Phytate Dephosphorylation Products Also Act as Potent Inhibitors of Calcium Oxalate Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of InsP6 Hydrolysates
2.2. Identification of InsPs Using MS/MS
2.3. Crystallization Experiments
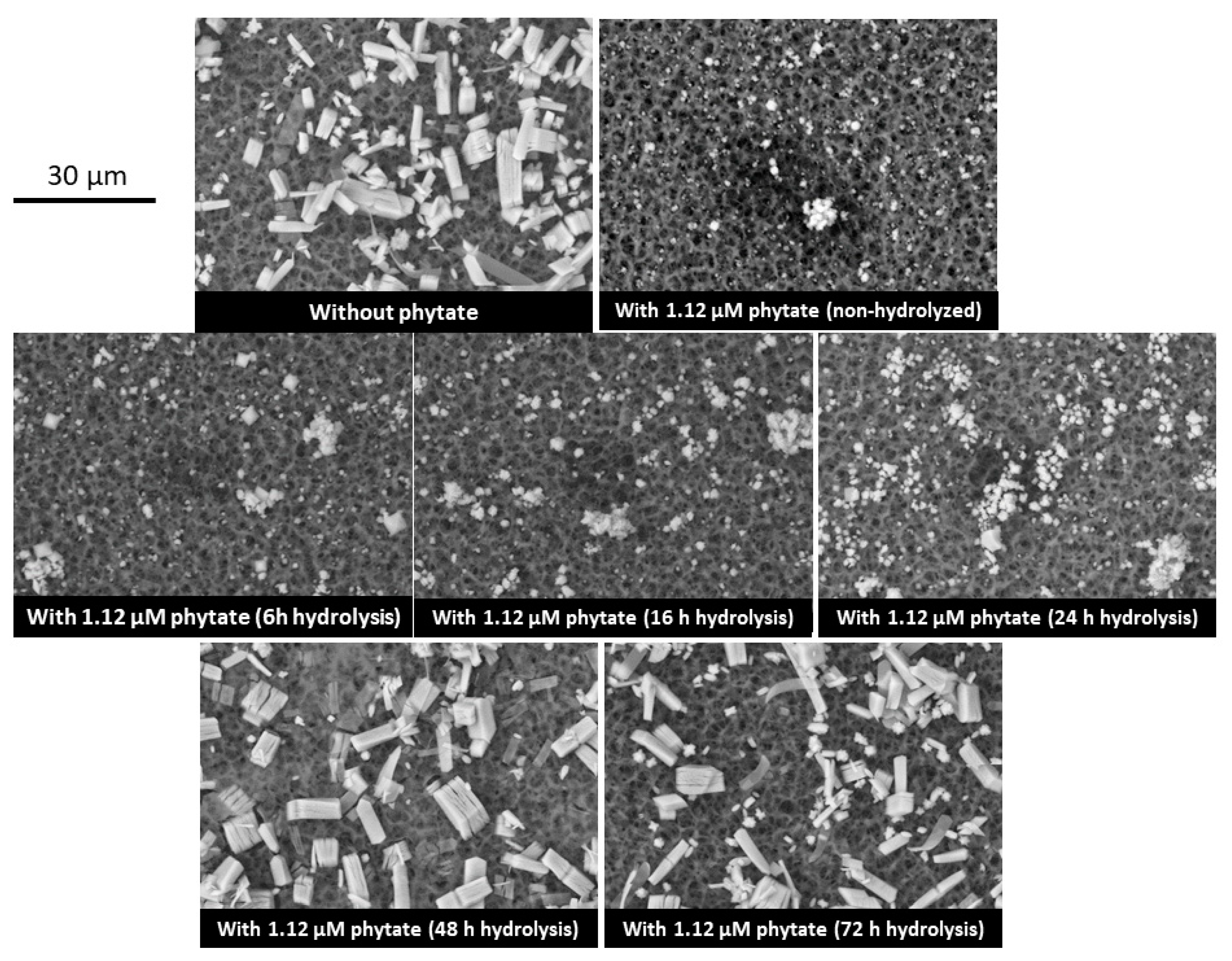
2.4. Scanning Electron Microscopy
2.5. Nonspecific Quantification of InsPs
2.6. Quantification of Inorganic Phosphate
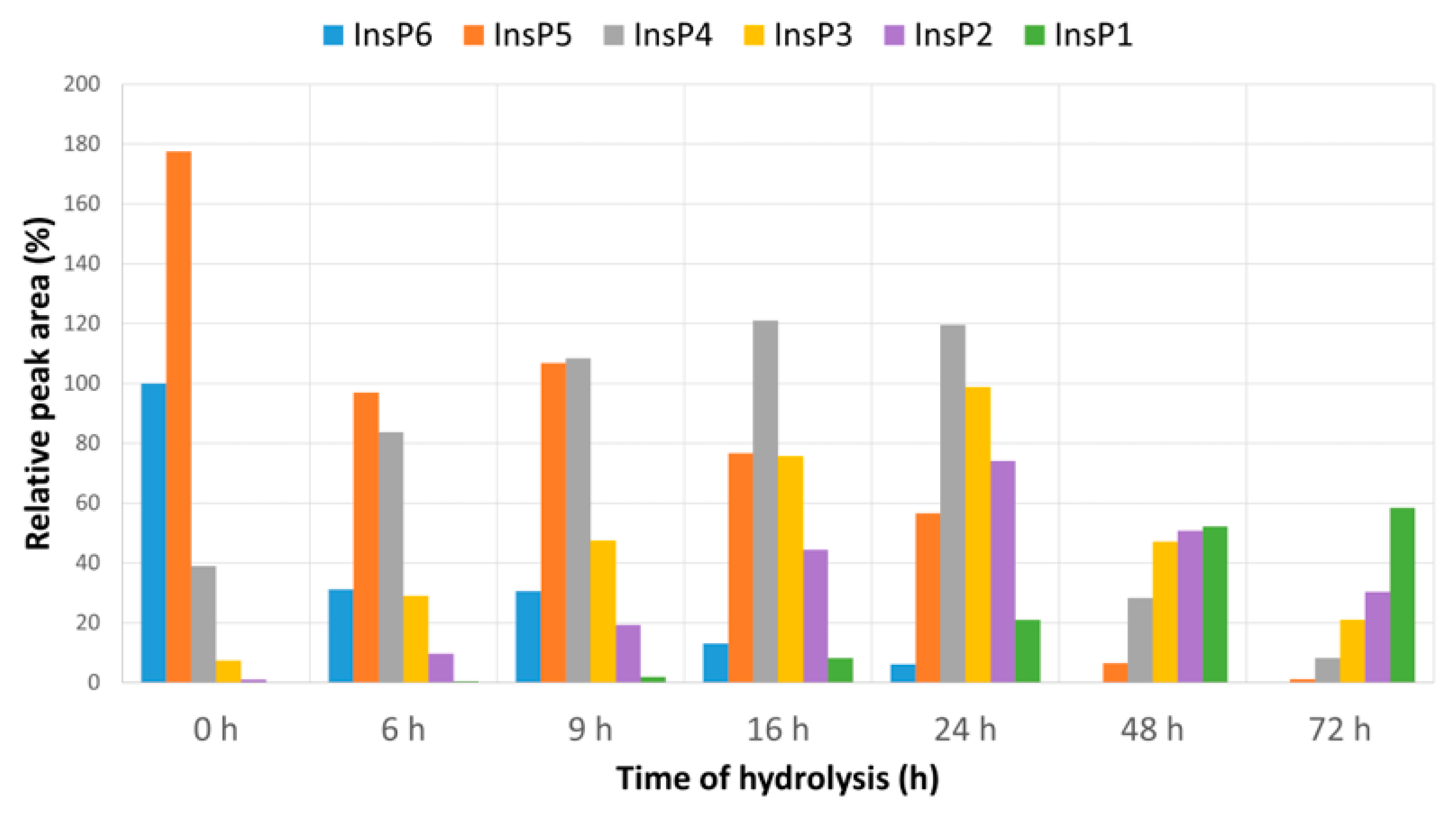
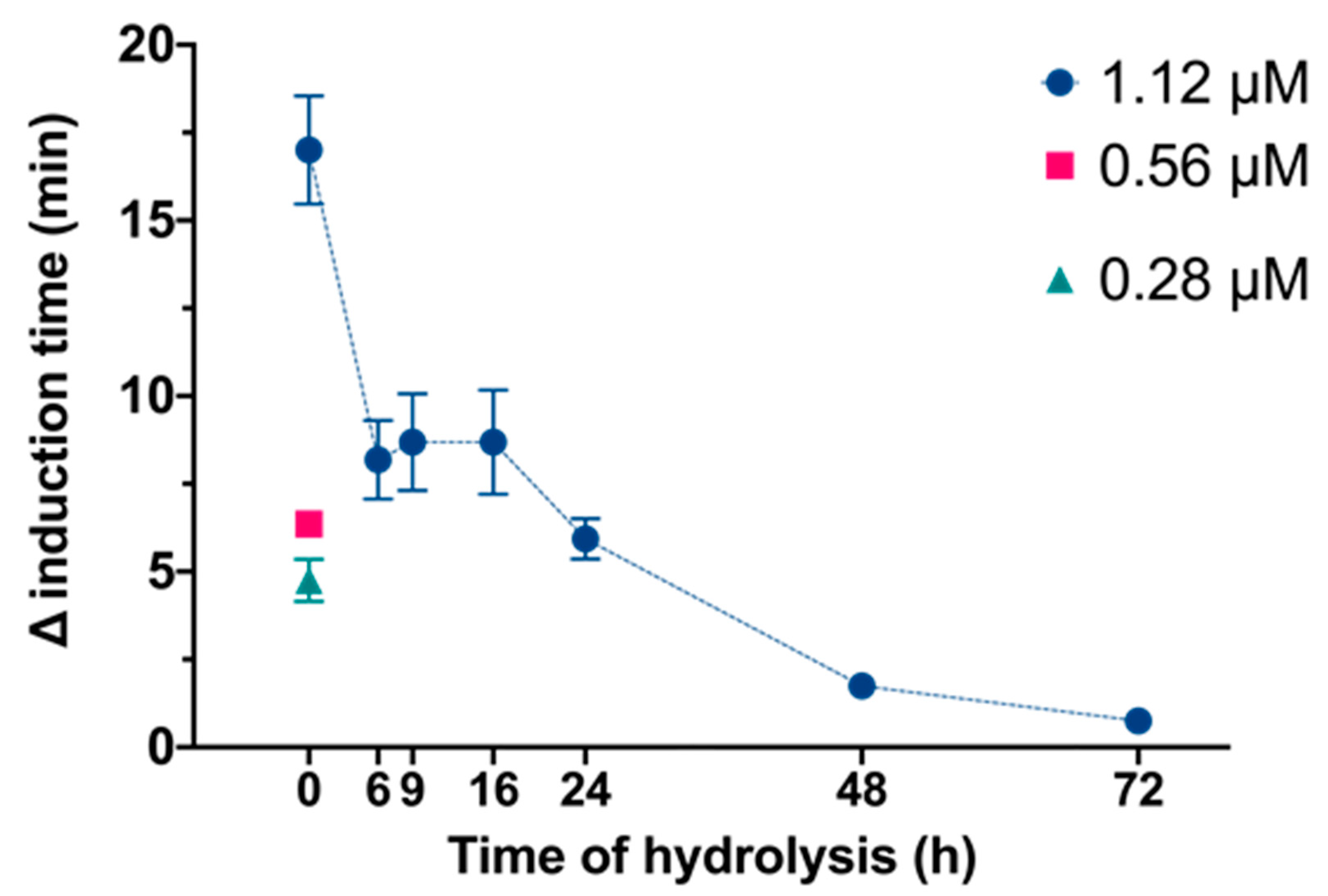
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellanby, E. The rickets-producing and anti-calcifying action of phytate. J. Physiol. 1949, 109, 488–533. [Google Scholar] [CrossRef]
- Forbes, R.M.; Parker, H.M.; Erdman, J.W. Effects of dietary phytate, calcium and magnesium levels on zinc bioavailability to rats. J. Nutr. 1984, 114, 1421–1425. [Google Scholar] [CrossRef]
- Khokhar, S.; Pushpanjali; Fenwick, G.R. Phytate Content of Indian Foods and Intakes by Vegetarian Indians of Hisar Region, Haryana State. J. Agric. Food. Chem. 1994, 42, 2440–2444. [Google Scholar] [CrossRef]
- Zhou, J.R.; Fordyce, E.J.; Raboy, V.; Dickinson, D.B.; Wong, M.-S.; Burns, R.A.; Erdman, J.W. Reduction of phytic acid in soybean products improves zinc bioavailability in rats. J. Nutr. 1992, 122, 2466–2473. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Dietary phytate and mineral bioavailability. J. Trace. Elem. Med. Biol. 2001, 15, 221–228. [Google Scholar] [CrossRef]
