Abstract
A series of N-substituted iminosugar C-glycosides were synthesized and tested for α-glucosidase inhibition. The results suggested that 6e is a promising and potent α-glucosidase inhibitor. Enzymatic kinetic assays indicated that compound 6e may be classified as an uncompetitive inhibitor. The study of structure-activity relationships of those iminosugars provided a starting point for the discovery of new α-glucosidase inhibitors.
1. Introduction
Diabetes mellitus (DM) is one of the most common chronic diseases with 1.5 million deaths each year [1]. According to the International Diabetes Federation (IDF), the prevalence of DM was estimated to be 783 million by 2045 [2]. Type 2 diabetes mellitus (T2DM) is the most common form of this disease and almost 80–90% of all diabetic patients are T2DM [3]. Generally, diabetic pathogeny mostly focuses on genetic, lifestyle, environmental toxins, and advancing age [4]. Current antidiabetic drugs to treat T2DM include incretin mimetic, biguanides, sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide 1 agonists, and α-glucosidase inhibitors [5,6]. α-Glucosidase (α-D-glucoside glucohydrolase; EC3.2.1.20) is the glycoside hydrolase specifically hydrolyzing 1,4-α-glucopyranosides bond to produce α-glucose, located in the brush border of the small intestine [7]. α-Glucosidase inhibitors are thought to be the most efficient agents to reduce postprandial hyperglycemia [8]. Thus, it has been considered as one of the most popular targets for the treatment of diabetes [9]. At present, α-glucosidase inhibitors have been marketed as therapeutic drugs for T2DM, including acarbose [10], voglibose [10], and miglitol [11], and have been used in reducing plasma glucose levels and postprandial hyperglycemia (Figure 1) [12]. However, their widespread application has been limited by the complicated multistep procedures needed for their synthesis and undesirable side effects such as gastrointestinal intolerability, diarrhoea and flatulence [13]. Therefore, the search for new small molecules possessing potent α-glucosidase inhibitory activity and minimal side effects has attracted significant attention for many years.
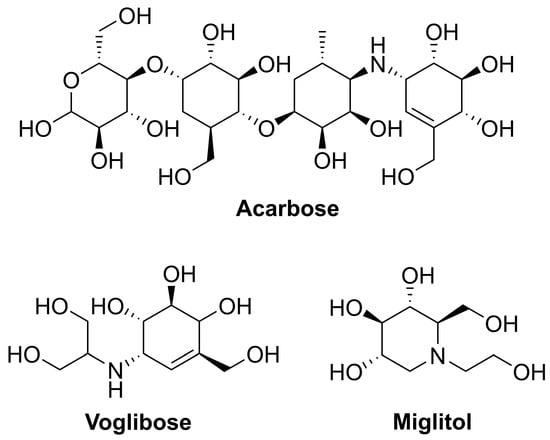
Figure 1.
Important clinically used α-glucosidase inhibitors for the treatment of T2DM.
Iminosugars [14], formed by the replacement of sugar ring oxygen with nitrogen, are well known for their ability to selectively inhibit glycosidases [15]. This kind of scaffold inhibits glycosidases by mimicking the substrate transition states with oxacarbenium ion character during the hydrolysis reaction catalyzed by glycosidases [16]. In the past two decades, more than 100 iminosugars have been isolated from plants and microorganisms [17,18,19]. Besides, hundreds of their analogues and derivatives were synthesized and evaluation of their biological activity was assayed, especially as glucosidase inhibitors [20]. However, various comparative studies on simple glycolipid analogues have demonstrated a marked dependence of the potency of the inhibitors upon the position of the alkyl chain (1-C- or N-alkyl derivatives) [21]. Meanwhile, Butters, T. D. and co-workers also found that the presence of a hydrophobic N-alkyl chain of iminosugars provided an increase in inhibitory potency to glucosidases [22]. As a part of our continuing interest in the synthesis of novel iminosugars and their α-glucosidase inhibition, we report a library of N-substituted iminosugar C-glycosides and their structure-activity relationships against α-glucosidase.
2. Results and Discussion
2.1. Synthesis of Iminosugar C-Glycosides
The synthesis of the compound 1-C-Acetylmethyl-5-deoxy-5-amino-α-D-ribopyranoside was based on our PREVIOUS synthetic strategy (Scheme 1) [23]. Nucleophilic substitution of known mesylate 1 with NaN3 generated 5-azido-C-riboside 2. Reduction of the azido group to amine after terminal olefin oxidation, which was immediately treated with the saturated sodium methoxide-methanol solution at room temperature overnight. Purified the crude with silica gel flash column chromatography (ethyl acetate/methanol, 2:1, Rf = 0.2) to afford the compound 4.
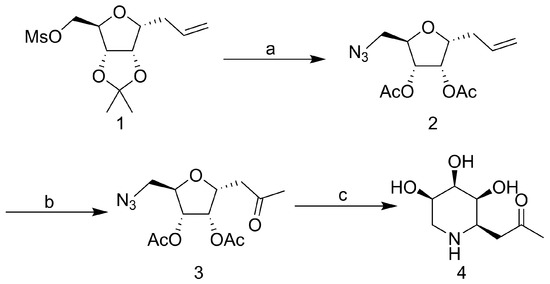
Scheme 1.
The synthesis of the compound 1-C-Acetylmethyl-5-deoxy-5-amino-α-D-ribopyranoside. Reagents and conditions: (a) NaN3, DMF, 80 °C, overnight; 6 N HCl, THF, 50 °C, 18 h; Ac2O, DMAP, 5 h. (b) Acetone/H2O:4/1, Hg(OAc)2, Jones reagent, 0 °C–r. t., overnight. (c) Pd/C, H2, MeOH, 5 h; 1% MeONa/MeOH, 8 h; Saturated MeONa/MeOH, overnight.
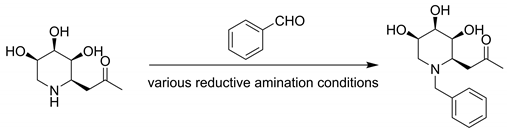
Based on previous work, the inhibitory potency of C-glycosides 4 has been demonstrated for the α-glucosidase [23]. To obtain higher activity compounds, the compound 4 was structurally modified by introducing alkyl side chains on its nitrogen atom. The reaction conditions were screened (Table 1). Under the conditions of −20 °C–r. t., NaBH(OAc)3 and MeOH, good yield was obtained by reductive amination. Finally, a variety of aldehydes could be attached to the structure 4 through reductive amination and obtained N-substituted iminosugar C-glycosides 5 (Scheme 2, Figure 2).

Table 1.
Screening of synthesis conditions of N-substituted iminosugar C-glycosides.

Scheme 2.
Synthesis of N-substituted iminosugar C-glycosides.
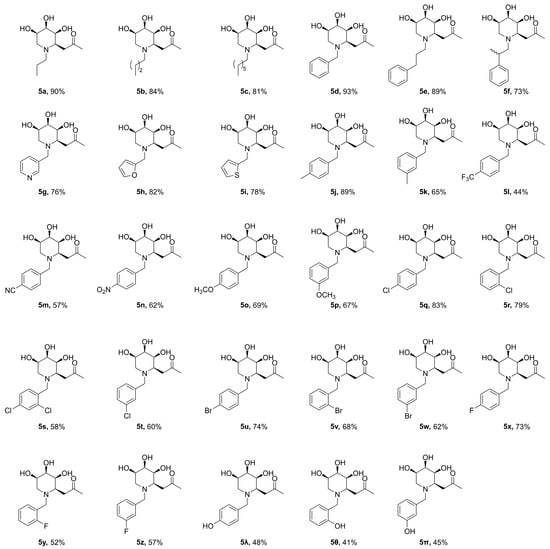
Figure 2.
Synthesized N-substituted iminosugar C-glycosides (5a–5π).
2.2. α-Glucosidase Inhibitory Assays
The inhibitory potency of the synthesized N-substituted iminosugar C-glycosides 5 was assessed by testing the compounds in assays for the α-glucosidase from yeast [24]. Compared to the positive control Acarbose (Glucobay®), the lead compound (4) suggests weak inhibitory activity against α-glucosidase (Figure 3). To improve the inhibitory potency against α-glucosidase of the lead compound, construction of the N-functionalized iminosugars was carried out. Installation of the N atom with aliphatic chain (5a, 5b, 5c) showed no improvement of α-glucosidase inhibition, where shortened or lengthened N-alkylated chain was invalid. Alternatively, N atom was modified with various benzaldehydes, giving a library of iminosugar C-glycosides containing phenyl. It was fascinating to discover that the introduction of a hydroxyl group at C4-position (5λ) of the aromatic ring improved the enzyme inhibition, whereas introducing a hydroxyl at C2 or C3 (5θ, 5π) reduced the inhibitory potency significantly. This indicates that a hydroxy in C4-position of the phenyl contributed to improving the enzyme inhibitory activity. These findings demonstrate that subtle changes in the iminosugar N-functionalized region may result in remarkable enzyme specificity. To identify more potent inhibitors, we also introduced halogen atoms onto the aromatic ring, and discovered that positioning Cl or Br at C2 (5r, 5v) enhanced the enzyme inhibition. However, the location of the halogen atom at C3 or C4-position (5q, 5t, 5u, 5w, 5x, 5y, 5z) of the phenyl doesn’t show any significant improvement for the inhibitory activity on the enzyme. The same result was obtained when introducing electron-donating groups as CH3(5j, 5k), OCH3(5o, 5p) or electron-withdrawing groups such as CF3(5l), NO2(5n), CN(5m).
