Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice
Abstract
1. Introduction
2. Results
2.1. Gastric Mucosal Lesions
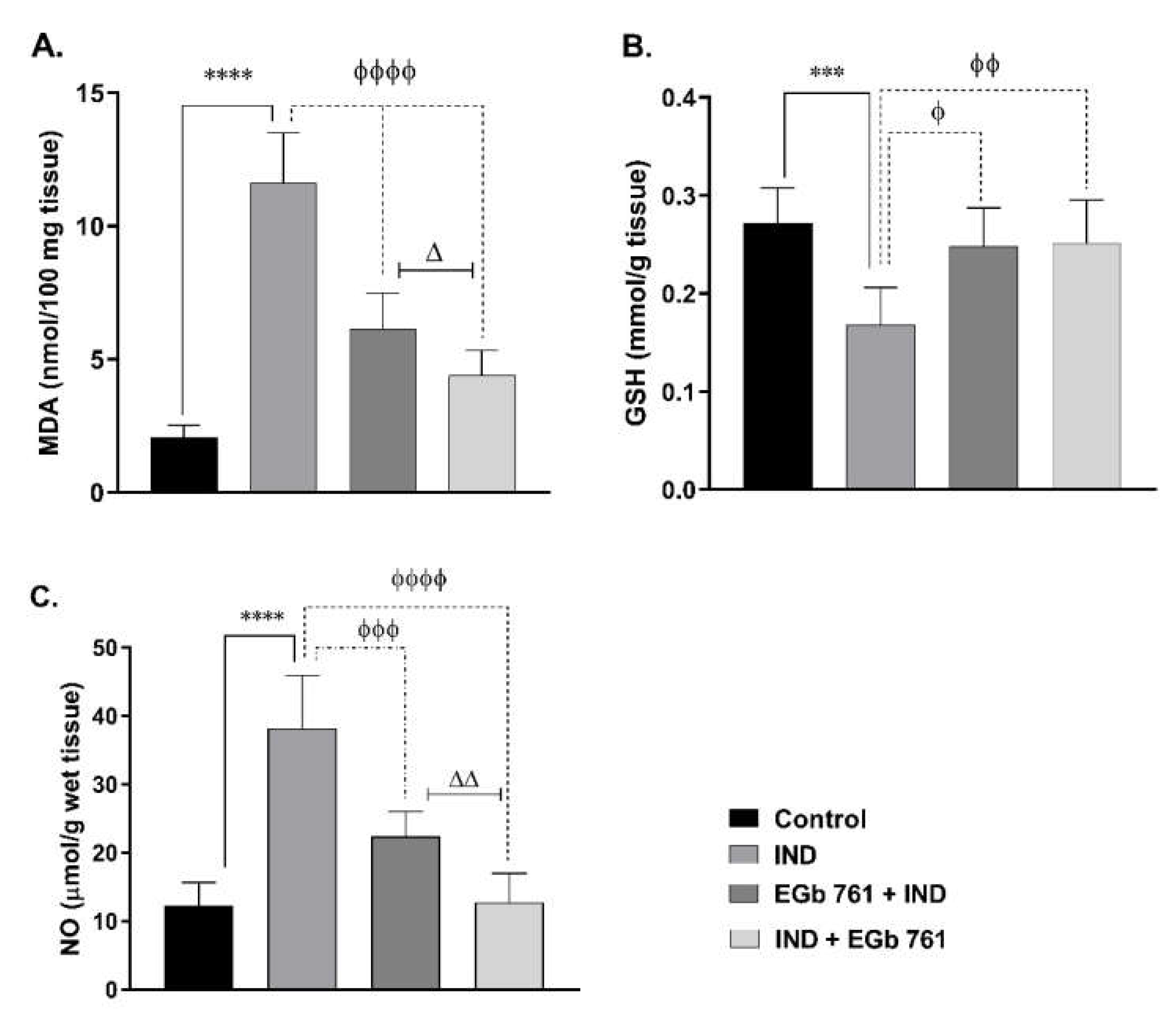
2.2. Lipid Peroxidation (MDA Level)
2.3. Gastric GSH Concentration
2.4. NO Level
2.5. Cytokine Levels
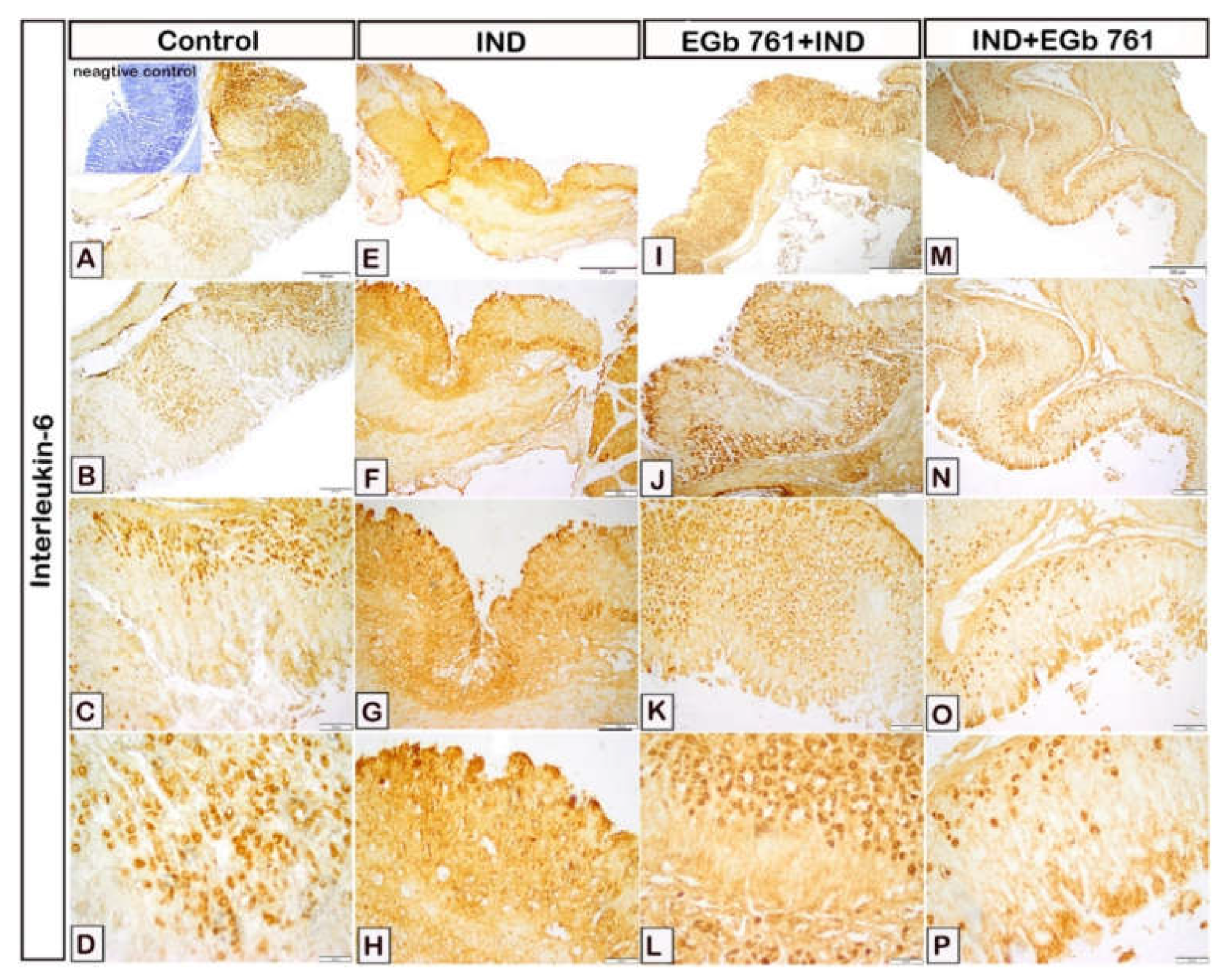
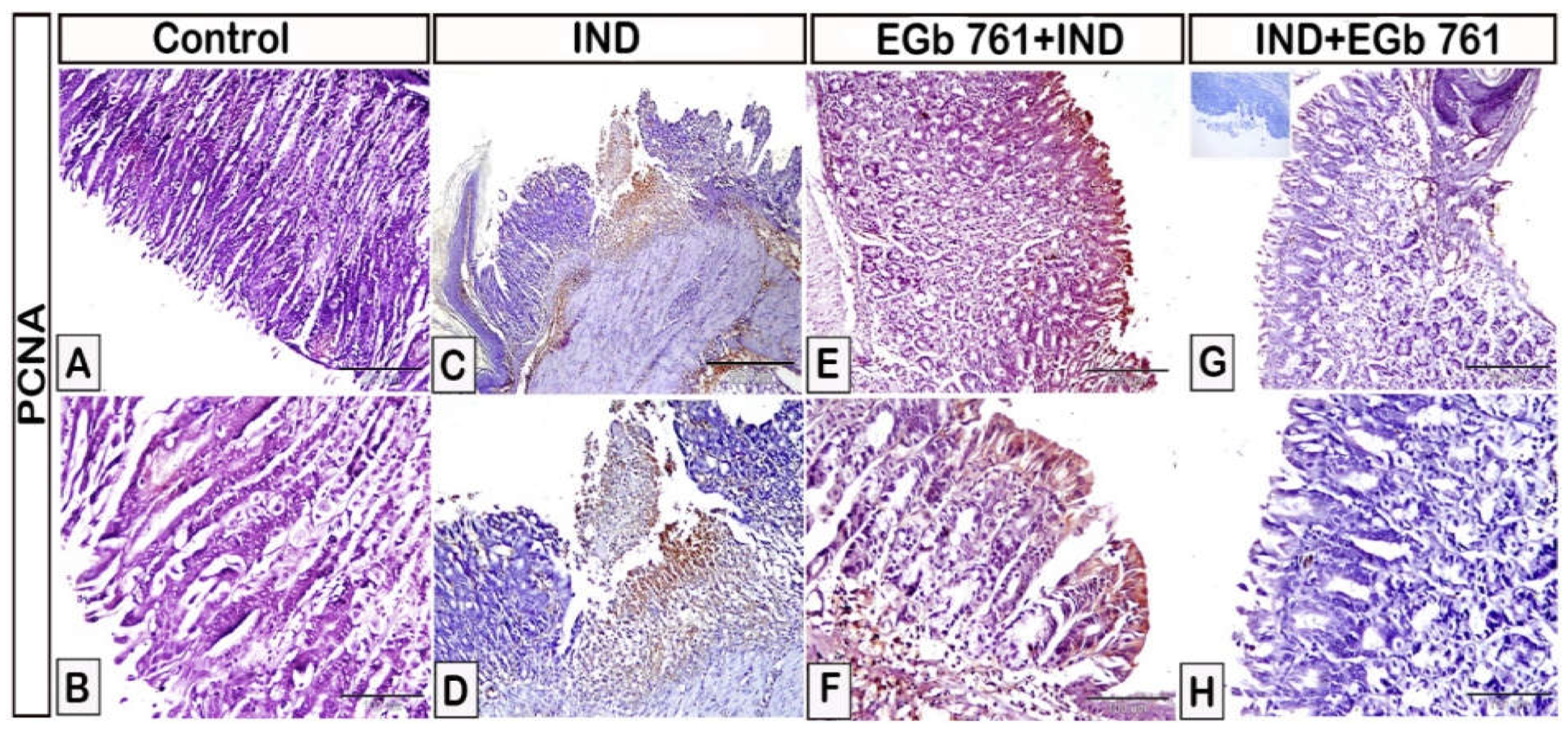
2.6. Histological Assessment
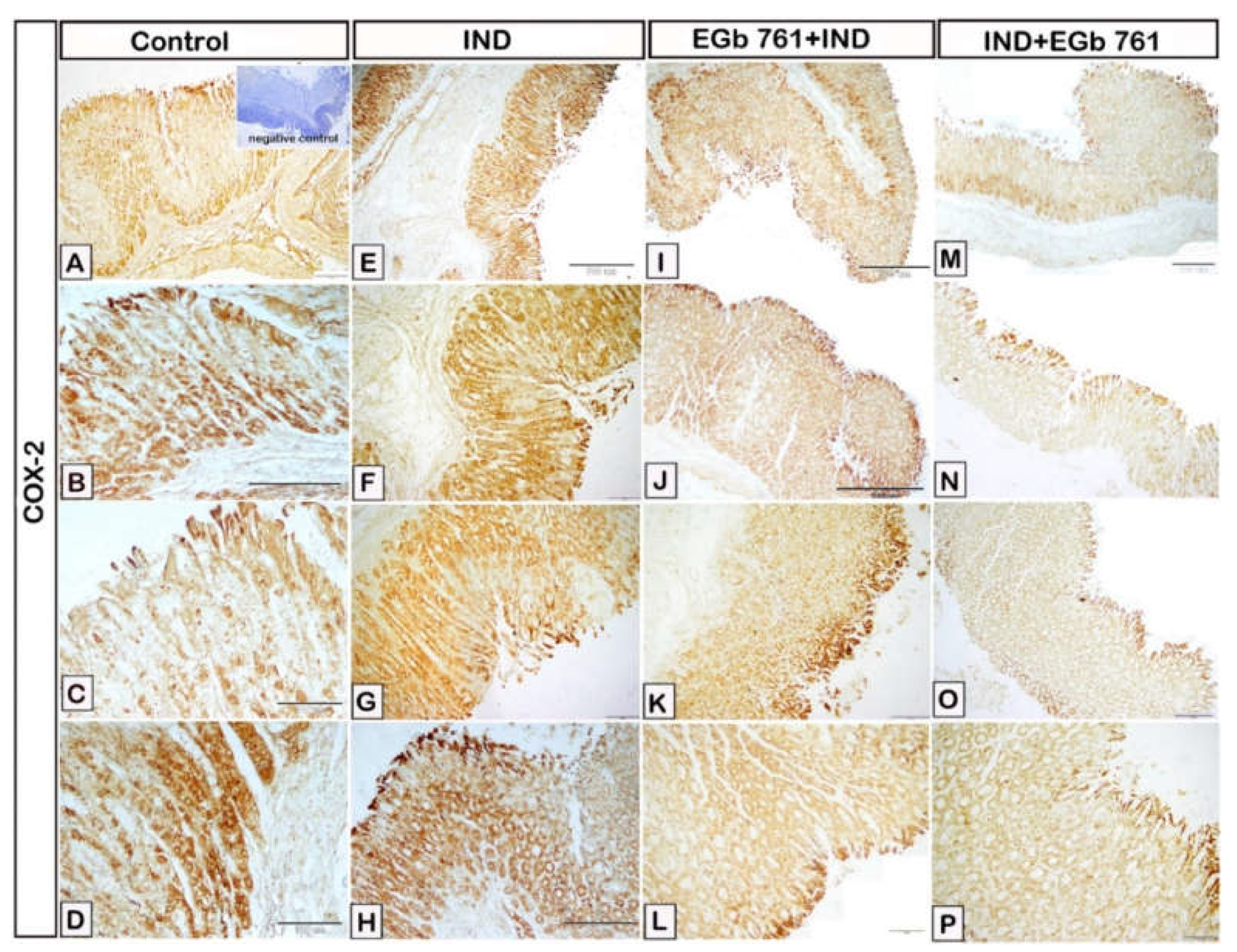
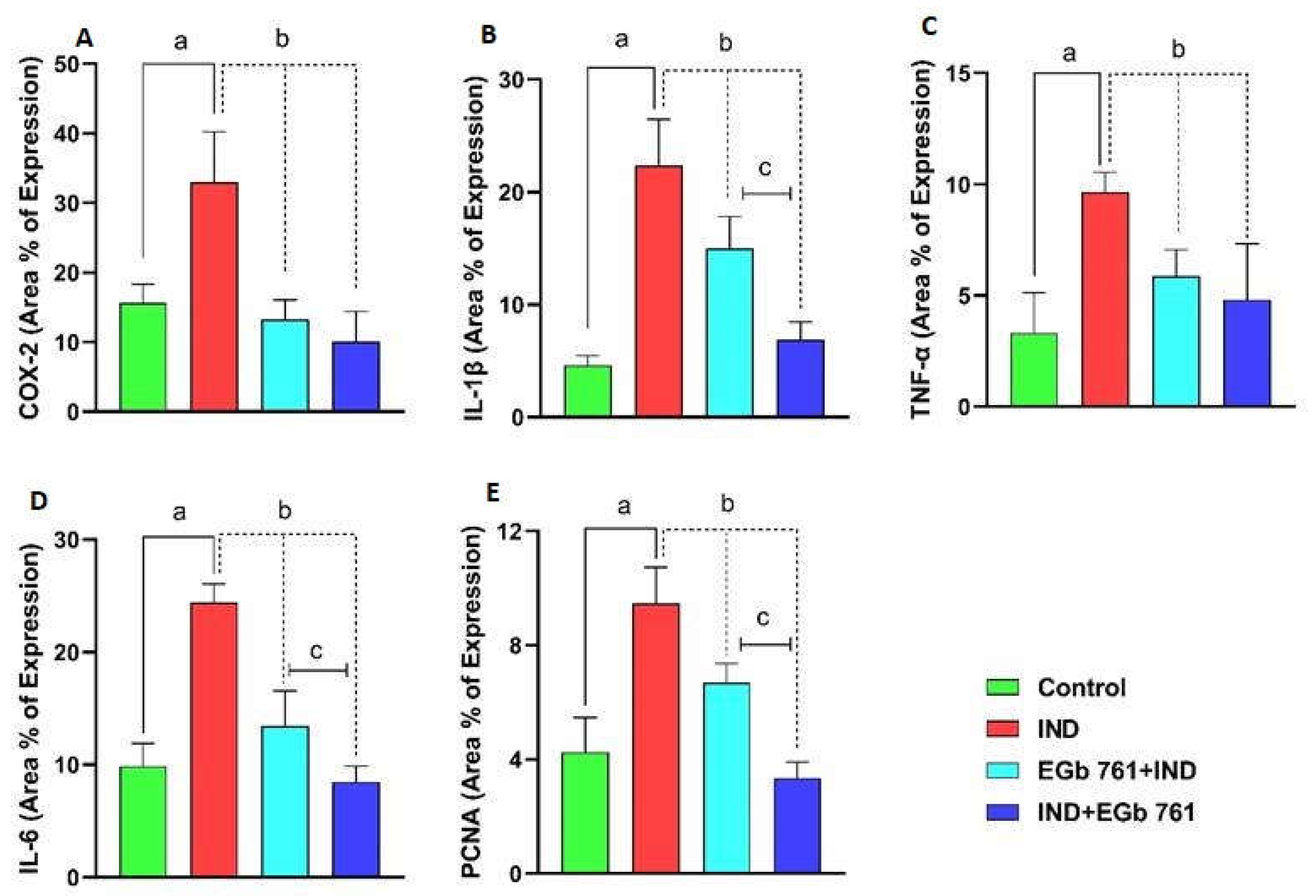
2.7. Immunohistochemical Expression of COX2, IL-1β, TNF-α, IL-6, and PCNA
2.8. Statistical Analysis of Area Percentage of Immunoreactivity of COX2, IL-1β, TNF-α, IL-6, and PCNA
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Animals and Study Design
4.3. Assessment of Gastric Mucosal Lesions
4.4. Determination of Lipid Peroxidation (TBARS)
4.5. Determination of Gastric Reduced Glutathione Concentration
4.6. Nitric Oxide (NO) Measurement
4.7. Determination of Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-6 (IL-6)
4.8. Histopathological and Immunohistochemical Evaluation
4.8.1. Collection of Samples and Preparation for Paraffin Embedding
4.8.2. The Immunohistochemical Procedure of COX-2, IL-1β, TNF-α, IL-6, and PCNA
4.8.3. Detection of Area Percentage of Immune Expression of COX-2, IL-1β, TNF-α, IL6, PCNA, and statistical analysis
4.8.4. Color Segmentation by CMEIAS (The Negative Images in the Supplementary Figures)
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vane, J.R. Inhibition of Prostaglandin Synthesis as a Mechanism of Action for Aspirin-like Drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.J.; Currier, B.L. Analgesic Pharmacology: II. Specific Analgesics. J. Am. Acad. Orthop. Surg. 2004, 12, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Robb, C.T.; Goepp, M.; Rossi, A.G.; Yao, C. Non-Steroidal Anti-Inflammatory Drugs, Prostaglandins, and COVID-19. Br. J. Pharmacol. 2020, 177, 4899–4920. [Google Scholar] [CrossRef] [PubMed]
- Chaiamnuay, S.; Allison, J.J.; Curtis, J.R. Risks versus Benefits of Cyclooxygenase-2-Selective Nonsteroidal Antiinflammatory Drugs. Am. J. Health Syst. Pharm. 2006, 63, 1837–1851. [Google Scholar] [CrossRef]
- Tomić, M.; Micov, A.; Pecikoza, U.; Stepanović-Petrović, R. Clinical Uses of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Potential Benefits of NSAIDs Modified-Release Preparations. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs; Challenges Potential Benefits; Academic Press: Cambridge, MA, USA, 2017; pp. 1–29. [Google Scholar] [CrossRef]
