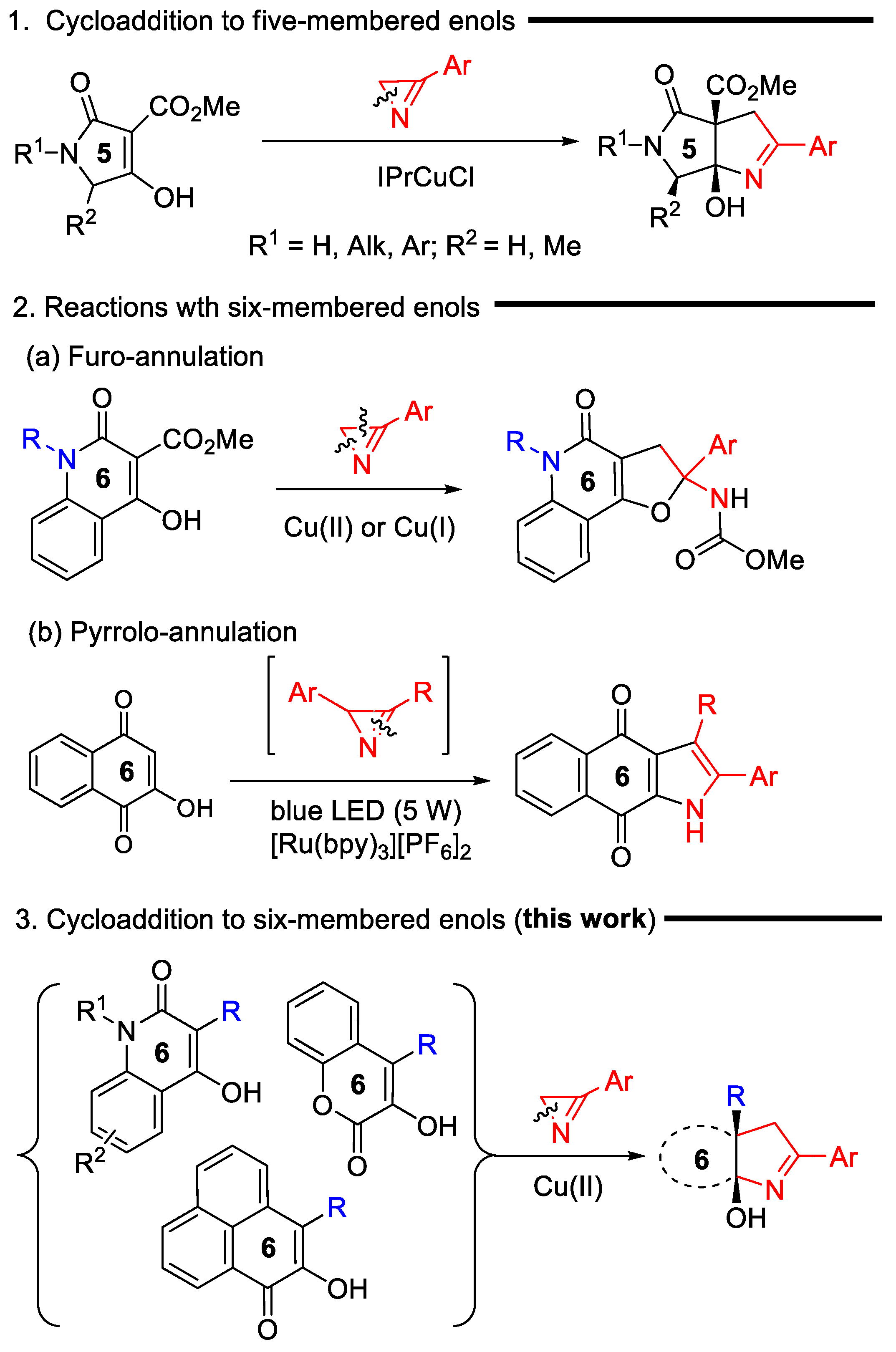
:1. Introduction
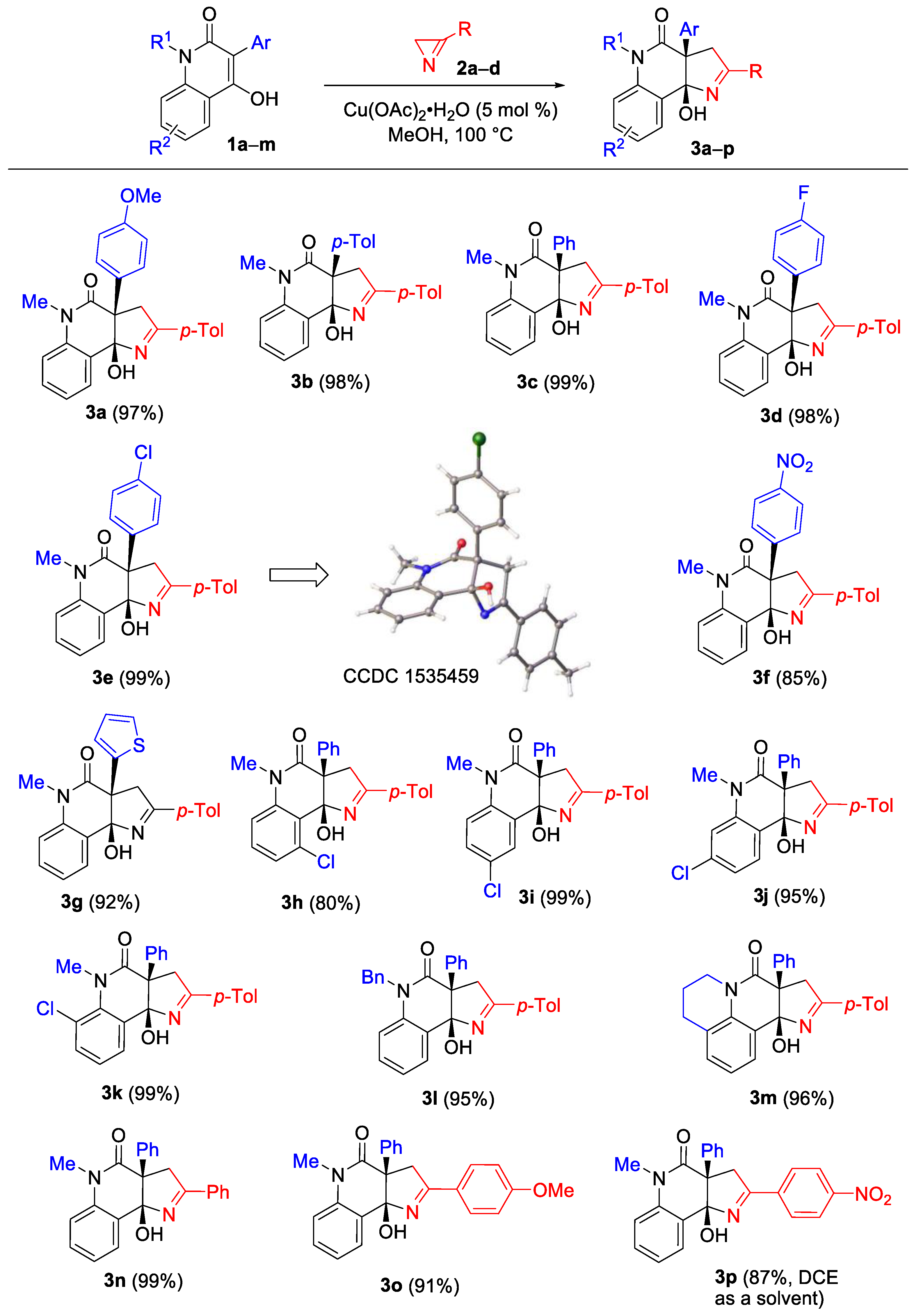
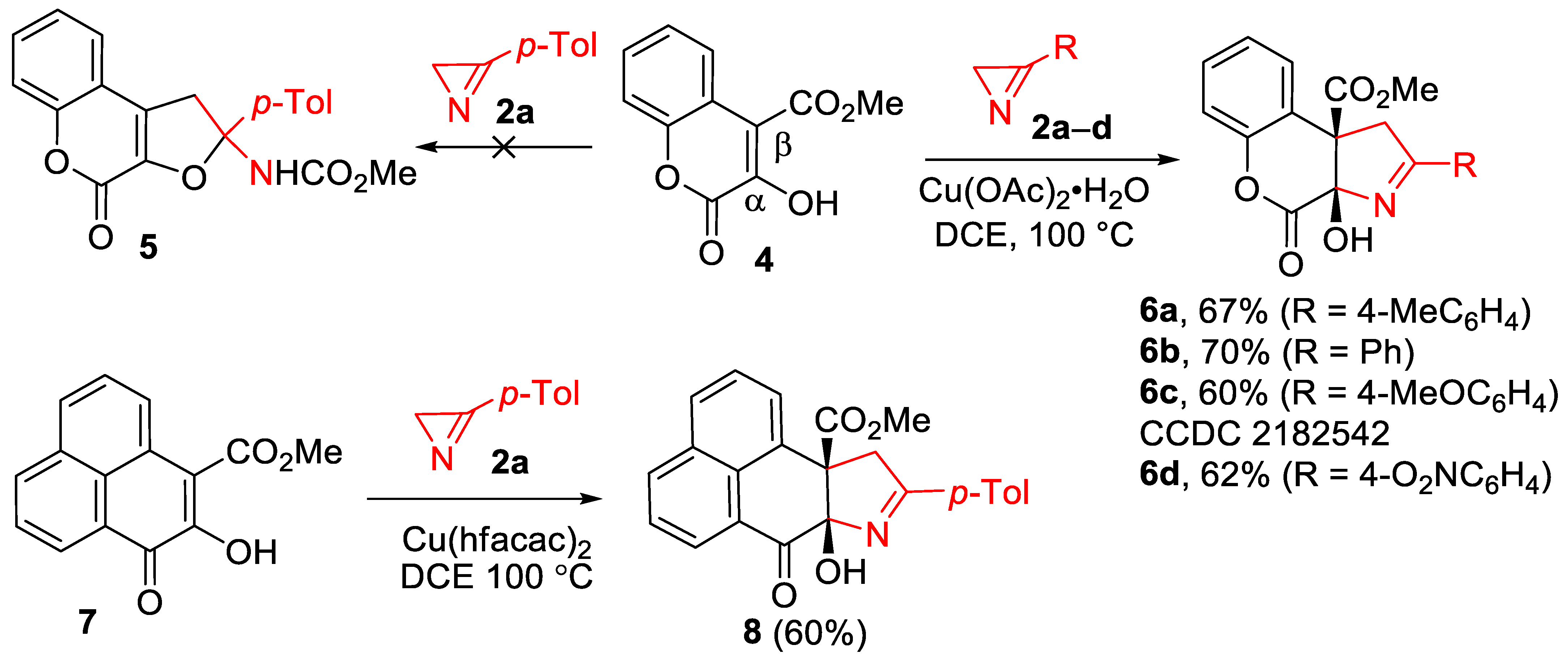
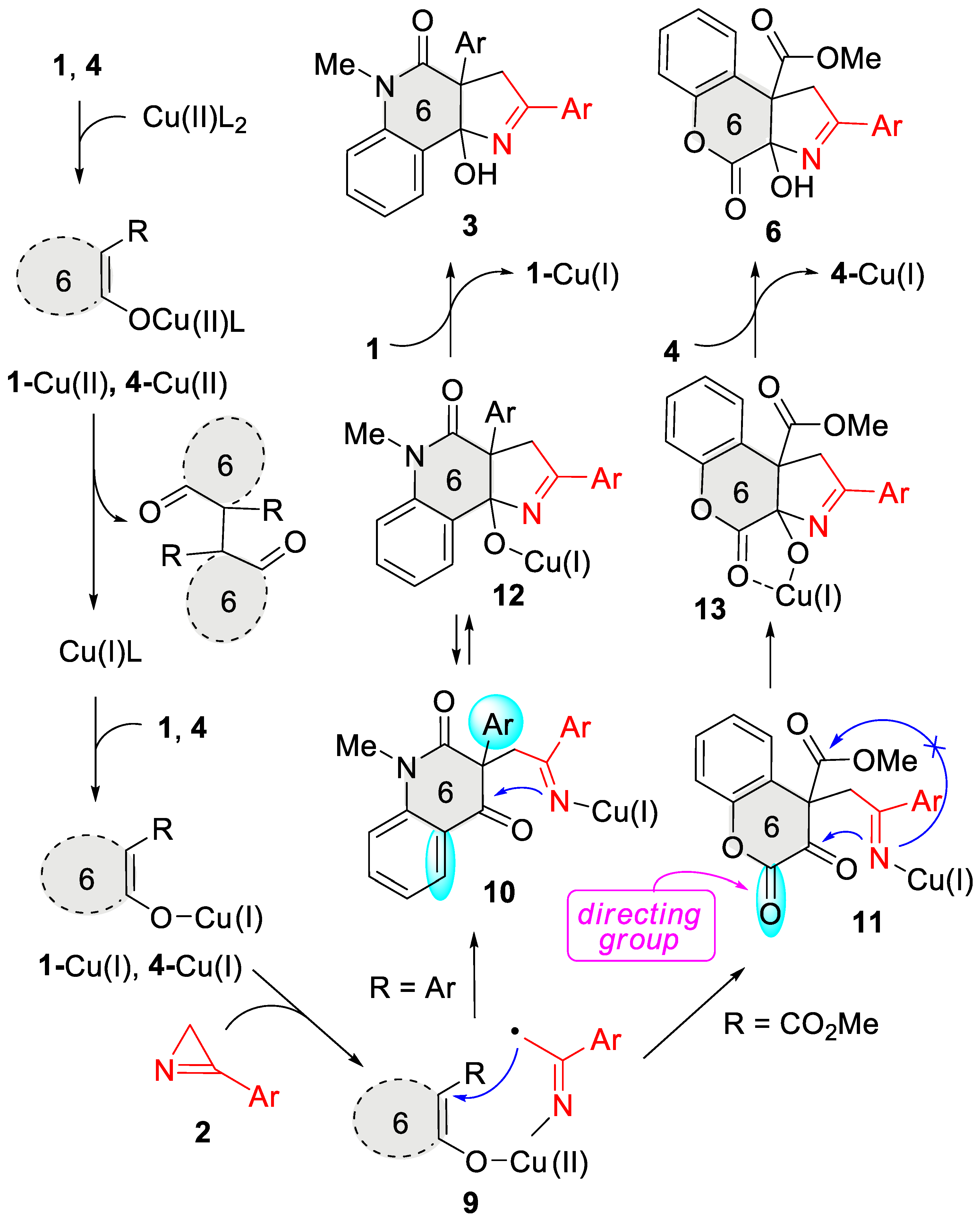
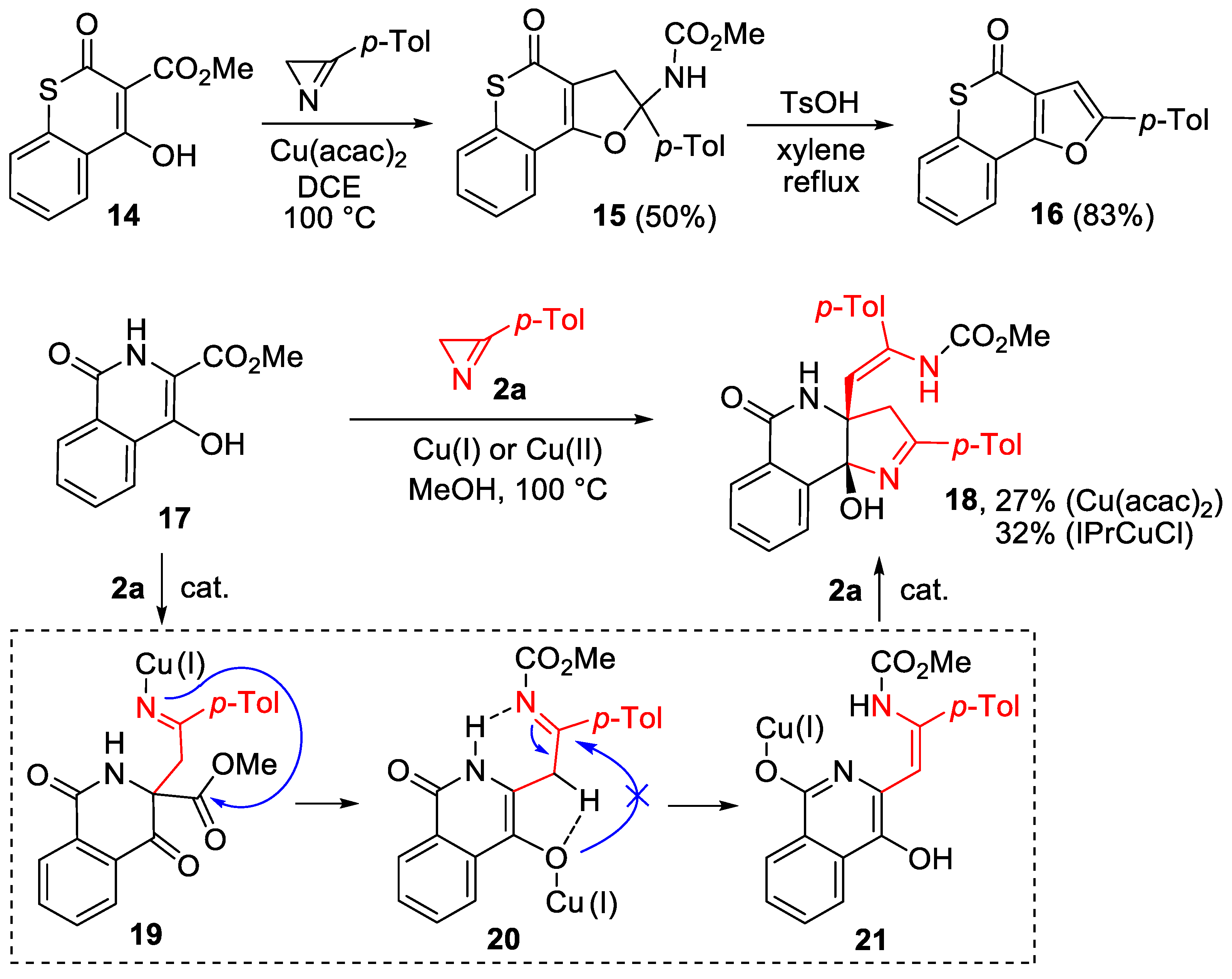
2. Results and Discussion
3. Materials and Methods
3.1. General Instrumentation
3.2. Synthesis and Characterization of Quinolones
3.3. Synthesis of Pyrrolo[3,2-c]quinolin-4-ones 3
3.4. Synthesis of Chromenopyrroles 6
3.5. Synthesis of Methyl Rac-(7aR,10aR)-7a-hydroxy-9-(4-methylphenyl)-7oxo-7a,10-dihydronaphtho[1,8-ef]indole-10a(7H)-carboxylate (8)
3.6. Synthesis of Methyl (2-(4-Methylphenyl)-4-oxo-2,3-dihydro-4H-thiochromeno[4,3-b]furan-2-yl)carbamate (15)
3.7. Synthesis of 2-(4-Methylphenyl)-4H-thiochromeno[4,3-b]furan-4-one (16)
3.8. Synthesis of Methyl (2-(rac-(3aR,9bR)-9b-Hydroxy-2-(4-methylphenyl)-5-oxo-3,4,5,9b-tetrahydro-3aH-pyrrolo[3,2-c]isoquinolin-3a-yl)-1-(4-methylphenyl)vinyl)carbamate (18)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khlebnikov, A.F.; Novikov, M.S.; Rostovskii, N.V. Advances in 2H-azirine chemistry: A seven-year update. Tetrahedron 2019, 75, 2555–2624. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Doyle, A.G. The Chemistry of Transition Metals with Three-Membered Ring Heterocycles. Chem. Rev. 2014, 114, 8153–8198. [Google Scholar] [CrossRef] [PubMed]
- Smetanin, I.A.; Novikov, M.S.; Agafonova, A.V.; Rostovskii, N.V.; Khlebnikov, A.F.; Kudryavtsev, I.V.; Terpilowski, M.A.; Serebriakova, M.K.; Trulioff, A.S.; Goncharov, N.V. A novel strategy for the synthesis of thermally stable and apoptosis-inducing 2,3-dihydroazetes. Org. Biomol. Chem. 2016, 14, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Pokhriyal, A.; Karki, B.S.; Kant, R.; Rastogi, N. Redox-Neutral 1,3-Dipolar Cycloaddition of 2H-Azirines with 2,4,6-Triarylpyrylium Salts under Visible Light Irradiation. J. Org. Chem. 2021, 86, 4661–4670. [Google Scholar] [CrossRef]
