Exploring the Mechanism of Ionic Liquids to Improve the Extraction Efficiency of Essential Oils Based on Density Functional Theory and Molecular Dynamics Simulation
Abstract
Highlights
- According to the design of the experiment (DoE), multivariate analysis models were used to optimize the critical process parameters combined with multi-objective optimization.
- Based on the optimized operating conditions, the MILT-HD method not only enhances the extraction efficiency from Amomi fructus but also reduces energy demands and CO2 emissions.
- Based on the density functional theoretical (DFT) and molecular dynamics (MD) simulations, the mechanisms for ionic liquids (ILs) to improve the extraction efficiency of essential oil was comprehensively revealed.
Abstract
1. Introduction
2. Experiment
2.1. Materials and Chemicals
2.2. Extraction Process
2.3. Experimental Design of Extraction Process
2.3.1. Kinetic Model
2.3.2. DoE for MILT-HD
2.4. GC-MS Analysis of Essential Oil
2.5. Fourier Transforms Infrared Spectroscopy (FTIR)
2.6. Scanning Electron Microscopy (SEM)
2.7. Calculation Method of Quantum Chemical
2.8. Molecular Dynamics Simulation
2.9. Data Analysis
3. Results and Discussion
3.1. Determination of the Key Operating Parameters Affecting the Extraction Process
3.1.1. Key Parameters Affecting the Yt50
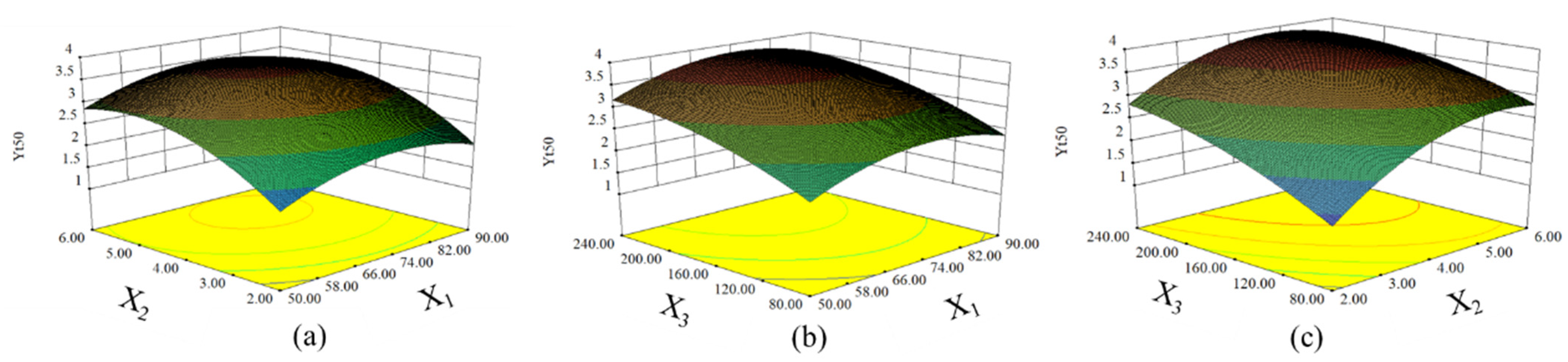
3.1.2. Analysis of Response Surface for the Yt50
3.1.3. Multiple Response Optimization and Verification
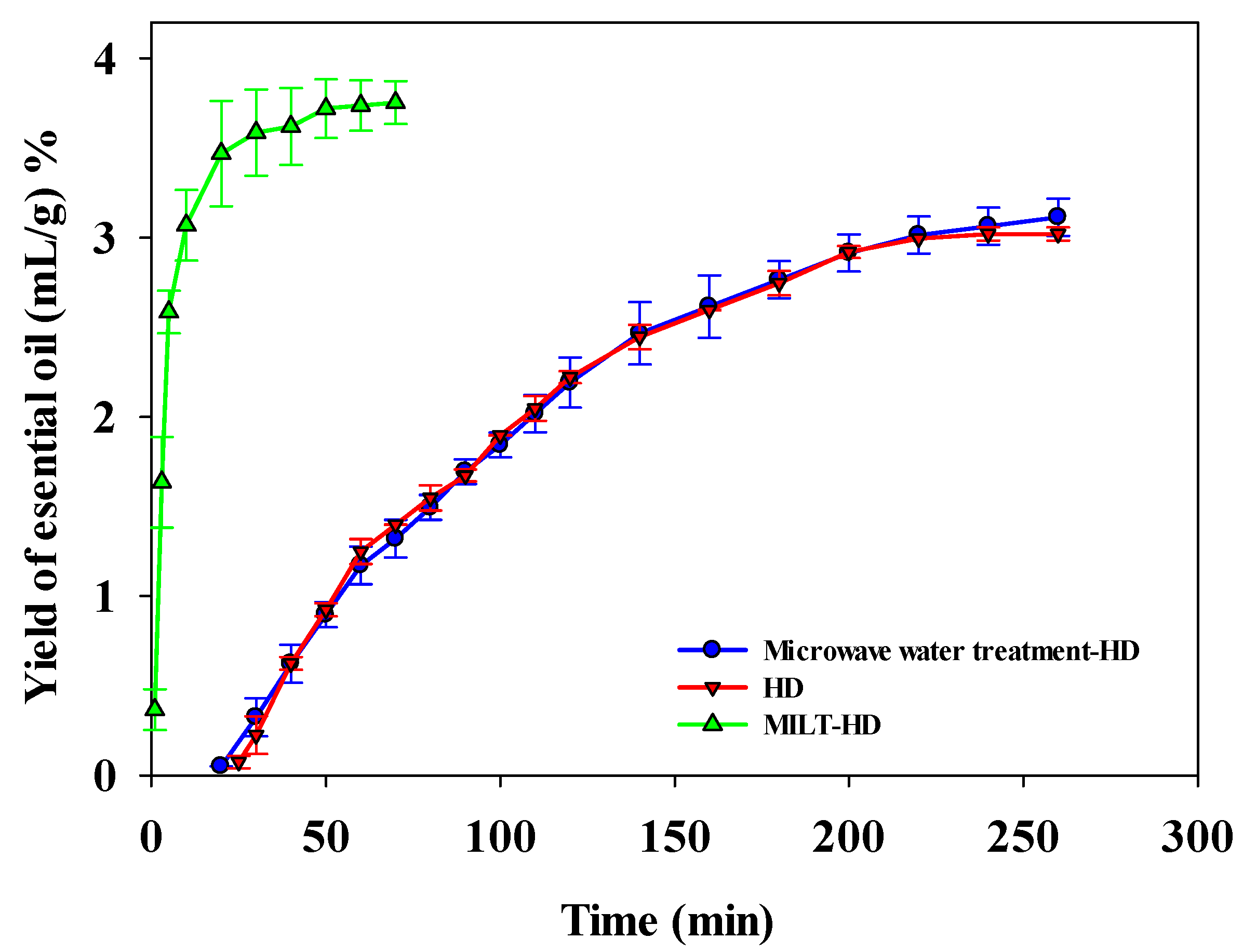
3.2. Comparison of Extraction Efficiency
3.3. GC-MS Analysis
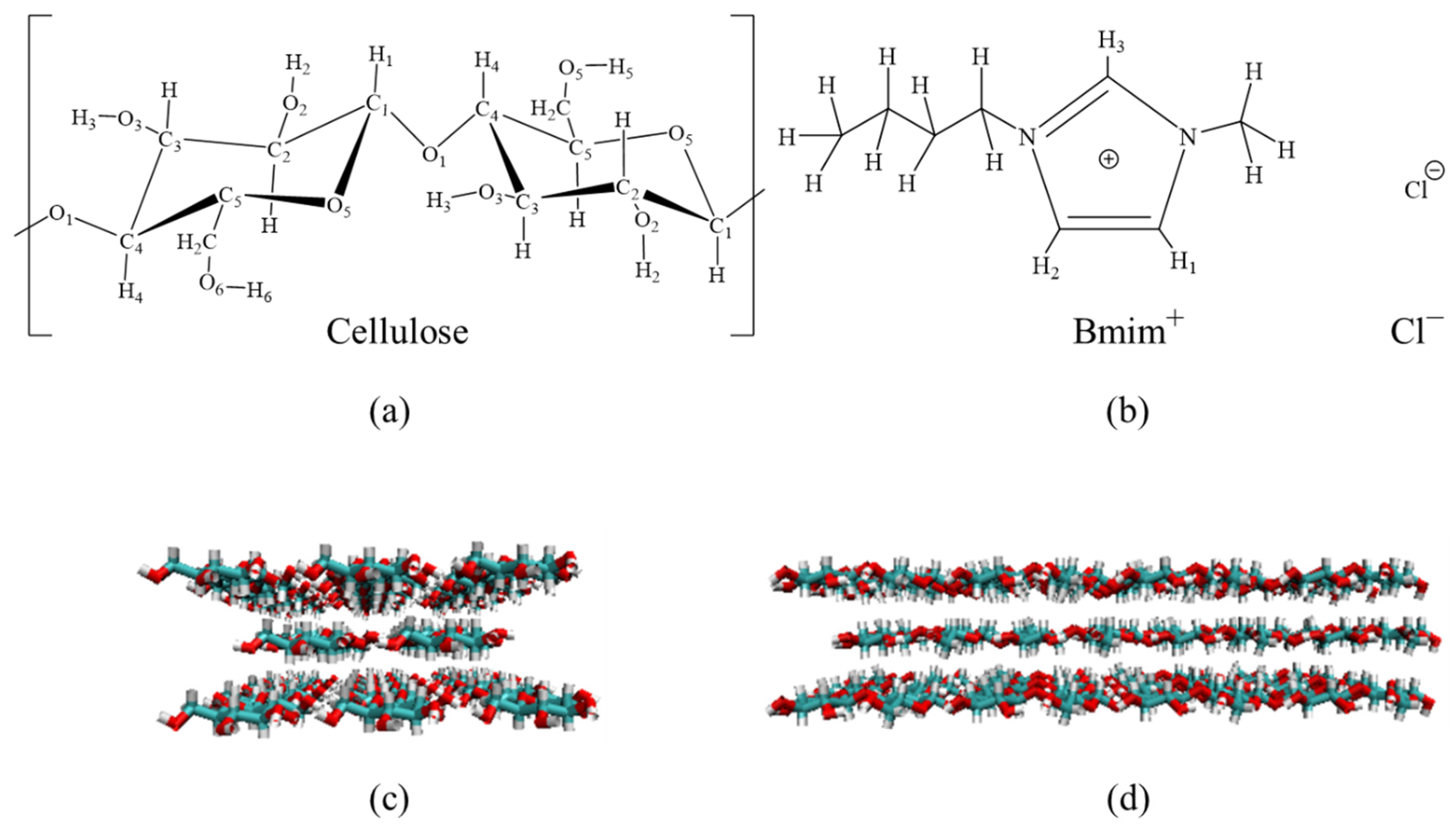
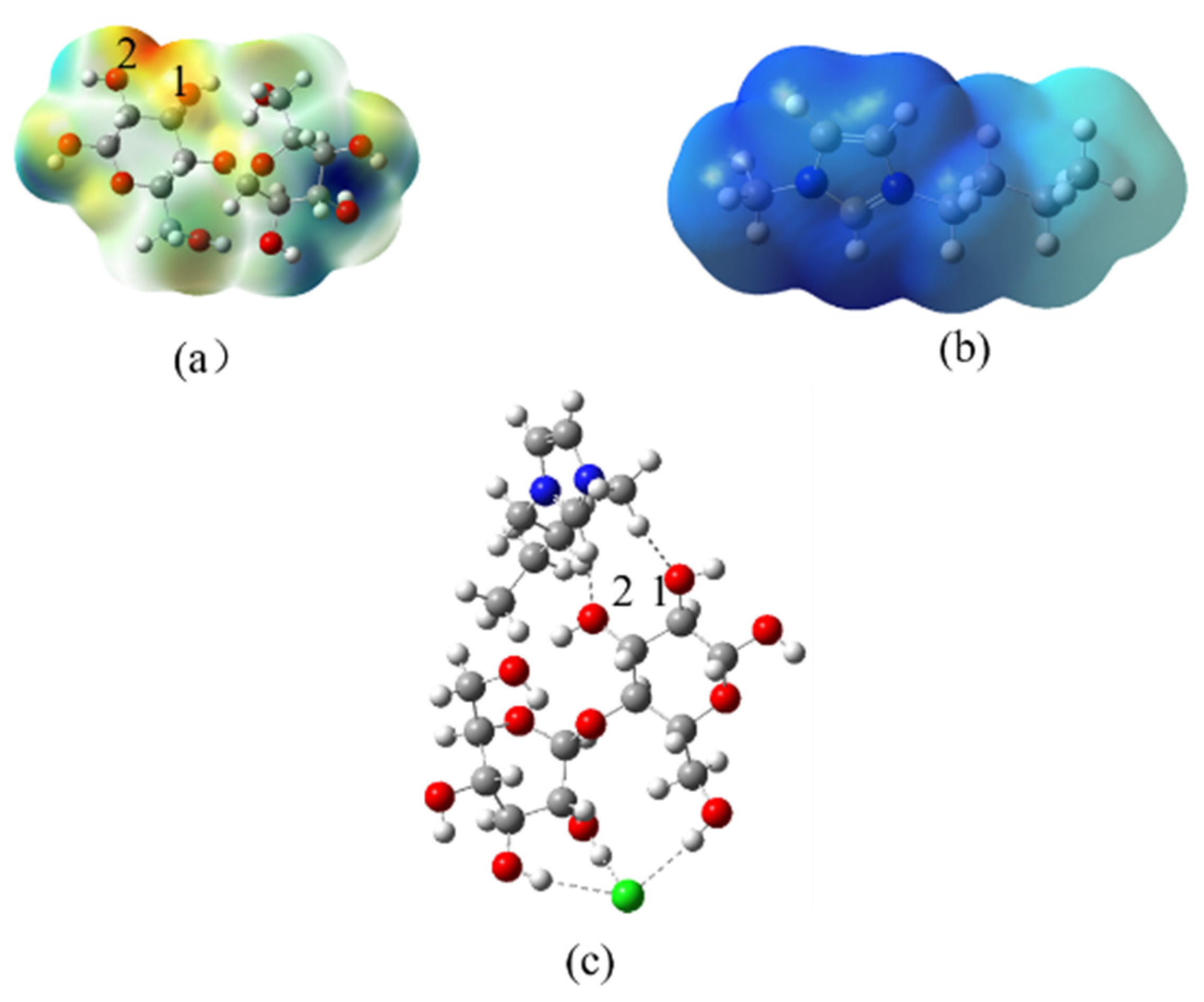
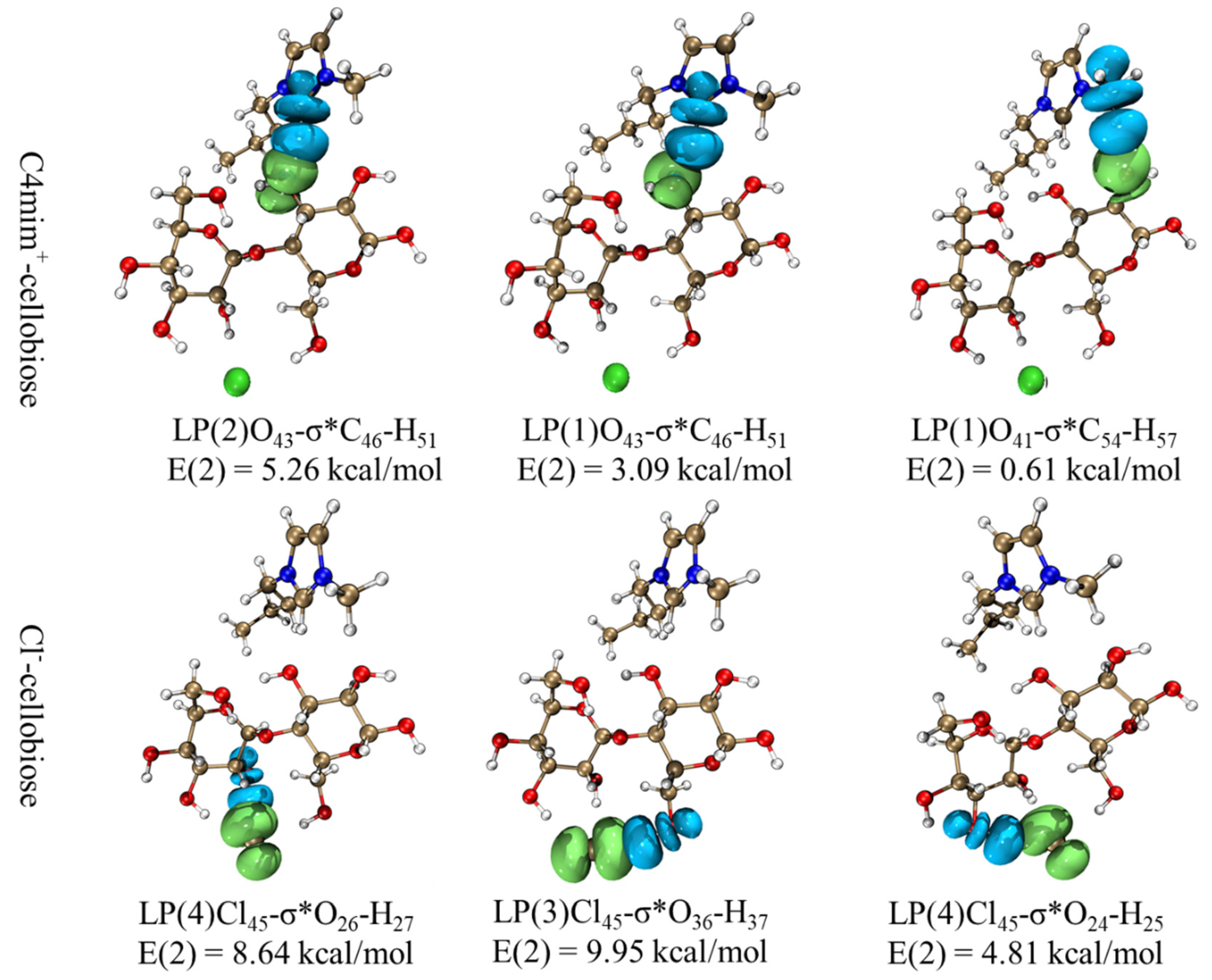
3.4. Mechanism Analysis of ILs