- Shin, S.J.; Noh, C.K.; Lim, S.G.; Lee, K.M.; Lee, K.J. Non-Steroidal Anti-Inflammatory Drug-Induced Enteropathy. Intest. Res. 2017, 15, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Warner, T.D. Cyclo-Oxygenase-2: Pharmacology, Physiology, Biochemistry and Relevance to NSAID Therapy. Br. J. Pharmacol. 1999, 128, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Drini, M. Peptic Ulcer Disease and Non-Steroidal Anti-Inflammatory Drugs. Aust. Prescr. 2017, 40, 91–93. [Google Scholar] [CrossRef]
- Sigthorsson, G.; Crane, R.; Simon, T.; Hoover, M.; Quan, H.; Bolognese, J.; Bjarnason, I. COX-2 Inhibition with Rofecoxib Does Not Increase Intestinal Permeability in Healthy Subjects: A Double Blind Crossover Study Comparing Rofecoxib with Placebo and Indomethacin. Gut 2000, 47, 527–532. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Milosavljevic, T.; Kostić-Milosavljević, M.; Jovanović, I.; Krstić, M. Complications of Peptic Ulcer Disease. Dig. Dis. 2011, 29, 491–493. [Google Scholar] [CrossRef]
- Najm, W.I. Peptic Ulcer Disease. Prim. Care 2011, 38, 383–394. [Google Scholar] [CrossRef]
- Strong, V.E. Progress in Gastric Cancer. Updates Surg. 2018, 70, 157–159. [Google Scholar] [CrossRef]
- Xie, C.; Liu, L.; Zhu, S.; Wei, M. Effectiveness and Safety of Chinese Medicine Combined with Omeprazole in the Treatment of Gastric Ulcer: A Protocol for Systematic Review and Meta-Analysis. Medicine 2021, 100, e25744. [Google Scholar] [CrossRef]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese Sumac (Rhus Chinensis Mill.) Fruits Alleviate Indomethacin-Induced Gastric Ulcer in Mice by Improving Oxidative Stress, Inflammation and Apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef]
- Pereira, A.C.H.; Lenz, D.; Nogueira, B.V.; Scherer, R.; Andrade, T.U.; Da Costa, H.B.; Romão, W.; Pereira, T.M.C.; Endringer, D.C. Gastroprotective Activity of the Resin from Virola Oleifera. Pharm. Biol. 2017, 55, 472–480. [Google Scholar] [CrossRef]
- Eraslan, E.; Tanyeli, A.; Güler, M.C.; Kurt, N.; Yetim, Z. Agomelatine Prevents Indomethacin-Induced Gastric Ulcer in Rats. Pharmacol. Rep. 2020, 72, 984–991. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Nathan, C. Inducible Nitric Oxide Synthase: What Difference Does It Make? J. Clin. Invest. 1997, 100, 2417–2423. [Google Scholar] [CrossRef]