- Zhao, M.-N.; Ren, Z.-H.; Yang, D.-S.; Guan, Z.-H. Iron-Catalyzed Radical Cycloaddition of 2H-Azirines and Enamides for the Synthesis of Pyrroles. Org. Lett. 2018, 20, 1287–1290. [Google Scholar] [CrossRef]
- Zhao, M.-N.; Ning, G.-W.; Yang, D.-S.; Gao, P.; Fan, M.-J.; Zhao, L.-F. Nickel-catalyzed formal [3+2]-cycloaddition of 2H-azirines with 1,3-dicarbonyl compounds for the synthesis of pyrroles. Tetrahedron Lett. 2020, 61, 152319. [Google Scholar] [CrossRef]
- Zeng, T.-T.; Xuan, J.; Ding, W.; Wang, K.; Lu, L.-Q.; Xiao, W.-J. [3+2] Cycloaddition/Oxidative Aromatization Sequence via Photoredox Catalysis: One-Pot Synthesis of Oxazoles from 2H-Azirines and Aldehydes. Org. Lett. 2015, 17, 4070–4073. [Google Scholar] [CrossRef]
- Duan, X.; Yang, K.; Lu, J.; Kong, X.; Liu, N.; Ma, J. Base-Mediated Cascade Substitution–Cyclization of 2H-Azirines: Access to Highly Substituted Oxazoles. Org. Lett. 2017, 19, 3370–3373. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Otani, Y.; Ohwada, T. Base-Induced Transformation of 2-Acyl-3-alkyl-2H-azirines to Oxazoles: Involvement of Deprotonation-Initiated Pathways. J. Org. Chem. 2017, 82, 6313–6326. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, J.; Yuan, C.; Wang, G.; Guo, D.; Wang, J. Switchable assembly of substituted pyrimidines and 2H-imidazoles via Cu(I)-catalysed ring expansion of 2-methoxyl-2H-azirines. Org. Chem. Front. 2022, 9, 3006–3011. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. Acid-Catalyzed 1,3-Dipolar Cycloaddition of 2H-Azirines with Nitrones: An Unexpected Access to 1,2,4,5-Tetrasubstituted Imidazoles. J. Org. Chem. 2020, 85, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Rossa, T.A.; Fantinel, M.; Bortoluzzi, A.J.; Sá, M.M. Multicomponent Synthesis of Structurally Diverse Imidazoles Featuring Azirines, Amines and Aldehydes. Eur. J. Org. Chem. 2018, 2018, 4171–4177. [Google Scholar] [CrossRef]
