Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review
Abstract
:1. Introduction
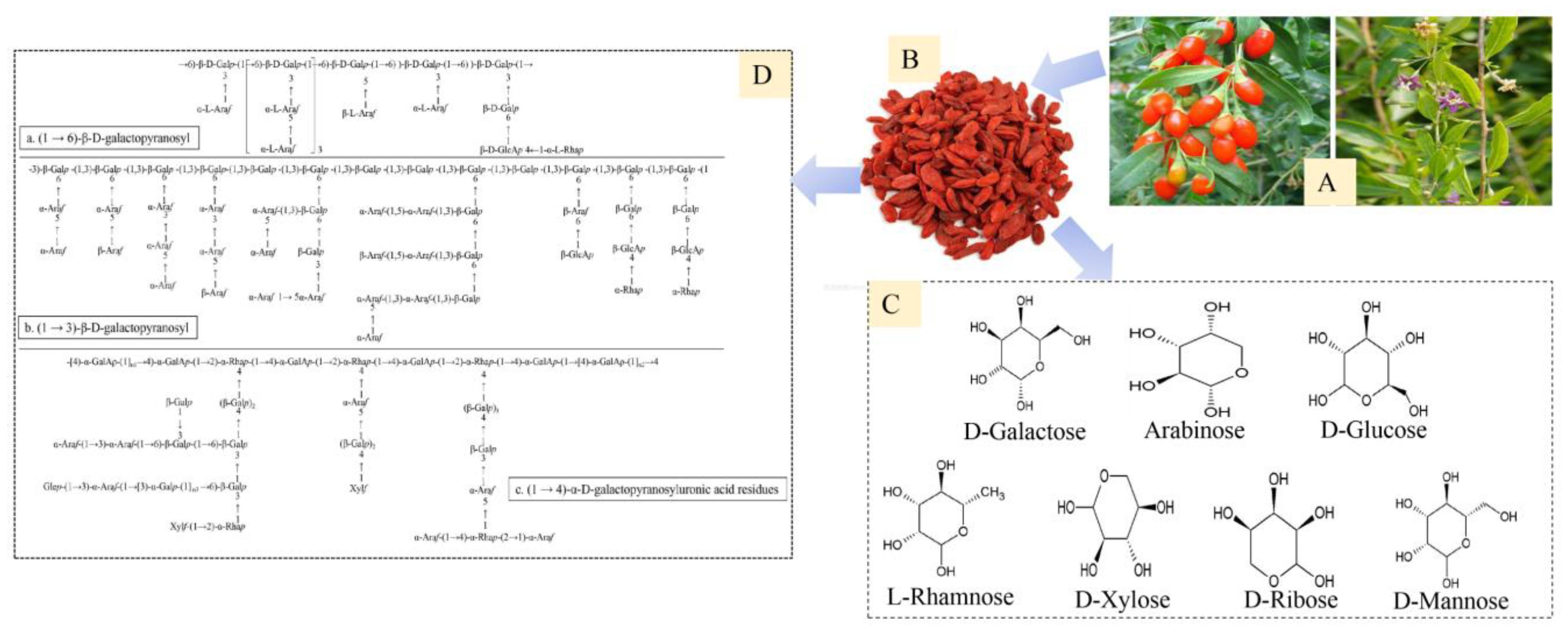
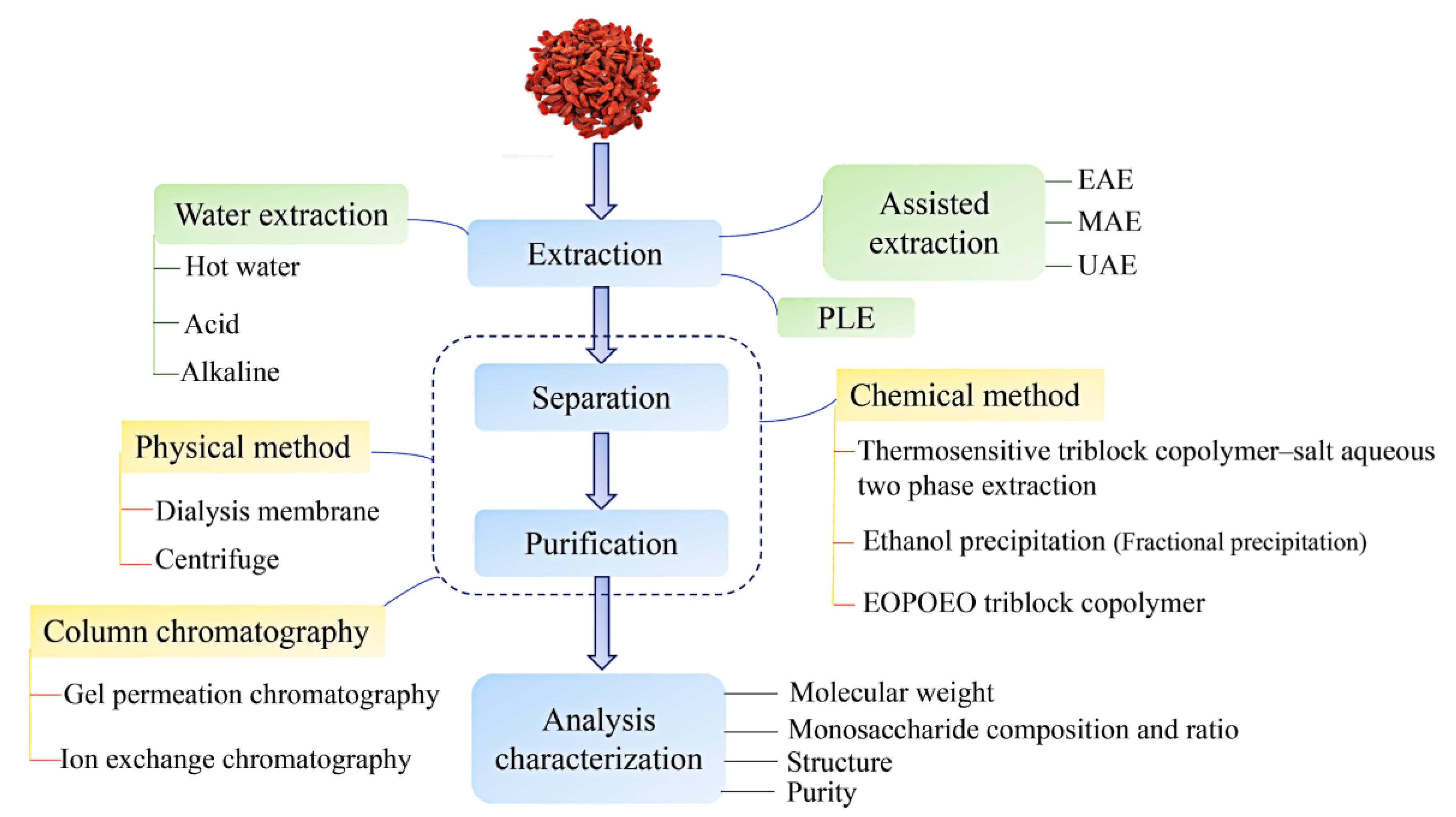
2. Bioinformatics of LBPs
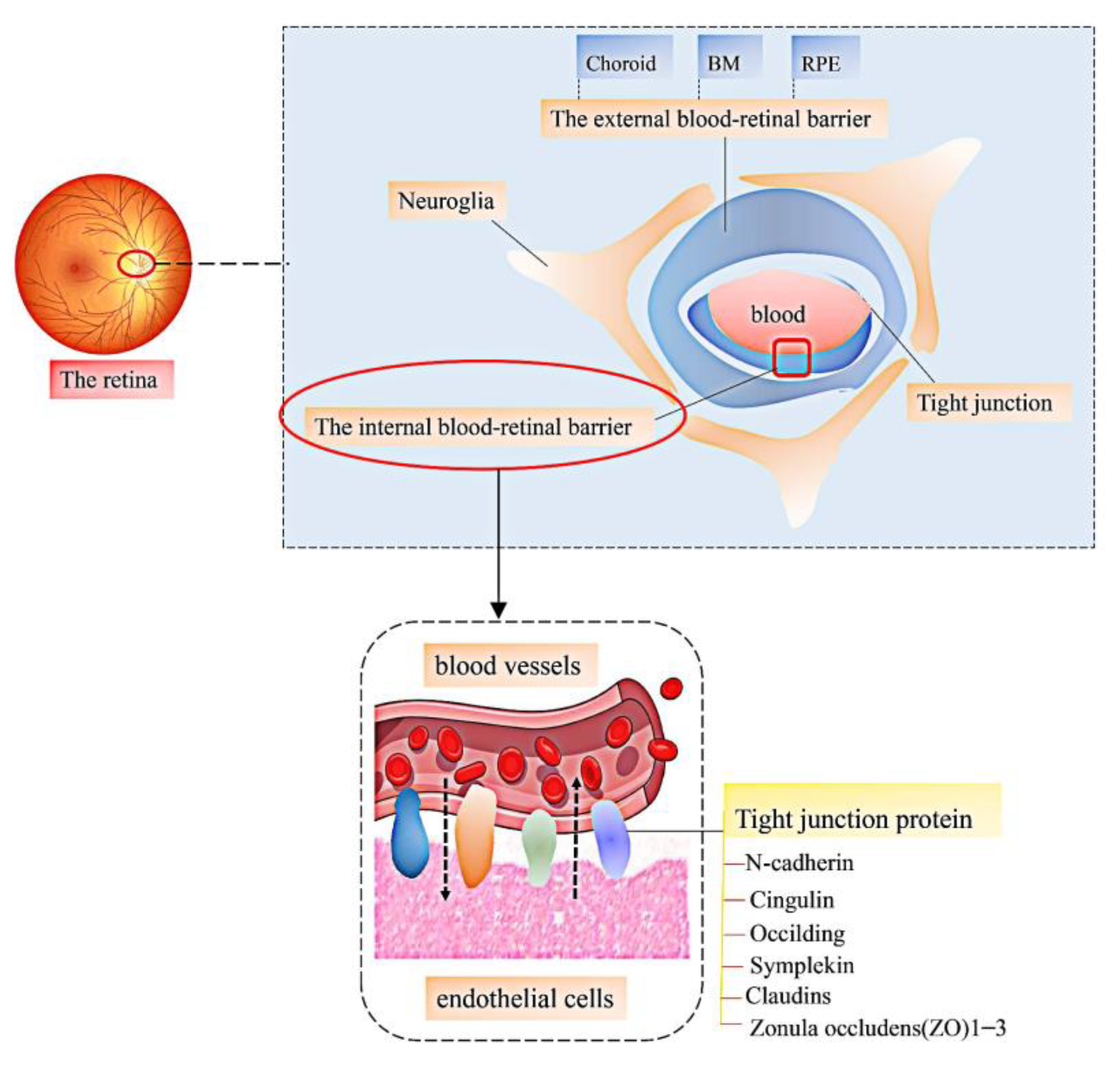
