Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols
Abstract
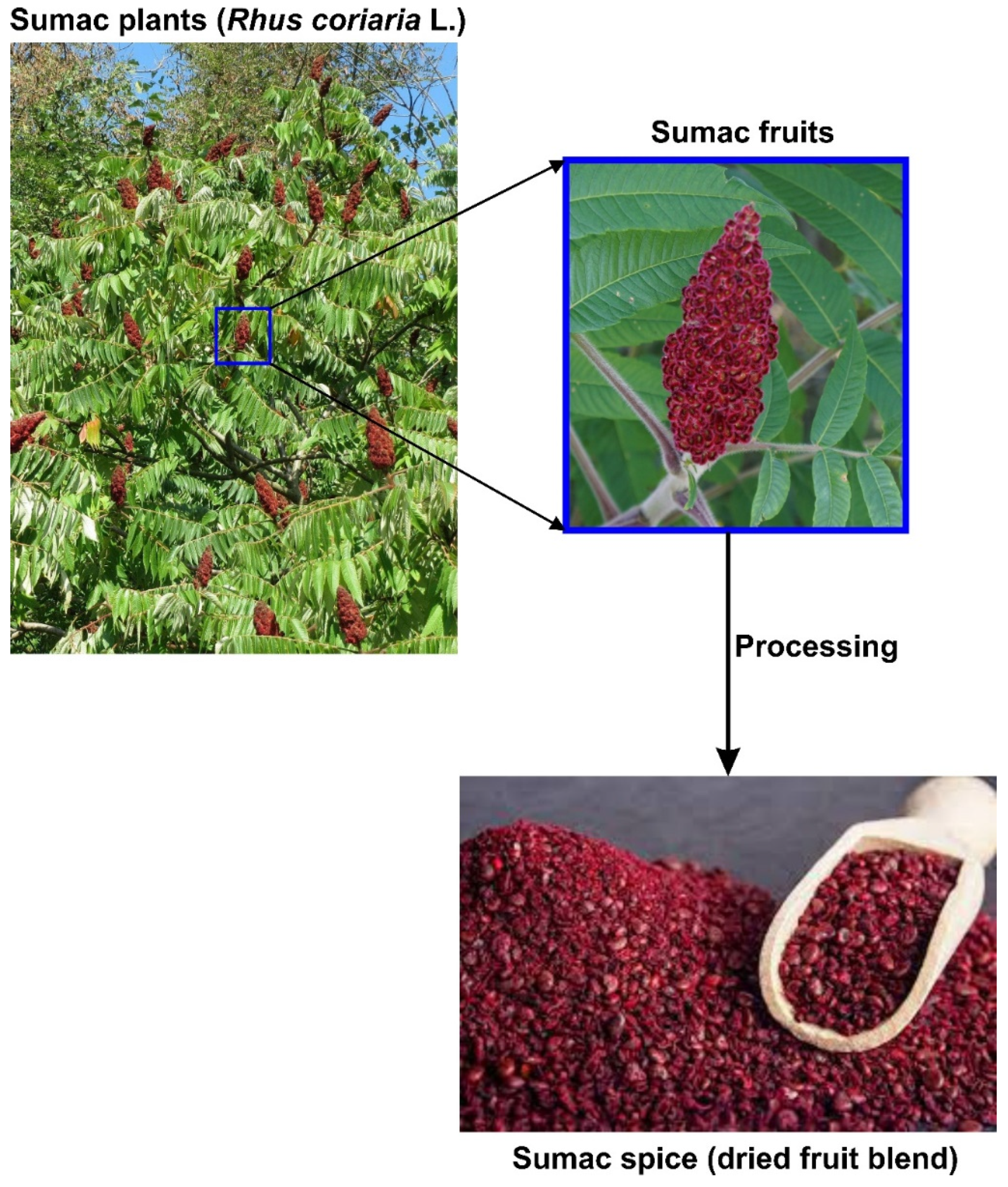
:1. Introduction
2. Sumac as a Functional Food
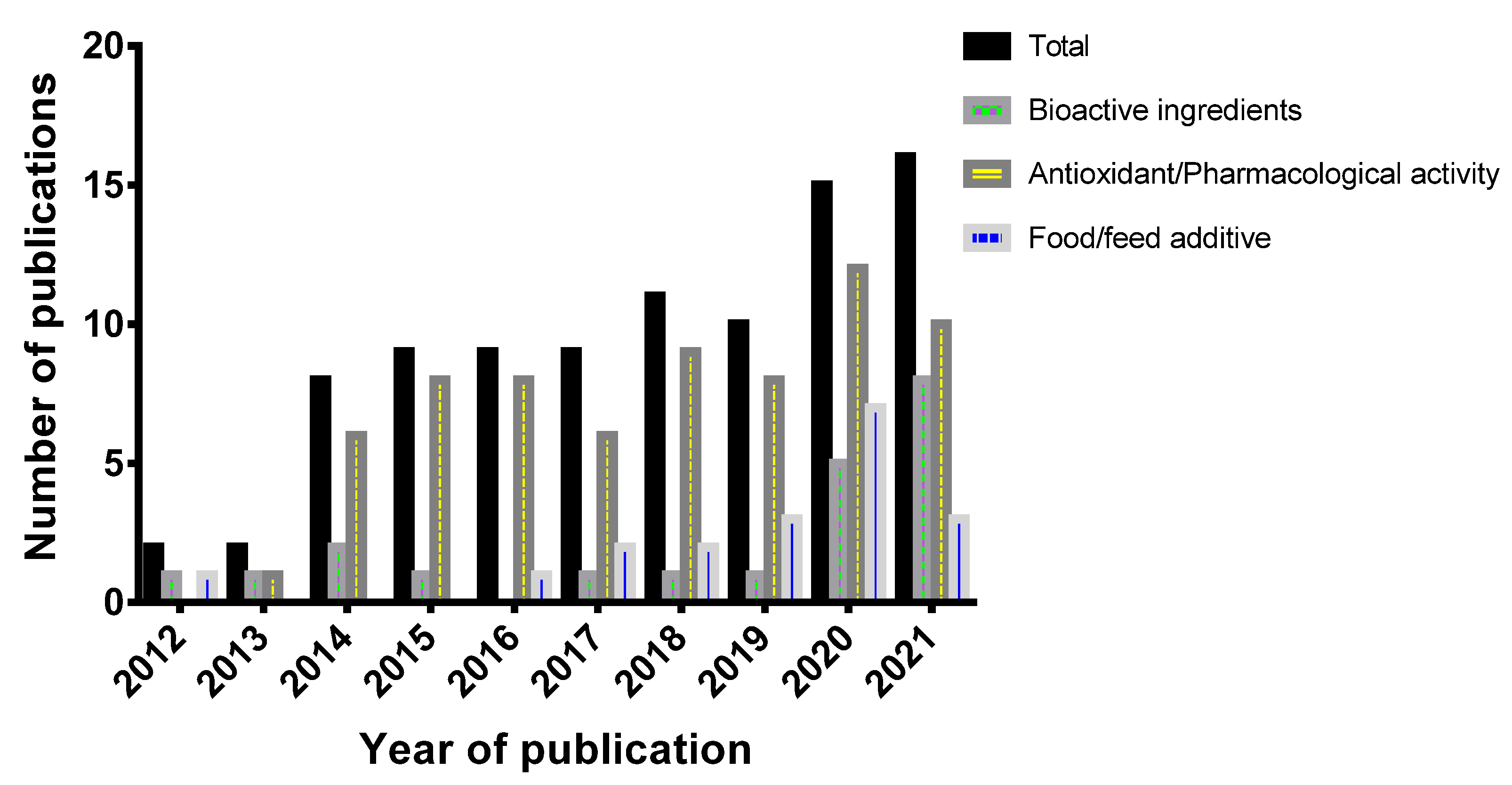
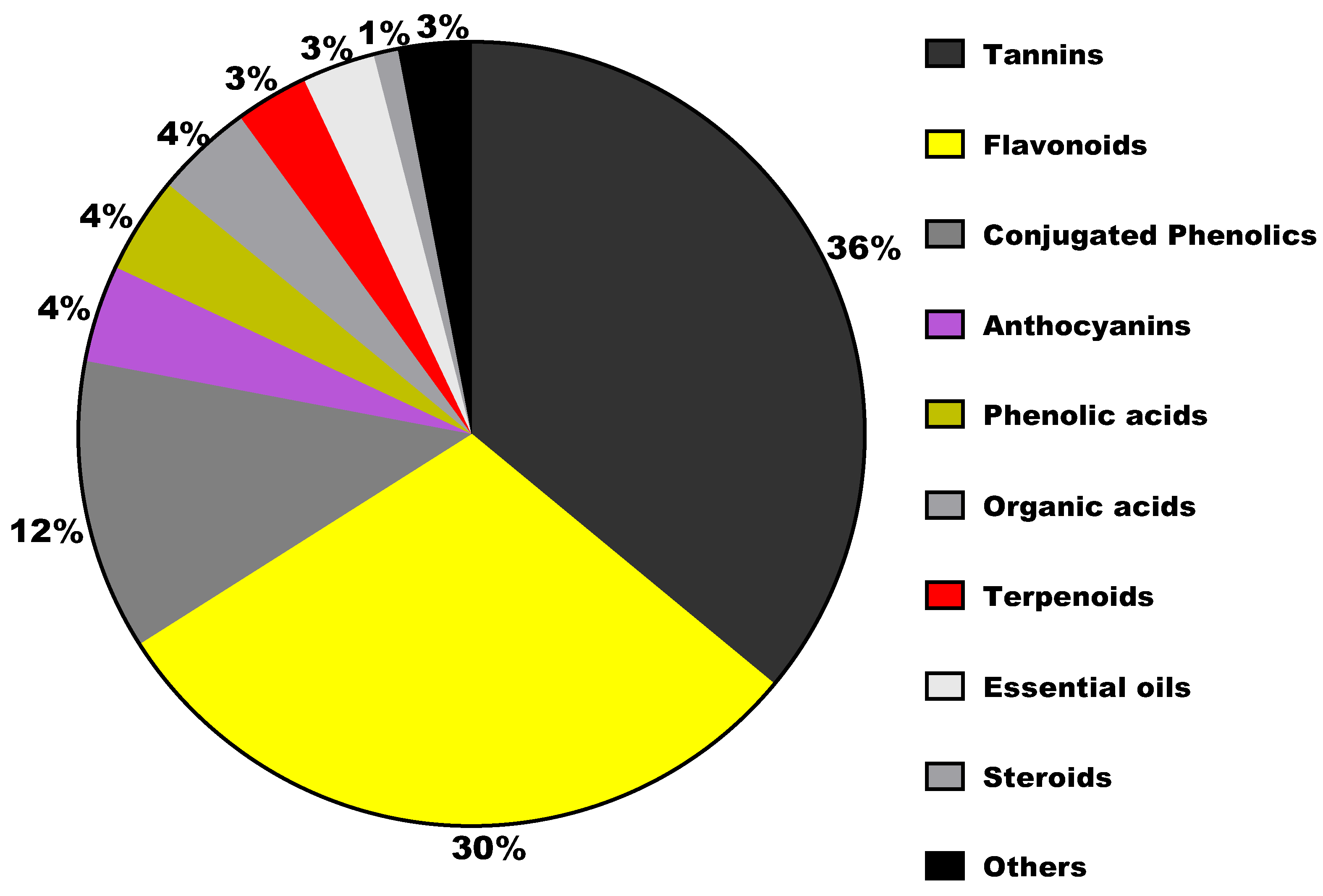
3. Bioactive Ingredients in Sumac
4. Antioxidant Potential of Sumac
5. Pharmacological Potential of Sumac
5.1. Anti-Obesity and Anti-Hyperlipidemic Potential of Sumac
5.2. Antidiabetic Potential of Sumac
5.3. Potential of Sumac in Cardiovascular Health
5.4. Neuroprotective Potential of Sumac
5.5. Role of Sumac in Cancer
5.6. Antimicrobial Potential of Sumac
6. Potential of Sumac in Food Industry
6.1. Natural Antioxidant
6.2. Natural Food Colorant
6.3. Natural Food Preservative
6.4. Food Fortifier
6.5. Natural Feed Additive
7. Safety Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Nasar-Abbas, S.M.; Halkman, A.K. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int. J. Food Microbiol. 2004, 97, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sakhr, K.; El Khatib, S. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac-Rhus coriaria): A review. Heliyon 2020, 6, e03207. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Fayek, N.M.; Reidah, I.A. Volatile profiling in Rhus coriaria fruit (sumac) from three different geographical origins and upon roasting as analyzed via solid-phase microextraction. PeerJ 2018, 6, e5121. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Cioni, P.L.; Majo, L.; Pistelli, L. Evolution of Volatile Emission in Rhus coriaria Organs During Different Stages of Growth and Evaluation of the Essential Oil Composition. Chem. Biodivers. 2017, 14, e1700270. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Pillai, G.S.; Divakaran, M. Other herbs and spices: Mango ginger to wasabi. Handb. Herbs Spices 2012, 2, 557–582. [Google Scholar]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Eid, A.H.; Iratni, R. Pharmacological and Antioxidant Activities of Rhus coriaria L. (Sumac). Antioxidants 2021, 10, 73. [Google Scholar] [CrossRef]
- Riddle, J.M. Book Review: De materia medica. Med. Hist. 2006, 50, 553–554. [Google Scholar] [CrossRef]
- Abdul-Jalil, T.Z. Rhus Coriaria (Sumac): A Magical Spice, in Herbs and Spices; Akram, M., Ahmad, R.S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lev, E.; Amar, Z. “Fossils” of practical medical knowledge from medieval Cairo. J. Ethnopharmacol. 2008, 119, 24–40. [Google Scholar] [CrossRef]
- Khalil, M.; Hayek, S.; Khalil, N.; Serale, N.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Role of Sumac (Rhus coriaria L.) in the management of metabolic syndrome and related disorders: Focus on NAFLD-atherosclerosis interplay. J. Funct. Foods 2021, 87, 104811. [Google Scholar] [CrossRef]