- Feng, F.-F.; Li, J.-K.; Liu, X.-Y.; Zhang, F.-G.; Cheung, C.W.; Ma, J.-A. General Synthesis of Tri-Carbo-Substituted N2-Aryl-1,2,3-triazoles via Cu-Catalyzed Annulation of Azirines with Aryldiazonium Salts. J. Org. Chem. 2020, 85, 10872–10883. [Google Scholar] [CrossRef]
- Sujatha, C.; Bhatt, C.S.; Ravva, M.K.; Suresh, A.K.; Namitharan, K. Copper-Catalyzed Ring-Expansion Cascade of Azirines with Alkynes: Synthesis of Multisubstituted Pyridines at Room Temperature. Org. Lett. 2018, 20, 3241–3244. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S.; Petrovskii, P.P.; Stoeckli-Evans, H. An Aza Cyclopropylcarbinyl-Homoallyl Radical Rearrangement–Radical Cyclization Cascade. Synthesis of Dibenzoimidazoazepine and Oxazepine Derivatives. J. Org. Chem. 2011, 76, 5384–5391. [Google Scholar] [CrossRef]
- Borra, S.; Chandrasekhar, D.; Adhikary, S.; Rasala, S.; Gokulnath, S.; Maurya, R.A. Visible-Light Driven Photocascade Catalysis: Union of N,N-Dimethylanilines and α-Azidochalcones in Flow Microreactors. J. Org. Chem. 2017, 82, 2249–2256. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Harmat, V.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. 1,3-Dipolar Cycloaddition of Isatin-Derived Azomethine Ylides with 2H-Azirines: Stereoselective Synthesis of 1,3-Diazaspiro[bicyclo[3.1.0]hexane]oxindoles. J. Org. Chem. 2019, 84, 4273–4281. [Google Scholar] [CrossRef]
- Colin, A.R.; Risberg, E.; Somfai, P. Diastereoselective Lewis acid-catalysed [4+2] cycloadditions of 3-alkyl-, 3-aryl- and 3-carboxyl-2H-azirines: A route to aziridine containing azabicyclo[4.1.0]heptanes and azatricyclo[2.2.1.0]nonanes. Tetrahedron 2002, 58, 5983–5987. [Google Scholar] [CrossRef]