3. LBPs and Vascular Retinopathy
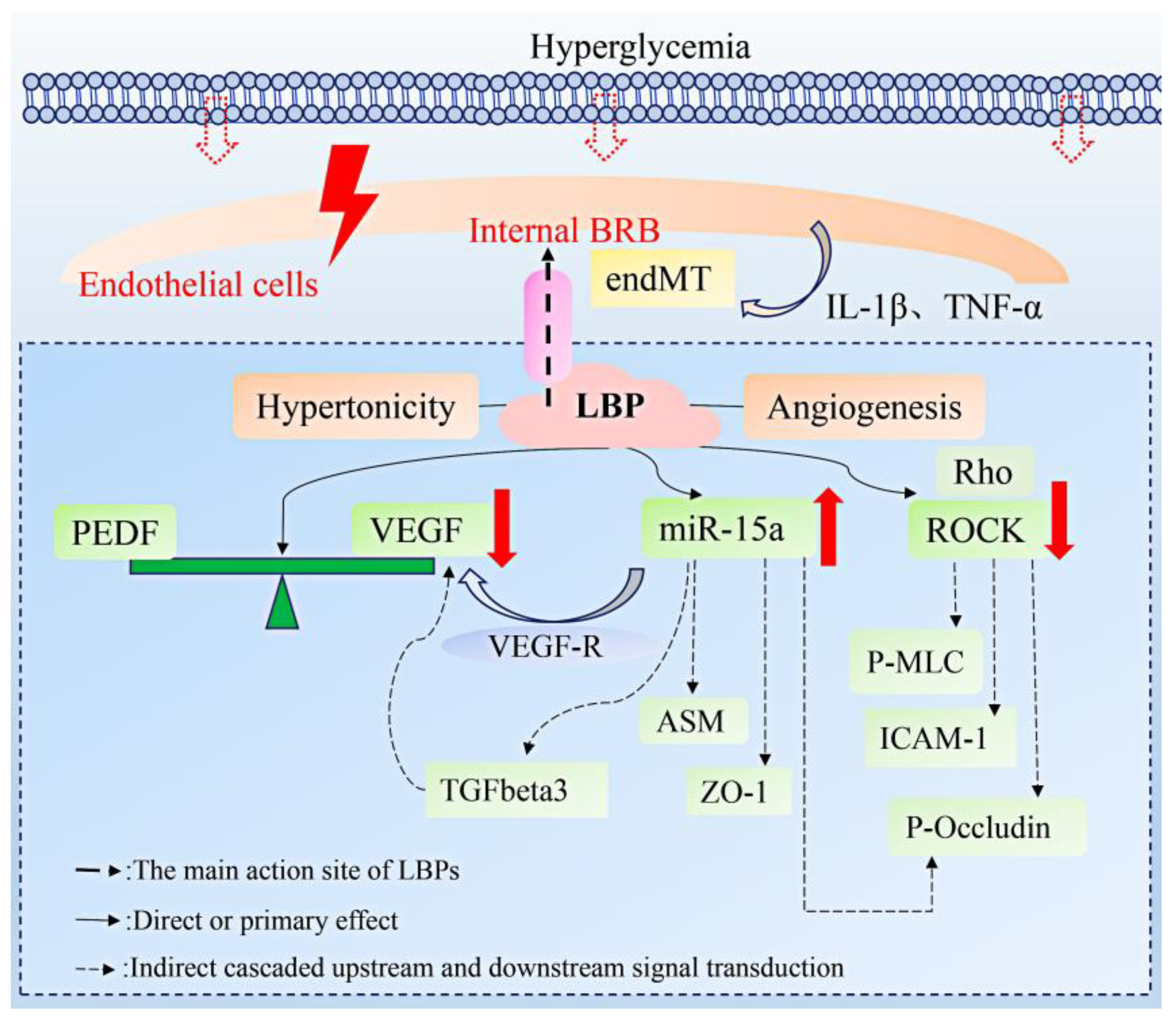
3.1. Diabetes Retinopathy
3.2. Age-Related Macular Degeneration
3.3. Hypertensive Retinopathy
3.4. Ischemia-Reperfusion Injury
4. Pharmacokinetics
5. Application Prospect
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Global Health Data Exchange. Global Burden of Disease Study 2019 Data Resources [EB/OL]. Available online: http://ghdx.healthdata.org/gbd-2019 (accessed on 20 December 2021).