- Kizil, S.; Turk, M. Microelement contents and fatty acid compositions of Rhus coriaria L. and Pistacia terebinthus L. fruits spread commonly in the south eastern Anatolia region of Turkey. Nat. Prod. Res. 2010, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, C.; Ogunyemi, O.; Karigidi, K. Biotechnological strategies for enhancing the nutritive and nutraceutical values of tomato (Solanum lycopersicon). Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2015, 39, 9–19. [Google Scholar]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS Pharm. Sci. 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Khoshkharam, M.; Shahrajabian, M.H.; Singh, R.B.; Sun, W.; Magadlela, A.; Khatibi, M.; Cheng, Q. Chapter 17-Sumac: A Functional Food and Herbal Remedy in Traditional Herbal Medicine in the Asia, in Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 261–266. [Google Scholar]
- Özcan, M.; Hacıseferoǧulları, H.A. Condiment (Sumac (Rhus Coriaria L.) Fruits): Some Physico-Chemical Properties. J. Plant Physiol. 2004, 30, 3–4. [Google Scholar]
- Dogan, M.; Akgul, A. Characteristics and Fatty Acid Compositions of Rhus coriaria Cultivars from Southeast Turkey. Chem. Nat. Compd. 2005, 41, 724–725. [Google Scholar] [CrossRef]
- Kossah, R.; Nsabimana, C.; Zhang, H.; Chen, W. Optimization of Extraction of Polyphenols from Syrian Sumac (Rhus coriaria L.) and Chinese Sumac (Rhus typhina L.) Fruits. Res. J. Phytochem. 2010, 4, 146–153. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Ardalani, H.; Amiri, F.H.; Hadipanah, A.; Kongstad, K.T. Potential antidiabetic phytochemicals in plant roots: A review of in vivo studies. J. Diabetes Metab. Disord. 2021, 20, 1837–1854. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Shabana, M.M.; El Sayed, A.M.; Yousif, M.F.; El Sayed, A.M.; Sleem, A.A.l. Bioactive constituents from Harpephyllum caffrum Bernh. and Rhus coriaria L. Pharmacogn. Mag. 2011, 7, 298–306. [Google Scholar]
- Perkin, A.G.; Allen, G.Y. Colouring matter of Ssicilian sumach, Rhus coriaria. J. Chem. Soc. Trans. 1896, 69, 1299–1303. [Google Scholar] [CrossRef]
- Fröhlich, B.; Niemetz, R.; Gross, G.G. Gallotannin biosynthesis: Two new galloyltransferases from Rhus typhina leaves preferentially acylating hexa- and heptagalloylglucoses. Planta 2002, 216, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Arlandini, E.; Garzon, D.; Santagati, N.A.; Beretta, G.; Maffei Facino, R. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J. Pharm. Biomed. Anal. 2013, 72, 202–207. [Google Scholar] [CrossRef]