- Alves, M.J.; Lemos, A.; Rodriguez-Borges, J.E.; García-Mera, X.; Fortes, A.G. Ethyl 2-(diisopropoxyphosphoryl)-2H-azirine-3-carboxylate: Reactions with nucleophilic 1,3-dienes. Synthesis 2009, 2009, 3263–3266. [Google Scholar] [CrossRef]
- Ding, H.; Wang, Z.; Bai, S.; Lu, P.; Wang, Y. Rh-Catalyzed Conversion of 3-Diazoindolin-2-imines to 5H-Pyrazino[2,3-b]indoles with Photoluminescent Properties. Org. Lett. 2017, 19, 6514–6517. [Google Scholar] [CrossRef]
- Baek, Y.; Maeng, C.; Kim, H.; Lee, P.H. Regioselective Synthesis of Indolopyrazines through a Sequential Rhodium-Catalyzed Formal [3+3] Cycloaddition and Aromatization Reaction of Diazoindolinimines with Azirines. J. Org. Chem. 2018, 83, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Ruvinskaya, J.O.; Rostovskii, N.V.; Filippov, I.P.; Khlebnikov, A.F.; Novikov, M.S. A novel approach to 5H-pyrazino[2,3-b]indoles via annulation of 3-diazoindolin-2-imines with 2H-azirines or 5-alkoxyisoxazoles under Rh(II) catalysis. Org. Biomol. Chem. 2018, 16, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wu, M.-F.; Liao, J.-H.; Hong, B.-C. Formal [6+3] cycloaddition of fulvenes with 2H-azirine: A facile approach to the [2]pyrindines system. Tetrahedron Lett. 2004, 45, 1663–1666. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Li, L.; Wang, X.; Sun, R.; Zhou, M.-D.; Wang, H. Visible-Light-Promoted [3 + 2] Cycloaddition of 2H-Azirines with Quinones: Access to Substituted Benzo[f]isoindole-4,9-diones. Chin. J. Chem. 2022, 40, 719–724. [Google Scholar] [CrossRef]