- Chen, B.; Lou, L.; Ye, J. Eye diseases burden in China in the past 30 years. J. Zhejiang Univ. Med. Sci. 2021, 50, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, Y.C. Retinal vascular development in an immature retina at 33–34 weeks postmenstrual age predicts retinopathy of prematurity. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Kaczmarek, R.; Gajdzis, P.; Gajdzis, M. Eph Receptors and Ephrins in Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 6207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Tao, J.W.; Li, L.; Mao, J.B.; Zhu, C.T.; Lao, J.M.; Yang, Y.; Shen, L.-J. Feasibility study on robot-assisted retinal vascular bypass surgery in an ex vivo porcine model. Acta Ophthalmol. 2017, 95, e462–e467. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Q.; Wang, H.; Hu, Y.; Ren, B.; Li, X. Recent advances in plant polysaccharide-mediated nano drug delivery systems. Int. J. Biol. Macromol. 2020, 165, 2668–2683. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii Boiss. Carbohydr. Polym. 2020, 248, 116783. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Lelekami, A.K.; Khedmat, L. Plant/algal polysaccharides extracted by microwave: A review on hypoglycemic, hypolipidemic, prebiotic, and immune-stimulatory effect. Carbohydr. Polym. 2021, 266, 118134. [Google Scholar] [CrossRef]
- Sen, I.K.; Chakraborty, I.; Mandal, A.K.; Bhanja, S.K.; Patra, S.; Maity, P. A review on antiviral and immunomodulatory polysaccharides from Indian medicinal plants, which may be beneficial to COVID-19 infected patients. Int. J. Biol. Macromol. 2021, 181, 462–470. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2021, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Lin, C.-Y.; Hsu, C.-L.; Fan, K.-H.; Huang, S.-F.; Liao, C.-T.; Lee, L.-Y.; Ng, S.-K.; Yen, T.-C.; Chang, J.T.-C.; et al. Incorporation of Astragalus polysaccharides injection during concurrent chemoradiotherapy in advanced pharyngeal or laryngeal squamous cell carcinoma: Preliminary experience of a phase II double-blind, randomized trial. J. Cancer Res. Clin. Oncol. 2019, 146, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydrates Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.-L.; Lam, H.-I.; Choi, K.-Y.; Li, S.Z.-C.; Lakshmanan, Y.; Yu, W.-Y.; Chang, R.C.-C.; Lai, J.S.-M.; So, K.-F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Gong, G.; Song, S.; Ma, F.; Huang, L.; et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019, 10, 4231–4241. [Google Scholar] [CrossRef]
- Wong, H.L.; Bu, Y.; Chan, Y.K.; Shih, K.C. Lycium barbarum polysaccharide promotes corneal Re-epithelialization after alkaline injury. Exp. Eye Res. 2022, 221, 109007. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Medica 2009, 76, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Liu, J.; Cheng, H.; Peng, D.; Xing, L.; Shi, S.; Yu, N. Determination of monosaccharides in Lycium barbarum fruit polysaccharide by an efficient UHPLC-QTRAP-MS/MS method. Phytochem. Anal. 2021, 32, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; de Oliveira, W.F.; Silva, P.M.D.S.; Correia, M.T.D.S.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2021, 277, 118824. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Dong, H.; Ma, C.; Qiao, Y.; Zheng, Z. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: A comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef]
- Hao, W.; Wang, S.-F.; Zhao, J.; Li, S.-P. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef]
- Gong, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.L.; Zou, Z.H.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.J.; Wang, Z.F. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. In. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Han, J.; Ni, L.; Tang, X.; Hu, Y.; Chen, T. Integrated method of thermosensitive triblock copolymer–salt aqueous two phase extraction and dialysis membrane separation for purification of lycium barbarum polysaccharide. Food Chem. 2016, 194, 257–264. [Google Scholar] [CrossRef]
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 2021, 229, 107921. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; So, K.-F.; Lo, A.C. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin. Exp. Ophthalmol. 2017, 45, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-L.; Li, Y.-X.; Ma, J.-M.; Guo, Y.-Q.; Li, L.; Gao, Q.-H.; Fan, Y.-N.; Zhang, M.-W.; Tao, X.-J.; Yu, J.-Q.; et al. Effect of Lycium barbarum polysaccharide supplementation in non-alcoholic fatty liver disease patients: Study protocol for a randomized controlled trial. Trials 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.; Lo, A.C.-Y.; Chan, T.C.-Y.; So, K.F.; Lai, J.S.-M.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium barbarum Polysaccharides in Disease. BioMed Res. Int. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef]
- Smith, R.O.; Ninchoji, T.; Gordon, E.; André, H.; Dejana, E.; Vestweber, D.; Kvanta, A.; Claesson-Welsh, L. Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin. eLife 2020, 9, e54056. [Google Scholar] [CrossRef]
