Curcumin-Loaded Microspheres Are Effective in Preventing Oxidative Stress and Intestinal Inflammatory Abnormalities in Experimental Ulcerative Colitis in Rats
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Plasma Antioxidant Response
2.1.1. Catalase (CAT)
2.1.2. Total Antioxidant Capacity (TAC)
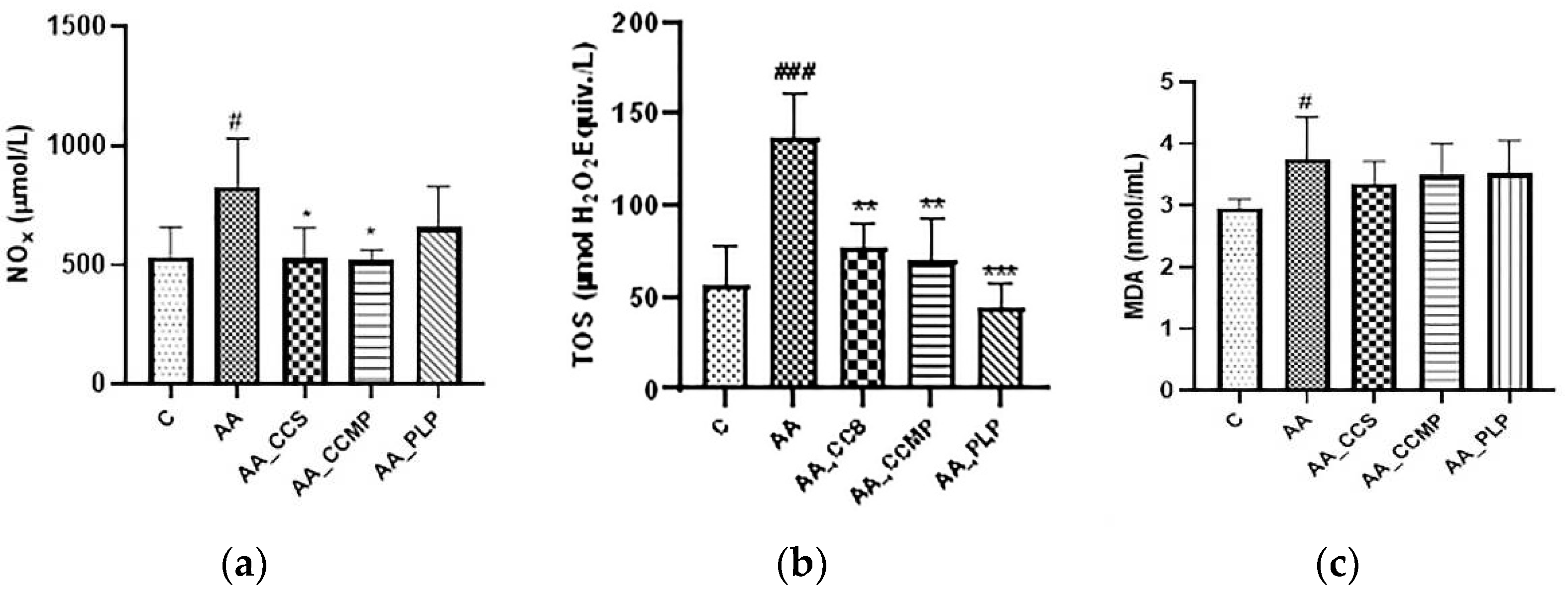
2.2. Evaluation of Plasma Oxidative Stress
2.2.1. Total NO Metabolites (Nitrites and Nitrates, NOx)
2.2.2. Total Oxidant Status (TOS)
2.2.3. Lipid Peroxidation (Malondialdehyde, MDA)
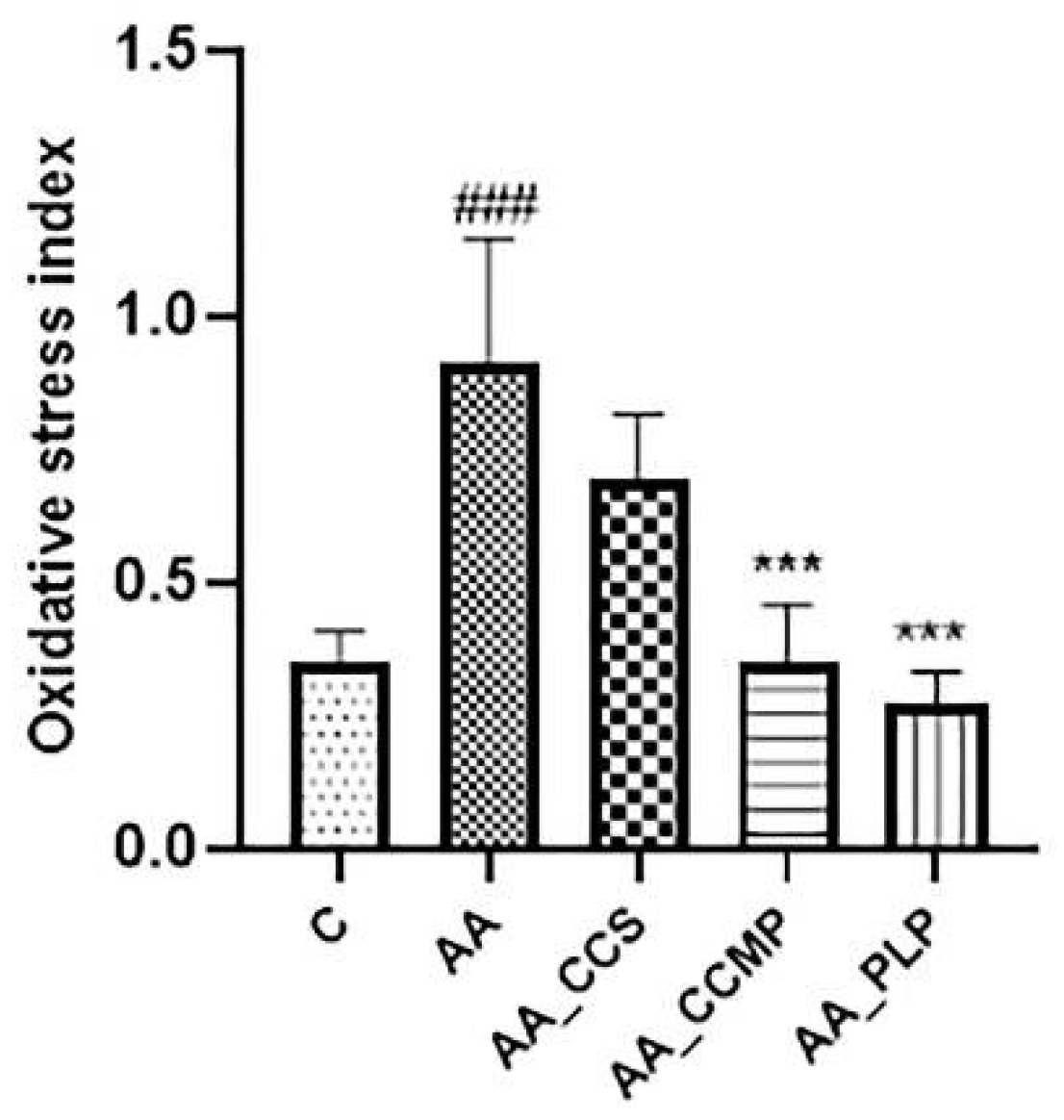
2.3. Evaluation of Oxidative Stress Index (OSI)
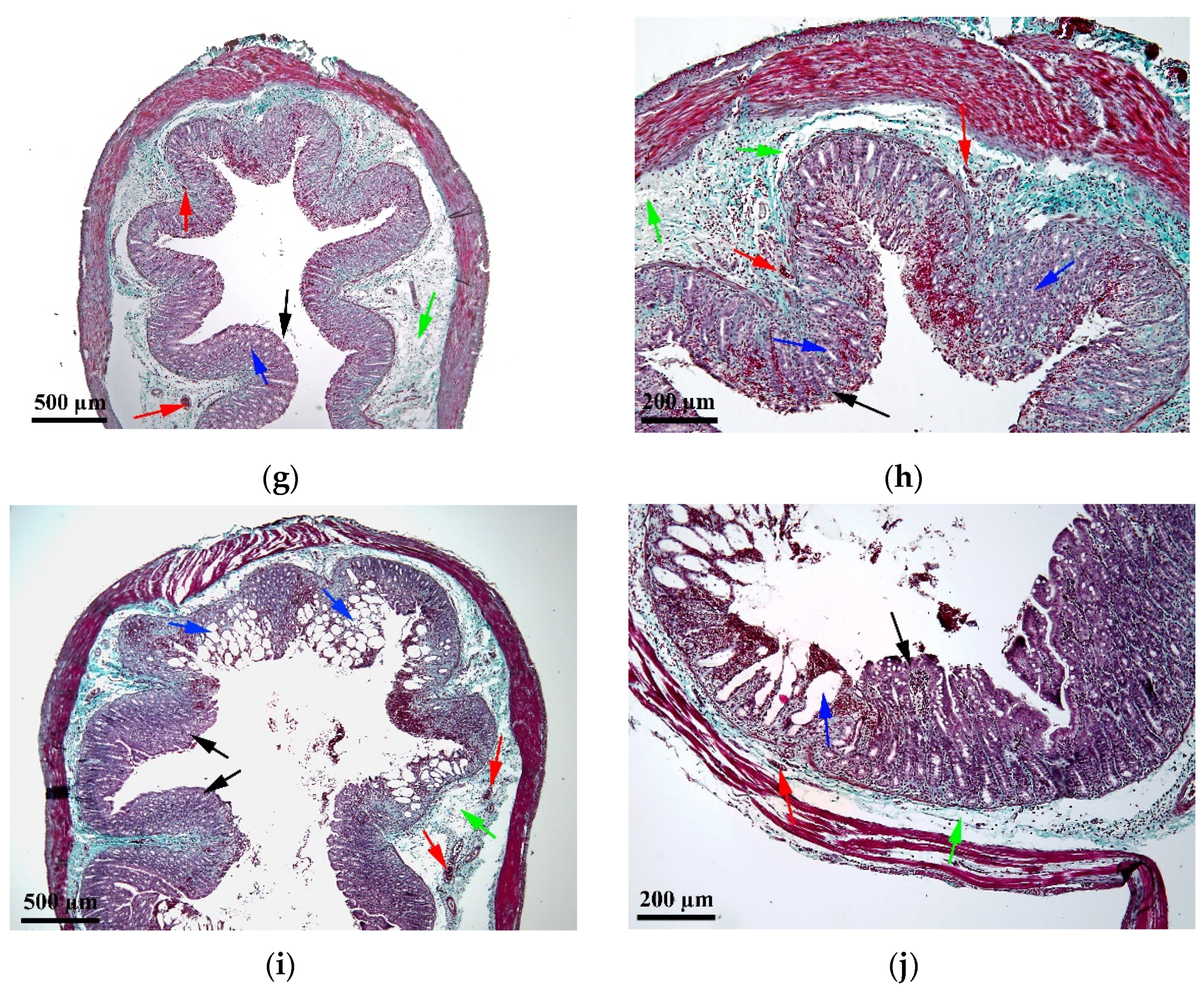
2.4. Histopathological Assessment
3. Discussion
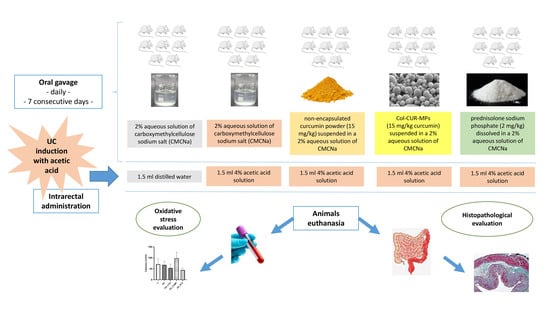
4. Materials and Methods
4.1. Reagents
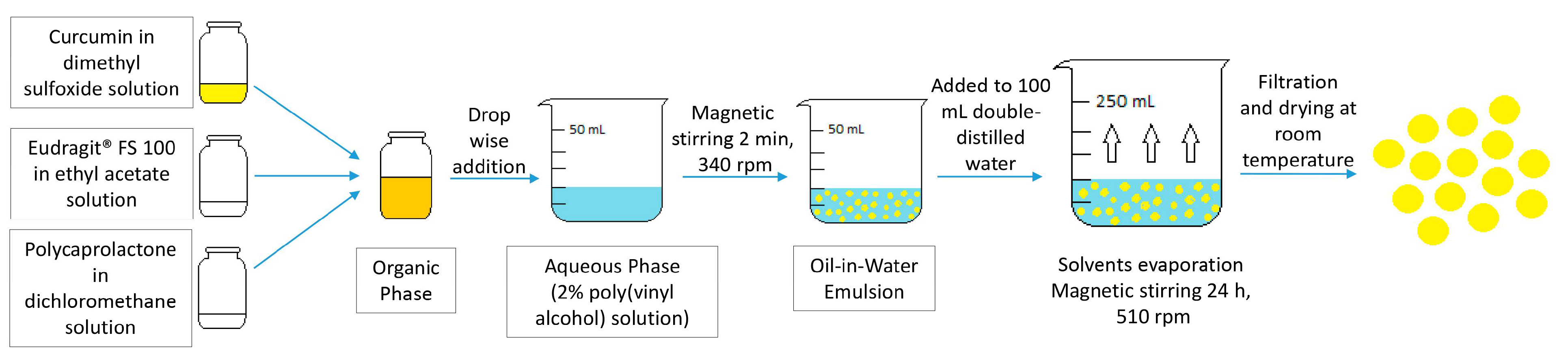
4.2. Preparation of Curcumin-Loaded Microspheres
4.3. Animals and Experimental Protocol
4.4. Evaluation of Plasma Antioxidant Response
4.4.1. Catalase (CAT)
4.4.2. Total Antioxidant Capacity (TAC)
4.5. Evaluation of Plasma Oxidative Stress
4.5.1. Total NO Metabolites (Nitrites and Nitrates, NOx)
4.5.2. Total Oxidant Status (TOS)
4.5.3. Lipid Peroxidation (Malondialdehyde, MDA)
4.6. Evaluation of Oxidative Stress Index (OSI)