- Ding, Y.; Nguyen, H.T.; Choi, E.M.; Bae, K.H.; Kim, Y.H. Rhusonoside A, a New Megastigmane Glycoside from Rhus sylvestris, Increases the Function of Osteoblastic MC3T3-E1 Cells. Planta Med. 2009, 75, 158–162. [Google Scholar] [CrossRef]
- Kosar, M.; Bozan, B.; Temelli, F.; Baser, K.H.C. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007, 103, 952–959. [Google Scholar] [CrossRef]
- Romeo, F.V.; Ballistreri, G.; Fabroni, S.; Pangallo, S.; Nicosia, M.G.; Schena, L.; Rapisarda, P. Chemical Characterization of Different Sumac and Pomegranate Extracts Effective against Botrytis cinerea Rots. Molecules 2015, 20, 11941–11958. [Google Scholar] [CrossRef]
- Dalar, A.; Dogan, A.; Bengu, A.S.; Mukemre, M.; Celik, I. Screening in vivo antioxidant and haematological properties of sumac and acorn bioactive rich extracts. Ind. Crops Prod. 2018, 124, 20–27. [Google Scholar] [CrossRef]
- Abdallah, S.; Abu-Reidah, I.; Mousa, A.; Abdel-Latif, T. Rhus coriaria (sumac) extract reduces migration capacity of uterus cervix cancer cells. Rev. Bras. Farmacogn. 2019, 29, 591–596. [Google Scholar] [CrossRef]
- Gyebi, G.A.; Ocheje, J.O.; Ogunyemi, O.M. Molecular docking studies on bioactive compounds from clove (Syzygium aromaticum) on metabolic regulators in cancer. Salem Univ. J. Life Sci. 2019, 1, 1–18. [Google Scholar]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential Oils from Herbs and Spices as Natural Antioxidants: Diversity of Promising Food Applications in the past Decade. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems–A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Kurucu, S.; Koyuncu, M.; Güvenç, A.; Baser, K.H.C.; Özek, T. The Essential Oils of Rhus coriaria L. (Sumac). J. Essent. Oil Res. 1993, 5, 481–486. [Google Scholar] [CrossRef]
- Gharaei, A.; Khajeh, M.; Ghaffari, M.; Choopani, A. Iranian Rhus coriaria (sumac) Essential Oils Extraction. J. Essent. Oil Bear. Plants 2013, 16, 270–273. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M. Fatty acid composition, tocopherol, and sterol contents of sumac (Rhus coriaria L.) fruit oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1301–1302. [Google Scholar] [CrossRef]
- Giovanelli, S.; Giusti, G.; Cioni, P.L.; Minissale, P.; Ciccarelli, D.; Pistelli, L. Aroma profile and essential oil composition of Rhus coriaria fruits from four Sicilian sites of collection. Ind. Crops Prod. 2017, 97, 166–174. [Google Scholar] [CrossRef]