- Kim, O.-H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.-S.; Choi, H.J.; Kim, H.; et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. eLife 2020, 9, e52709. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Vucenik, I.; Druzijanic, A.; Druzijanic, N. Inositol Hexaphosphate (IP6) and Colon Cancer: From Concepts and First Experiments to Clinical Application. Molecules 2020, 25, 5931. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A. Key aspects of myo-inositol hexaphosphate (phytate) and pathological calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef]
- Sanchis, P.; Rivera, R.; Berga, F.; Fortuny, R.; Adrover, M.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci. Rep. 2018, 8, 9619. [Google Scholar] [CrossRef] [Green Version]
- Omoruyi, F.O.; Stennett, D.; Foster, S.; Dilworth, L. New frontiers for the use of ip6 and inositol combination in treating diabetes mellitus: A review. Molecules 2020, 25, 1720. [Google Scholar] [CrossRef]
- Silva, E.O.; Bracarense, A.P.F. Phytic Acid: From Antinutritional to Multiple Protection Factor of Organic Systems. J. Food Sci. 2016, 81, R1357–R1362. [Google Scholar]
- McIntyre, C.A.; Arthur, C.J.; Evershed, R.P. High-resolution mass spectrometric analysis of myo-inositol hexakisphosphate using electrospray ionisation Orbitrap. Rapid Commun. Mass Spectrom. 2017, 31, 1681–1689. [Google Scholar] [CrossRef]
- Yu, S.; Cai, C.; Wang, Y.; Sheng, C.; Jiang, K. Quantification of phytic acid in baby foods by derivatization with (trimethylsilyl)diazomethane and liquid chromatography–mass spectrometry analysis. Rapid Commun. Mass Spectrom. 2021, 35, e9194. [Google Scholar] [CrossRef]