4.7. Histopathological Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Akkol, E.K.; Dereli, F.T.G.; Taștan, H.; Sobarzo-Sánchez, E.; Khan, H. Effect of Sorbus domestica and its active constituents in an experimental model of colitis rats induced by acetic acid. J. Ethnopharmacol. 2020, 251, 112521. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gou, S.; Huang, Y.; Zhou, X.; Li, Q.; Han, M.K.; Kang, Y.; Xiao, B. Facile fabrication of bowl-shaped microparticles for oral curcumin delivery to ulcerative colitis tissue. Colloids Surf. B Biointerfaces 2018, 169, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.L.; Liu, G.X.; Chen, X.; Yang, K.; Yang, Y.X.; Xie, Q.; Gan, H.K.; Huang, X.L.; Gan, H.T. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int. Immunopharmacol. 2013, 17, 314–320. [Google Scholar] [CrossRef]
- Țiburcă, L.; Bembea, M.; Zaha, D.C.; Jurca, A.D.; Vesa, C.M.; Rațiu, I.A.; Jurca, C.M. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Curr. Issues Mol. Biol. 2022, 44, 1851–1866. [Google Scholar] [CrossRef]
- Liu, M.; Rao, H.; Liu, J.; Li, X.; Feng, W.; Gui, L.; Tang, H.; Xu, J.; Gao, W.Q.; Li, L. The histone methyltransferase SETD2 modulates oxidative stress to attenuate experimental colitis. Redox Biol. 2021, 43, 102004. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The Implications of Oxidative Stress and Antioxidant Therapies in Inflammatory Bowel Disease: Clinical Aspects and Animal Models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Ilktac, H.Y.; Kiziltan, G.; Ozansoy, M.; Kilic, U.; Togay, S.O.; Keskin, I.; Ozdemir, E.M.; Gunal, M.Y. The Effect of Probiotic and Omega-3 Supplements on Total Oxidant and Total Antioxidant Levels in Experimental Colitis. Clin. Exp. Health Sci. 2021, 11, 362–366. [Google Scholar] [CrossRef]