- Bahar, B.; Altug, T. Flavour Characterization of Sumach (Rhus coriaria L.) by Means of GC/MS and Sensory Flavour Profile Analysis Techniques. Int. J. Food Prop. 2009, 12, 379–387. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Ebadi, A.; Maggi, F.; Fattahi, R.; Yazdani, D.; Jafari, M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crops Prod. 2015, 76, 565–573. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Maxwell, S.R.; Lip, G.Y. Free radicals and antioxidants in cardiovascular disease. Br. J. Clin. Pharmacol. 1997, 44, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Oso, B.J.; Ogunyemi, O.M. Assessment of in vitrobiological properties of aqueous extracts of Murraya koenigii (L.) Spreng, Thymus vulgaris L.; and Ocimum gratissimum L. leaves. Croat. J. Food Sci. Technol. 2020, 12, 238–248. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Shafiei, M.; Nobakht, M.; Moazzam, A.A. Lipid-lowering effect of Rhus coriaria L. (sumac) fruit extract in hypercholesterolemic rats. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 988–992. [Google Scholar]
- Aliakbarlu, J.; Mohammadi, S.; Khalili, S. A Study on Antioxidant Potency and Antibacterial Activity of Water Extracts of Some Spices Widely Consumed in Iranian Diet. J. Food Biochem. 2014, 38, 159–166. [Google Scholar] [CrossRef]
- Baştürk, A.; Ceylan, M.M.; Çavuş, M.; Boran, G.; Javidipour, I. Effects of some herbal extracts on oxidative stability of corn oil under accelerated oxidation conditions in comparison with some commonly used antioxidants. LWT 2018, 89, 358–364. [Google Scholar] [CrossRef]
- Candan, F.; Sökmen, A. Effects of Rhus coriaria L (Anacardiaceae) on lipid peroxidation and free radical scavenging activity. Phytother. Res. 2004, 18, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.Y.; El-Khateeb, A.Y.; Mohamed, A.H. Rhus and Safflower Extracts as Potential Novel Food Antioxidant, Anticancer, and Antimicrobial Agents Using Nanotechnology. Foods 2019, 8, 139. [Google Scholar]
- Bursal, E.; Köksal, E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res. Int. 2011, 44, 2217–2221. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Grassi, G.; Gambacorta, E. Effect of α(S1)-casein genotype on phenolic compounds and antioxidant activity in goat milk yogurt fortified with Rhus coriaria leaf powder. J. Dairy Sci. 2018, 101, 7691–7701. [Google Scholar] [CrossRef]
- Majd, N.S.; Coe, S.; Thondre, S.; Lightowler, H. Determination of the antioxidant activity and polyphenol content of different types of Rhus coriaria Linn (sumac) from different regions. Proc. Nutr. Soc. 2017, 76, E137. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ferk, F.; Simić, T.; Brantner, A.; Dusinská, M.; Kundi, M.; Hoelzl, C.; Nersesyan, A.; Knasmüller, S. DNA-protective effects of sumach (Rhus coriaria L.), a common spice: Results of human and animal studies. Mutat. Res. 2009, 661, 10–17. [Google Scholar] [CrossRef]
