The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner
Abstract
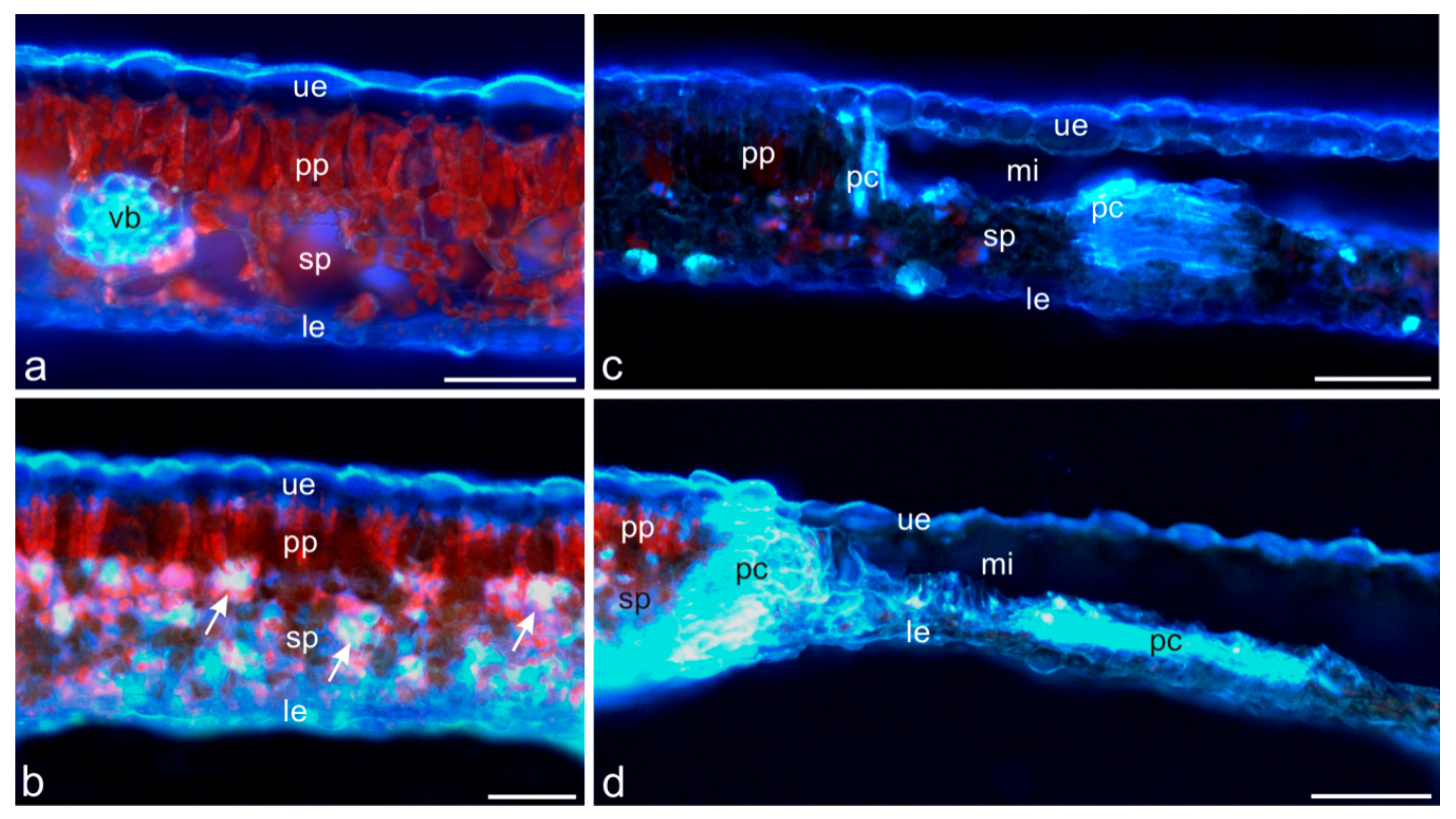
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Total Tannins (TT)
3.3. Total Phenolics (Folin)
3.4. Total Flavonoids (TF)
3.5. Antiradical Activity (AA)
- AA—antioxidant activity of the analyzed sample
- Ap—absorbance of the analyzed sample
- Ab—absorbance of the blank sample
3.6. HPLC Analysis
3.7. Microscopic Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ordano, M.; Blendinger, P.G.; Lomáscolo, S.B.; Chacoff, N.P.; Sánchez, M.S.; Montellano, M.G.N.; Jiménez, J.; Ruggera, R.A.; Valoy, M. The role of trait combination in the conspicuousness of fruit display among bird-dispersed plants. Funct. Ecol. 2017, 31, 1718–1727. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, G.; Constabel, C.P. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Deschka, G.; Dimic, N. Cameraria ohridella sp.n. (Lep., Lithocolletidae) from Macedonia, Yugoslavia. Acta Entomol. Jugosl. 1986, 22, 11–23. [Google Scholar]
- Łabanowski, G.; Sojka, G. Szrotówek kasztanowcowiaczek zagraża kasztanowcom w Polsce. Ochr. Roślin 1998, 12, 12. [Google Scholar]
- Weryszko-Chmielewska, E.; Haratym, W. Changes in leaf tissues of common horse chestnut (Aesculus hippocastanum L.) colonised by the horse-chestnut leaf miner (Cameraria ochridella Deschka and Dimić). Acta Agrobot. 2011, 64, 11–22. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Haratym, W. Leaf micromorphology of Aesculus hippocastanum L. and damage caused by leaf-mining larvae of Cameraria ohridella Deschka and Dimić. Acta Agrobot. 2012, 65, 25–34. [Google Scholar] [CrossRef]