- Gupta, S.; Kunti, S.; Chatterjee, S.; Dutta, S.; Nath, S.; Nath Das, H. Oxidative stress index as a biochemical parameter in major depressive disorder. Asian J. Med. Sci. 2016, 7, 31–35. [Google Scholar] [CrossRef]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Rieux, A.; Préat, V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, A.G.; Cunningham, G.; Irving, P.M.; Samaan, M.A. Recent advances in monoclonal antibody therapy in IBD: Practical issues. Frontline Gastroenterol. 2019, 10, 409–416. [Google Scholar] [CrossRef]
- Archer, R.; Tappenden, P.; Ren, S.; Martyn-St James, M.; Harvey, R.; Basarir, H.; Stevens, J.; Carroll, C.; Cantrell, A.; Lobo, A.; et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): Clinical effectiveness systematic review and economic model. Health Technol. Assess. 2016, 20, 1–326. [Google Scholar] [CrossRef]
- Dorai, T.; Aggarwal, B.B. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004, 215, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Chen, Q.; Gou, S.; Ma, P.; Song, H.; Zhou, X.; Huang, Y.; Han, M.K.; Wan, Y.; Kang, Y.; Xiao, B. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy. Int. J. Pharm. 2019, 557, 135–144. [Google Scholar] [CrossRef]