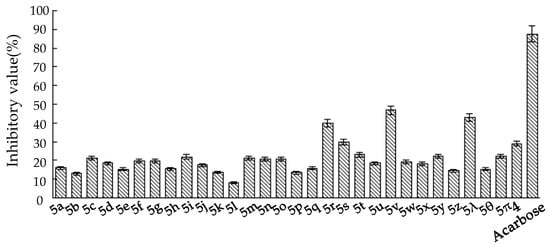
Figure 3.
The inhibitory value of the compounds against α-glucosidase from yeast at 1 µg/mL.
Given the above results, and in order to ascertain whether hydroxyl and halogen atoms would have a synergistic effect on the enhancement of the enzyme inhibition, we synthesized a series of iminosugar C-glycosides 6 containing hydroxyl at C4-position on phenyl and introducing halogen atoms at different positions (Scheme 3, Figure 4). The assay of their inhibition against α-glucosidase was listed in Table 2. Compared with compound 5λ, the installation of halogen atoms at C3-position (6a, 6b, 6c and 6i) can slightly improve the inhibition potency on the basis of the presence of hydroxyl at C4 position. However, introducing an alkoxyl at C3-position (6f, 6g) severely diminishes the inhibition, which may be due to the fact that the substituents are too large to allow the guest to fit into the enzyme pocket. Clearly, iminosugars substituted with chlorine at the C2, 6-position (or at C2) provide greater enhancement of inhibition than others, such as 6e, which has a 2-fold stronger inhibitory potency than the positive control Acarbose. The reason for this improvement in inhibition provided by additional chlorine atoms at C2, 6-position (or C2-position) and hydroxyl at C4-position on phenyl is not known but may be due to structural and spatial features of the enzyme, which allow the hydroxyl to bind with active site and chlorine atoms matching the pocket well.
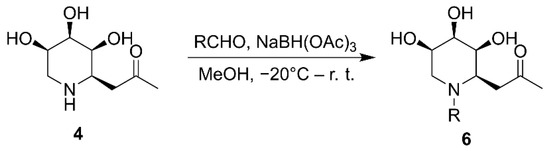
Scheme 3.
Synthesis of N-substituted iminosugar C-glycosides.
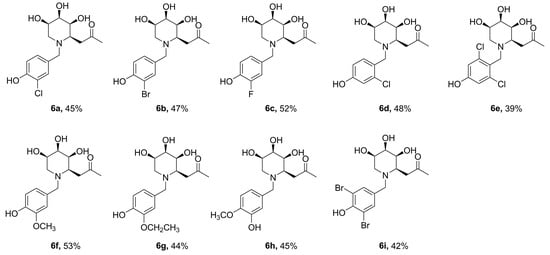
Figure 4.
Synthesized N-substituted iminosugar C-glycosides (6a–6i).

Table 2.
The IC50 value of the compounds against α-glucosidase from yeast.
The inhibitory mechanisms of glucosidases were divided into two distinct mechanisms: reversible inhibitors that have a high affinity for the enzyme, and irreversible inhibitors that react with carboxylic acid of the active site of the enzyme [25]. To determine which mechanism 6e belonged to, we removed the unreacted inhibitor from the enzyme solution by ultrafiltration [26], and examined whether the activity would be recovered or not by the method described in the literatures. When an enzyme solution containing 6e was subjected to ultrafiltration and then redissolved with the same concentration, the α-glucosidase activity was substantially recovered (Figure 5A). From this result, we can conclude that 6e is a reversible inhibitor against α-glucosidase.
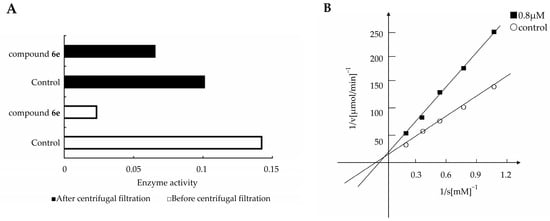
Figure 5.
(A) Recovery of enzymatic activity by elimination of 6e by centrifugal filtration. (B) Lineweaver–Burk plots of α-glucosidase without (○) and with (■) 6e.
Further analysis of the compound 6e, in order to reveal the type of enzyme inhibition on α-glucosidase, was done with different substrate concentration [7]. Based on Lineweaver-Burk plots (Figure 5B), compound 6e may be classified as an uncompetitive inhibitor with Ki = 8.6 µM. These results indicate that 6e may reversibly combine with only the E–S (enzyme–substrate) complex and inhibit the activity of this enzyme. It is specifically mentioned that uncompetitive inhibitors have a benefit over competitive inhibitors such as therapeutic drugs, since the inhibition is not overcome even when the substrate concentration reaches saturation.
2.3. Homology Modeling and Molecular Docking
Since the three-dimensional structure of α-glucosidase MAL12 in Saccharomyces cerevisiae is unknown, the FASTA format sequence of α-glucosidase is downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/ (accessed on 28 December 2021)). Furthermore, the three-dimensional structure of α-glucosidase was obtained by SWISS-MODEL [27,28,29,30,31] homology modeling method using 3AXH_A [32] (72% similarity) as template. The ligand was optimized using B3LYP [33,34] functional in gas phase, which was performed with in Gaussian 16 program [35]. Next, molecular docking was achieved employing AutoDock Vina [36]. PyMol and Ligplot [37] were applied to visualize the interactions between the ligand and the protein.
As shown in Figure 6, compound 6e forms hydrogen bonds with amino acid residues Lys155, Phe157, Asn241 and Arg312, as well as hydrophobic interactions with amino acid residues Leu176, Phe177, Leu218, His239, Pro240 and Pro309, which were close to the catalytic active site (Asp214) [38] of α-glucosidase, supporting the experimental conclusion that it is a noncompetitive inhibitor.
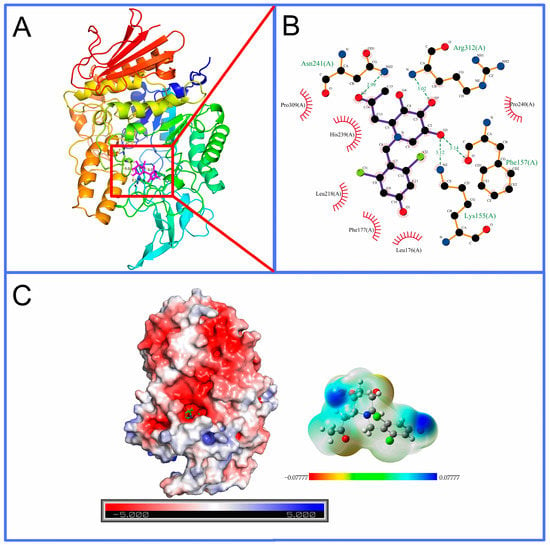
Figure 6.
Binding interactions results of molecular docking between compound 6e and α-glucosidase. (A) The ligand and protein are shown as magenta-colored stick models and colored cartoons, respectively. (B) Interactions between compound 6e and its surrounding amino acid residues. (C) The electrostatic potential (ESP) map of α-glucosidase and compound 6e. The scale of the ESP of α-glucosidase is −5.000 (negative, red) to 5.000 (positive, blue). The isovalue of 0.0004 electron/bohr3 was selected for the definition of the density surface of 6e with a scale of −0.07777 (red) to 0.07777 hartree (blue).
2.4. In Silico Analysis
Because some compounds showed good inhibitory activities, we chose to conduct in silico researches to evaluate their drug-likeness and pharmacokinetic properties that were carried out using the SwissADME [39] and the admetSAR [40] platforms. Satisfyingly, all compounds were found to have excellent obedience (75–100%) with different drug-likeness filters (Lipinski [41], Ghose [42], Veber [43], Egan [44], and Muegge [45]) (Table 3). In addition, the compounds 6d and 6e were found to have great average ADMET scores [46,47] (0.77, 0.79) in respect to human intestinal absorption, blood-brain barrier penetration, Caco-2 permeability, Ames mutagenicity, carcinogenicity, and acute oral toxicity class (Table 4).