- Myśkow, E.; Sokołowska, K.; Słupianek, A.; Gryc, V. Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella. Forests 2021, 12, 1537. [Google Scholar] [CrossRef]
- Percival, G.C.; Barrow, I.; Noviss, K.; Keary, I.; Pennington, P. The impact of horse chestnut leaf miner (Cameraria ohridella Deschka and Dimic; HCLM) on vitality, growth and reproduction of Aesculus hippocastanum L. Urban For. Urban Green. 2011, 10, 11–17. [Google Scholar] [CrossRef]
- Holoborodko, K.K.; Seliutina, O.V.; Ivanko, I.A.; Alexeyeva, A.A.; Shulman, M.V.; Pakhomov, O.Y. Effect of Cameraria ohridella feeding on Aesculus hippocastanum photosynthesis. Regul. Mech. Biosyst. 2021, 12, 346–352. [Google Scholar] [CrossRef]
- Tyapkina, A.P.; Silaeva, Z.G.; Kiseleva, L.L.; Shiryaeva, N.A.; Parakhina, E.A. Ecological characteristics of horse chestnut (Aesculus hippocastanum L.) plantings of the city of Orel. In Proceedings of the BIO Web of Conferences, Orel, Russia, 23–24 March 2022; Volume 47, p. 11001. [Google Scholar] [CrossRef]
- Arnold, C.; Sengonca, C. Bedeutung von gängigen gartenbaulichen Maßnahmen für die Reduktion des Befallsdrucks der Rosskastanien-Miniermotte Cameraria ohridella Deschka & Dimic (Lep., Gracillariidae). Gesunde Pflanz. 2002, 54, 1–5. [Google Scholar] [CrossRef]
- Pavan, F.; Barro, P.; Bernardinelli, I.; Gambon, N.; Zandigiacomo, P. Cultural control of Cameraria ohridella on horsechestnut in urban areas by removing fallen leaves in autumn. Arboric. Urban For. 2003, 29, 253–258. [Google Scholar] [CrossRef]