- Tuntiyasawasdikul, S.; Sripanidkulchai, B. Development and clinical trials on anti-inflammatory effect of transdermal patch containing a combination of Kaempferia parviflora and Curcuma longa extracts. J. Drug Deliv. Sci. Technol. 2022, 68, 103093. [Google Scholar] [CrossRef]
- Thbayh, D.K.; Fiser, B. Computational study of synthetic and natural polymer additives—Antioxidant potential of BHA, TBHQ, BHT, and curcumin. Polym. Degrad. Stab. 2022, 201, 109979. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Huang, Y.; Ma, Y.; Lv, J.; Xiao, B. Mucus-penetrating polymeric nanoparticles for oral delivery of curcumin to inflamed colon tissue. J. Drug Deliv. Sci. Technol. 2019, 52, 157–164. [Google Scholar] [CrossRef]
- Xiao, B.; Si, X.; Zhang, M.; Merlin, D. Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids Surf. B Biointerfaces 2015, 135, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Shaal, L.; Müller, R.H.; Keck, C.M. Development of Curcumin Nanocrystal: Physical Aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kong, D.; Hu, Q.; Gao, N.; Pang, S. Evaluation of High-Performance Curcumin Nanocrystals for Pulmonary Drug Delivery Both In Vitro and In Vivo. Nanoscale Res. Lett. 2015, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.; Lee, J.; Naeem, M.; Hasan, N.; Kim, J.; Kim, H.K.; Lee, E.H.; Jung, Y.; Yoo, J.W. Curcumin Nanocrystal/pH-Responsive Polyelectrolyte Multilayer Core–Shell Nanoparticles for Inflammation-Targeted Alleviation of Ulcerative Colitis. Biomacromolecules 2020, 21, 3571–3581. [Google Scholar] [CrossRef]