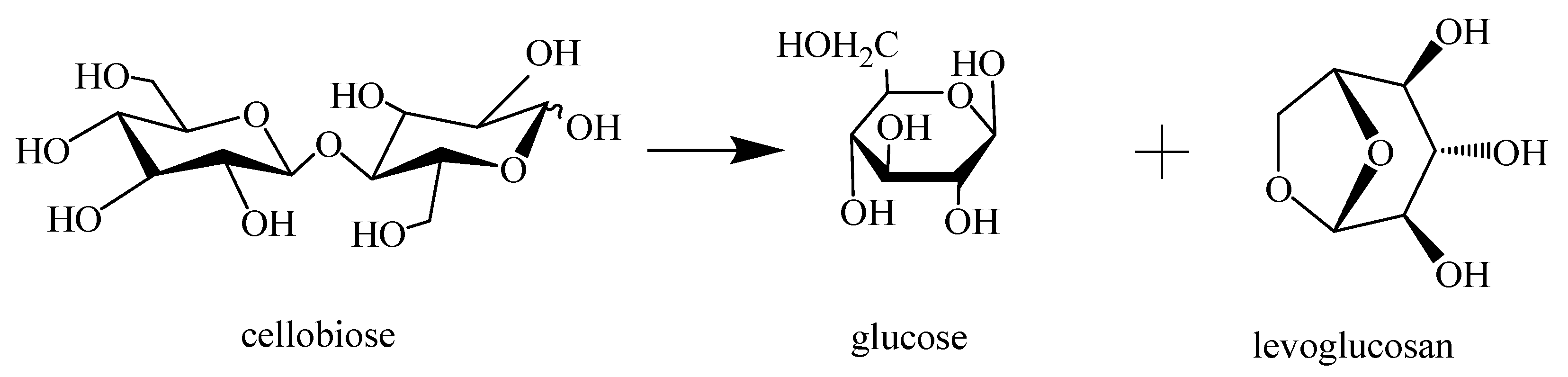
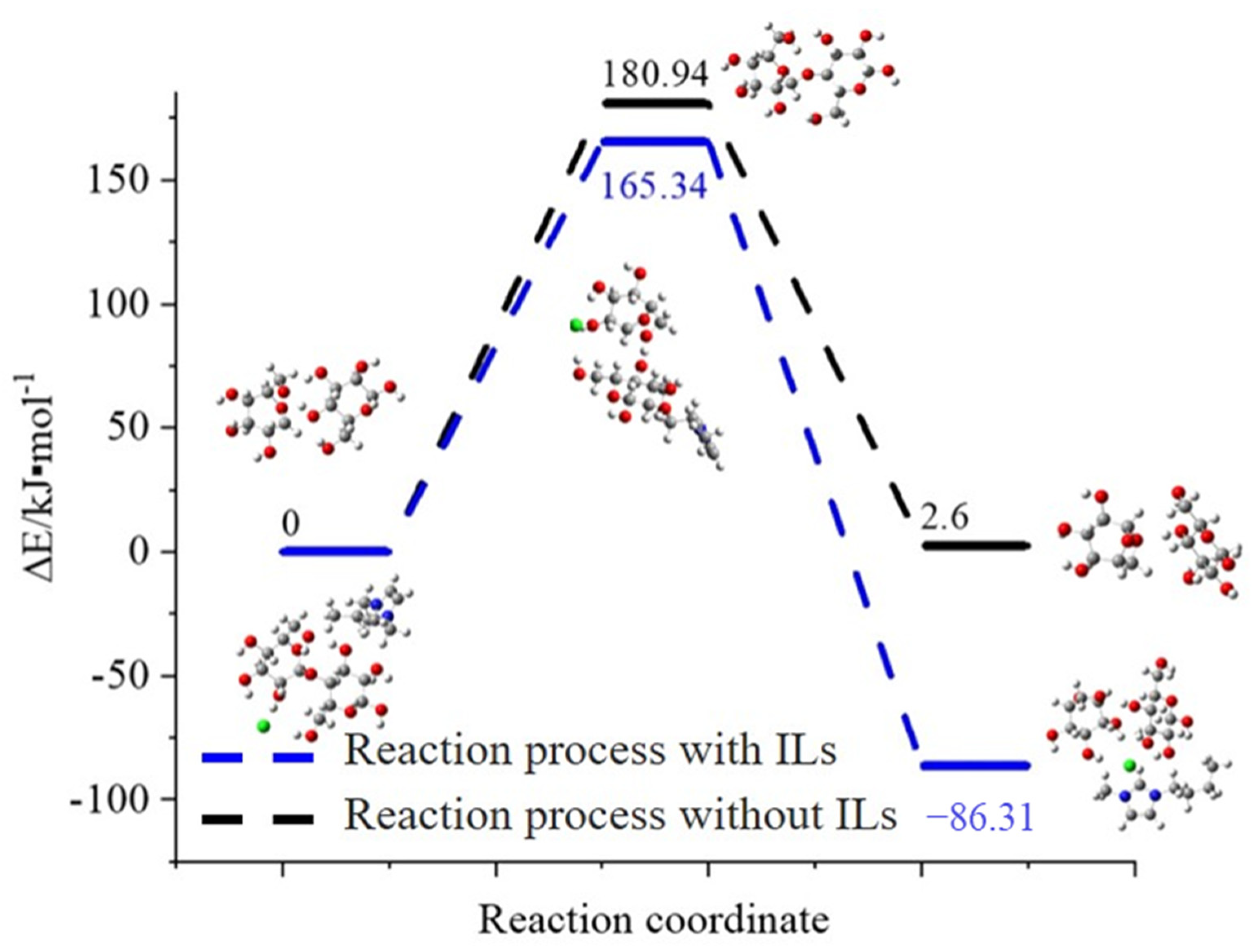
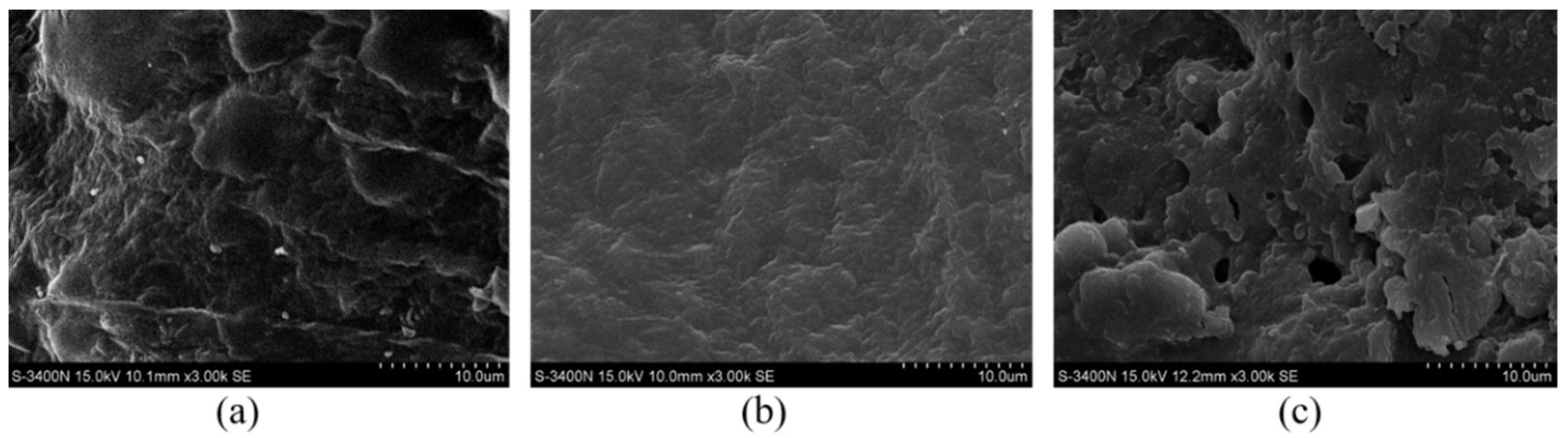
3.4.1. Cleavage Cellulose Chain
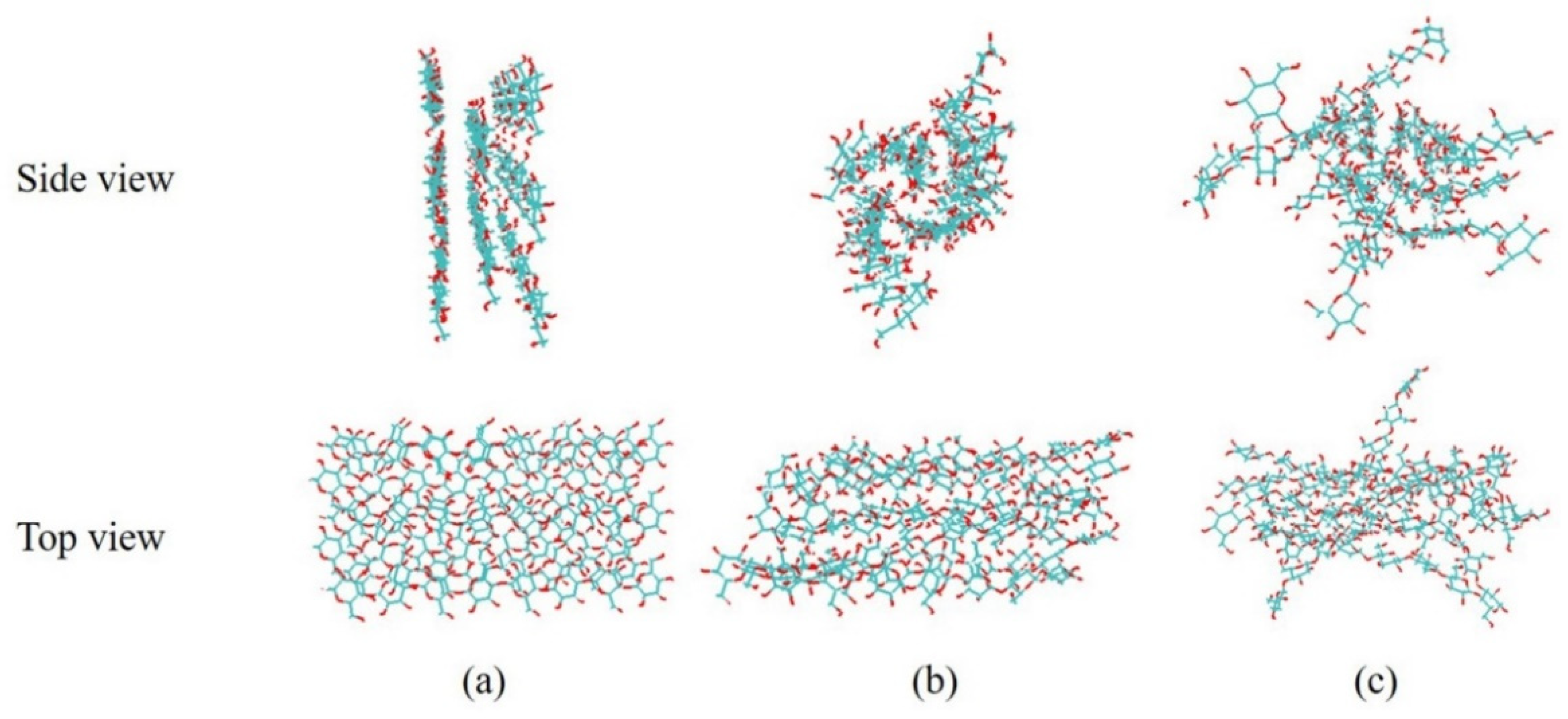
3.4.2. Structure Change of Cellulose in the BmimCl and Water
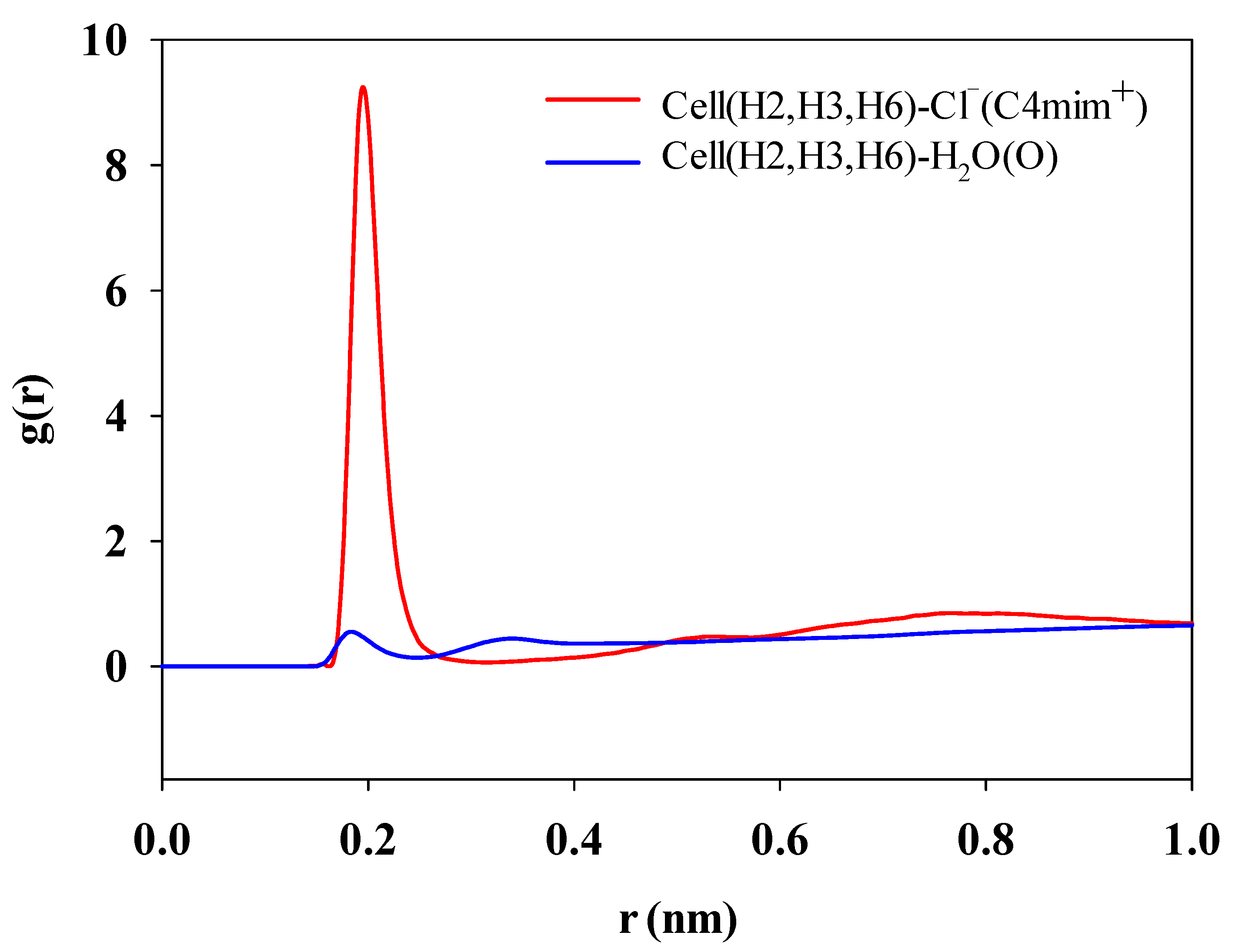
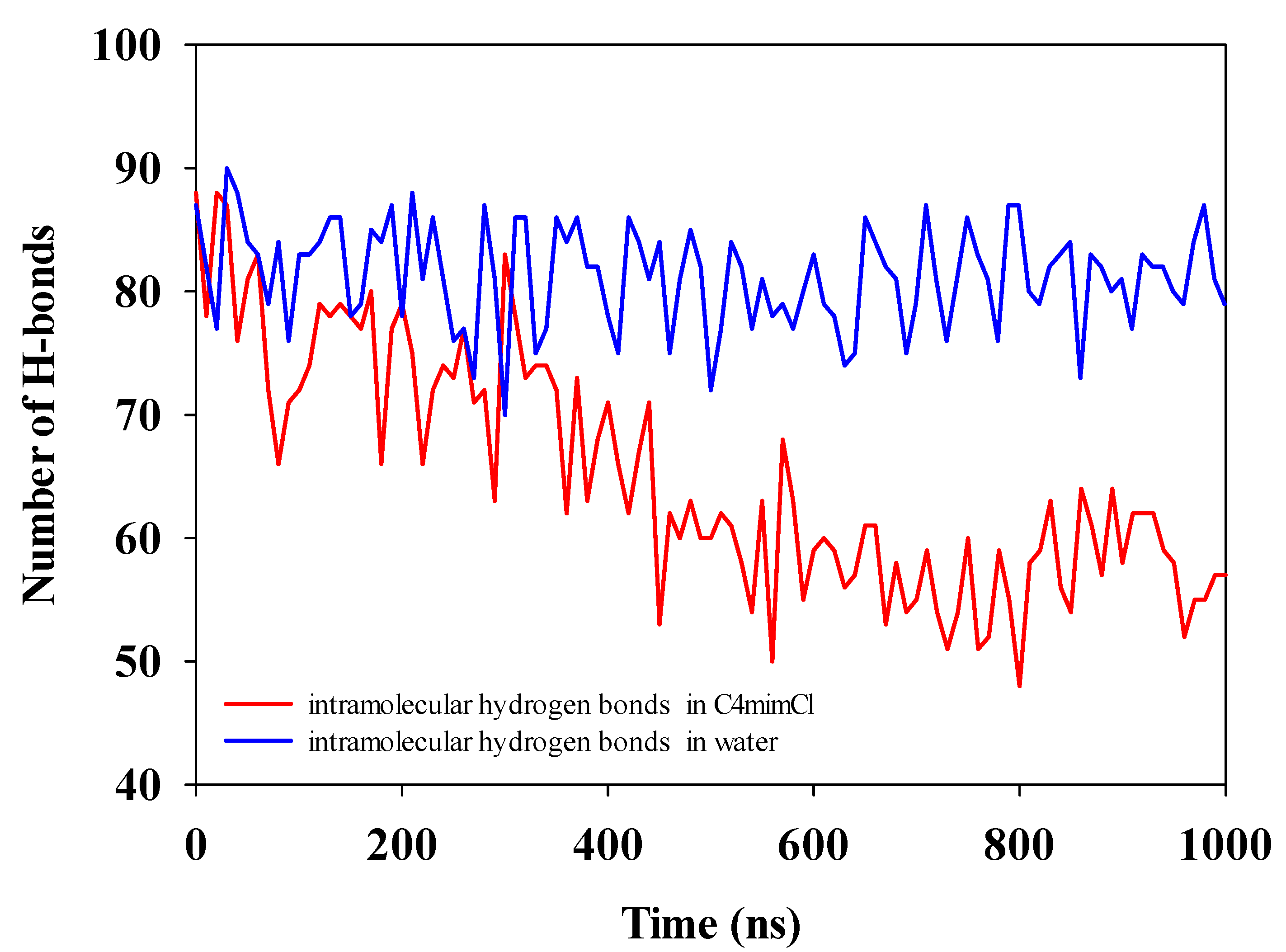
3.4.3. H-Bond Change in the Cellulose Dissolving Process
3.4.4. Supposed Mechanism of Ionic Liquids in Improving the Extraction Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xiao, Y.; Olatunde, O.Z.; Yong, J.; Lu, C. Progress of chemical components and biological activities of fructus amomi. Arch. Biotechnol. Biomed. 2020, 4, 001–004. [Google Scholar]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile oil from amomi fructus attenuates 5-fluorouracil-induced intestinal mucositis. Front. Pharmacol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Suo, S.; Lai, Y.; Li, M.; Song, Q.; Cai, J.; Zhao, J.; Yang, Q.; Ung, C.O.L.; Hu, H. Phytochemicals, pharmacology, clinical application, patents, and products of amomi fructus. Food Chem. Toxicol. 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Wang, T.; Li, M.; Lin, J. Composition and antimicrobial activities of essential oil of fructus amomi. Nat. Prod. Res. Dev. 2011, 23, 464–472. [Google Scholar]
- Jafri, M.; Javed, K.; Singh, S. Evaluation of the gastric antiulcerogenic effect of large cardamom (fruits of amomum subulatum roxb). J. Ethnopharmacol. 2001, 75, 89–94. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, W.; Yang, C.; Zhang, T.; Lu, S.; Zhao, R.; Mao, X.; Yu, J. Therapeutic effect of amomum villosum on inflammatory bowel disease in rats. Front. Pharmacol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Xiao, F.; Zhang, Z.; Xu, Z.; Wang, H. Studies on the analgesic and anti-inflammatory effect of bornyl acetate in volatile oil from amomum villosum. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2004, 27, 438. [Google Scholar]
- Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Zu, Y.-G.; Luo, M.; Zhao, C.-J.; Li, C.-Y. Microwave-assisted ionic liquids treatment followed by hydro-distillation for the efficient isolation of essential oil from fructus forsythiae seed. Sep. Purif. Technol. 2013, 107, 228–237. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Zu, Y.-G.; Luo, M.; Wang, W.; Zhao, C.-J. Microwave-assisted ionic liquids pretreatment followed by hydro-distillation for the efficient extraction of essential oil from dryopteris fragrans and evaluation of its antioxidant efficacy in sunflower oil storage. J. Food Eng. 2013, 117, 477–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation. Carbohydr. Polym. 2013, 94, 723–730. [Google Scholar] [CrossRef]
- Bloino, J.; Zheng, G.; Sonnenberg, J.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T. Gaussian 09, Revision d. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yao, Y.; Li, Y.; Liu, X.; Zhang, X.; Wang, J.; Yao, X.; Zhang, S. Mechanistic study on the cellulose dissolution in ionic liquids by density functional theory. Chin. J. Chem. Eng. 2015, 23, 1894–1906. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.C.; Skaf, M.S. Cellulose-builder: A toolkit for building crystalline structures of cellulose. J. Comput. Chem. 2012, 33, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. Glycam06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Berendsen, H.; Grigera, J.; Straatsma, T. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. Lincs: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Salam, M.A.; Abdullah, B.; Ramli, A.; Mujtaba, I.M. Structural feature based computational approach of toxicity prediction of ionic liquids: Cationic and anionic effects on ionic liquids toxicity. J. Mol. Liq. 2016, 224, 393–400. [Google Scholar] [CrossRef]
- Yu, G.-W.; Nie, J.; Song, Z.-Y.; Li, Z.-G.; Lee, M.-R.; Wang, S.-P. Microwave-assisted simplified simultaneous distillation coupled with ionic liquid pretreatment for the analysis of essential oil in schisandra sphenanthera. J. Chromatogr. Sci. 2017, 55, 1051–1058. [Google Scholar] [CrossRef][Green Version]
- Xia, R.; Li, B.; Wang, X.; Li, T.; Yang, Z. Measurement and calibration of the discrete element parameters of wet bulk coal. Measurement 2019, 142, 84–95. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Yan, F.; Xu, Y.; Yang, M.; Gao, Y.; Aihemaiti, A.; Zou, Q. Optimization of simultaneous production of volatile fatty acids and bio-hydrogen from food waste using response surface methodology. RSC Adv. 2018, 8, 10457–10464. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.; Duan, J.-a.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (rsm) for rapid analysis of bioactive compounds in the flower head of chrysanthemum morifolium ramat. Ind. Crops Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Yansheng, C.; Zhida, Z.; Changping, L.; Qingshan, L.; Peifang, Y.; Welz-Biermann, U. Microwave-assisted extraction of lactones from ligusticum chuanxiong hort. Using protic ionic liquids. Green Chem. 2011, 13, 666–670. [Google Scholar] [CrossRef]