- Ferk, F.; Kundi, M.; Brath, H.; Szekeres, T.; Al-Serori, H.; Mišík, M.; Saiko, P.; Marculescu, R.; Wagner, K.-H.; Knasmueller, S. Gallic Acid Improves Health-Associated Biochemical Parameters and Prevents Oxidative Damage of DNA in Type 2 Diabetes Patients: Results of a Placebo-Controlled Pilot Study. Mol. Nutr. Food Res. 2018, 62, 1700482. [Google Scholar] [CrossRef]
- Elagbar, Z.A.; Shakya, A.K.; Barhoumi, L.M.; Al-Jaber, H.I. Phytochemical Diversity and Pharmacological Properties of Rhus coriaria. Chem. Biodivers. 2020, 17, e1900561. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Sezik, E.; Yeşilada, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J. Ethnopharmacol. 2001, 75, 95–115. [Google Scholar] [CrossRef]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J. Ethnopharmacol. 2002, 82, 131–145. [Google Scholar] [CrossRef]
- Tuzlaci, E.; Aymaz, P.E. Turkish folk medicinal plants, Part IV: Gönen (Balikesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Balaji, M.; Ganjayi, M.S.; Hanuma Kumar, G.E.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Ige-Elegbede, J.O.; Ezejimofor, M.; Owolabi, E.O.; Ezeigwe, N.; Omoyele, C.; Mpazanje, R.G.; Dewan, M.T.; Agogo, E.; Gadanya, M.A.; et al. Estimating the prevalence of overweight and obesity in Nigeria in 2020: A systematic review and meta-analysis. Ann. Med. 2021, 53, 495–507. [Google Scholar] [CrossRef]
- Ogunyemi, O.M.; Gyebi, G.A.; Ibrahim, I.M.; Esan, A.M.; Olaiya, C.O.; Soliman, M.M.; Batiha, G.E.-S. Identification of promising multi-targeting inhibitors of obesity from Vernonia amygdalina through computational analysis. Mol. Divers. 2022. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Lillich, F.F.; Imig, J.D.; Proschak, E. Multi-Target Approaches in Metabolic Syndrome. Front. Pharmacol. 2021, 11, 554961. [Google Scholar] [CrossRef]
- Ghaeni Pasavei, A.; Mohebbati, R.; Jalili-Nik, M.; Mollazadeh, H.; Ghorbani, A.; Nosrati Tirkani, A.; Taraz Jamshidi, S.; Hashemy, S.I.; Heidarian Miri, H.; Soukhtanloo, M. Effects of Rhus coriaria L. hydroalcoholic extract on the lipid and antioxidant profile in high fat diet-induced hepatic steatosis in rats. Drug Chem. Toxicol. 2021, 44, 75–83. [Google Scholar] [CrossRef]
- Capcarova, M.; Slamecka, J.; Abbas, K.; Kolesarova, A.; Kalafova, A.; Valent, M.; Filipejova, T.; Chrastinova, L.; Ondruska, L.; Massanyi, P. Effects of dietary inclusion of Rhus coriaria on internal milieu of rabbits. J. Anim. Physiol. Anim. Nutr. 2012, 96, 459–465. [Google Scholar] [CrossRef]