- Cludius-Brandt, S.; Kupracz, L.; Kirschning, A. [3+2]-Cycloadditions of nitrile ylides after photoactivation of vinyl azides under flow conditions. Beilstein J. Org. Chem. 2013, 9, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, C.; Lai, H.; Wang, S.; Ni, H.; Yu, W.; Cao, P. The Fe(III)-catalyzed decarboxylative cycloaddition of β-ketoacids and 2H-azirines for the synthesis of pyrrole derivatives. Org. Chem. Front. 2020, 7, 3686–3691. [Google Scholar] [CrossRef]
- Rostovskii, N.V.; Sakharov, P.A.; Novikov, M.S.; Khlebnikov, A.F.; Starova, G.L. Cu(I)–NHC-Catalyzed (2+3)-Annulation of Tetramic Acids with 2H-Azirines: Stereoselective Synthesis of Functionalized Hexahydropyrrolo[3,4-b]pyrroles. Org. Lett. 2015, 17, 4148–4151. [Google Scholar] [CrossRef]
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Annulation of five-membered cyclic enols with 3-aryl-2H-azirines: Catalytic versus non-catalytic cycloaddition. Tetrahedron 2017, 73, 4663–4670. [Google Scholar] [CrossRef]
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Panikorovskii, T.L.; Novikov, M.S. 2H-Azirines as C–C Annulation Reagents in Cu-Catalyzed Synthesis of Furo[3,2-c]quinolone Derivatives. Org. Lett. 2019, 21, 3615–3619. [Google Scholar] [CrossRef]
- Borra, S.; Chandrasekhar, D.; Newar, U.D.; Maurya, R.A. Access to 2,3-Fused Pyrroles via Visible Light Driven Coupling of α-Azidochalcones with 1/2-Naphthols, or 2-Hydroxy-1,4-Naphthoquinone. J. Org. Chem. 2019, 84, 1042–1052. [Google Scholar] [CrossRef]
- Mahto, P.; Shukla, K.; Das, A.; Singh, V.K. Organocatalytic asymmetric synthesis of pyrrolo[3,2-c]quinolines via a formal [3+2] cycloaddition-lactamization cascade reaction using a bifunctional squaramide catalyst. Tetrahedron 2021, 87, 132115. [Google Scholar] [CrossRef]