Table 3.
Physiochemical properties of the compounds predicted by SwissADME and admetSAR.

Table 4.
ADMET score for human intestinal absorption, Caco-2 permeability, blood brain barrier, carcinogenicity, ames mutagenesis and acute oral toxicity as calculated by admetSAR.
3. Experimental
3.1. Chemistry
3.1.1. General Procedures
All reactions sensitive to air or moisture were carried out under nitrogen or argon with anhydrous solvents. All reagents were purchased from commercial suppliers and used without further purification unless otherwise noted. TLC was performed by using silica gel GF254 precoated plates (0.20–0.25 mm thickness) with a fluorescent indicator. Visualization of TLC plates was achieved by UV light (254 nm) and a typical TLC indicator solution (10 % sulfuric acid/ethanol solution). Column chromatography was performed on silica gel 90, 200–300 mesh. Optical rotations were measured with a Perkin–Elmer M341 digital polarimeter. 1H and 13C NMR spectra (600 and 150 MHz, respectively) were recorded with a Bruker Avance 600 spectrometer. 1H NMR chemical shifts are reported in ppm (δ) relative to tetramethylsilane (TMS) with the solvent resonance employed as the internal standard (DMSO-d6 or CD3OD). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration, and coupling constants [Hz]. 13C NMR chemical shifts are reported in ppm from TMS with the solvent resonance as the internal standard (DMSO-d6 or CD3OD). ESI-HRMS data were recorded with a BioTOF Q instrument.
3.1.2. Synthetic Procedures
A suspension of compound 4 (40 mg, 0.2 mmol) containing activated 4Å molecular sieves in anhydrous methanol (3 mL) was stirred at room temperature for 10 min. After cooling to −20 °C, the corresponding aldehydes (0.6 mmol, 3eq) and NaBH(OAc)3 (0.6 mmol, 3eq) were added, and the solution was stirred for 3 h under an argon atmosphere. The reaction solution was concentrated in vacuo and purified by silica gel flash column chromatography (dichloromethane/methanol, 25:1→5:1) to afford colorless syrupy products.
Detailed physicochemical properties of novel N-substituted iminosugar C-glycosides (see the supporting information for details):
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-n-propylpiperidine (5a)
colorless syrup, yield 90%, −55.0 (c 0.08, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 3.67 (s, 1H), 3.51–3.43 (m, 2H), 3.09 (dd, J = 8.5, 2.4 Hz, 1H), 2.92 (d, J = 5.7 Hz, 1H), 2.62 (dd, J = 16.7, 4.7 Hz, 1H), 2.47–2.41 (m, 1H), 2.37–2.28 (m, 1H), 2.28–2.23 (m, 1H), 2.15–2.10 (m, 1H), 2.09 (s, 3H), 1.37–1.28 (m, 2H), 0.78–0.72 (m, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 72.3, 70.7, 67.7, 56.9, 54.5, 52.0, 40.5, 30.6, 19.7, 12.1. ESI-HRMS: m/z calcd for C11H22NO4 [M+H]+: 232.1543; found: 232.1551.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-n-butylpiperidine (5b)
colorless syrup, yield 84%, −52.0 (c 0.01, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 3.66 (s, 1H), 3.45 (dd, J = 15.7, 9.9 Hz, 2H), 3.09 (d, J = 8.5 Hz, 1H), 2.91 (s, 1H), 2.63 (dd, J = 16.7, 4.6 Hz, 1H), 2.47–2.24 (m, 4H), 2.09 (s, 3H), 1.33–1.26 (m, 2H), 1.17 (ddd, J = 28.3, 14.0, 7.4 Hz, 2H), 0.83 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 72.3, 70.7, 67.7, 57.0, 52.3, 52.0, 40.5, 30.6, 28.8, 20.4, 14.3. ESI-HRMS: m/z calcd for C12H24O4NNa [M+Na]+: 246.1700; found: 246.1712.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-n-heptylpiperidine (5c)
colorless syrup, yield 81%, −31.3 (c 0.16, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 3.66 (s, 1H), 3.44 (s, 1H), 3.08 (d, J = 7.1 Hz, 1H), 2.90 (s, 1H), 2.62 (dd, J = 16.7, 4.3 Hz, 1H), 2.46–2.40 (m, 1H), 2.38–2.28 (m, 2H), 2.08 (d, J = 10.7 Hz, 3H), 1.37–1.09 (m, 12H), 0.84 (t, J = 7.0 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.1, 72.3, 70.7, 67.7, 57.0, 52.6, 52.0, 40.6, 31.7, 30.6, 29.0, 27.2, 26.5, 22.5, 14.4. ESI-HRMS: m/z calcd for C15H29O4NNa [M+Na]+: 310.1989; found: 310.1998.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-benzylpiperidine (5d)
colorless syrup, yield 93%, −34.4 (c 0.34, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.42–7.15 (m, 5H), 3.69 (d, J = 15.0 Hz, 1H), 3.65 (d, J = 13.5 Hz, 1H), 3.41 (s, 1H), 3.19 (d, J = 8.5 Hz, 1H), 3.11 (d, J = 13.5 Hz, 1H), 3.00 (s, 1H), 2.80 (dd, J = 17.0, 3.7 Hz, 1H), 2.62 (dd, J = 17.0, 6.4 Hz, 1H), 2.30 (dd, J = 11.0, 3.9 Hz, 1H), 2.22 (t, J = 10.4 Hz, 1H), 2.08 (d, J = 10.2 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 140.2, 128.9, 128.9, 128.6, 128.6, 127.2, 72.0, 72.0, 67.4, 57.3, 57.0, 52.1, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H22NO4 [M+H]+: 280.1543; found: 280.1552.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-benzylpropylpiperidine (5e)
colorless syrup, yield 89%, −25.5 (c 0.40, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.25 (t, J = 7.5 Hz, 2H), 7.21–7.10 (m, 3H), 3.66 (s, 1H), 3.46 (s, 1H), 3.08 (d, J = 7.7 Hz, 1H), 2.91 (s, 1H), 2.59 (dd, J = 17.0, 4.3 Hz, 1H), 2.50-2.45 (m, 2H), 2.40–2.27 (m, 3H), 2.12 (dt, J = 15.7, 8.4 Hz, 1H), 1.97 (d, J = 17.3 Hz, 3H), 1.71–1.57 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 208.1, 142.5, 128.8, 128.8, 128.7, 128.7, 126.1, 72.3, 70.6, 67.9, 56.8, 56.8, 51.9, 40.5, 33.0, 30.5, 28.2. ESI-HRMS: m/z calcd for C17H26NO4 [M+Na]+: 308.1856; found: 308.1863.