- Akl, M.A.; Kartal-Hodzic, A.; Oksanen, T.; Ismael, H.R.; Afouna, M.M.; Yliperttula, M.; Samy, A.M.; Viitala, T. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016, 32, 10–20. [Google Scholar] [CrossRef]
- Hales, D.; Tefas, L.R.; Tomuță, I.; Moldovan, C.; Gulei, D.; Munteanu, R.; Porfire, A. Development of a Curcumin-Loaded Polymeric Microparticulate Oral Drug Delivery System for Colon Targeting by Quality-by-Design Approach. Pharmaceutics 2020, 12, 1027. [Google Scholar] [CrossRef]
- Catinean, A.; Neag, M.A.; Krishnan, K.; Muntean, D.M.; Bocsan, C.I.; Pop, R.M.; Mitre, A.O.; Melincovici, C.S.; Buzoianu, A.D. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients 2020, 12, 3607. [Google Scholar] [CrossRef]
- Wei, T.T.; Lin, Y.T.; Tseng, R.Y.; Shun, C.T.; Lin, Y.C.; Wu, M.S.; Fang, J.M.; Chen, C.C. Prevention of Colitis and Colitis-Associated Colorectal Cancer by a Novel Polypharmacological Histone Deacetylase Inhibitor. Clin. Cancer Res. 2016, 22, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.L.M.; Ferreira, A.V.M.; de Oliveira, M.C.; Rachid, M.A.; da Cunha Sousa, L.F.; Dos Santos Martins, F.; Gomes-Santos, A.C.; Vieira, A.T.; Teixeira, M.M. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice. Eur. J. Nutr. 2017, 56, 179–191. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/09/WC500132403.pdf (accessed on 10 August 2015).