- Peskar, B.M.; Maricic, N.; Gretzer, B.; Schuligoi, R.; Schmassmann, A. Role of Cyclooxygenase-2 in Gastric Mucosal Defense. Life Sci. 2001, 69, 2993–3003. [Google Scholar] [CrossRef]
- Marcin Magierowski, C.; Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Ginter, G.; Pajdo, R.; Chmura, A.; Kwiecien, S.; Brzozowski, T. Carbon Monoxide Released from Its Pharmacological Donor, Tricarbonyldichlororuthenium (II) Dimer, Accelerates the Healing of Pre-Existing Gastric Ulcers. Br. J. Pharmacol. 2017, 174, 3654. [Google Scholar] [CrossRef]
- Sayed Abdel-Tawab, M.; Mostafa Tork, O.; Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Azmy Elberry, D. Protective Effects of Quercetin and Melatonin on Indomethacin Induced Gastric Ulcers in Rats. Reports Biochem. Mol. Biol. 2020, 9, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Tanaka, A.; Hayashi, Y.; Kubo, Y. Functional Mechanism Underlying COX-2 Expression Following Administration of Indomethacin in Rat Stomachs: Importance of Gastric Hypermotility. Dig. Dis. Sci. 2004, 49, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Tanaka, A.; Kato, S.; Amagase, K.; Satoh, H. Roles of COX Inhibition in Pathogenesis of NSAID-Induced Small Intestinal Damage. Clin. Chim. Acta 2010, 411, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Komoike, Y.; Matsumoto, M.; Tanaka, A.; Takeuchi, K. Healing of Duodenal Ulcers Is Not Impaired by Indomethacin or Rofecoxib, the Selective COX-2 Inhibitor, in Rats. Digestion 2002, 66, 145–153. [Google Scholar] [CrossRef]
- Poonam, D.; Vinay, C.S.; Gautam, P. Cyclo-Oxygenase-2 Expression and Prostaglandin E2 Production in Experimental Chronic Gastric Ulcer Healing. Eur. J. Pharmacol. 2005, 519, 277–284. [Google Scholar] [CrossRef]
- Gudis, K.; Sakamoto, C. The Role of Cyclooxygenase in Gastric Mucosal Protection. Dig. Dis. Sci. 2005, 50 (Suppl. 1), S16–S23. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chatterjee, S.; Das, S.; Saha, A.; Chattopadhyay, S.; Bandyopadhyay, S.K. Ellagic Acid Facilitates Indomethacin-Induced Gastric Ulcer Healing via COX-2 up-Regulation. Acta Biochim. Biophys. Sin. 2012, 44, 565–576. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.H.; Song, J.; Kim, H. Gastroprotective Effects of Inulae Flos on HCl/Ethanol-Induced Gastric Ulcers in Rats. Molecules 2020, 25, 5623. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Salinas, R.C. Peptic Ulcer Disease. Am. Fam. Physician 2007, 76, 1005–1012. [Google Scholar]