- Dou, X.; Duerfeldt, A.S. Small-Molecule Modulation of PPARs for the Treatment of Prevalent Vascular Retinal Diseases. Int. J. Mol. Sci. 2020, 21, 9251. [Google Scholar] [CrossRef]
- Sheskey, S.R.; Antonetti, D.A.; Rentería, R.C.; Lin, C.-M. Correlation of Retinal Structure and Visual Function Assessments in Mouse Diabetes Models. Investig. Opthalmology Vis. Sci. 2021, 62, 20. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, N.; Masai, I. Mechanisms underlying microglial colonization of developing neural retina in zebrafish. eLife 2021, 10, e70550. [Google Scholar] [CrossRef] [PubMed]
- Jäckle, A.; Ziemssen, F.; Kuhn, E.-M.; Kampmeier, J.; Lang, G.K.; Lang, G.E.; Deissler, H.; Deissler, H.L. Sitagliptin and the Blood-Retina Barrier: Effects on Retinal Endothelial Cells Manifested Only after Prolonged Exposure. J. Diabetes Res. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.K.; Qi, X.; Goldwaser, E.L.; A Godsey, G.; Wu, H.; Kosciuk, M.C.; A Freeman, T.; Macphee, C.H.; Wilensky, R.L.; Venkataraman, V.; et al. Retinal pathology is associated with increased blood–retina barrier permeability in a diabetic and hypercholesterolaemic pig model: Beneficial effects of the LpPLA2 inhibitor Darapladib. Diabetes Vasc. Dis. Res. 2017, 14, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Kurauchi, Y.; Asano, D.; Morita, A.; Sakamoto, K.; Nakahara, T. Pharmacological inhibition of Na+/K+-ATPase induces neurovascular degeneration and glial cell alteration in the rat retina. Exp. Eye Res. 2022, 220, 109107. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, Y.; Lu, X.; Zhang, Q.; Luo, M.; Li, P.A.; Pan, Y. Lycium barbarum Polysaccharides Improve Retinopathy in Diabetic Sprague-Dawley Rats. Evid.-Based Complement. Alternat. Med. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Jiang, Y. Lycium barbarum polysaccharides attenuates high glucose-induced diabetic retinal angiogenesis by rescuing the expression of miR-15a-5p in RF/6A cells. J. Ethnopharmacol. 2021, 283, 114652. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Y.; Liu, X.; Wang, K.; Zhou, Q.; Tang, Y. Protective effects of lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am. J. Transl. Res. 2019, 11, 6304–6315. [Google Scholar]
- Zhou, J.; Wang, F.; Jia, L.; Chai, R.; Wang, H.; Wang, X.; Li, J.; Wang, K.; Zhang, P.; Yang, H. 2,4-dichlorophenoxyacetic acid induces ROS activation in NLRP3 inflammatory body-induced autophagy disorder in microglia and the protective effect of Lycium barbarum polysaccharide. Environ. Toxicol. 2022, 1136–1151. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Huang, J.; Li, W.-J.; Yu, Y. Effects of Lycium barbarum polysaccharide on the photoinduced autophagy of retinal pigment epithelium cells. Int. J. Ophthalmol. 2022, 15, 23–30. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, Q.; Zhu, Q.; He, X.; Gao, M.; Wang, Y. Lycium barbarum polysaccharide protects ARPE-19 cells against H2O2-induced oxidative stress via the Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- So, K.-F.; Mi, X.-S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.-C.; Chung, S.K. Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen. Res. 2020, 15, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, T.; Wan, F.; Wang, J.; Li, X.; Li, W.; Ma, L. Structural characterization of a polysaccharide from Lycium barbarum and its neuroprotective effect against β-amyloid peptide neurotoxicity. Int. J. Biol. Macromol. 2021, 176, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, Y.; Wong, F.S.-Y.; Yu, W.-Y.; Li, S.Z.-C.; Choi, K.-Y.; So, K.-F.; Chan, H.H.-L. Lycium Barbarum Polysaccharides Rescue Neurodegeneration in an Acute Ocular Hypertension Rat Model Under Pre- and Posttreatment Conditions. Investig. Opthalmology Vis. Sci. 2019, 60, 2023–2033. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.-C.; So, K.-F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 Antioxidant Pathway Contributes to the Protective Effects of Lycium Barbarum Polysaccharides in the Rodent Retina after Ischemia-Reperfusion-Induced Damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Cui, Z.; Wang, S. CD93 and GIPC expression and localization during central nervous system inflammation. Neural Regen. Res. 2014, 9, 1995–2001. [Google Scholar] [CrossRef]
- Pang, B.; Ni, Q.; Di, S.; Du, L.-J.; Qin, Y.-L.; Li, Q.-W.; Li, M.; Tong, X.-L. Luo Tong Formula Alleviates Diabetic Retinopathy in Rats Through Micro-200b Target. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Q.; Zheng, F.; Wei, Y.; Wu, Q. Investigation of Influencing Factors on the Prevalence of Retinopathy in Diabetic Patients Based on Medical Big Data. Comput. Math. Methods Med. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, H.; Bao, S.; Wang, N.; Gillies, M.C. Diabetic macular edema: New concepts in patho-physiology and treatment. Cell Biosci. 2014, 4, 27. [Google Scholar] [CrossRef]