- Jeon, M.; Rahman, N.; Kim, Y.-S. Wnt/β-catenin signaling plays a distinct role in methyl gallate–mediated inhibition of adipogenesis. Biochem. Biophys. Res. Commun. 2016, 479, 22–27. [Google Scholar] [CrossRef]
- Motamedrad, M.; Shokouhifar, A.; Hemmati, M.; Moossavi, M. The regulatory effect of saffron stigma on the gene expression of the glucose metabolism key enzymes and stress proteins in streptozotocin-induced diabetic rats. Res. Pharm. Sci. 2019, 14, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, O.M.; Gyebi, A.G.; Adebayo, J.O.; Oguntola, J.A.; Olaiya, C.O. Marsectohexol and other pregnane phytochemicals derived from Gongronema latifolium as α-amylase and α-glucosidase inhibitors: In vitro and molecular docking studies. SN Appl. Sci. 2020, 2, 2119. [Google Scholar] [CrossRef]
- Eruygur, N.; Koçyiğit, U.M.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Anwer, T.; Sharma, M.; Khan, G.; Iqbal, M.; Ali, M.S.; Alam, M.S.; Safhi, M.M.; Gupta, N. Rhus coriaria ameliorates insulin resistance in non-insulin-dependent diabetes mellitus (NIDDM) rats. Acta Pol. Pharm. 2013, 70, 861–867. [Google Scholar] [PubMed]
- Mohammadi, S.; Kouhsari, S.M.; Feshani, A.M. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2010, 18, 270–275. [Google Scholar]
- Giancarlo, S.; Rosa, L.M.; Nadjafi, F.; Francesco, M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Nat. Prod. Res. 2006, 20, 882–886. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Junghani, M.S.; Absari, R.; Khoogar, M.; Ghaedi, E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res. Pharm. Sci. 2017, 12, 416–424. [Google Scholar]
- Doğan, A.; Çelik, İ. Healing effects of sumac (Rhus coriaria) in streptozotocin-induced diabetic rats. Pharm. Biol. 2016, 54, 2092–2102. [Google Scholar] [CrossRef]
- Abedi Gaballu, F.; Abedi Gaballu, Y.; Moazenzade Khyavy, O.; Mardomi, A.; Ghahremanzadeh, K.; Shokouhi, B.; Mamandy, H. Effects of a triplex mixture of Peganum harmala, Rhus coriaria, and Urtica dioica aqueous extracts on metabolic and histological parameters in diabetic rats. Pharm. Biol. 2015, 53, 1104–1109. [Google Scholar] [CrossRef]
- Ghorbanian, B.; Mohammadi, H.; Azali, K. Effects of 10-weeks aerobic training with Rhus coriaria. L. supplementation on TAC, insulin resistance and anthropometric indices in women with type 2 diabetes. CMJA 2017, 7, 1805–1815. [Google Scholar]
- Shidfar, F.; Rahideh, S.T.; Rajab, A.; Khandozi, N.; Hosseini, S.; Shidfar, S.; Mojab, F. The Effect of Sumac (Rhus coriaria L.) Powder on Serum Glycemic Status, ApoB, ApoA-I and Total Antioxidant Capacity in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2014, 13, 1249–1255. [Google Scholar] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- NCD. NCD Countdown 2030: Pathways to achieving Sustainable Development Goal target 3.4. Lancet 2020, 396, 918–934. [Google Scholar] [CrossRef]