- Lau, J.Y.; Sung, J.J.; Metz, D.C.; Howden, C.W. 187 Systematic Review of the Epidemiology of Complicated Peptic Ulcer: Incidence, Recurrence, Risk Factors and Mortality. Gastroenterology 2008, 134, A-32. [Google Scholar] [CrossRef]
- Chanudom, L.; Tangpong, J. Anti-Inflammation Property of Syzygium Cumini (L.) Skeels on Indomethacin-Induced Acute Gastric Ulceration. Gastroenterol. Res. Pract. 2015, 2015, 343642. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Nabil, M.; Abdo, W.; Abdelfattah, M.A.O.; El-Shazly, A.M.; El Kharrassi, Y.; Sobeh, M. Syzygium Samarangense Leaf Extract Mitigates Indomethacin-Induced Gastropathy via the NF-ΚB Signaling Pathway in Rats. Biomed. Pharmacother. 2021, 139, 111675. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, S.; Wang, Y.; Yang, Y.; Yao, L.; Chu, L.; Du, H.; Fu, F. Protective Effects of Escin against Indomethacin-Induced Gastric Ulcer in Mice. Toxicol. Mech. Methods 2014, 24, 560–566. [Google Scholar] [CrossRef]
- Sabiu, S.; Garuba, T.; Sunmonu, T.; Ajani, E.; Sulyman, A.; Nurain, I.; Balogun, A. Indomethacin-Induced Gastric Ulceration in Rats: Protective Roles of Spondias Mombin a Nd Ficus Exasperata. Toxicol. Rep. 2015, 2, 261–267. [Google Scholar] [CrossRef]
- Barboza, K.R.M.; Coco, L.Z.; Alves, G.M.; Peters, B.; Vasquez, E.C.; Pereira, T.M.C.; Meyrelles, S.S.; Campagnaro, B.P. Gastroprotective Effect of Oral Kefir on Indomethacin-Induced Acute Gastric Lesions in Mice: Impact on Oxidative Stress. Life Sci. 2018, 209, 370–376. [Google Scholar] [CrossRef]
- Crane, P.R. An Evolutionary and Cultural Biography of Ginkgo. Plants People Planet 2019, 1, 32–37. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted Therapeutic Benefits of Ginkgo Biloba L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar] [CrossRef]
- Serrano-García, N.; Pedraza-Chaverri, J.; Mares-Sámano, J.J.; Orozco-Ibarra, M.; Cruz-Salgado, A.; Jiménez-Anguiano, A.; Sotelo, J.; Trejo-Solís, C. Antiapoptotic Effects of EGb 761. Evid. Based. Complement. Alternat. Med. 2013, 2013, 495703. [Google Scholar] [CrossRef]
- DeFeudis, F.V. Ginkgo Biloba Extract (EGb 761) : From Chemistry to the Clinic. LibraryThing. Available online: https://www.librarything.com/work/8440193 (accessed on 14 July 2022).