- El Rami, H.; Barham, R.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Retinopathy. Semin. Ophthalmol. 2016, 32, 67–74. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Honoré, B.; Vorum, H. A Review: Proteomics in Retinal Artery Occlusion, Retinal Vein Occlusion, Diabetic Retinopathy and Acquired Macular Disorders. Int. J. Mol. Sci. 2017, 18, 907. [Google Scholar] [CrossRef] [PubMed]
- Mahjoob, G.; Ahmadi, Y.; Fatima Rajani, H.; Khanbabaei, N.; Abolhasani, S. Circulating microRNAs as predictive biomarkers of coronary artery diseases in type 2 diabetes patients. J Clin Lab Anal. 2022, 36, e24380. [Google Scholar] [CrossRef]
- Kady, N.; Yan, Y.; Salazar, T.; Wang, Q.; Chakravarthy, H.; Huang, C.; Beli, E.; Navitskaya, S.; Grant, M.; Busik, J. Increase in acid sphingomyelinase level in human retinal endothelial cells and CD34+ circulating angiogenic cells isolated from diabetic individuals is associated with dysfunctional retinal vasculature and vascular repair process in diabetes. J. Clin. Lipidol. 2017, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Navitskaya, S.; Chakravarthy, H.; Huang, C.; Kady, N.; Lydic, T.A.; Chen, Y.E.; Yin, K.-J.; Powell, F.L.; Martin, P.M.; et al. Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy. eBioMedicine 2016, 11, 138–150. [Google Scholar] [CrossRef]
- Ye, E.-A.; Liu, L.; Steinle, J.J. miR-15a/16 inhibits TGF-beta3/VEGF signaling and increases retinal endothelial cell barrier proteins. Vis. Res. 2017, 139, 23–29. [Google Scholar] [CrossRef]
- Yemanyi, F.; Bora, K.; Blomfield, A.K.; Wang, Z.; Chen, J. Wnt Signaling in Inner Blood–Retinal Barrier Maintenance. Int. J. Mol. Sci. 2021, 22, 11877. [Google Scholar] [CrossRef]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell. Signal. 2019, 66, 109485. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, C.; Yang, Q.; Xie, H.; Liu, D.; Tian, H.; Lu, L.; Xu, J.; Li, W.; Xu, G.; et al. Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-κB pathway in diabetic retinopathy. J. Cell. Physiol. 2021, 236, 5848–5864. [Google Scholar] [CrossRef]
- Qin, Y.-J.; Xiao, K.; Zhong, Z.; Zhao, Y.; Yu, T.; Sun, X.-F. LECT2 Ameliorates Blood–Retinal Barrier Impairment Secondary to Diabetes Via Activation of the Tie2/Akt/mTOR Signaling Pathway. Investig. Opthalmol. Vis. Sci. 2022, 63, 7. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; De Hertogh, G.; Eynde, K.V.D.; Alam, K.; Van Raemdonck, K.; Opdenakker, G.; Van Damme, J.; Geboes, K.; Struyf, S. Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT). Exp. Eye Res. 2015, 132, 179–189. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood–Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int. J. Mol. Sci. 2020, 21, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lu, X.; Wei, L.; Ye, D.; Lin, J.; Tang, X.; Cui, K.; Yu, S.; Xu, Y.; Liang, X. PHD2 attenuates high-glucose-induced blood retinal barrier breakdown in human retinal microvascular endothelial cells by regulating the Hif-1α/VEGF pathway. Agents Actions 2021, 71, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Nakao, S.; Arima, M.; Wada, I.; Kaizu, Y.; Hao, F.; Yoshida, S.; Sonoda, K.-H. Rho-Kinase/ROCK as a Potential Drug Target for Vitreoretinal Diseases. J. Ophthalmol. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Paulus, Y.M.; Sodhi, A. Anti-angiogenic Therapy for Retinal Disease. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2016; pp. 271–307. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Fields, M.A.; Del Priore, L.V.; Adelman, R.A.; Rizzolo, L.J. Interactions of the choroid, Bruch’s membrane, retinal pigment epithelium, and neurosensory retina collaborate to form the outer blood-retinal-barrier. Prog. Retin. Eye Res. 2019, 76, 100803. [Google Scholar] [CrossRef]
- Tangeman, J.A.; Pérez-Estrada, J.R.; Van Zeeland, E.; Liu, L.; Danciutiu, A.; Grajales-Esquivel, E.; Smucker, B.; Liang, C.; Del Rio-Tsonis, K. A Stage-Specific OTX2 Regulatory Network and Maturation-Associated Gene Programs Are Inherent Barriers to RPE Neural Competency. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, H.; He, D.; Wang, Y.; Cai, B.; Chen, J.; Ma, J.; Liu, Z.; Wu, Y. Retinal Pigment Epithelium Cell Death Is Associated With NLRP3 Inflammasome Activation by All-trans Retinal. Investig. Opthalmology Vis. Sci. 2019, 60, 3034–3045. [Google Scholar] [CrossRef]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.-D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef]
- Yang, M.; So, K.-F.; Lo, A.C.Y.; Lam, W.C. The Effect of Lycium barbarum Polysaccharides on Pyroptosis-Associated Amyloid β1-40 Oligomers-Induced Adult Retinal Pigment Epithelium 19 Cell Damage. Int. J. Mol. Sci. 2020, 21, 4658. [Google Scholar] [CrossRef]