(1R, 2S, 3R, 4R, 2’R/S)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2’-benzyl-2’-methylethylpiperidine (5f)
colorless syrup, yield 73%, −53.3 (c 0.02, CH2Cl2). (R) 1H NMR (600 MHz, DMSO-d6) δ 7.25 (t, J = 7.5 Hz, 2H), 7.16 (dd, J = 7.5, 4.7 Hz, 2H), 3.65 (s, 1H), 3.52–3.44 (m, 1H), 3.14 (dd, J = 15.6, 8.4 Hz, 2H), 3.02 (s, 1H), 2.79 (dd, J = 13.9, 7.0 Hz, 1H), 2.65–2.58 (m, 2H), 2.56–2.39 (m, 2H), 2.35 (dd, J = 12.5, 9.9 Hz, 1H), 2.23 (dd, J = 12.9, 7.1 Hz, 1H), 2.07 (d, J = 2.1 Hz, 3H), 1.11 (d, J = 6.9 Hz, 2H). (S) 1H NMR (600 MHz, DMSO-d6) δ 7.25 (t, J = 7.5 Hz, 2H), 7.16 (dd, J = 7.5, 4.7 Hz, 2H), 3.59 (s, 1H), 3.41 (s, 1H), 3.20 (m, 2H), 2.91 (s, 1H), 2.79 (dd, J = 13.9, 7.0 Hz, 1H), 2.72–2.65 (m, 2H), 2.56–2.39 (m, 2H), 2.30 (t, J = 10.3 Hz, 1H), 2.15 (dd, J = 12.5, 5.0 Hz, 1H), 2.05 (s, 3H), 1.00 (d, J = 6.1 Hz, 2H). ESI-HRMS: m/z calcd for C17H25O4NNa [M+Na]+: 330.1676; found: 330.1685.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-pyridinemethylenepiperidine (5g)
colorless syrup, yield 76%, −10.8 (c 0.12, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 8.47–8.38 (m, 2H), 7.65 (d, J = 7.8 Hz, 1H), 7.31 (dd, J = 7.7, 4.8 Hz, 1H), 3.70 (s, 1H), 3.66 (d, J = 13.8 Hz, 1H), 3.42 (dd, J = 6.8, 4.3 Hz, 1H), 3.19 (dd, J = 8.4, 6.1 Hz, 2H), 3.03 (s, 1H), 2.80 (dd, J = 17.1, 4.3 Hz, 1H), 2.65 (dd, J = 17.1, 6.3 Hz, 1H), 2.25 (d, J = 6.9 Hz, 2H), 2.10 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 150.3, 148.5, 136.7, 135.5, 123.8, 71.9, 70.7, 67.3, 57.2, 54.1, 51.9, 40.5, 30.6. ESI-HRMS: m/z calcd for C14H20O4N2Na [M+Na]+: 303.1326; found: 303.1315.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-furanmethylenepiperidine (5h)
colorless syrup, yield 82%, −38.8 (c 0.26, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.54 (s, 1H), 6.36 (d, J = 1.9 Hz, 1H), 6.23 (d, J = 3.0 Hz, 1H), 3.67 (s, 1H), 3.57 (t, J = 12.3 Hz, 1H), 3.44–3.10 (m, 3H, overlap), 3.13 (dd, J = 9.1, 2.6 Hz, 1H), 2.89–2.80 (m, 1H), 2.66 (dd, J = 9.8, 5.3 Hz, 1H), 2.39 (dd, J = 11.0, 4.5 Hz, 1H), 2.32 (t, J = 10.5 Hz, 1H), 2.09 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 208.2, 152.7, 142.6, 110.7, 109.0, 72.3, 71.0, 67.4, 56.6, 52.5, 49.5, 40.5, 30.7. ESI-HRMS: m/z calcd for C13H19O5NNa [M+Na]+: 292.1165; found: 292.1155.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-thiophenemethylenepiperidine (5i)
colorless syrup, yield 78%, −40.0 (c 0.16, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.37 (t, J = 10.4 Hz, 1H), 6.98–6.87 (m, 2H), 3.77 (d, J = 14.3 Hz, 1H), 3.70 (s, 1H), 3.50 (d, J = 14.3 Hz, 1H), 3.43 (s, 1H), 3.21–3.12 (m, 1H), 2.97 (d, J = 4.4 Hz, 1H), 2.76 (dd, J = 16.9, 4.4 Hz, 1H), 2.65–2.55 (m, 1H), 2.43 (dd, J = 11.0, 4.2 Hz, 1H), 2.29 (t, J = 10.4 Hz, 1H), 2.08 (d, J = 14.7 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 143.8, 126.9, 126.0, 125.5, 72.0, 70.8, 67.3, 56.8, 52.2, 51.7, 44.6, 30.6. ESI-HRMS: m/z calcd for C13H20NO4S [M+H]+: 286.1108; found: 286.1106.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-methylbenzylpiperidine (5j)
colorless syrup, yield 89%, −31.8 (c 0.27, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.10 (dd, J = 24.3, 7.8 Hz, 4H), 3.69 (s, 1H), 3.59 (d, J = 13.3 Hz, 1H), 3.42–3.36 (m, 1H), 3.23–3.15 (m, 1H), 3.05 (d, J = 13.3 Hz, 1H), 2.96 (s, 1H), 2.78 (dd, J = 17.0, 3.9 Hz, 1H), 2.61 (dd, J = 17.0, 6.3 Hz, 1H), 2.28 (dd, J = 16.1, 6.9 Hz, 1H), 2.25 (s, 3H), 2.18 (t, J = 10.5 Hz, 1H), 2.08 (d, J = 10.5 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 137.0, 136.2, 129.1, 129.1, 129.0, 129.0, 71.9, 70.8, 67.3, 57.3, 56.7, 51.9, 44.6, 30.6, 21.1. ESI-HRMS: m/z calcd for C16H24NO4 [M+H]+: 294.1700; found: 294.1714.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-methylbenzylpiperidine (5k)
colorless syrup, yield 65%, −56.6 (c 0.06, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.16 (t, J = 7.5 Hz, 1H), 7.03 (dd, J = 18.8, 9.4 Hz, 3H), 3.69 (d, J = 23.2 Hz, 1H), 3.61 (d, J = 13.3 Hz, 1H), 3.40 (s, 1H), 3.18 (t, J = 7.1 Hz, 1H), 3.05 (d, J = 13.4 Hz, 1H), 2.97 (s, 1H), 2.80 (dd, J = 17.2, 4.0 Hz, 1H), 2.61 (dd, J = 17.0, 6.4 Hz, 1H), 2.29 (dd, J = 12.1, 5.8 Hz, 2H), 2.26 (s, 3H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 140.1, 137.6, 129.6, 128.4, 127.8, 126.1, 72.1, 71.0, 67.4, 57.3, 57.1, 52.1, 44.8, 30.6, 21.5. ESI-HRMS: m/z calcd for C16H24NO4 [M+H]+: 294.1700; found: 294.1705.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-trifluoromethylbenzylpiperidine (5l)
colorless syrup, yield 44%, −40.0 (c 0.02, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.60 (s, 1H), 7.57 (d, J = 7.8 Hz, 2H), 7.54–7.50 (m, 1H), 3.72 (d, J = 14.2 Hz, 2H), 3.44 (s, 1H), 3.28 (d, J = 14.1 Hz, 1H, overlap), 3.22 (d, J = 6.4 Hz, 1H), 3.05 (t, J = 9.5 Hz, 1H), 2.80 (dd, J = 17.1, 4.2 Hz, 1H), 2.64 (dd, J = 17.1, 6.4 Hz, 1H), 2.30–2.24 (m, 2H), 2.08 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 142.0, 133.0, 133.0, 129.6, 129.6, 125.2, 123.9, 72.0, 70.7, 67.4, 57.2, 56.2, 52.2, 44.2, 40.5, 30.6. ESI-HRMS: m/z calcd for C16H20NF3O4Na [M+Na]+: 370.1237; found: 370.1246.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-cyanobenzylpiperidine (5m)
colorless syrup, yield 57%, 0 (c 0.09, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.58 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.2 Hz, 2H), 3.70 (s, 1H), 3.64 (s, 1H), 3.44 (s, 1H), 3.21 (d, J = 8.5 Hz, 1H), 3.10 (d, J = 7.3 Hz, 1H), 3.04 (s, 1H), 2.79 (dd, J = 17.2, 4.5 Hz, 1H), 2.60 (dd, J = 17.0, 6.4 Hz, 1H), 2.26 (dt, J = 11.1, 9.0 Hz, 2H), 2.08 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 146.7, 145.9, 132.7, 132.7, 132.5, 132.5, 129.7, 73.4, 72.0, 71.3, 56.4, 52.3, 49.1, 40.5, 30.6. ESI-HRMS: m/z calcd for C16H20N2O4Na [M+Na]+: 327.1315; found: 327.1329.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-nitrobenzylpiperidine (5n)