- Nieman, J.A.; Ennis, M.D. Enantioselective Synthesis of the Pyrroloquinoline Core of the Martinellines. Org. Lett. 2000, 2, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Yugandar, S.; Misra, N.C.; Parameshwarappa, G.; Panda, K.; Ila, H. Reaction of Cyclic α-Oxoketene Dithioacetals with Methylene Isocyanides: A Novel Pyrrole Annulation–Ring-Expansion Domino Process. Org. Lett. 2013, 15, 5250–5253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, J.; Ding, K.; Liu, J.; Cai, Q. Copper-Catalyzed Tandem Reaction of Isocyanides with N-(2-Haloaryl)propiolamides for the Synthesis of Pyrrolo[3,2-c]quinolin-4-ones. J. Org. Chem. 2011, 76, 5346–5353. [Google Scholar] [CrossRef]
- Ma, D.; Xia, C.; Jiang, J.; Zhang, J. First Total Synthesis of Martinellic Acid, a Naturally Occurring Bradykinin Receptor Antagonist. Org. Lett. 2001, 3, 2189–2191. [Google Scholar] [CrossRef]
- Witherup, K.M.; Ransom, R.W.; Graham, A.C.; Bernard, A.M.; Salvatore, M.J.; Lumma, W.C.; Anderson, P.S.; Pitzenberger, S.M.; Varga, S.L. Martinelline and Martinellic Acid, Novel G-Protein Linked Receptor Antagonists from the Tropical Plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 1995, 117, 6682–6685. [Google Scholar] [CrossRef]
- Heidempergher, F.; Pevarello, P.; Pillan, A.; Pinciroli, V.; Della Torre, A.; Speciale, C.; Marconi, M.; Cini, M.; Toma, S.; Greco, F.; et al. Pyrrolo[3,2-c]quinoline derivatives: A new class of kynurenine-3-hydroxylase inhibitors. Farmaco 1999, 54, 152–160. [Google Scholar] [CrossRef]
- Ohashi, T.; Oguro, Y.; Tanaka, T.; Shiokawa, Z.; Shibata, S.; Sato, Y.; Yamakawa, H.; Hattori, H.; Yamamoto, Y.; Kondo, S.; et al. Discovery of pyrrolo[3,2-c]quinoline-4-one derivatives as novel hedgehog signaling inhibitors. Bioorg. Med. Chem. 2012, 20, 5496–5506. [Google Scholar] [CrossRef]
- Grychowska, K.; Satała, G.; Kos, T.; Partyka, A.; Colacino, E.; Chaumont-Dubel, S.; Bantreil, X.; Wesołowska, A.; Pawłowski, M.; Martinez, J.; et al. Novel 1H-Pyrrolo[3,2-c]quinoline Based 5-HT6 Receptor Antagonists with Potential Application for the Treatment of Cognitive Disorders Associated with Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 7, 972–983. [Google Scholar] [CrossRef]