- Widler, L.; Jahnke, W.; Green, J.R. The Chemistry of Bisphosphonates: From Antiscaling Agents to Clinical Therapeutics. Anticancer Agents Med. Chem. 2012, 12, 95–101. [Google Scholar] [CrossRef]
- Kovacevic, L.; Lu, H.; Kovacevic, N.; Lakshmanan, Y. Effect of bisphosphonates on the crystallization of stone-forming salts in synthetic urine. Investig. Clin. Urol. 2020, 61, 310–315. [Google Scholar] [CrossRef]
- Berga, F.; Rodriguez, A.; Costa-Bauzá, A.; Grases, F. Novel Colorimetric Determination of Phytate in Urine. Anal. Lett. 2016, 49, 307–318. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Nancollas, G.H. The mechanism of formation of renal stone crystals. Proc. Eur. Dial. Transpl. Assoc. 1983, 20, 386–397. [Google Scholar]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar]
- Eiseman, J.; Lan, J.; Guo, J.; Joseph, E.; Vucenik, I. Pharmacokinetics and tissue distribution of inositol hexaphosphate in C.B17 SCID mice bearing human breast cancer xenografts. Metabolism 2011, 60, 1465–1474. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauzá, A.; Berga, F.; Rodriguez, A.; Gomila, R.; Martorell, G.; Martínez-Cignoni, M. Evaluation of inositol phosphates in urine after topical administration of myo-inositol hexaphosphate to female Wistar rats. Life Sci. 2018, 192, 33–37. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauzá, A.; Berga, F.; Gomila, R.M.; Martorell, G.; Martínez-Cignoni, M.R. Intake of myo-inositol hexaphosphate and urinary excretion of inositol phosphates in Wistar rats: Gavage vs. Oral administration with sugar. PLoS ONE 2019, 14, e0223959. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Kenéz, A.; Rodehutscord, M.; Huber, K. Dietary phytase and myo-inositol supplementation are associated with distinct plasma metabolome profile in broiler chickens. Animal 2020, 14, 549–559. [Google Scholar] [CrossRef]
- Foster, S.R.; Dilworth, L.L.; Thompson, R.K.; Alexander-Lindo, R.L.; Omoruyi, F.O. Effects of combined inositol hexakisphosphate and inositol supplement on antioxidant activity and metabolic enzymes in the liver of streptozotocin-induced type 2 diabetic rats. Chem. Biol. Interact. 2017, 275, 108–115. [Google Scholar] [CrossRef]
- Chen, C.; Yang, F.; Liu, C.; Cui, L.; Fu, M.; Song, Y. Inositol hexaphosphate hydrolysate competitively binds to AKT to inhibit the proliferation of colon carcinoma. Oncol. Rep. 2017, 38, 2901–2910. [Google Scholar] [CrossRef]
- López-González, A.A.; Grases, F.; Roca, P.; Mari, B.; Vicente-Herrero, M.T.; Costa-Bauza, A. Phytate (myo-inositol hexaphosphate) and risk factors for osteoporosis. J. Med. Food 2008, 11, 747–752. [Google Scholar] [CrossRef]
- Thomas, W.C.; Tilden, M.T. Inhibition of mineralization by hydrolysates of phytic acid. Johns Hopkins Med. J. 1972, 131, 133–142. [Google Scholar]
- Van den Berg, C.J.; Hill, L.F.; Stanbury, S.W. Inositol phosphates and phytic acid as inhibitors of biological calcification in the rat. Clin. Sci. 1972, 43, 377–383. [Google Scholar] [CrossRef] [Green Version]
| Solution A | Solution B | ||
|---|---|---|---|
| Na2SO4 · 10H2O | 19.34 mM | NaH2PO4 · 2H2O | 15.45 mM |