colorless syrup, yield 62%, −30.0 (c 0.10, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.59 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 8.2 Hz, 2H), 3.70 (s, 1H), 3.64 (s, 1H), 3.44 (s, 1H), 3.21 (d, J = 8.5 Hz, 1H), 3.10 (d, J = 7.3 Hz, 1H), 3.04 (s, 1H), 2.79 (dd, J = 17.2, 4.5 Hz, 1H), 2.60 (dd, J = 17.0, 6.4 Hz, 1H), 2.26 (dt, J = 11.1, 9.0 Hz, 2H), 2.08 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 148.1, 147.8, 129.8, 129.7, 123.9, 123.7, 73.4, 72.7, 58.6, 57.2, 56.2, 49.1, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H20N2O6Na [M+Na]+: 347.1315; found: 347.1328.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-methoxylbenzylpiperidine (5o)
colorless syrup, yield 69%, +10.0 (c 0.12, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.15 (t, J = 8.6 Hz, 2H), 6.84 (d, J = 8.5 Hz, 2H), 3.73 (s, 1H), 3.70 (m, 1H, overlap), 3.57 (d, J = 13.3 Hz, 1H), 3.36 (d, J = 11.8 Hz, 1H), 3.17 (d, J = 8.5 Hz, 1H), 3.03 (d, J = 13.1 Hz, 1H), 2.96 (s, 1H), 2.78 (dd, J = 17.0, 4.0 Hz, 1H), 2.62 (dd, J = 17.0, 6.4 Hz, 1H), 2.36 (s, 1H), 2.28 (dd, J = 11.1, 4.1 Hz, 1H), 2.17 (t, J = 10.4 Hz, 1H), 2.08 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 158.6, 131.8, 131.8, 131.8, 114.0, 114.0, 79.6, 73.0, 71.9, 57.3, 56.3, 55.5, 51.8, 40.5, 30.6. ESI-HRMS: m/z calcd for C16H24NO5 [M+H]+: 310.1649; found: 310.1659.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-methoxylbenzylpiperidine (5p)
colorless syrup, yield 67%, −47.5 (c 0.08, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.20 (dd, J = 18.7, 11.0 Hz, 1H), 6.82 (d, J = 8.6 Hz, 2H), 6.77 (d, J = 7.6 Hz, 1H), 3.72 (s, 1H), 3.71 (s, 2H), 3.61 (d, J = 13.3 Hz, 1H), 3.43 (s, 1H), 3.20 (s, 1H), 3.15 (d, J = 5.0 Hz, 1H), 3.11 (d, J = 13.6 Hz, 1H), 3.00 (s, 1H), 2.79 (d, J = 13.6 Hz, 1H), 2.66–2.55 (m, 1H), 2.31 (d, J = 7.4 Hz, 1H), 2.23 (d, J = 10.2 Hz, 1H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 159.7, 141.9, 129.6, 121.1, 114.4, 112.5, 72.1, 70.8, 67.5, 57.3, 56.9, 55.4, 52.1, 49.1, 30.6. ESI-HRMS: m/z calcd for C16H24NO5 [M+H]+: 310.1649; found: 310.1658.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-chlorobenzylpiperidine (5q)
colorless syrup, yield 83%, −9.6 (c 0.26, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.33 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.3 Hz, 2H), 3.70 (s, 1H), 3.62 (d, J = 13.8 Hz, 1H), 3.42 (d, J = 4.8 Hz, 1H), 3.20 (d, J = 8.1 Hz, 1H), 3.17–3.11 (m, 1H), 3.00 (t, J = 9.6 Hz, 1H), 2.79 (dd, J = 17.1, 4.3 Hz, 1H), 2.61 (dd, J = 17.1, 6.3 Hz, 1H), 2.27–2.19 (m, 2H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 139.6, 131.6, 130.7, 130.7, 128.5, 128.5, 79.6, 73.2, 72.0, 67.4, 56.1, 52.0, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H20O4NClNa [M+Na]+: 336.0973; found: 336.0985.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-chlorobenzylpiperidine (5r)
colorless syrup, yield 79%, −31.2 (c 0.24, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.46 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 7.9 Hz, 1H), 7.28 (t, J = 7.3 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 3.70 (s, 1H), 3.63 (d, J = 14.4 Hz, 1H), 3.42–3.36 (m, 1H), 3.20 (d, J = 11.1 Hz, 1H), 3.09 (d, J = 21.0 Hz, 1H), 2.80 (dd, J = 17.3, 4.0 Hz, 1H), 2.62 (dd, J = 17.3, 6.3 Hz, 1H), 2.35–2.29 (m, 2H), 2.06 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.1, 137.3, 133.5, 131.1, 129.5, 128.8, 127.3, 72.1, 72.0, 67.5, 57.6, 54.1, 52.2, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H21NClO4 [M+H]+: 314.1154; found: 314.1163.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2,4-dichlorobenzylpiperidine (5s)
colorless syrup, yield 58%, +16.7 (c 0.09, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.52 (d, J = 2.1 Hz, 1H), 7.50 (d, J = 8.3 Hz, 1H), 7.39 (dd, J = 8.3, 2.0 Hz, 1H), 3.70 (s, 1H), 3.64–3.59 (m, 1H), 3.44 (s, 1H), 3.32 (d, J = 7.9 Hz, 1H), 3.19 (d, J = 11.0 Hz, 1H), 3.12–3.03 (m, 1H), 2.78 (dt, J = 17.2, 8.7 Hz, 1H), 2.61 (dd, J = 17.2, 6.2 Hz, 1H), 2.34 (dd, J = 20.5, 9.3 Hz, 1H), 2.28 (dd, J = 11.2, 4.0 Hz, 1H), 2.08 (d, J = 5.8 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 136.7, 134.3, 134.1, 132.4, 128.9, 127.6, 73.6, 72.8, 72.1, 55.3, 53.5, 52.3, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H19O4NCl2Na [M+Na]+: 370.0583; found: 370.0599.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-chlorobenzylpiperidine (5t)
colorless syrup, yield 60%, −31.3 (c 0.08, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.31 (d, J = 4.0 Hz, 2H), 7.26 (d, J = 8.2 Hz, 1H), 7.21 (d, J = 7.4 Hz, 1H), 3.71 (d, J = 3.0 Hz, 1H), 3.64 (d, J = 13.9 Hz, 1H), 3.49–3.41 (m, 1H), 3.23–3.17 (m, 2H), 3.01 (d, J = 13.1 Hz, 1H), 2.79 (dd, J = 17.1, 4.3 Hz, 1H), 2.62 (dd, J = 17.2, 6.4 Hz, 1H), 2.30–2.22 (m, 2H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 143.1, 133.4, 130.4, 128.5, 127.6, 127.1, 72.0, 70.8, 67.4, 57.2, 56.2, 52.1, 49.1, 30.6. ESI-HRMS: m/z calcd for C15H21ClNO4 [M+ H]+: 314.1154; found: 314.1155.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-bromobenzylpiperidine (5u)
colorless syrup, yield 74%, −19.5 (c 0.20, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.47 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 8.2 Hz, 2H), 3.70 (s, 1H), 3.59 (t, J = 14.2 Hz, 1H), 3.39 (d, J = 23.1 Hz, 1H), 3.18 (t, J = 12.2 Hz, 1H), 3.12 (d, J = 14.4 Hz, 1H), 3.03–2.97 (m, 1H), 2.79 (dd, J = 17.1, 4.4 Hz, 1H), 2.61 (dd, J = 17.1, 6.3 Hz, 1H), 2.28–2.20 (m, 1H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 139.8, 131.4, 131.4, 131.1, 131.1, 120.1, 72.0, 72.0, 67.4, 57.3, 56.1, 52.1, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H21O4NBrNa [M+Na]+: 358.0648; found: 358.0649.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-bromobenzylpiperidine (5v)
colorless syrup, yield 68%, −19.2 (c 0.12, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.55 (t, J = 7.8 Hz, 1H), 7.47 (d, J = 7.4 Hz, 1H), 7.34 (t, J = 7.4 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 3.70 (d, J = 12.1 Hz, 1H), 3.61 (d, J = 14.4 Hz, 1H), 3.44 (s, 1H), 3.31 (d, J = 8.1 Hz, 2H), 3.21 (s, 1H), 3.16 (s, 1H), 3.08 (s, 1H), 2.80 (dd, J = 17.2, 4.1 Hz, 1H), 2.64 (dd, J = 17.3, 6.3 Hz, 1H), 2.07 (d, J = 15.6 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 138.9, 132.8, 131.3, 129.2, 128.0, 124.0, 72.2, 70.6, 67.7, 57.6, 56.6, 55.4, 52.3, 30.6. ESI-HRMS: m/z calcd for C15H20NBrO5Na [M+Na]+: 380.0468; found: 380.0459.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-bromobenzylpiperidine (5w)