- Ohki, S.; Yoshino, M. Synthesis of nitrogen-containing heterocyclic compounds through nitrilium salt. I. Reaction of nitriles with 2-(α-hydroxy-methyl, -ethyl, and isopropyl)cyclohexanol in the presence of acid. Chem. Pharm. Bull. 1969, 17, 2142–2150. [Google Scholar] [CrossRef] [Green Version]
- Maini, P.N.; Sammes, M.P. The synthesis and chemistry of azolenines. Part 8. The Paal-Knorr reaction with cyclic 2-(acylmethyl)-2-alkyl-l,3-diketones: Isolation of 1-acyl-1H-pyrroles. J. Chem. Soc. Perkin Trans. 1 1988, 161–168. [Google Scholar] [CrossRef]
- Coppola, G.M. An Efficient Synthesis of Arboricine. Synth. Commun. 1985, 15, 135–139. [Google Scholar] [CrossRef]
- Choppakatla, S.; Dachepally, A.K.; Bollikolla, H.B. Palladium-Catalyzed Double C–H Functionalization of 2-Aryl-1,3-Dicarbonyl Compounds: A Facile Access to Alkenylated Benzopyrans. Tetrahedron Lett. 2016, 57, 2488–2491. [Google Scholar] [CrossRef]
- Hortmann, A.G.; Robertson, D.A.; Gillard, B.K. A Convenient Procedure for the Preparation of 2-Arylazirines. J. Org. Chem. 1972, 37, 322–324. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, X.; Tang, Y. Rh(II)-catalyzed cycloadditions of 1-tosyl 1,2,3-triazoles with 2H-azirines: Switchable reactivity of Rh-azavinylcarbene as [2C]- or aza-[3C]-synthon. Chem. Commun. 2015, 51, 4507–4510. [Google Scholar] [CrossRef]
- Eistert, B.; Selzer, H. Umsetzungen Einiger Diazoalkane Mit Isatin, N-Methyl-Isatin, Cumarandion Und Thionaphthenchinon. Chem. Ber. 1963, 96, 1234–1255. [Google Scholar] [CrossRef]
- Eistert, B.; Selzer, H. Ringerweiterung von Acenaphthenchinon Mit Diazoalkanen Unter Abfangen Der Produkte Als Enolate Bzw. Zinkchelate. Chem. Ber. 1963, 96, 314–319. [Google Scholar] [CrossRef]