- Sharma, I.; Ahmad, P. Chapter 4. Catalase. A Versatile Antioxidant in Plants. In Oxidative Damage to Plants. Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, A.; Jamehdar, S.A.; Eidgahi, M.R.A.; Ghazvini, K. Evaluation of the therapeutic effect of melittin peptide on the ulcerative colitis mouse model. Int. Immunopharmacol. 2022, 108, 108810. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.R.; Yuan, L.X.; Stryker, S.J.; Ratliff, P.; Telford, G.L.; Opara, E.C. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig. Dis. Sci. 2000, 45, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Peres Rubio, C.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Kao, N.J.; Hu, J.H.; Wu, C.S.; Kong, Z.W. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Int. Immunopharmacol. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef]
- Karimi, S.; Tabataba-Vakili, S.; Yari, Z.; Alborzi, F.; Hedayati, M.; Ebrahimi-Daryani, N.; Hekmatdoost, A. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J. 2019, 18, 16. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Porfire, A.; Bolboacă, S.D.; Nicula, C.A.; Feștilă, D.G.; Roman, A.; Râjnoveanu, R.M.; Râjnoveanu, A.; Dogaru, G.; Boarescu, P.M.; et al. Protective Effects of Liposomal Curcumin on Oxidative Stress/Antioxidant Imbalance, Metalloproteinases 2 and -9, Histological Changes and Renal Function in Experimental Nephrotoxicity Induced by Gentamicin. Antioxidants 2021, 10, 325. [Google Scholar] [CrossRef]
- Djordjević, A.; Kotnik, P.; Horvat, D.; Knez, Ž.; Antonič, M. Pharmacodynamics of malondialdehyde as indirect oxidative stress marker after arrested-heart cardiopulmonary bypass surgery. Biomed. Pharmacother. 2020, 132, 110877. [Google Scholar] [CrossRef]
- Pop, C.; Ștefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef]
- Porfire, A.S.; Leucuța, S.E.; Kiss, B.; Loghin, F.; Pârvu, A.E. Investigation into the role of Cu/Zn-SOD delivery system on its antioxidant and antiinflammatory activity in rat model of peritonitis. Pharmacol. Rep. 2014, 66, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Boarescu, P.M.; Porfire, A.S.; Dogaru, G.; Barbalata, C.; Valeanu, M.; Munteanu, C.; Râjnoveanu, R.M.; Nicula, C.A.; Stanescu, I.C. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants 2020, 9, 172. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Bolboacă, S.D.; Bulboacă, A.C.; Porfire, A.S.; Tefas, L.R.; Suciu, Ș.M.; Dogaru, G.; Stănescu, I.C. Liposomal Curcumin Enhances the Effect of Naproxen in a Rat Model of Migraine. Med. Sci. Monit. 2019, 25, 5087–5097. [Google Scholar] [CrossRef]
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.G.; Muntean, D.M.; Mirel, S.; Vostinaru, O.; Kiss, B.; Popa, D.S. Antitussive, Antioxidant, and Anti-Inflammatory Effects of a Walnut (Juglans regia L.) Septum Extract Rich in Bioactive Compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Bulboacă, A.E.; Bolboacă, S.D.; Stănescu, I.C.; Sfrângeu, C.A.; Porfire, A.S.; Tefas, L.; Bulboacă, A.C. The effect of intravenous administration of liposomal curcumin in addition to sumatriptan treatment in an experimental migraine model in rats. Int. J. Nanomed. 2018, 13, 3093–3103. [Google Scholar] [CrossRef]
- Licarete, E.; Rauca, V.F.; Luput, L.; Drotar, D.; Stejerean, I.; Patras, L.; Dume, B.; Toma, V.A.; Porfire, A.; Gherman, C.; et al. Overcoming Intrinsic Doxorubicin Resistance in Melanoma by Anti-Angiogenic and Anti-Metastatic Effects of Liposomal Prednisolone Phosphate on Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2968. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 23 June 2022).