- Liu, L.; Lao, W.; Ji, Q.-S.; Yang, Z.-H.; Yu, G.-C.; Zhong, J.-X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Dey, S.; Sim, R.; Lee, J.; Eong, K.-G.A. Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases. Nutrients 2021, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Tsujinaka, H.; Hirai, H.; Yamashita, M.; Ueda, T.; Ogata, N. Effects of concentration of amyloid β (Aβ) on viability of cultured retinal pigment epithelial cells. BMC Ophthalmol. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Liong, E.C.; So, K.-F.; Tipoe, G.L. Lycium barbarum polysaccharides improve hepatic injury through NFkappa-B and NLRP3/6 pathways in a methionine choline deficient diet steatohepatitis mouse model. Int. J. Biol. Macromol. 2018, 120, 1480–1489. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Zhang, H.-D.; Liu, X.-Y.; Xu, Y. Attenuation of hyperoxic acute lung injury by Lycium barbarum polysaccharide via inhibiting NLRP3 inflammasome. Arch. Pharmacal Res. 2019, 42, 902–908. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.-H.; Won, Y.; Lee, M.-W.; Shin, Y.-I.; Jo, Y.-J.; Kim, J.-Y. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Kupersmith, M.J.; Sibony, P.A. Retinal and Optic Nerve Deformations Due to Orbital Versus Intracranial Venous Hypertension. J. Neuro-Ophthalmology 2020, 41, 321–328. [Google Scholar] [CrossRef]
- Matteucci, A.; Ricceri, L.; Fabbri, A.; Fortuna, A.; Travaglione, S.; Guidotti, M.; Martinelli, A.; Villa, M.; Pricci, F.; Maroccia, Z.; et al. Eye Drop Instillation of the Rac1 Modulator CNF1 Attenuates Retinal Gliosis and Ameliorates Visual Performance in a Rat Model of Hypertensive Retinopathy. Neuroscience 2019, 411, 119–129. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, Q.; Zeng, Y.; Zhang, Y.; Zhang, M. Regulations of Retinal Inflammation: Focusing on Müller Glia. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef]
- Halder, S.K.; Milner, R. The GFAP Monoclonal Antibody GA-5 Identifies Astrocyte Remodeling and Glio-Vascular Uncoupling During the Evolution of EAE. Cell. Mol. Neurobiol. 2021, 42, 1615–1622. [Google Scholar] [CrossRef]
- Huang, R.; Liang, S.; Fang, L.; Wu, M.; Cheng, H.; Mi, X.; Ding, Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018, 24, 367–378. [Google Scholar] [PubMed]
- Chiu, K.; Chan, H.-C.; Yeung, S.-C.; Yuen, W.-H.; Zee, S.-Y.; Chang, R.C.-C.; So, K.-F. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J. Ocul. Biol. Dis. Informatics 2009, 2, 127–136. [Google Scholar] [CrossRef]
- Bie, M.; Lv, Y.; Ren, C.; Xing, F.; Cui, Q.; Xiao, J.; So, K.F. Lycium barbarum Polysaccharide Improves Bipolar Pulse Current-Induced Microglia Cell Injury Through Modulating Autophagy. Cell Transplant. 2015, 24, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Verkman, A.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef]
- Shah, Z.A.; Li, R.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef]
- Li, S.-Y.; Yang, D.; Yeung, C.-M.; Yu, W.Y.; Chang, R.C.-C.; So, K.-F.; Wong, D.; Lo, A.C.Y. Lycium Barbarum Polysaccharides Reduce Neuronal Damage, Blood-Retinal Barrier Disruption and Oxidative Stress in Retinal Ischemia/Reperfusion Injury. PLoS ONE 2011, 6, e16380. [Google Scholar] [CrossRef]
- Koide, R.; Xi, J.; Hamanaka, Y.; Shiga, S. Mapping PERIOD-immunoreactive cells with neurons relevant to photoperiodic response in the bean bug Riptortus pedestris. Cell Tissue Res. 2021, 385, 571–583. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Heneberg, P.; Mao, Y.; Jiang, Q.; Loor, J.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2021, 100. [Google Scholar] [CrossRef]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Zhou, L.; Huang, R. Fraction From Lycium barbarum Polysaccharides Reduces Immunotoxicity and Enhances Antitumor Activity of Doxorubicin in Mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Jin, H.; Yang, J.; Kang, S.; Liu, Y.; Yang, S.; Ma, S.; Ni, J. Effects of Lycium barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Pan, X.; Xu, J.; Wu, Z.; Zhang, Y.; Wang, K. Advances in tracking of polysaccharides in vivo: Labeling strategies, potential factors and applications based on pharmacokinetic characteristics. Int. J. Biol. Macromol. 2020, 163, 1403–1420. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, H.; Tang, H.; Yang, L.; Sun, G. Tissue distribution of Lycium barbarum polysaccharides in rat tissue by fluorescein isothiocyanate labeling. Food Sci. Hum. Wellness 2022, 11, 837–844. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Ji, T.; Wen, C.; Ye, Z.; Liu, X.; Liang, L.; Liu, G.; Xu, X. Digestion and absorption properties of Lycium barbarum polysaccharides stabilized selenium nanoparticles. Food Chem. 2021, 373, 131637. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2018, 125, 751–760. [Google Scholar] [CrossRef]
- Xia, H.; Yang, C.; Zhou, B.; Tang, H.; Yang, L.; Liao, W.; Sun, G. Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods 2021, 10, 2851. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef]
- Chen, R.; Lin, M.L.; Wang, H.; Zhai, M.X.; Liang, Y.; Zhao, B.; Yu, M.Z.; Li, M.K.; Shen, W. Effects of Morphologies of Thermosensitive Electrospun Nanofibers on Controllable Drug Release. Tissue Eng. Part A 2021, 27, 724–732. [Google Scholar] [CrossRef]