- Marcus, M.E.; Manne-Goehler, J.; Theilmann, M.; Farzadfar, F.; Moghaddam, S.S.; Keykhaei, M.; Hajebi, A.; Tschida, S.; Lemp, J.M.; Aryal, K.K.; et al. Use of statins for the prevention of cardiovascular disease in 41 low-income and middle-income countries: A cross-sectional study of nationally representative, individual-level data. Lancet Glob. Health 2022, 10, e369–e379. [Google Scholar] [CrossRef]
- Ferk, F.; Chakraborty, A.; Simic, T.; Kundi, M.; Knasmüller, S. Antioxidant and free radical scavenging activities of sumac (Rhus coriaria) and identification of gallic acid as its active principle. BMC Pharmacol. 2007, 7, A71. [Google Scholar] [CrossRef]
- Beretta, G.; Rossoni, G.; Santagati, N.A.; Facino, R.M. Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta. Planta Med. 2009, 75, 1482–1488. [Google Scholar] [CrossRef]
- Zargham, H.; Zargham, R. Tannin extracted from Sumac inhibits vascular smooth muscle cell migration. McGill J. Med. MJM Int. Forum Adv. Med. Sci. Stud. 2008, 11, 119–123. [Google Scholar] [CrossRef]
- Asgary, S.; Salehizadeh, L.; Keshvari, M.; Taheri, M.; Spence, N.D.; Farvid, M.S.; Rafieian-Kopaei, M.; Sarrafzadegan, N. Potential Cardioprotective Effects of Sumac Capsule in Patients with Hyperlipidemia: A Triple-Blind Randomized, Placebo-Controlled Crossover Trial. J. Am. Coll. Nutr. 2018, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Sabzghabaee, A.M.; Kelishadi, R.; Golshiri, K.; Ghannadi, A.; Badri, S. Clinical Effects of Rhus coriaria Fruits on Dyslipidemia308 in Adolescents: A Triple-blinded Randomized Placebo-controlled Trial. Med. Arch. 2014, 68, 308–312. [Google Scholar] [CrossRef]
- Anwar, M.A.; Samaha, A.A.; Baydoun, S.; Iratni, R.; Eid, A.H. Rhus coriaria L. (Sumac) Evokes Endothelium-Dependent Vasorelaxation of Rat Aorta: Involvement of the cAMP and cGMP Pathways. Front. Pharmacol. 2018, 9, 688. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current Trends in Neurodegeneration: Cross Talks between Oxidative Stress, Cell Death, and Inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Bozoki, A.; Giordani, B.; Heidebrink, J.L.; Berent, S.; Foster, N.L. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch. Neurol. 2001, 58, 411–416. [Google Scholar] [CrossRef]
- Khalilpour, S.; Behnammanesh, G.; Suede, F.; Ezzat, M.O.; Muniandy, J.; Tabana, Y.; Ahamed, M.K.; Tamayol, A.; Majid, A.M.S.; Sangiovanni, E.; et al. Neuroprotective and Anti-Inflammatory Effects of Rhus coriaria Extract in a Mouse Model of Ischemic Optic Neuropathy. Biomedicines 2018, 6, 48. [Google Scholar] [CrossRef]