colorless syrup, yield 62%, −16.0 (c 0.10, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.45 (s, 1H), 7.41–7.38 (m, 1H), 7.25 (d, J = 5.7 Hz, 2H), 3.70 (s, 1H), 3.64 (t, J = 11.4 Hz, 1H), 3.45–3.40 (m, 1H), 3.19 (dd, J = 14.9, 6.3 Hz, 2H), 3.02 (dd, J = 25.2, 7.1 Hz, 1H), 2.79 (dd, J = 17.1, 4.2 Hz, 1H), 2.62 (dd, J = 17.1, 6.4 Hz, 1H), 2.32–2.20 (m, 2H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 143.4, 131.4, 130.7, 130.0, 128.0, 122.1, 72.0, 70.8, 67.4, 57.2, 56.2, 52.2, 49.1, 30.6. ESI-HRMS: m/z calcd for C15H21BrNO4 [M+ H]+: 358.0648; found: 358.0659.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-fluorobenzylpiperidine (5x)
colorless syrup, yield 73%, −25.9 (c 0.22, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.27 (dd, J = 8.4, 5.8 Hz, 2H), 7.09 (t, J = 8.7 Hz, 2H), 3.68 (d, J = 20.6 Hz, 1H), 3.61 (d, J = 13.5 Hz, 1H), 3.43–3.39 (m, 1H), 3.19 (dd, J = 8.7, 2.3 Hz, 1H), 3.12 (d, J = 13.4 Hz, 1H), 3.00 (dd, J = 11.8, 7.4 Hz, 1H), 2.79 (dd, J = 17.0, 4.4 Hz, 1H), 2.62 (dd, J = 17.1, 6.4 Hz, 1H), 2.27 (dd, J = 11.1, 4.3 Hz, 1H), 2.21 (t, J = 10.4 Hz, 1H), 2.09 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 162.4, 136.3, 130.7, 130.7, 115.3, 115.2, 79.6, 71.9, 67.3, 57.3, 56.0, 51.9, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H21NFO4 [M+H]+: 298.1449; found: 298.1459.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-fluorobenzylpiperidine (5y)
colorless syrup, yield 52%, −30.0 (c 0.06, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.39 (t, J = 7.0 Hz, 1H), 7.27 (dd, J = 13.3, 5.8 Hz, 1H), 7.20–7.05 (m, 2H), 3.69 (d, J = 19.8 Hz, 1H), 3.62 (d, J = 13.8 Hz, 1H), 3.41 (s, 1H), 3.22 (d, J = 13.7 Hz, 1H), 3.20–3.14 (m, 1H), 3.04–2.95 (m, 1H), 2.81 (dd, J = 17.2, 4.3 Hz, 1H), 2.64 (dd, J = 17.2, 6.2 Hz, 1H), 2.35–2.23 (m, 2H), 2.09 (d, J = 15.9 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 162.0, 131.6, 129.2, 126.5, 124.6, 115.4, 72.1, 70.8, 67.4, 57.4, 52.1, 49.8, 40.6, 30.6. ESI-HRMS: m/z calcd for C15H21FNO4 [M+H]+: 298.1449; found: 298.1455.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-fluorobenzylpiperidine (5z)
colorless syrup, yield 57%, −35.0 (c 0.10, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.31 (dd, J = 14.2, 7.8 Hz, 1H), 7.11–7.06 (m, 2H), 7.02 (t, J = 7.5 Hz, 1H), 3.69 (d, J = 18.6 Hz, 1H), 3.65 (d, J = 14.0 Hz, 1H), 3.43 (d, J = 5.5 Hz, 1H), 3.20 (dd, J = 17.3, 8.7 Hz, 2H), 3.03 (d, J = 5.4 Hz, 1H), 2.79 (dd, J = 17.1, 4.2 Hz, 1H), 2.61 (dd, J = 17.1, 6.3 Hz, 1H), 2.32–2.22 (m, 2H), 2.08 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 161.9, 143.6, 130.4, 124.8, 115.2, 113.8, 72.0, 70.8, 67.4, 57.2, 56.3, 52.2, 49.1, 30.6. ESI-HRMS: m/z calcd for C15H21FNO4 [M+H]+: 298.1449; found: 298.1457.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-hydroxylbenzylpiperidine (5λ)
colorless syrup, yield 48%, +22.2 (c 0.18, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.03 (t, J = 15.2 Hz, 2H), 6.67 (t, J = 8.7 Hz, 2H), 3.70 (d, J = 14.8 Hz, 1H), 3.51 (t, J = 13.2 Hz, 1H), 3.38 (s, 1H), 3.17 (t, J = 14.1 Hz, 1H), 3.02–2.89 (m, 1H), 2.77 (d, J = 21.1 Hz, 1H), 2.64–2.55 (m, 1H), 2.29 (dd, J = 11.0, 4.2 Hz, 1H), 2.15 (t, J = 10.5 Hz, 1H), 2.08 (d, J = 12.3 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.4, 156.6, 130.1, 130.1, 130.1, 130.1, 115.3, 79.6, 72.0, 67.5, 57.3, 56.5, 51.8, 40.6, 30.6. ESI-HRMS: m/z calcd for C15H22NO5 [M+H]+: 296.1492; found: 296.1496.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-hydroxylbenzylpiperidine (5θ)
colorless syrup, yield 41%, −34.4 (c 0.16, CH2Cl2). 1H NMR (600 MHz, DMSO-d6) δ 7.08–7.00 (m, 2H), 6.73–6.66 (m, 2H), 3.75 (d, J = 13.9 Hz, 1H), 3.70 (s, 1H), 3.46 (s, 1H), 3.41–3.10 (m, 2H, overlap),3.04 (s, 1H), 2.71 (dd, J = 20.8, 5.8 Hz, 1H), 2.60 (s, 1H), 2.43 (dd, J = 11.4, 3.8 Hz, 1H), 2.34 (dd, J = 20.1, 10.6 Hz, 1H), 2.07 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 207.9, 157.2, 129.5, 128.5, 123.6, 119.2, 115.8, 79.6, 71.6, 67.5, 57.3, 54.8, 52.0, 40.5, 30.6. ESI-HRMS: m/z calcd for C15H21O5NNa [M+Na]+: 318.1318; found: 318.1312.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-hydroxylbenzylpiperidine (5π)
colorless syrup, yield 45%, +21.1 (c 0.08, MeOH). 1H NMR (600 MHz, CD3OD) δ 7.13 (t, J = 8.0 Hz, 1H), 6.80 (d, J = 6.6 Hz, 2H), 6.70 (d, J = 7.6 Hz, 1H), 3.91 (s, 1H), 3.80 (d, J = 13.0 Hz, 1H), 3.72 (s, 1H), 3.51 (s, 1H), 3.41 (d, J = 13.6 Hz, 1H), 2.89 (d, J = 5.4 Hz, 1H), 2.70–2.62 (m, 1H), 2.57 (s, 1H), 2.19 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.0, 157.3, 129.1, 129.1, 120.1, 115.7, 114.2, 71.3, 67.2, 63.7, 57.6, 57.2, 50.8, 48.4, 29.0. ESI-HRMS: m/z calcd for C15H21NO4Na [M+Na]+: 318.1312; found: 318.1308.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-chloro-4-hydroxylbenzylpiperidine (6a)
colorless syrup, yield 45%, +13.3 (c 0.12, MeOH). 1H NMR (600 MHz, CD3OD) δ 7.25 (d, J = 1.9 Hz, 1H), 7.05 (dd, J = 8.2, 1.9 Hz, 1H), 6.84 (d, J = 8.3 Hz, 1H), 3.86 (s, 1H), 3.64 (dd, J = 10.9, 7.4 Hz, 2H), 3.42 (d, J = 8.0 Hz, 1H), 3.26–3.15 (m, 2H), 2.89–2.82 (m, 1H), 2.78 (dd, J = 17.2, 5.7 Hz, 1H), 2.54 (dd, J = 11.5, 4.0 Hz, 1H), 2.47–2.39 (m, 1H), 2.18 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.2, 152.0, 130.9, 130.0, 128.2, 120.1, 115.9, 72.0, 70.1, 67.6, 57.3, 56.0, 50.9, 48.4, 28.9. ESI-HRMS: m/z calcd for C15H21ClNO5 [M+H]+: 330.1103; found: 330.1108.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-bromo-4-hydroxylbenzylpiperidine (6b)
colorless syrup, yield 47%, +11.5 (c 0.13, MeOH). 1H NMR (600 MHz, CD3OD) δ 7.42 (d, J = 1.9 Hz, 1H), 7.09 (dd, J = 8.2, 1.9 Hz, 1H), 6.83 (d, J = 8.2 Hz, 1H), 3.86 (s, 1H), 3.64 (t, J = 10.9 Hz, 2H), 3.42 (d, J = 5.7 Hz, 1H), 3.25–3.17 (m, 2H), 2.88–2.83 (m, 1H), 2.78 (dd, J = 17.2, 5.8 Hz, 1H), 2.55 (dd, J = 11.6, 4.0 Hz, 1H), 2.47–2.38 (m, 1H), 2.18 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.2, 153.1, 133.1, 131.3, 128.9, 115.6, 109.3, 72.0, 70.1, 67.6, 57.2, 55.9, 50.9, 48.2, 29.0. ESI-HRMS: m/z calcd for C15H21BrNO5 [M+H]+: 374.0598; found: 374.0598.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-fluoro-4-hydroxylbenzylpiperidine (6c)