- Maclennan, K.M.; Darlington, C.L.; Smith, P.F. The CNS Effects of Ginkgo Biloba Extracts and Ginkgolide B. Prog. Neurobiol. 2002, 67, 235–257. [Google Scholar] [CrossRef]
- EGb 761: Ginkgo Biloba Extract, Ginkor. Drugs R. D. 2003, 4, 188–193. [CrossRef]
- Smith, J.V.; Luo, Y. Studies on Molecular Mechanisms of Ginkgo Biloba Extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar] [CrossRef]
- Liu, X.G.; Wu, S.Q.; Li, P.; Yang, H. Advancement in the Chemical Analysis and Quality Control of Flavonoid in Ginkgo Biloba. J. Pharm. Biomed. Anal. 2015, 113, 212–225. [Google Scholar] [CrossRef]
- El-Sheikh, M.M. The Role of Some Natural Products in Protecting against the Effect of Gamma Irradiation on the Gastrointestinal Tract in Rats. Available online: http://thesis.mandumah.com/Record/264905/Details (accessed on 15 March 2022).
- El-Tanbouly, G.; Mahmoud, M.; Mohamed, M. Gastroprotective Effect of Standardized Ginkgo Biloba Extract (Egb761) against Indomethacin-Induced Gastric Ulcers in Rats. Delta Univ. Sci. J. 2020, 3, 142–152. [Google Scholar]
- Mahmoud, M. Gastroprotective Effects of Standardized Ginkgo Biloba Extract (EGb761), Aniseed, Quercetin and Trans–Anethole on Ethanol–Induced Ulcers in Rats. Available online: https://austinpublishinggroup.com/pharmacology-therapeutics/fulltext/ajpt-v2-id1031.php (accessed on 15 March 2022).
- Wang, Q.; Zhao, W.Z.; Ma, C.G. Protective Effects of Ginkgo Biloba Extract on Gastric Mucosa. Acta Pharmacol. Sin. 2000, 21, 1153–1156. [Google Scholar]
- Özdemir, Ö.M.A.; Ergin, H.; Yenisey, Ç.; Türk, N.Ş. Protective Effects of Ginkgo Biloba Extract in Rats with Hypoxia/Reoxygenation–Induced Intestinal Injury. J. Pediatr. Surg. 2011, 46, 685–690. [Google Scholar] [CrossRef]
- Pehlivan, M.; Dalbeler, Y.; Hazinedaroglu, S.; Arikan, Y.; Erkek, A.B.; Günal, O.; Türkçapar, A.G. An Assessment of the Effect of Ginkgo Biloba EGb 761 on Ischemia Reperfusion Injury of Intestine. Hepato-Gastroenterology 2002, 49, 201–204. [Google Scholar] [PubMed]
- Rai, D.; Bhatia, G.; Sen, T.; Palit, G. Anti-Stress Effects of Ginkgo Biloba and Panax Ginseng: A Comparative Study. J. Pharmacol. Sci. 2003, 93, 458–464. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Baiuomy, A.R.; El-batran, S.; Arbid, M.S. Evaluation of the Anti-Inflammatory, Anti-Nociceptive and Gastric Effects of Ginkgo Biloba in the Rat. Pharmacol. Res. 2004, 49, 133–142. [Google Scholar] [CrossRef]
- El-Medany, A.; Guemei, A.A.S.; Abdel Twab, R.; Al-Matrafi, T.; El-Medany, J. What Is the Possible Therapeutic Effect of Ginkgo Biloba on Gastric Ulcer Induced by Ammonia in Albino Rats? Environ. Sci. Pollut. Res. Int. 2020, 27, 25082–25092. [Google Scholar] [CrossRef]
- Kleijnen, J.; Knipschild, P. Ginkgo Biloba. Lancet 1992, 340, 1136–1139. [Google Scholar] [CrossRef]
- Chao, J.C.; Chu, C.C. Effects of Ginkgo Biloba Extract on Cell Proliferation and Cytotoxicity in Human Hepatocellular Carcinoma Cells. World J. Gastroenterol. 2004, 10, 37–41. [Google Scholar] [CrossRef]
- Mei, N.; Guo, X.; Ren, Z.; Kobayashi, D.; Wada, K.; Guo, L. Review of Ginkgo Biloba-Induced Toxicity, from Experimental Studies to Human Case Reports. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2017, 35, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ugan, R.A.; Un, H. The Protective Roles of Butein on Indomethacin Induced Gastric Ulcer in Mice. Eurasian J. Med. 2020, 52, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Nandi, J.; Saud, B.; Zinkievich, J.M.; Yang, Z.J.; Levine, R.A. TNF-Alpha Modulates INOS Expression in an Experimental Rat Model of Indomethacin-Induced Jejunoileitis. Mol. Cell. Biochem. 2010, 336, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different Mechanisms in Formation and Prevention of Indomethacin-Induced Gastric Ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Blandizzi, C.; Fornai, M.; Colucci, R.; Natale, G.; Lubrano, V.; Vassalle, C.; Antonioli, L.; Lazzeri, G.; Del Tacca, M. Lansoprazole Prevents Experimental Gastric Injury Induced by Non-Steroidal Anti-Inflammatory Drugs through a Reduction of Mucosal Oxidative Damage. World J. Gastroenterol. 2005, 11, 4052–4060. [Google Scholar] [CrossRef]