- Głowacka, B.; Lipiński, S.; Tarwacki, G. Possibilities of protection of horse chestnut Aesculus hippocastanum L. against horse chestnut leaf-miner Cameraria ohridella Deschka et Dimić. For. Res. Pap. 2009, 70, 317–328. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kalisz, S.; Wojdyło, A. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Biernat, A. The content of phenolic compounds in leaf tissues of Aesculus glabra and Aesculus parviflora Walt. Molecules 2015, 20, 2176–2189. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Schultz, J.C. Impact of Variable Plant Defensive Chemistry on Susceptibility of Insects to Natural Enemies; Hein, P.A., Ed.; American Chemistry Society: Washington, DC, USA, 1983; pp. 37–54. [Google Scholar]
- Paterska, M.; Bandurska, H.; Wysłouch, J.; Molińska-Glura, M.; Moliński, K. Chemical composition of horse-chestnut (Aesculus) leaves and their susceptibility to chestnut leaf miner Cameraria ohridella Deschka & Dimić. Acta Physiol. Plant. 2017, 39, 105. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; p. 354. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Szczodrak, J. Taniny i ich rozkład enzymatyczny. Biotechnologia 2005, 68, 152–165. [Google Scholar]
- Padayachee, A.; Netzelb, G.; Netzelb, M.; Day, L.; Zabarase, D.; Mikkelsena, D.; Gidleya, M.J. Binding of polyphenols to plant cell wall analogues—Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A.; Grochowska, M.; Haratym, H.; Tietze, M.; Weryszko-Chmielewska, E.; Lechowski, L. Changes in Aesculus hippocastanum leaves during development of Cameraria ohridella. Urban For. Urban Green. 