- Bacanlı, M.; Eşim, Ö.; Erdoğan, H.; Sarper, M.; Erdem, O.; Özkan, Y. Evaluation of cytotoxic and genotoxic effects of paclitaxel-loaded PLGA nanoparticles in neuroblastoma cells. Food Chem. Toxicol. 2021, 154, 112323. [Google Scholar] [CrossRef]
- Soleimannejad, M.; Ebrahimi-Barough, S.; Nadri, S.; Riazi-Esfahani, M.; Soleimani, M.; Tavangar, S.M.; Ai, J. Retina tissue engineering by conjunctiva mesenchymal stem cells encapsulated in fibrin gel: Hypotheses on novel approach to retinal diseases treatment. Med Hypotheses 2017, 101, 75–77. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; He, L.; Chen, N.; Ramakrishna, S.; So, K.-F.; Mo, X. Lycium barbarum polysaccharide encapsulated Poly lactic-co-glycolic acid Nanofibers: Cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci. Rep. 2018, 8, 8669. [Google Scholar] [CrossRef]
- Bo, R.; Liu, Z.; Zhang, J.; Gu, P.; Ou, N.; Sun, Y.; Hu, Y.; Liu, J.; Wang, D. Mechanism of Lycium barbarum polysaccharides liposomes on activating murine dendritic cells. Carbohydr. Polym. 2018, 205, 540–549. [Google Scholar] [CrossRef]
- Bo, R.; Zheng, S.; Xing, J.; Luo, L.; Niu, Y.; Huang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016, 85, 294–301. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hsueh, H.T.; Shin, M.D.; Berlinicke, C.A.; Han, H.; Anders, N.M.; Hemingway, A.; Leo, K.T.; Chou, R.T.; Kwon, H.; et al. A hypotonic gel-forming eye drop provides enhanced intraocular delivery of a kinase inhibitor with melanin-binding properties for sustained protection of retinal ganglion cells. Drug Deliv. Transl. Res. 2021, 12, 826–837. [Google Scholar] [CrossRef]
- Deguchi, S.; Ogata, F.; Yamaguchi, M.; Minami, M.; Otake, H.; Kanai, K.; Kawasaki, N.; Nagai, N. In Situ Gel Incorporating Disulfiram Nanoparticles Rescues the Retinal Dysfunction via ATP Collapse in Otsuka Long–Evans Tokushima Fatty Rats. Cells 2020, 9, 2171. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shetty, K.H.; Desai, D.T.; Shah, D.O.; Willcox, M.D. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. Int. J. Pharm. 2021, 608, 121105. [Google Scholar] [CrossRef]

| The Experimental Model | Dosage of Administration | Application | Mechanism/Pathway (Major Molecular Change) | Reference |
|---|---|---|---|---|
| STZ induced -Male Sprague-Dawley rats (250 ± 20 g) | 200, 400 mg/kg/d orally for 20 weeks | in vivo | Decreasing the immune intensity of GFAP and VEGF overexpression, increasing PEDF expression | [47] |
| Monkey retinal vascular endothelial (RF/6A) cells | 600 mg/L for 48 h | in vitro | Decreasing VEGFA, VEGFR2, ANG2, ASM mRNA, and protein expression while increasing ANG1 protein expression | [48] |
| STZ induced-diabetic rat (8–12 weeks, 180–220 g) | 250 mg/kg/d for 12 weeks | in vivo | Increasing P-occludin, down-regulating ROCK1, and P-MLC | [49] |
| BV2 cells | 300 μg/mL | in vitro | Significantly reducing NLRP3, cleaved caspase-1, IL-1β, IL-18, and P62 | [50] |
| ARPE-19 cells | 10, 50, 100 mg/L for 24 h | in vitro | Increasing PI3K, P-mTOR/mTOR, and P-Akt/Akt levels | [51] |
| ARPE-19 cells | 0, 0.25, 0.5, 1 and 2 mg/mL for 2 h | in vitro | Decreasing ROS production of alleviating OxS increased Nrf2 nuclear translocation and HO-1 expression | [52] |
| Male C57BL/6N mice | 1 mg/kg/d for 7 days | in vivo | Decreasing the Aβ level of RAGE expression in RGC | [53] |
| N2a/APP695 cells | 0, 1.25, 2.5 and 5 μM for 24 h | in vitro | Decreasing in the ratio of Aβ42/Aβ40 in N2a/APP695 cells to protect nerves from damage | [54] |
| Adult female Sprague-Dawley rats (10-week-old,180–200 g) | 1, 10 mg/kg/d for 28 days | in vivo | Significantly reducing retinal inner thickness (IRLT) and positive dark threshold response (pSTR), inhibiting secondary degeneration | [55] |
| male Sprague-Dawley rats (8 weeks, 300–350 g) | 1 mg/kg/d for 1 week | in vivo | Inhibiting RGC loss and ROS production and enhancing Nrf2 and HO-1 immune reaction activities | [56] |
| C57BL/6N male mice (10–12 weeks old) | 1 mg/kg/d for 1 week | in vivo | Decreasing activation of GFAP and AQP4 and down-regulating levels of IgG exosmosis and PAR expression | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules 2022, 27, 5628. https://doi.org/10.3390/molecules27175628
Yang C, Zhao Q, Li S, Pu L, Yu L, Liu Y, Lai X. Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules. 2022; 27(17):5628. https://doi.org/10.3390/molecules27175628
Chicago/Turabian StyleYang, Chunhong, Qi Zhao, Shiling Li, Lili Pu, Liqiong Yu, Yaqin Liu, and Xianrong Lai. 2022. "Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review" Molecules 27, no. 17: 5628. https://doi.org/10.3390/molecules27175628
APA StyleYang, C., Zhao, Q., Li, S., Pu, L., Yu, L., Liu, Y., & Lai, X. (2022). Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules, 27(17), 5628. https://doi.org/10.3390/molecules27175628