- Halici, M.; Odabasoglu, F.; Suleyman, H.; Cakir, A.; Aslan, A.; Bayir, Y. Effects of Water Extract of Usnea Longissima on Antioxidant Enzyme Activity and Mucosal Damage Caused by Indomethacin in Rats. Phytomedicine 2005, 12, 656–662. [Google Scholar] [CrossRef]
- Chao, J.C.J.; Hung, H.C.; Chen, S.H.; Fang, C.L. Effects of Ginkgo Biloba Extract on Cytoprotective Factors in Rats with Duodenal Ulcer. World J. Gastroenterol. 2004, 10, 560. [Google Scholar] [CrossRef]
- Chen, S.-H.; Liang, Y.-C.; Chao, J.C.; Tsai, L.-H.; Chang, C.-C.; Wang, C.-C.; Pan, S. Protective Effects of Ginkgo Biloba Extract on the Ethanol-Induced Gastric Ulcer in Rats. World J. Gastroenterol. 2005, 11, 3746–3750. [Google Scholar] [CrossRef]
- Eli, R. Ginkgo Biloba, May Significantly Reduce Gastrointestinal Pain: It May Also Reduce the Risk of Stomach Cancer That Is Associated with the Wide-Spread Use of Proton Pump Inhibitors. Med. Hypotheses 2006, 66, 1244. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- DeFeudis, F.; Drieu, K. Ginkgo Biloba Extract (EGb 761) and CNS Functions: Basic Studies and Clinical Applications. Curr. Drug Targets 2000, 1, 25–58. [Google Scholar] [CrossRef]
- Rohde, F.; Schusser, B.; Hron, T.; Farkašová, H.; Plachý, J.; Härtle, S.; Hejnar, J.; Elleder, D.; Kaspers, B. Characterization of Chicken Tumor Necrosis Factor-α, a Long Missed Cytokine in Birds. Front. Immunol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Li, C.; Liu, K.; Liu, S.; Aerqin, Q.; Wu, X. Role of Ginkgolides in the Inflammatory Immune Response of Neurological Diseases: A Review of Current Literatures. Front. Syst. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential Therapeutic Anti-Inflammatory and Immunomodulatory Effects of Dihydroflavones, Flavones, and Flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Fan, Y.; Zhuang, S.; Liu, Z. Kaempferol Inhibits Multiple Pathways Involved in the Secretion of Inflammatory Mediators from LPS-induced Rat Intestinal Microvascular Endothelial Cells. Mol. Med. Rep. 2019, 19, 1958–1964. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated Analysis of COX-2 and INOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium Plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of Interleukin-6 Gene Expression through the NF-Kappa B Transcription Factor. Mol. Cell. Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of Ginkgo Biloba Extract (EGb 761) and Quercetin on Lipopolysaccharide-Induced Release of Nitric Oxide. Chem. Biol. Interact. 2001, 137, 43–58. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, G.; Yao, L.; Xue, P.; Yu, D.; Xu, R.; Shi, W.; Yao, X.; Yan, Z.; Duan, J. ao Protective Effect of Ginkgo Biloba Leaves Extract, EGb761, on Myocardium Injury in Ischemia Reperfusion Rats via Regulation of TLR-4/NF-ΚB Signaling Pathway. Oncotarget 2017, 8, 86671–86680. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Jackson, L.M.; Wu, C.; Mahida, Y.R.; Jenkins, D.; Hawkey, C.J. Cyclooxygenase (COX) 1 and 2 in Normal, Inflamed, and Ulcerated Human Gastric Mucosa-Ate Prostaglandin Dependent Gastric Protection. However, Gastric Mucosa Con-Tains Cells Capable of Expressing Inducible COX-2. We Therefore Investigated COX-1 and COX-2 Expression, Localisation, and Activity in Normal and Abnormal Human Gastric Mucosa. Methods-COX-1 and COX-2 Distribution. Gut 2000, 47, 762–770. [Google Scholar]
- Miura, S.; Tatsuguchi, A.; Wada, K.; Takeyama, H.; Shinji, Y.; Hiratsuka, T.; Futagami, S.; Miyake, K.; Gudis, K.; Mizokami, Y.; et al. Cyclooxygenase-2-Regulated Vascular Endothelial Growth Factor Release in Gastric Fibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G444–G451. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, Y.I.; Abd El-Ghffar, E.A. Spirulina Ameliorates Aspirin-Induced Gastric Ulcer in Albino Mice by Alleviating Oxidative Stress and Inflammation. Biomed. Pharmacother. 2019, 109, 314–321. [Google Scholar] [CrossRef]