2020, 56, 126793. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function and Developmnent; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Théroux-Rancourt, G.; Roddy, A.B.; Earles, J.M.; Gilbert, M.E.; Zwieniecki, M.A.; Boyce, C.K.; Tholen, D.; McElrone, A.J.; Simonin, K.A.; Brodersen, C.R. Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. Proc. R. Soc. B Boil. Sci. 2021, 288, 20203145. [Google Scholar] [CrossRef] [PubMed]
- Borsuk, A.M.; Brodersen, C.R. The spatial distribution of chlorophyll in leaves. Plant Physiol. 2019, 180, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Dmitruk, M.; Sulborska, A.; Żuraw, B.; Stawiarz, E.; Weryszko-Chmielewska, E. Sites of secretion of bioactive compounds in leaves of Dracocephalum moldavica L.: Anatomical, histochemical, and essential oil study. Braz. J. Bot. 2019, 42, 701–715. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P.; Stavroulaki, V.; Sumbele, S. “Carbon gain vs. water saving, growth vs. defence”: Two dilemmas with soluble phenolics as a joker. Plant Sci. 2014, 227, 21–27. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 163–177. [Google Scholar] [CrossRef]
- Chernetskyy, M.; Woźniak, A.; Skalska-Kamińska, A.; Żuraw, B.; Blicharska, E.; Rejdak, R.; Donica, H.; Weryszko-Chmielewska, E. Structure of leaves and phenolic acids in Kalanchoë daigremontiana Raym.-Hamet & H. Perrier. Acta Sci. Pol. Hortorum Cultus 2018, 17, 137–155. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G. Phenolic compounds in plant cuticules: Physiological and ecological aspects. Adv. Plant Physiol. 2005, 8, 33–47. [Google Scholar]