- Hou, K.; Bao, M.; Wang, L.; Zhang, H.; Yang, L.; Zhao, H.; Wang, Z. Aqueous enzymatic pretreatment ionic liquid–lithium salt based microwave–assisted extraction of essential oil and procyanidins from pinecones of pinus koraiensis. J. Clean. Prod. 2019, 236, 117581. [Google Scholar] [CrossRef]
- Ascrizzi, R.; González-Rivera, J.; Pomelli, C.S.; Chiappe, C.; Margari, P.; Costagli, F.; Longo, I.; Tine, M.R.; Flamini, G.; Duce, C. Ionic liquids, ultra-sounds and microwaves: An effective combination for a sustainable extraction with higher yields. The cumin essential oil case. React. Chem. Eng. 2017, 2, 577–589. [Google Scholar] [CrossRef]
- Paré, J.; Bélanger, J.M. Instrumental Methods in Food Analysis; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Sheng-Mao, L.I.; Qiang, Y.E.; Hui, A.O.; Pharmacy, S.O. Relationship between the gc-ms fingerprints of essential oil from amomi fructus and its analgesia effect. Chin. Tradit. Pat. Med. 2016, 38, 346–350. [Google Scholar]
- Hui, A.O.; Hongmei, L.; Jiangrui, W.; Chengxin, F.; Li, X.U.; Li, G.; Cheng, P.; Qiang, Y.E. Determination of volatile oil by gc-ms and evaluation of heavy metals residue in fructus amomi from different producing areas. Tradit. Chin. Drug Res. Clin. Pharmacol. 2016, 27, 250–254. [Google Scholar]
- Chen, F.; Zu, Y.; Yang, L. A novel approach for isolation of essential oil from fresh leaves of magnolia sieboldii using microwave-assisted simultaneous distillation and extraction. Sep. Purif. Technol. 2015, 154, 271–280. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, S.; Yao, Y.; Yao, X.; Xu, J.; Lu, X. Dissolving process of a cellulose bunch in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 17894–17905. [Google Scholar] [CrossRef]