- Alazzouni, A.S.; Fathalla, A.S.; Gabri, M.S.; Dkhil, M.A.; Hassan, B.N. Role of Bone Marrow Derived-Mesenchymal Stem Cells against Gastric Ulceration: Histological, Immunohistochemical and Ultrastructural Study. Saudi J. Biol. Sci. 2020, 27, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, N.; Ma, C.H.; Tao, J.; Bao, J.A.; Cheng, Z.Q.; Chen, Z.T.; Miao, L.Y. Ginkgo Biloba Extracts Attenuate Lipopolysaccharide-Induced Inflammatory Responses in Acute Lung Injury by Inhibiting the COX-2 and NF-ΚB Pathways. Chin. J. Nat. Med. 2015, 13, 52–58. [Google Scholar] [CrossRef]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.H.; Fiebich, B.L. Anti-Neuroinflammatory Effects of Ginkgo Biloba Extract EGb761 in LPS-Activated Primary Microglial Cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tariq, L.; Kury, A.; Dayyan, F.; Shah, F.A.; Malik, Z.; Ali, A.; Khalil, K.; Alattar, A.; Alshaman, R.; Ali, A.; et al. Molecules Ginkgo Biloba Extract Protects against Methotrexate-Induced Hepatotoxicity: A Computational and Pharmacological Approach. Molecules 2020, 25, 2540. [Google Scholar] [CrossRef]
- Park, Y.-M.; Won, J.-H.; Yun, K.-J.; Ryu, J.-H.; Han, Y.-N.; Choi, S.-K.; Lee, K.-T. Preventive Effect of Ginkgo Biloba Extract (GBB) on the Lipopolysaccharide-Induced Expressions of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 via Suppression of Nuclear Factor-k KB in RAW 264.7 Cells. Biol. Pharm. Bull 2006, 29, 985–990. [Google Scholar] [CrossRef]
- El-Kerdasy, H.I.; Mousa, H.R. A Comparative Study Between The Therapeutic Role of Adipose Derived Mesenchymal Stem Cells and Omeprazole in Regeneration of Gastric Ulcer Induced by Aspirin in Albino Rats : Histological and Immunohistochemical Study. Egypt. J. Histol. 2021, 44, 993–1006. [Google Scholar] [CrossRef]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231. [Google Scholar] [CrossRef]
- Salema, M.Y.; El-Azaba, N.E.E.; Helala, O.K.; Metwalyb, H.G.; El-Halim Bayoumia, H.E.A. Does Selenium Improve the Stem Cell Therapeutic Effect on Isoproterenol-Induced Myocardial Infarction in Rats? A Histological and Immunohistochemical Study. Egypt. J. Histol. 2015, 38, 679–691. [Google Scholar] [CrossRef]
- Chassagne, F.; Huang, X.; Lyles, J.T.; Quave, C.L. Validation of a 16th Century Traditional Chinese Medicine Use of Ginkgo Biloba as a Topical Antimicrobial. Front. Microbiol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Athaydes, B.R.; Alves, G.M.; de Assis, A.L.E.M.; Gomes, J.V.D.; Rodrigues, R.P.; Campagnaro, B.P.; Nogueira, B.V.; Silveira, D.; Kuster, R.M.; Pereira, T.M.C.; et al. Avocado Seeds (Persea Americana Mill.) Prevents Indomethacin-Induced Gastric Ulcer in Mice. Food Res. Int. 2019, 119, 751–760. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Wang, X.P.; Xie, Y.N.; Wu, L.L.; Liu, H. Protective Effect of Ginkgo Biloba Extract on Paracetamol-Induced Acute Hepatic Injury in Mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2018, 34, 432–435. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, P.; Wang, Q.; Sheng, X.; Zhang, J.; Lu, X.; Fan, X. Protective Effects of Ginkgo Biloba Dropping Pills against Liver Ischemia/Reperfusion Injury in Mice. Chin. Med. 2020, 15, 122. [Google Scholar] [CrossRef]
- Till, M.; Gáti, T.; Rábai, K.; Szombath, D.; Székely, J.I. Effect of [D-Met2,Pro5]Enkephalinamide on Gastric Ulceration and Transmucosal Potential Difference. Eur. J. Pharmacol. 1988, 150, 325–330. [Google Scholar] [CrossRef]
- Rofaeil, R.R.; Gaber, S.S. Gastroprotective Effect of Memantine in Indomethacin-Induced Peptic Ulcer in Rats, a Possible Role for Potassium Channels. Life Sci. 2019, 217, 164–168. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Siracusa, R.; Genovese, T.; D’Amico, R.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S.; et al. Protective Effect of Snail Secretion Filtrate against Ethanol-Induced Gastric Ulcer in Mice. Sci. Rep. 2021, 11, 3638. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium Occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Abd-Eldayem, A.M. Systemic and Topical Ginkgo Biloba Leaf Extract (Egb-761) Ameliorated Rat Paw Inflammation in Comparison to Dexamethasone. J. Ethnopharmacol. 2022, 282, 114619. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Abd-Elhafeez, H.H.; Moustafa, M.; Zayed, A.; Sayed, R. The Development of the Intratesticular Excurrent Duct System of Donkey (Equus Asinus) from Birth to Maturity. Histol. Cytol. Embryol. 