- Kariotia, A.; Bilia, A.R.; Skaltsa, H. Quercus ilex L.: A rich source of polyacylated flavonoid glucosides. Food Chem. 2010, 123, 131–142. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef]
- Feucht, W.; Treutter, D.; Polster, J. Flavanol binding of nuclei from tree species. Plant Cell Rep. 2004, 22, 430–436. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Price, M.L.; Socoyoc, S.V.; Butler, L.G. A critical evaluation of vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Osman, M.A. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem. 2004, 88, 129–134. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Zhisten, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure: Principles and Selected Methods; Termarcarphi Pty Ltd.: Melbourne, Australia, 1981. [Google Scholar]
- Harris, P.J.; Hartley, R.D. Detection of bound ferulic acid in cell walls of the Gramineae by ultraviolet fluorescence microscopy. Nature 1978, 259, 508–510. [Google Scholar] [CrossRef]
- De Pinna, G.F.A.M.; Kraus, J.E.; De Menezes, N.L. Morphology and anatomy of leaf mine in Richterago riparia Roque (Asteraceae) in the campos rupestres of Serra do Cipó, Brazil. Braz. J. Biol. 2002, 62, 179–185. [Google Scholar] [CrossRef] [PubMed]
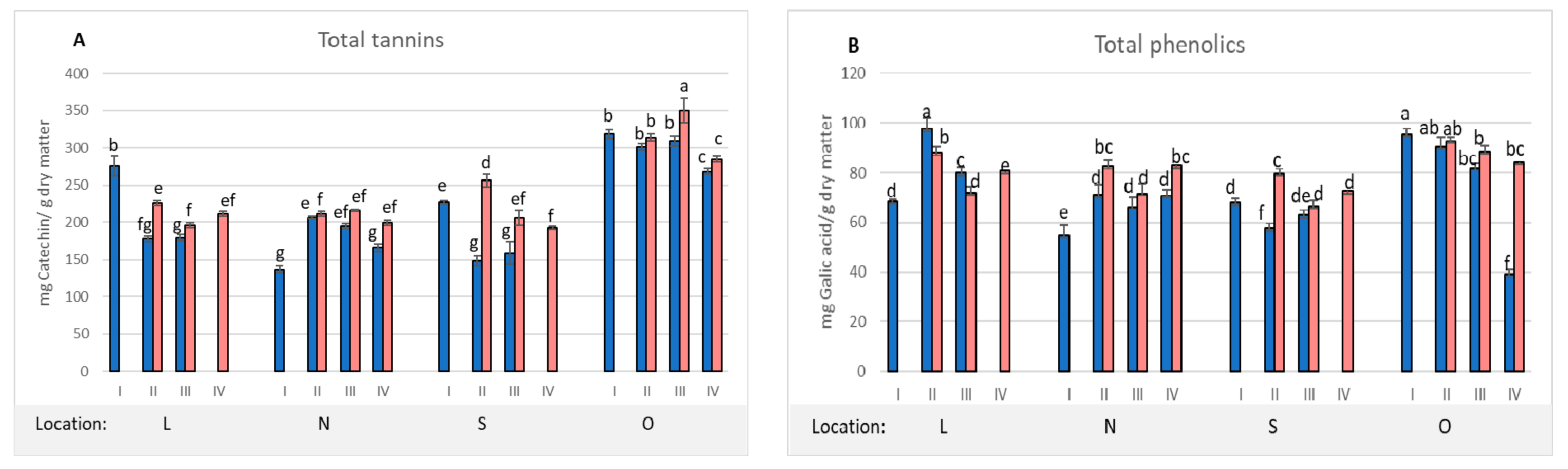
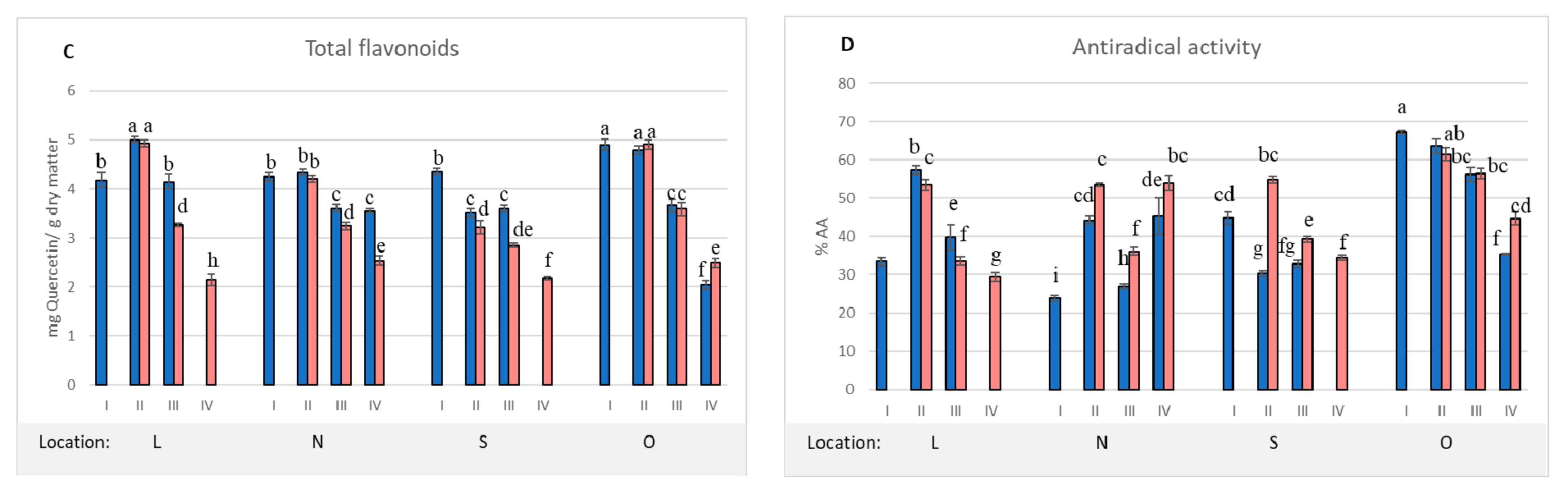

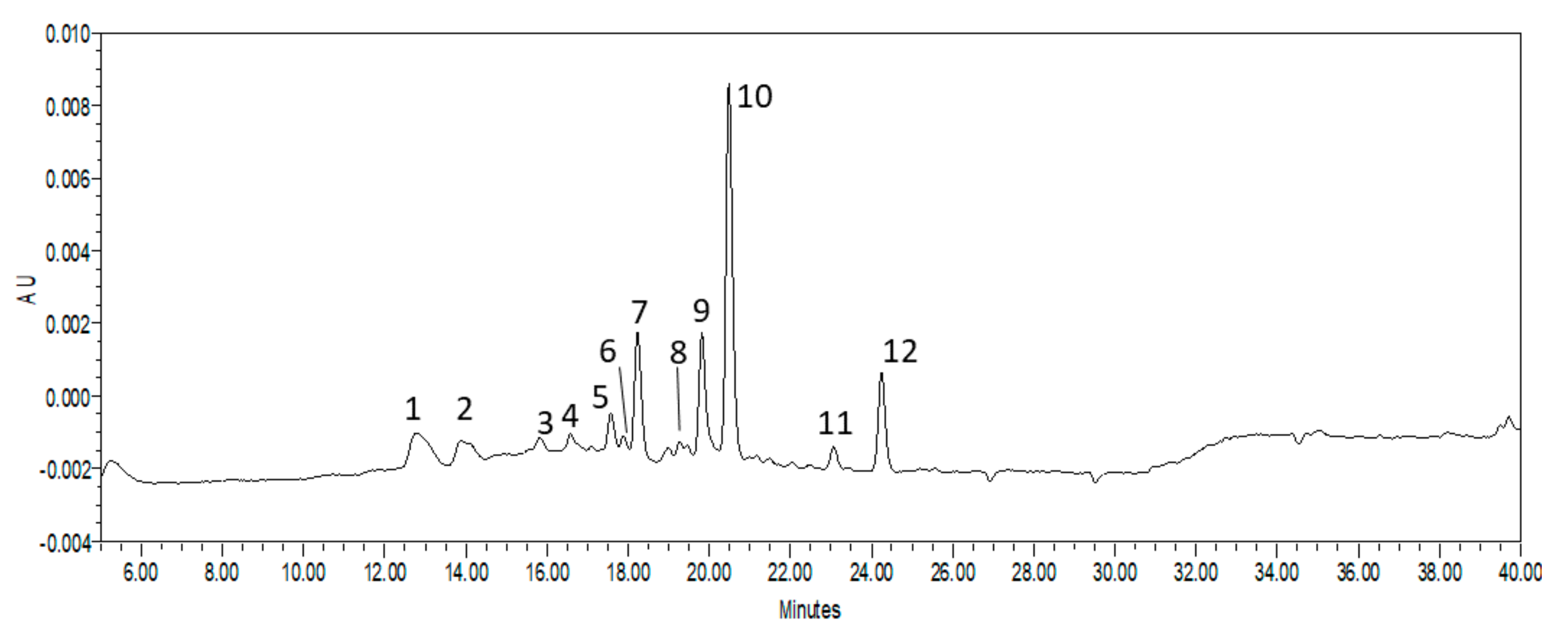
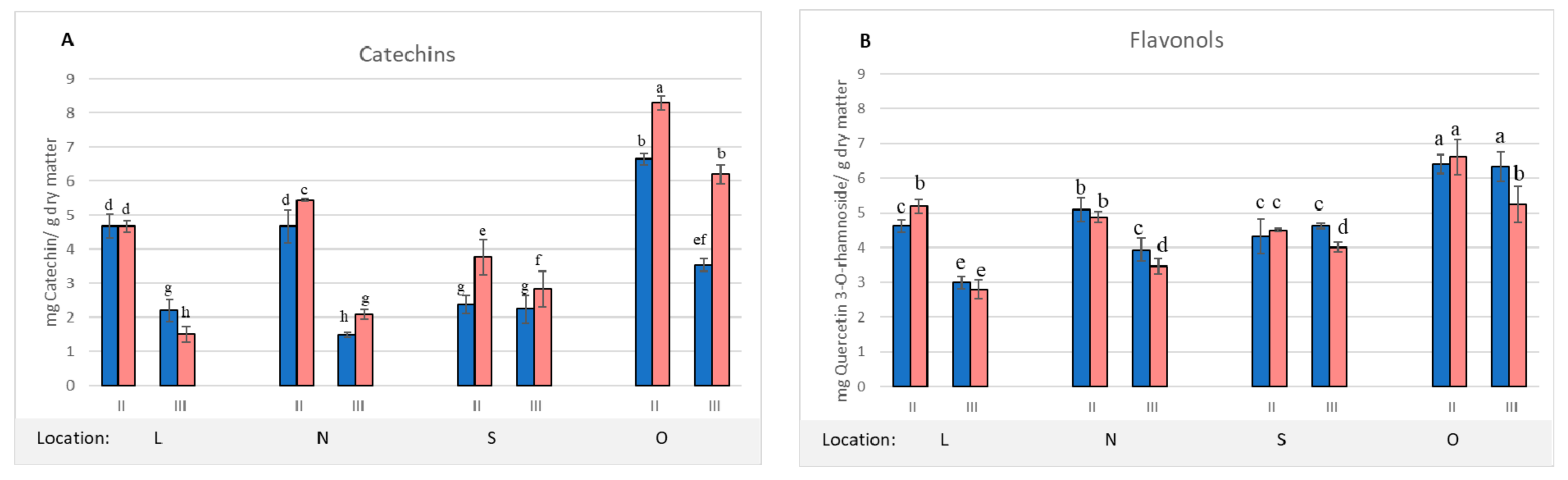

| Location | Dry Matter (%) | Total Tannins 1 | Total Phenolics 2 | Total Flavonoids 3 | Antioxidant Activity 4 |
|---|---|---|---|---|---|