| MgSO4 · 7H2O | 5.92 mM | Na2HPO4 · 12H2O | 15.64 mM |
| NH4Cl | 86.75 mM | NaCl | 223.31 mM |
| KCl | 162.69 mM | Na2C2O4 | 0.6 mM |
| CaCl2 | 10 mM | ||
| Time of Hydrolysis (h) | InsPs (mM) | Pi (mM) |
|---|---|---|
| 0 | 0.82 | 0 |
| 6 | 0.71 | 0.45 |
| 9 | 0.60 | 0.74 |
| 16 | 0.53 | 1.3 |
| 24 | 0.39 | 2.01 |
| 48 | 0.16 | 4.18 |
| 72 | 0.10 | 5.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grases, F.; Costa-Bauzá, A.; Calvó, P.; Julià, F.; Dietrich, J.; Gomila, R.M.; Martorell, G.; Sanchis, P. Phytate Dephosphorylation Products Also Act as Potent Inhibitors of Calcium Oxalate Crystallization. Molecules 2022, 27, 5463. https://doi.org/10.3390/molecules27175463
Grases F, Costa-Bauzá A, Calvó P, Julià F, Dietrich J, Gomila RM, Martorell G, Sanchis P. Phytate Dephosphorylation Products Also Act as Potent Inhibitors of Calcium Oxalate Crystallization. Molecules. 2022; 27(17):5463. https://doi.org/10.3390/molecules27175463
Chicago/Turabian StyleGrases, Felix, Antonia Costa-Bauzá, Paula Calvó, Francesca Julià, Jaume Dietrich, Rosa Maria Gomila, Gabriel Martorell, and Pilar Sanchis. 2022. "Phytate Dephosphorylation Products Also Act as Potent Inhibitors of Calcium Oxalate Crystallization" Molecules 27, no. 17: 5463. https://doi.org/10.3390/molecules27175463
APA StyleGrases, F., Costa-Bauzá, A., Calvó, P., Julià, F., Dietrich, J., Gomila, R. M., Martorell, G., & Sanchis, P. (2022). Phytate Dephosphorylation Products Also Act as Potent Inhibitors of Calcium Oxalate Crystallization. Molecules, 27(17), 5463. https://doi.org/10.3390/molecules27175463