2017, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Abdelhafeez, H.H.; Moustafa, M.N.K.; Zayed, A.E.; Sayed, R. Morphological and Morphometric Study of the Development of Leydig Cell Population of Donkey (Equus Asinus) Testis from Birth to Maturity. Cell Biol. Res. Ther. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Moustafa, M. Morphological and Morphometric Study of the Development of Seminiferous Epithelium of Donkey (Equus Asinus) from Birth to Maturity. J. Cytol. Histol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Kämmerer, U.; Kapp, M.; Gassel, A.; Richter, T.; Tank, C.; Dietl, J.; Ruck, P. A New Rapid Immunohistochemical Staining Technique Using the EnVision Antibody Complex. J. Histochem. Cytochem. 2001, 49, 623–630. [Google Scholar] [CrossRef]
- Abd-Elhafeez, H.H.; Soliman, S.A.; Attaai, A.H.; Abdel-Hakeem, S.S.; El-Sayed, A.M.; Abou-Elhamd, A.S. Endocrine, Stemness, Proliferative, and Proteolytic Properties of Alarm Cells in Ruby-Red-Fin Shark (Rainbow Shark), Epalzeorhynchos Frenatum (Teleostei: Cyprinidae). Microsc. Microanal. 2021, 27, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
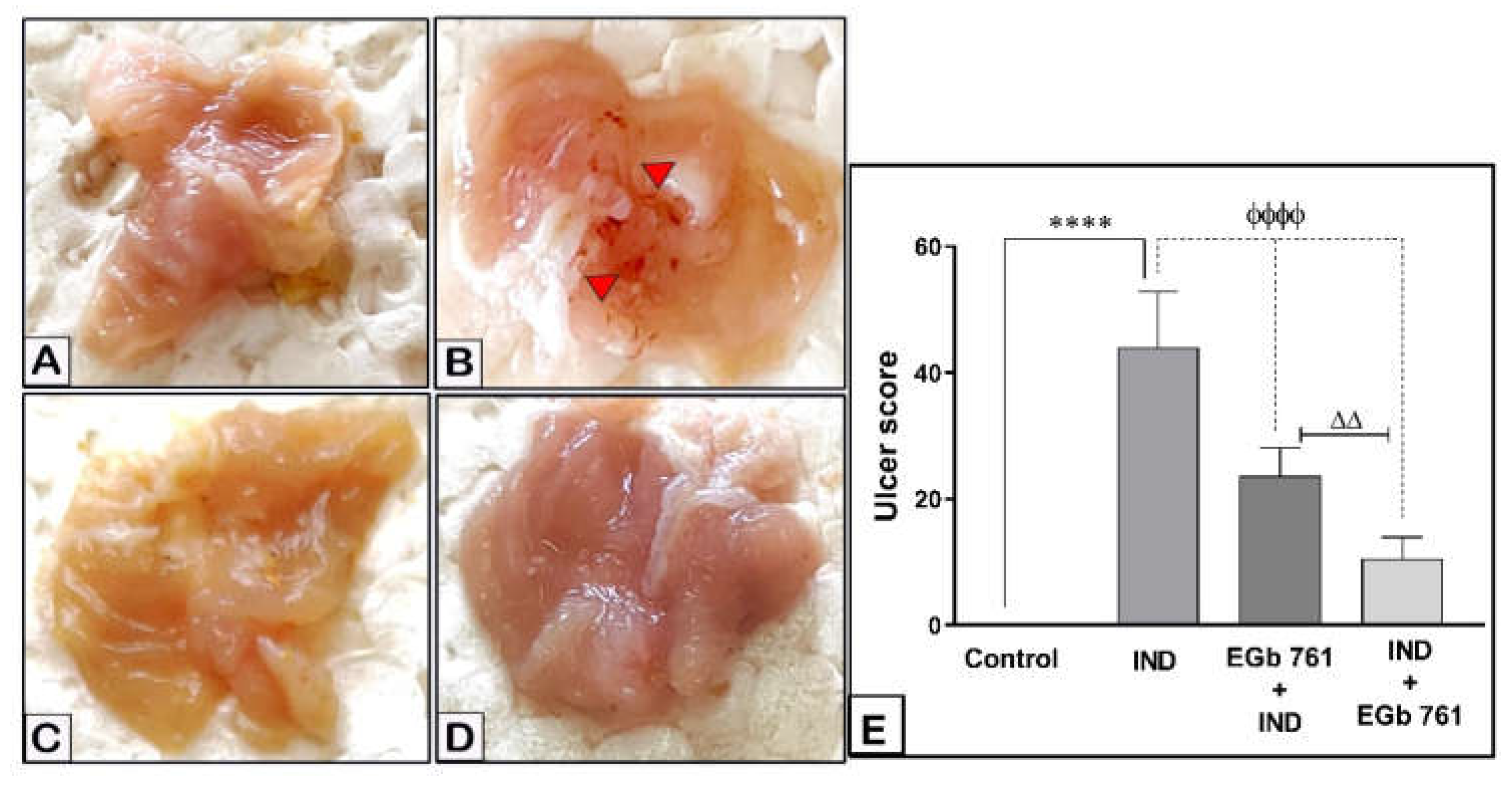

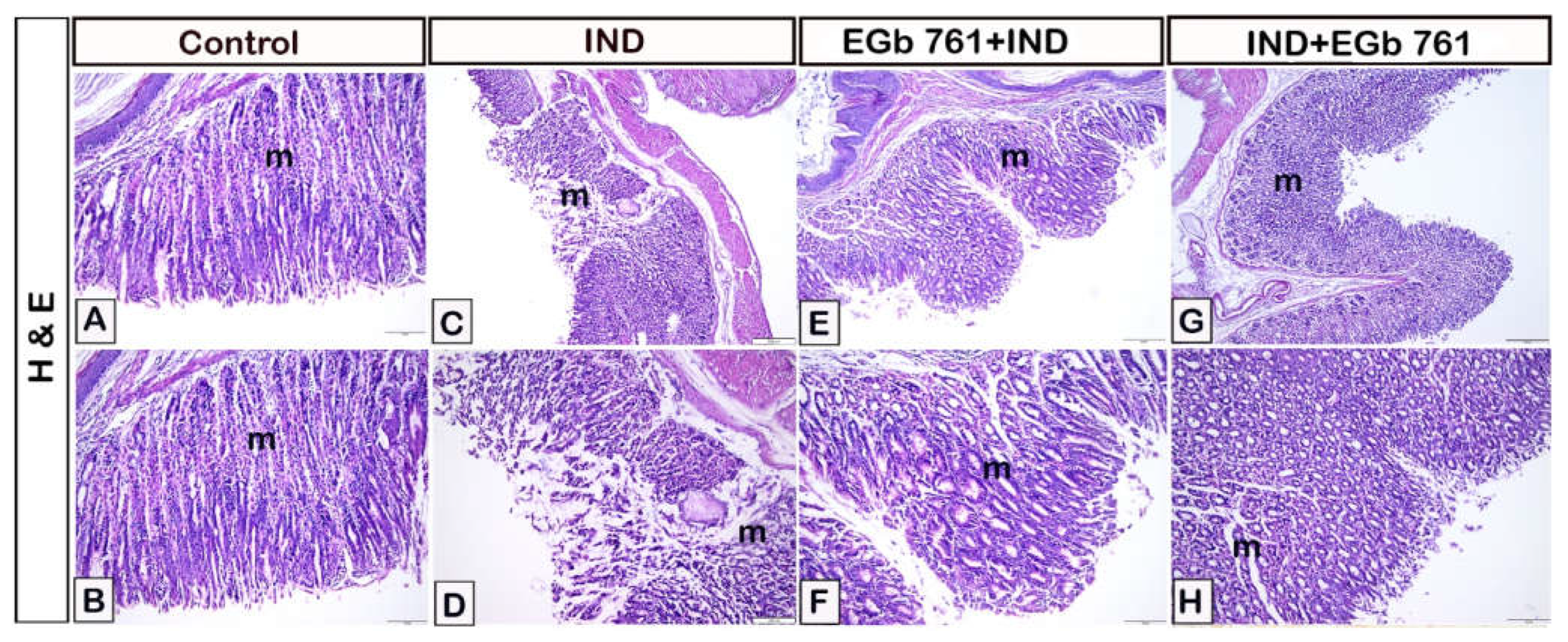


| Histopathological Lesions | Control | Indomethacin-Induced Gastric Ulceration | EGb 761 Pre-Treated Mice | EGb 761 Treatment after Indomethacin |
|---|---|---|---|---|
| Necrosis of Gastric Mucosa | 00 | 04 | 02 | 01 |
| Mucosal Inflammatory Cells | 00 | 03 | 02 | 01 |
| Submucosal Oedema | 00 | 04 | 02 | 01 |
| Hemorrhage | 00 | 04 | 01 | 01 |
| Total Score | 00 | 15 | 07 | 04 |
| Fixative | Components | Amount |
|---|---|---|
| N a-Phosphate buffer (0.1 M, pH 7.4) | Solution A | |
| Na2HPO4 2H2O | 17.02 gm | |
| Distilled water | 600 mL | |
| Solution B | ||
| NaH2PO4 H2 | 6 gm | |
| Distilled water | 200 mL | |
| Using solution | ||
| Solution A | 580 mL | |
| Solution B | 219 mL | |
| Citrate-buffer (pH 6.0) | Solution A | |
| Citrate C6H8O7 H2O | 21 g | |
| Distilled water | 1 L | |
| Solution B | ||
| Sodium citrate Na3C6H5O7 2H2O | 29.41 g | |
| Distilled water | 1 L | |
| Using solution | ||
| Solution A | 9 mL | |
| Solution B | 41 mL | |
| Distilled water | Add 500 mL | |
| Target | Primary Antibody Supplier | Origin (Catalog No) | Dilution | Incubation | Antigen Retrieval | Secondary Antibody-Incubation Time |
|---|---|---|---|---|---|---|
| COX-2 | Abcam | Rabbit anti-mouse monoclonal [EPR12012] to COX2 | 1:100 | Overnight | Boiling in citrate buffer (pH 6.0), 20 min Goat | Goat anti-Mouse IgG (H+L) Secondary Antibody Catalog # 31569 Dilution; 1:100 One hour at room temperature |
| IL- 1β | Bio-Rad | Rabbit anti-Mouse Interleukin-1 beta Clone: AAM13G Polyclonal | 1:200 | Overnight | Boiling in citrate buffer (pH 6.0), 20 min | |
| IL-6 | Abcam | Mouse monoclonal Anti-IL-6 antibody [1.2-2B11-2G10] (ab9324) | 1:100 | 45-min incubation at room temperature | Boiling in citrate buffer (pH 6.0), 20 min | |
| TNF-α | (Novus Biologicals, Littleton, CO, USA), | TNF-alpha Antibody [NBP1-19532] | 1:300 | Overnight | Boiling in citrate buffer (pH 6.0), 20 min | |
| PCNA | Anti PCNA Santa Cruz Biotechnology, Inc. | (PC10 sc-56 mouse anti-rat IgG2a monoclonal antibody | 1:500 | 2 h at room temperature | Boiling in citrate buffer (pH 6.0), 20 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Eldayem, A.M.; Alnasser, S.M.; Abd-Elhafeez, H.H.; Soliman, S.A.; Abdel-Emam, R.A. Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice. Molecules 2022, 27, 5598. https://doi.org/10.3390/molecules27175598
Abd-Eldayem AM, Alnasser SM, Abd-Elhafeez HH, Soliman SA, Abdel-Emam RA. Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice. Molecules. 2022; 27(17):5598. https://doi.org/10.3390/molecules27175598
Chicago/Turabian StyleAbd-Eldayem, Ahmed M., Sulaiman Mohammed Alnasser, Hanan H. Abd-Elhafeez, Soha A. Soliman, and Rania A. Abdel-Emam. 2022. "Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice" Molecules 27, no. 17: 5598. https://doi.org/10.3390/molecules27175598
APA StyleAbd-Eldayem, A. M., Alnasser, S. M., Abd-Elhafeez, H. H., Soliman, S. A., & Abdel-Emam, R. A. (2022). Therapeutic Versus Preventative Use of Ginkgo biloba Extract (EGb 761) against Indomethacin-Induced Gastric Ulcer in Mice. Molecules, 27(17), 5598. https://doi.org/10.3390/molecules27175598