- Paavilainen, S.; Róg, T.; Vattulainen, I. Analysis of twisting of cellulose nanofibrils in atomistic molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 3747–3755. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Jiang, Z.-T.; Tan, J.; Tang, S.-H.; Li, T.-T.; Liang, L.-L.; He, H.-J.; Liu, Y.-M.; Li, J.-T.; et al. Green and solvent-free simultaneous ultrasonic-microwave assisted extraction of essential oil from white and black peppers. Ind. Crops Prod. 2018, 114, 164–172. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why only ionic liquids with unsaturated heterocyclic cations can dissolve cellulose: A simulation study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- He, H.; Zhang, S.; Liu, X.; Wang, J.; Yao, X.; Zhang, X. Structures and hydrogen bonds of biodegradable naphthenate ionic liquids. Fluid Phase Equilib. 2013, 360, 169–179. [Google Scholar] [CrossRef]
- Cao, B.; Du, J.; Cao, Z.; Sun, X.; Sun, H.; Fu, H. Dft study on the dissolution mechanisms of α-cyclodextrin and chitobiose in ionic liquid. Carbohydr. Polym. 2017, 169, 227–235. [Google Scholar] [CrossRef] [PubMed]
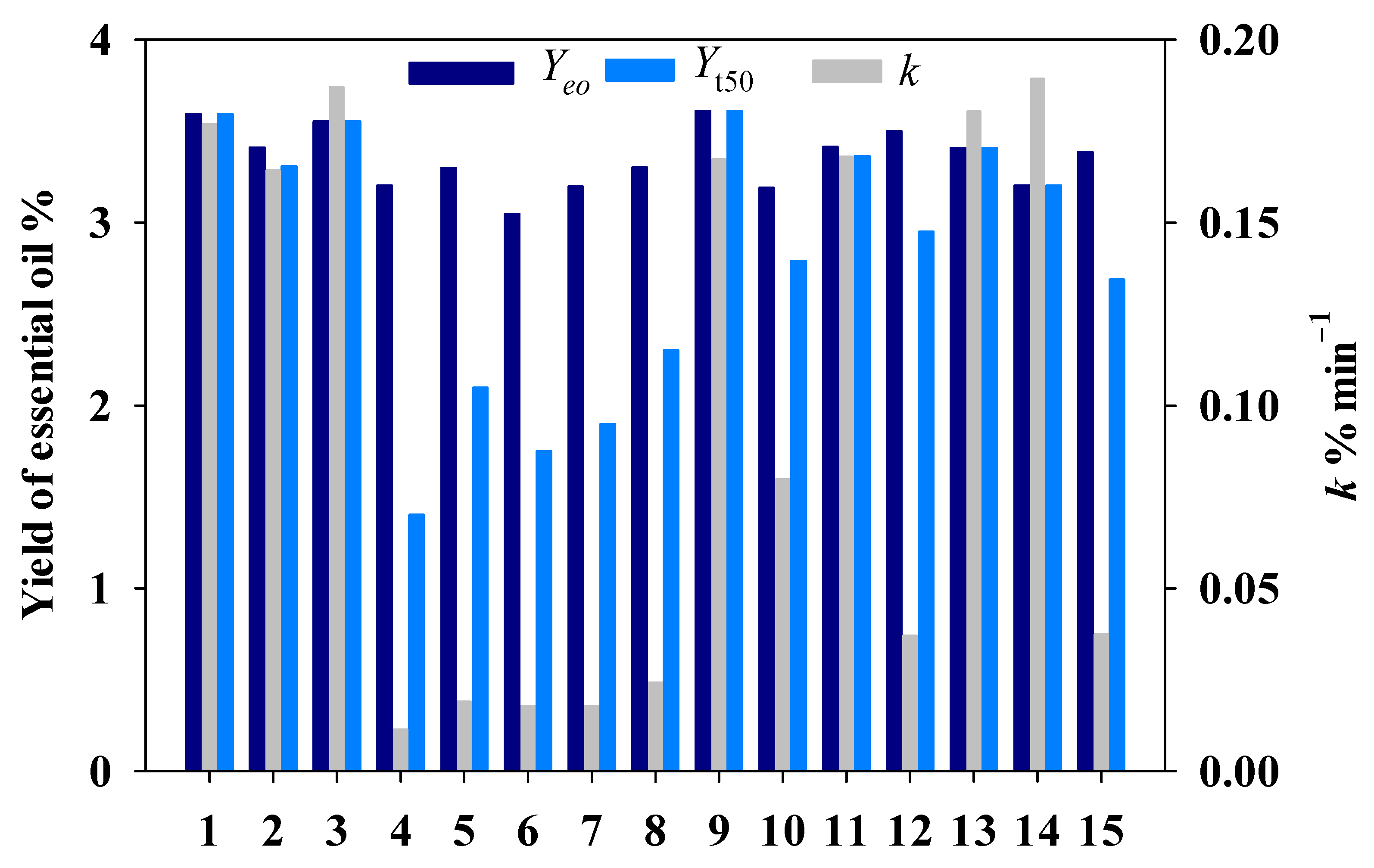

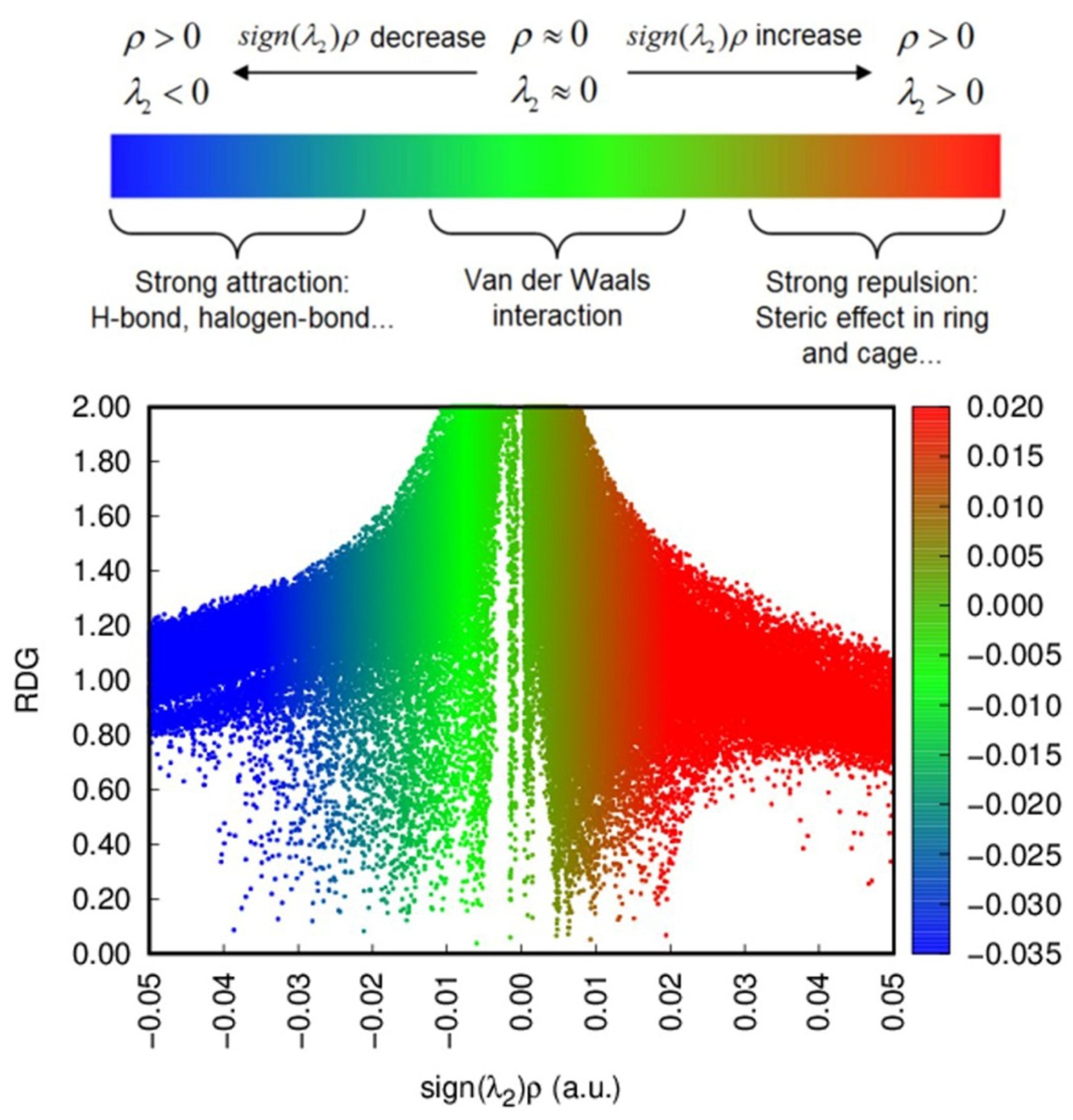
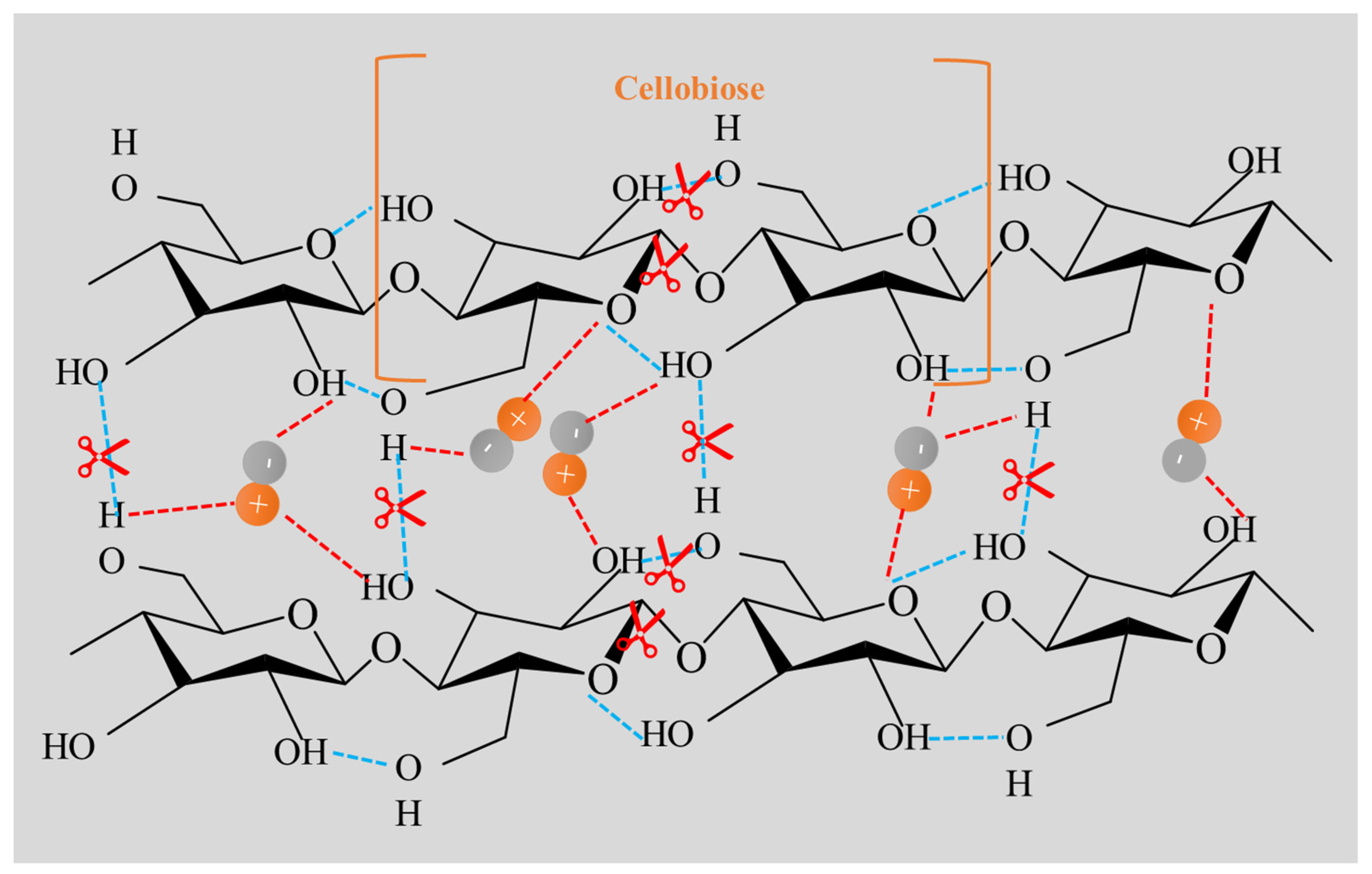
| Runs | Process Parameters | ||
|---|---|---|---|
| X1 (%) | X2 (min) | X3 (W) | |
| 1 | 90 | 4 | 240 |
| 2 | 50 | 4 | 240 |
| 3 | 70 | 4 | 160 |
| 4 | 70 | 2 | 80 |
| 5 | 90 | 2 | 160 |
| 6 | 50 | 2 | 160 |
| 7 | 50 | 4 | 80 |
| 8 | 90 | 4 | 80 |
| 9 | 70 | 6 | 240 |
| 10 | 50 | 6 | 160 |
| 11 | 70 | 4 | 160 |
| 12 | 70 | 6 | 80 |
| 13 | 70 | 4 | 160 |
| 14 | 90 | 6 | 160 |
| 15 | 70 | 2 | 240 |
| Source | Sum of Square | DF | Mean Square | F Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 7.50 | 7 | 1.07 | 68.78 | <0.0001 | Significant |