| L | 70.7 b ± 0.2 | 275.1 a ± 26.9 | 68.5 b ±1.4 | 4.2 b ± 0.3 | 33.4 c ± 2.1 |
| N | 69.8 b ± 0.9 | 136.6 c ± 10.4 | 54.9 c ± 8.2 | 4.2 b ± 0.2 | 23.9 d ± 1.8 |
| S | 78.7 a ± 0.3 | 217.4 b ± 4.2 | 67.9 b ± 3.3 | 4.4 b ± 0.1 | 44.7 b ± 3.3 |
| O | 67.5 bc ± 0.2 | 318.9 a ± 12.6 | 95.6 a ± 4.5 | 4.9 a ± 0.2 | 67.3 a ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Materska, M.; Pabich, M.; Sachadyn-Król, M.; Konarska, A.; Weryszko-Chmielewska, E.; Chilczuk, B.; Staszowska-Karkut, M.; Jackowska, I.; Dmitruk, M. The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner. Molecules 2022, 27, 5471. https://doi.org/10.3390/molecules27175471
Materska M, Pabich M, Sachadyn-Król M, Konarska A, Weryszko-Chmielewska E, Chilczuk B, Staszowska-Karkut M, Jackowska I, Dmitruk M. The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner. Molecules. 2022; 27(17):5471. https://doi.org/10.3390/molecules27175471
Chicago/Turabian StyleMaterska, Małgorzata, Marzena Pabich, Monika Sachadyn-Król, Agata Konarska, Elżbieta Weryszko-Chmielewska, Barbara Chilczuk, Monika Staszowska-Karkut, Izabella Jackowska, and Marta Dmitruk. 2022. "The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner" Molecules 27, no. 17: 5471. https://doi.org/10.3390/molecules27175471
APA StyleMaterska, M., Pabich, M., Sachadyn-Król, M., Konarska, A., Weryszko-Chmielewska, E., Chilczuk, B., Staszowska-Karkut, M., Jackowska, I., & Dmitruk, M. (2022). The Secondary Metabolites Profile in Horse Chestnut Leaves Infested with Horse-Chestnut Leaf Miner. Molecules, 27(17), 5471. https://doi.org/10.3390/molecules27175471