colorless syrup, yield 52%, +13.6 (c 0.16, MeOH). 1H NMR (600 MHz, CD3OD) δ 7.06–7.01 (m, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.83 (t, J = 8.6 Hz, 1H), 3.85 (d, J = 14.6 Hz, 1H), 3.65 (t, J = 10.1 Hz, 2H), 3.47–3.39 (m, 1H), 3.25 (d, J = 13.4 Hz, 1H), 3.20 (d, J = 19.1 Hz, 1H), 2.85 (dd, J = 17.4, 5.3 Hz, 1H), 2.78 (dd, J = 17.1, 5.7 Hz, 1H), 2.55 (dd, J = 11.6, 4.0 Hz, 1H), 2.48–2.40 (m, 1H), 2.17 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.8, 154.5, 150.7, 142.9, 130.5, 126.2, 117.9, 71.9, 70.0, 67.6, 56.1, 53.8, 50.9, 48.5, 28.9. ESI-HRMS: m/z calcd for C15H20NFO5Na [M+Na]+: 336.1218; found: 336.1209.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2-chloro-4-hydroxylbenzylpiperidine (6d)
colorless syrup, yield 48%, +14.2 (c 0.14, MeOH). 1H NMR (600 MHz, CD3OD) δ 7.27 (d, J = 8.4 Hz, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.71–6.68 (m, 1H), 3.85 (s, 1H), 3.72–3.67 (m, 1H), 3.66–3.61 (m, 1H), 3.43 (d, J = 5.3 Hz, 1H), 3.39 (d, J = 13.6 Hz, 1H), 3.27 (d, J = 6.8 Hz, 1H), 2.85 (d, J = 5.6 Hz, 2H), 2.58 (dd, J = 11.6, 4.0 Hz, 1H), 2.54–2.47 (m, 1H), 2.18 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.2, 157.2, 134.3, 131.8, 126.4, 115.7, 113.8, 71.9, 69.9, 67.7, 57.6, 53.5, 50.9, 48.5, 28.9. ESI-MS: m/z calcd for C15H20NClO5Na [M+ Na]+: 352.0922; found: 352.0924.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-2, 6-dichloro-4-hydroxylbenzylpiperidine (6e)
colorless syrup, yield 39%, +6.67 (c 0.06, MeOH). 1H NMR (600 MHz, DMSO-d6) δ 6.80 (s, 1H), 6.78 (s, 1H), 3.71 (s, 1H), 3.65 (s, 1H), 3.56 (dd, J = 9.6, 5.8 Hz, 1H), 3.51 (d, J = 12.5 Hz, 1H), 3.15 (s, 1H), 3.13–3.04 (m, 2H), 2.99 (s, 1H), 2.80 (d, J = 6.5 Hz, 1H), 2.39–2.27 (m, 2H), 2.14 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 158.3, 136.8, 124.5, 118.4, 116.1, 115.2, 72.9, 71.5, 68.0, 58.9, 51.9, 51.1, 48.5, 30.6. ESI-MS: m/z calcd for C15H24Cl2NO5 [M+H]+: 364.0713; found: 364.0704.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-4-hydroxyl-methoxylbenzylpiperidine (6f)
colorless syrup, yield 53%, +3.3 (c 0.24, MeOH). 1H NMR (600 MHz, CD3OD) δ 6.93 (s, 1H), 6.73 (s, 2H), 3.87 (s, 1H), 3.83 (d, J = 8.4 Hz, 2H), 3.71 (t, J = 14.8 Hz, 1H), 3.69–3.63 (m, 1H), 3.45 (d, J = 6.0 Hz, 1H), 3.34 (s, 3H), 3.24 (d, J = 20.5 Hz, 1H), 2.85 (t, J = 7.7 Hz, 1H), 2.61 (dd, J = 11.6, 4.0 Hz, 1H), 2.48 (t, J = 10.4 Hz, 1H), 2.18 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.1, 147.6, 145.6, 129.1, 121.6, 114.5, 112.4, 71.8, 70.0, 67.4, 56.9, 55.0, 55.0, 50.8, 48.5, 29.0. ESI-HRMS: m/z calcd for C16H23NO6Na [M+ Na]+: 348.1418; found: 348.1410.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-ethyoxyl-4-hydroxylbenzylpiperidine (6g)
colorless syrup, yield 44%, +11.0 (c 0.10, MeOH). 1H NMR (600 MHz, DMSO-d6) δ 6.78 (s, 1H), 6.71–6.65 (m, 1H), 6.60 (d, J = 7.5 Hz, 1H), 3.96 (dd, J = 13.0, 6.2 Hz, 2H), 3.69 (s, 1H), 3.52 (d, J = 13.2 Hz, 1H), 3.41 (s, 1H), 3.18 (s, 1H), 3.06–2.92 (m, 2H), 2.76 (dd, J = 16.9, 4.2 Hz, 1H), 2.61 (dd, J = 16.8, 6.2 Hz, 1H), 2.32 (dd, J = 11.2, 4.0 Hz, 1H), 2.18 (t, J = 10.2 Hz, 1H), 2.09 (s, 3H), 1.30 (t, J = 6.9 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.3, 146.9, 146.1, 130.7, 121.4, 115.6, 114.5, 72.1, 67.6, 64.3, 57.4, 56.7, 56.7, 51.8, 44.5, 30.6, 15.3. ESI-HRMS: m/z calcd for C17H25NO6Na [M+Na]+: 362.1574; found: 362.1573.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3-hydroxyl-4-methoxylbenzylpiperidine (6h)
colorless syrup, yield 45%, +21.6 (c 0.12, MeOH). 1H NMR (600 MHz, CD3OD) δ 6.86 (d, J = 8.2 Hz, 1H), 6.82 (d, J = 1.8 Hz, 1H), 6.75 (d, J = 8.2 Hz, 1H), 3.88 (s, 1H), 3.83 (s, 3H), 3.72 (d, J = 13.1 Hz, 1H), 3.66 (dd, J = 9.1, 3.4 Hz, 1H), 3.48–3.43 (m, 1H), 3.28 (d, J = 13.3 Hz, 1H), 3.26–3.17 (m, 1H), 2.89 (dd, J = 17.3, 5.0 Hz, 1H), 2.84 (dd, J = 17.3, 5.6 Hz, 1H), 2.61 (dd, J = 11.6, 4.0 Hz, 1H), 2.52–2.44 (m, 1H), 2.19 (s, 3H). 13C NMR (150 MHz, CD3OD) δ 209.2, 147.1, 146.2, 130.5, 120.2, 115.8, 111.2, 71.6, 68.7, 67.3, 57.4, 56.8, 55.1, 50.8, 48.2, 29.0. ESI-MS: m/z calcd for C16H23NO6Na [M+Na]+: 348.1418; found: 348.1410.
(1R, 2S, 3R, 4R)-1-Acetylmethyl-2, 3, 4-trihydroxyl-N-3, 5-dibromo-4-hydroxylbenzylpiperidine (6i)
colorless syrup, yield 42%, +13.8 (c 0.08, MeOH). 1H NMR (600 MHz, DMSO-d6) δ 7.40 (s, 2H), 3.69 (s, 1H), 3.53 (d, J = 13.7 Hz, 1H), 3.42 (s, 1H), 3.18 (s, 1H), 3.08 (d, J = 13.8 Hz, 1H), 2.97 (s, 1H), 2.77 (d, J = 17.3 Hz, 1H), 2.62 (dd, J = 17.1, 6.4 Hz, 1H), 2.28 (d, J = 7.5 Hz, 1H), 2.24 (d, J = 9.9 Hz, 1H), 2.08 (d, J = 6.7 Hz, 3H). 13C NMR (150 MHz, DMSO-d6) δ 208.2, 170.8, 149.8, 132.6, 132.6, 112.2, 112.2, 73.2, 72.0, 67.6, 60.2, 55.4, 51.9, 49.1, 30.6. ESI-HRMS: m/z calcd for C15H19NBr2O5Na [M+Na]+: 473.9522; found: 473.9515.
3.2. α-Glucosidase Inhibitory Activity Test
The extracts of each sample were dissolved in distilled water to prepare a certain concentration. 150 µL of the sample solution was mixed with 150 µL of maltose solution (prepared with 0.2 M, pH = 6.8 phosphate buffer) at a certain concentration, and cultured at 37 °C for 10 min. Then 150 µL of the enzyme solution at a certain concentration was added and mixed. The reaction was carried out at 37 °C for 60 min. The reaction was terminated in a boiling water bath for 10 min and centrifuged at 3000 r/min for 10 min. The supernatant was taken to determine the content of glucose with Glu kit. Acarbose solution was used as positive control, and blank control (distilled water instead of enzyme solution and sample solution) and negative control (distilled water instead of sample solution) were set. Enzyme activity inhibition rate (%) = (negative-sample)/(negative-blank) × 100%.
Enzyme kinetics experiment: The principle of the experimental method is the same as that of the above method, in which the micro-membrane centrifuge tube with a pore diameter of 3K is used for small molecule centrifugal separation. In the enzyme kinetics experiment, the concentration of enzyme was set at a fixed concentration, and five concentration gradients were designed for the solution of substrate maltose. The linear relationship was plotted using the obtained results combined with the Michaelis-Menten equation.