| X1 | 0.26 | 1 | 0.26 | 16.82 | 0.0046 | Significant |
| X2 | 2.67 | 1 | 2.67 | 171.10 | <0.0001 | Significant |
| X3 | 2.70 | 1 | 2.70 | 173.49 | <0.0001 | Significant |
| X2 X3 | 0.098 | 1 | 0.098 | 6.31 | 0.0403 | Significant |
| X12 | 0.70 | 1 | 0.70 | 44.63 | 0.0003 | Significant |
| X22 | 1.10 | 1 | 1.10 | 70.88 | <0.0001 | Significant |
| X32 | 0.20 | 1 | 0.20 | 12.69 | 0.0092 | Significant |
| Residual | 0.11 | 7 | 0.016 | Significant | ||
| Lack of fit | 0.089 | 5 | 0.018 | 1.8 | 0.3947 | Not significant |
| R2 | 0.9857 |
| X1 (%) | X2 (min) | X3 (W) | Yeo (%) | Yt50 (%) | k (% min−1) | Desirability | |
|---|---|---|---|---|---|---|---|
| Predicted | 74.00 | 4.24 | 233.12 | 3.611 | 3.842 | 0.194 | 1.00 |
| Experimental | 74.00 | 4.25 | 240.00 | 3.753 ± 0.119 | 3.720 ± 0.164 | 0.188 ± 0.0045 | |
| RE (%) | 3.78 | −3.28 | −3.19 |
| MILT-HD | HD | ||
|---|---|---|---|
| Pretreatment | Hydrodistillation | Hydrodistillation | |
| Heating Method | Microwave | Electric stove | Electric stove |
| Effective electric power (W) | 390 | 600 | 600 |
| Time consumption (h) | 0.0707 | 1.17 | 4 |
| Electricity consumption (kW·h) | 0.0276 | 0.702 | 2.4 |
| Total electricity consumption (kW·h) | 0.730 | 2.4 | |
| Yield of essential oil (mL/g) | 0.0375 | 0.0302 | |
| Yield of essential oil per kilowatt hour (mL/g/(kW·h)) | 0.0514 | 0.0126 | |
| Environmental impact (g CO2 emission) | 584.0 | 1920 | |
| No. | Components | Molecular Formula | Relative Contents (%) | |
|---|---|---|---|---|
| HD | MILT-HD | |||
| 1 | Pinene | C10H16 | 1.530 | 1.620 |
| 2 | Camphene | C10H16 | 8.333 | 7.323 |
| 3 | Myrcene | C10H16 | 2.927 | 2.853 |
| 4 | Phellandrene | C10H16 | 0.197 | 0.200 |
| 5 | Limonene | C10H16 | 7.540 | 7.437 |