- Kikionis, S.; McKee, V.; Markopoulos, J.; Igglessi-Markopoulou, O. A prominent C-acylation–cyclisation synthetic sequence and X-ray structure elucidation of benzothiopyranone derivatives. Tetrahedron 2008, 64, 5454–5458. [Google Scholar] [CrossRef]
- Gabriel, S.; Colman, J. Ueber Die Einwirkung von Natriumalkylaten Auf Phtalylglycinester Und Dessen Homologe. Ber. Dtsch. Chem. Ges. 1900, 33, 980–995. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

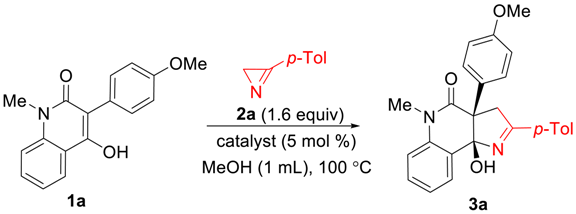 | |||
|---|---|---|---|
| Entry | Catalyst | Deviation from Standard Conditions | Yield of 3a, % a |
| 1 | - | 0 | |
| 2 | IPrCuCl b | 98 | |
| 3 | CuCl2·2H2O | 2a (2 equiv) | 95 |
| 4 | Cu(acac)2 | 98 | |
| 5 | Cu(OAc)2·H2O | 98 | |
| 6 | Cu(OAc)2·H2O | 2a (1 equiv) | 65 |
| 7 | Cu(OAc)2·H2O | solvent EtOH | 77 |
| 8 | Cu(OAc)2·H2O | temperature 20 °C | 53 c |
| 9 | Co(OAc)2·4H2O | 17 | |
| 10 | Ni(acac)2 | 64 | |
| 11 | Fe(acac)3 | 70 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds. Molecules 2022, 27, 5681. https://doi.org/10.3390/molecules27175681
Sakharov PA, Rostovskii NV, Khlebnikov AF, Novikov MS. Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds. Molecules. 2022; 27(17):5681. https://doi.org/10.3390/molecules27175681
Chicago/Turabian StyleSakharov, Pavel A., Nikolai V. Rostovskii, Alexander F. Khlebnikov, and Mikhail S. Novikov. 2022. "Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds" Molecules 27, no. 17: 5681. https://doi.org/10.3390/molecules27175681
APA StyleSakharov, P. A., Rostovskii, N. V., Khlebnikov, A. F., & Novikov, M. S. (2022). Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds. Molecules, 27(17), 5681. https://doi.org/10.3390/molecules27175681