4. Conclusions
In summary, a series of N-substituted iminosugar C-glycosides were synthesized, and their α-glucosidase inhibition was assessed. Compound 6e showed stronger inhibitory potency than positive control Acarbose. Besides, the inhibition of compound 6d was equivalent to positive control Acarbose. Enzymatic kinetic assays indicated that compound 6e may be classified as a reversible and uncompetitive inhibitor. The study of structure-activity relationships provided a starting point for the discovery of new more promising α-glucosidase inhibitors. Moreover, the experimental result was in good agreement with the docking result that presented evidence for the inhibition mechanism of compound 6e. Most importantly, compounds 6d and 6e were identified to have excellent performance in drug-likeness and pharmacokinetic properties in Silico Analysis, which revealed excellent prospects for the development of new α-glucosidase inhibitors. In addition, the influence of the iminosugar core on the inhibitory activity against α-glucosidase is being carried out.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175517/s1. Figures S1–S117: 1H NMR and 13C NMR spectra of N-substituted iminosugar C-glycosides (5a–5π); Figures S118–S153: 1H NMR and 13C NMR spectra of N-substituted iminosugar C-glycosides (6a–6i).
Author Contributions
Conceptualization, W.J. and H.S.; Data curation, H.W. and S.T.; Investigation, H.W.; Methodology, H.W. and Y.P.; Software, S.T.; Visualization, S.T.; Writing—original draft, H.W., S.T. and G.Z.; Writing—review and editing, W.J.; Funding acquisition, Project administration and Supervision, W.J. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Key Research and Development Program of Sichuan Province (No. 2022YFS0001), National Natural Science Foundation of China (No. 21672205) and Western Light Talent Culture Project of CAS (No. 2017XBZG_XBQNXZ_A1_006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The details of the data supporting the report results in this research, were included in the paper and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 16 February 2022).
- IDF Diabetes Atlas | Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 17 February 2022).
- Dhameja, M.; Gupta, P. Synthetic. Heterocyclic Candidates as Promising α-Glucosidase Inhibitors: An Overview. Eur. J. Med. Chem. 2019, 176, 343–377. [Google Scholar] [CrossRef] [PubMed]
- Minks, J.A. Theoretical Framework to Guide a Study for Exploring the Impact of Established and Potential Risk Factors for Type 2 Diabetes Mellitus. Appl. Nurs. Res. 2020, 53, 151267. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. Efficacy and Safety Profile Evaluation of Acarbose Alone and in Association With Other Antidiabetic Drugs: A Systematic Review. Clin. Ther. 2012, 34, 1221–1236. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting Hepatic Glucose Metabolism in the Treatment of Type 2 Diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef]
- Scheen, A.J. Is There a Role for α-Glucosidase Inhibitors in the Prevention of Type 2 Diabetes Mellitus? Drugs 2003, 63, 933–951. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Mini-Special Issue Paper Management of Diabetic Patients with Hypoglycemic Agents α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic Potential of α-Glucosidase Inhibitors in Type 2 Diabetes Mellitus: An Evidence-Based Review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Rosak, C.; Mertes, G. Critical Evaluation of the Role of Acarbose in the Treatment of Diabetes: Patient Considerations. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 357–367. [Google Scholar] [CrossRef]
- Sels, J.P.J.; Huijberts, M.S.; Wolffenbuttel, B.H. Miglitol, a New α-Glucosidase Inhibitor. Expert Opin. Pharmacother. 2005, 1, 149–156. [Google Scholar] [CrossRef]
- Saeedi, M.; Hadjiakhondi, A.; Mohammad Nabavi, S.; Manayi, A. Heterocyclic Compounds: Effective α-Amylase and α-Glucosidase Inhibitors. Curr. Top. Med. Chem. 2017, 17, 428–440. [Google Scholar] [CrossRef]
- Khan, M.A.; Javaid, K.; Wadood, A.; Jamal, A.; Batool, F.; Fazal-ur-Rehman, S.; Basha, F.Z.; Choudhary, M.I. In Vitro α-Glucosidase Inhibition by Non-Sugar Based Triazoles of Dibenzoazepine, Their Structure-Activity Relationship, and Molecular Docking. Med. Chem. 2017, 13, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Francis, S.; Dutta, D.; Gupta, V.; Yang, Y.; Zhu, J.Y.; Tash, J.S.; Schönbrunn, E.; Georg, G.I. Synthesis and Evaluation of Eight- and Four-Membered Iminosugar Analogues as Inhibitors of Testicular Ceramide-Specific Glucosyltransferase, Testicular β-Glucosidase 2, and Other Glycosidases. J. Org. Chem. 2012, 77, 3082–3098. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wadood, A.; Ghufran, M.; Khan, A.; Azam, S.S.; Jelani, M.; Uddin, R. Selective Glycosidase Inhibitors: A Patent Review (2012–Present). Int. J. Biol. Macromol. 2018, 111, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Igarashi, Y.; Ichikawa, M.; Suhara, Y. 1-N-Iminosugars: Potent and Selective Inhibitors of β-Glycosidases. J. Am. Chem. Soc. 1998, 120, 3007–3018. [Google Scholar] [CrossRef]
- Kato, A.; Hirokami, Y.; Kinami, K.; Tsuji, Y.; Miyawaki, S.; Adachi, I.; Hollinshead, J.; Nash, R.J.; Kiappes, J.L.; Zitzmann, N.; et al. Isolation and SAR Studies of Bicyclic Iminosugars from Castanospermum Australe as Glycosidase Inhibitors. Phytochemistry 2015, 111, 124–131. [Google Scholar] [CrossRef]
- Nash, R.J.; Bartholomew, B.; Penkova, Y.B.; Rotondo, D.; Yamasaka, F.; Stafford, G.P.; Jenkinson, S.F.; Fleet, G.W.J. Iminosugar IdoBR1 Isolated from Cucumber Cucumis Sativus Reduces Inflammatory Activity. ACS Omega 2020, 5, 16263–16271. [Google Scholar] [CrossRef]
- Wrodnigg, T.M.; Steiner, A.J.; Ueberbacher, B.J. Natural and Synthetic Iminosugars as Carbohydrate Processing Enzyme Inhibitors for Cancer Therapy. Anticancer. Agents Med. Chem. 2008, 8, 77–85. [Google Scholar] [CrossRef]
- Wang, G.N.; Yang, L.; Zhang, L.H.; Ye, X.S. A Versatile Approach to N-Alkylated 1,4-Dideoxy-1,4-Imino-D-Arabinitols and 1,4-Dideoxy-1,4-Imino-L-Xylitols. J. Org. Chem. 2011, 76, 2001–2009. [Google Scholar] [CrossRef]
- Compain, P. Iminosugar C-Glycosides: Synthesis and Biological Activity. In Iminosugars; Wiley-VCH: Weinheim, Germany, 2007; pp. 63–86. ISBN 9780470517437. [Google Scholar]
- Butters, T.D.; Dwek, R.A.; Platt, F.M. Inhibition of Glycosphingolipid Biosynthesis: Application to Lysosomal Storage Disorders. Chem. Rev. 2000, 100, 4683–4696. [Google Scholar] [CrossRef]
- Wang, H.; Luo, H.; Ma, X.; Zou, W.; Shao, H. Stereoselective Synthesis of a Series of New N-Alkyl-3-Hydroxypiperidine Derivatives Containing a Hemiketal. Eur. J. Org. Chem. 2011, 4834–4840. [Google Scholar] [CrossRef]
- Gao, H.; Kawabata, J. α-Glucosidase Inhibition of 6-Hydroxyflavones. Part 3: Synthesis and Evaluation of 2,3,4-Trihydroxybenzoyl-Containing Flavonoid Analogs and 6-Aminoflavones as α-Glucosidase Inhibitors. Bioorg. Med. Chem. 2005, 13, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Doi, U.; Osawa, T. Inhibitory Activity of Corn-Derived Bisamide Compounds against α-Glucosidase. J. Agric. Food Chem. 2002, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kawabata, J. 2-Aminoresorcinol Is a Potent α-Glucosidase Inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New Features and Functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated Comparative Protein Structure Modeling with SWISS-MODEL and Swiss-PdbViewer: A Historical Perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo—Distance Constraints Applied on Model Quality Estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling Protein Quaternary Structure of Homo- and Hetero-Oligomers beyond Binary Interactions by Homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Steric Hindrance by 2 Amino Acid Residues Determines the Substrate Specificity of Isomaltase from Saccharomyces Cerevisiae. J. Biosci. Bioeng. 2011, 112, 545–550. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01. Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A Program to Generate Schematic Diagrams of Protein-Ligand Interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- McCarter, J.D.; Withers, S.G. Unequivocal Identification of Asp-214 as the Catalytic Nucleophile of Saccharomyces Cerevisiae α-Glucosidase Using 5-Fluoro Glycosyl Fluorides. J. Biol. Chem. 1996, 271, 6889–6894. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug- Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yang, H. ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness. Med. Chem. Commun. 2019, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.K.; Jaiswal, S.; Singh, P.; Murarka, S. Multicomponent Synthesis of Biologically Relevant S-Aryl Dithiocarbamates Using Diaryliodonium Salts. Org. Lett. 2021, 23, 6401–6406. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).