| 6 | Terpinolene | C10H16 | 0.110 | 0.280 |
| 7 | Linalool | C10H18O | 0.467 | 0.403 |
| 8 | Camphor | C10H16O | 29.183 | 29.477 |
| 9 | Borneol | C10H18O | 2.517 | 2.347 |
| 10 | Bornyl acetate | C12H20O2 | 44.390 | 41.343 |
| 11 | Caryophyllene | C15H24 | 0.087 | 0.597 |
| 12 | Cadinene | C15H24 | 0.070 | 0.543 |
| Total% | 97.35 | 94.42 | ||
| E (kJ/mol) | Water | C4mim+ | Cl− |
|---|---|---|---|
| Ecoul | −5346.40 | −1058.52 | −6698.34 |
| EL-J | −681.03 | −3302.19 | 581.132 |
| Etotal | −6027.43 | −4360.71 | −6117.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wang, F.; Wang, G.; Li, H. Exploring the Mechanism of Ionic Liquids to Improve the Extraction Efficiency of Essential Oils Based on Density Functional Theory and Molecular Dynamics Simulation. Molecules 2022, 27, 5515. https://doi.org/10.3390/molecules27175515
Luo X, Wang F, Wang G, Li H. Exploring the Mechanism of Ionic Liquids to Improve the Extraction Efficiency of Essential Oils Based on Density Functional Theory and Molecular Dynamics Simulation. Molecules. 2022; 27(17):5515. https://doi.org/10.3390/molecules27175515
Chicago/Turabian StyleLuo, Xiaorong, Fen Wang, Guihua Wang, and Hui Li. 2022. "Exploring the Mechanism of Ionic Liquids to Improve the Extraction Efficiency of Essential Oils Based on Density Functional Theory and Molecular Dynamics Simulation" Molecules 27, no. 17: 5515. https://doi.org/10.3390/molecules27175515
APA StyleLuo, X., Wang, F., Wang, G., & Li, H. (2022). Exploring the Mechanism of Ionic Liquids to Improve the Extraction Efficiency of Essential Oils Based on Density Functional Theory and Molecular Dynamics Simulation. Molecules, 27(17), 5515. https://doi.org/10.3390/molecules27175515