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Negrea, G.; Rauca, V.F.; Meszaros, M.S.; Patras, L.; Luput, L.; Licarete, E.; Toma, V.A.; Porfire, A.; Muntean, D.; Sesarman, A.; et al. Active Tumor-Targeting Nano-formulations Containing Simvastatin and Doxorubicin Inhibit Melanoma Growth and Angiogenesis. Front. Pharmacol. 2022, 13, 870347. [Google Scholar] [CrossRef]
- Trolox Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trolox (accessed on 20 June 2022).
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [PubMed]
- Karasahin, T.; Alkan, H.; Satilmis, F.; Dursun, S.; Ozturk, C.; Bulut, G.; Aksoy, N.H.; Tekindal, M.A.; Caglayan, T.; Yesilkaya, O.F.; et al. Relationship between total antioxidant/oxidant status, and oxidative stress index and superovulation response in donor cows. Livest. Sci. 2021, 244, 104340. [Google Scholar] [CrossRef]
- Huțanu, E.; Damian, A.; Miclăuș, V.; Rațiu, I.A.; Rus, V.; Vlasiuc, I.; Gal, A.F. Morphometric Features and Microanatomy of the Lingual Filiform Papillae in the Wistar Rat. Biology 2022, 11, 920. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hales, D.; Muntean, D.-M.; Neag, M.A.; Kiss, B.; Ștefan, M.-G.; Tefas, L.R.; Tomuță, I.; Sesărman, A.; Rațiu, I.-A.; Porfire, A. Curcumin-Loaded Microspheres Are Effective in Preventing Oxidative Stress and Intestinal Inflammatory Abnormalities in Experimental Ulcerative Colitis in Rats. Molecules 2022, 27, 5680. https://doi.org/10.3390/molecules27175680
Hales D, Muntean D-M, Neag MA, Kiss B, Ștefan M-G, Tefas LR, Tomuță I, Sesărman A, Rațiu I-A, Porfire A. Curcumin-Loaded Microspheres Are Effective in Preventing Oxidative Stress and Intestinal Inflammatory Abnormalities in Experimental Ulcerative Colitis in Rats. Molecules. 2022; 27(17):5680. https://doi.org/10.3390/molecules27175680
Chicago/Turabian StyleHales, Dana, Dana-Maria Muntean, Maria Adriana Neag, Béla Kiss, Maria-Georgia Ștefan, Lucia Ruxandra Tefas, Ioan Tomuță, Alina Sesărman, Ioana-Adela Rațiu, and Alina Porfire. 2022. "Curcumin-Loaded Microspheres Are Effective in Preventing Oxidative Stress and Intestinal Inflammatory Abnormalities in Experimental Ulcerative Colitis in Rats" Molecules 27, no. 17: 5680. https://doi.org/10.3390/molecules27175680
APA StyleHales, D., Muntean, D.-M., Neag, M. A., Kiss, B., Ștefan, M.-G., Tefas, L. R., Tomuță, I., Sesărman, A., Rațiu, I.-A., & Porfire, A. (2022). Curcumin-Loaded Microspheres Are Effective in Preventing Oxidative Stress and Intestinal Inflammatory Abnormalities in Experimental Ulcerative Colitis in Rats. Molecules, 27(17), 5680. https://doi.org/10.3390/molecules27175680