- Mirian, M.; Behrooeian, M.; Ghanadian, M.; Dana, N.; Sadeghi-Aliabadi, H. Cytotoxicity and antiangiogenic effects of Rhus coriaria, Pistacia vera and Pistacia khinjuk oleoresin methanol extracts. Res. Pharm. Sci. 2015, 10, 233–240. [Google Scholar]
- El Hasasna, H.; Athamneh, K.; Al Samri, H.; Karuvantevida, N.; Al Dhaheri, Y.; Hisaindee, S.; Ramadan, G.; Al Tamimi, N.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria induces senescence and autophagic cell death in breast cancer cells through a mechanism involving p38 and ERK1/2 activation. Sci. Rep. 2015, 5, 13013. [Google Scholar] [CrossRef]
- El Hasasna, H.; Saleh, A.; Al Samri, H.; Athamneh, K.; Attoub, S.; Arafat, K.; Benhalilou, N.; Alyan, S.; Viallet, J.; Al Dhaheri, Y.; et al. Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways. Sci. Rep. 2016, 6, 21144. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M. Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma. Int. J. Mol. Sci. 2020, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, K.; Hasasna, H.E.; Samri, H.A.; Attoub, S.; Arafat, K.; Benhalilou, N.; Rashedi, A.A.; Dhaheri, Y.A.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria increases protein ubiquitination, proteasomal degradation and triggers non-canonical Beclin-1-independent autophagy and apoptotic cell death in colon cancer cells. Sci. Rep. 2017, 7, 11633. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Roy, J. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.E. Natural Antimicrobials from Plants, in New Methods of Food Preservation; Gould, G.W., Ed.; Springer: Boston, MA, USA, 1995; pp. 58–89. [Google Scholar]
- Zhaleh, M.; Sohrabi, N.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R.; Zhaleh, H. Chemical Composition and Antibacterial Effects of Essential Oil of Rhus coriaria Fruits in the West of Iran (Kermanshah). J. Essent. Oil-Bear. Plants 2018, 21, 493–501. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Vahid-Dastjerdi, E.; Sarmast, Z.; Abdolazimi, Z.; Mahboubi, A.; Amdjadi, P.; Kamalinejad, M. Effect of Rhus coriaria L. water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire. Iran. J. Microbiol. 2014, 6, 269–275. [Google Scholar]
- Vahid-Dastjerdi, E.E.; Monadi, E.; Khalighi, H.R.; Torshabi, M. Down-Regulation of Glycosyl Transferase Genes in Streptococcus Mutans by Punica granatum L. Flower and Rhus coriaria L. Fruit Water Extracts. Iran. J. Pharm. Res. 2016, 15, 513–519. [Google Scholar]
- Gregori, R.; Mari, M.; Bertolini, P.; Barajas, J.A.S.; Tian, J.B.; Labavitch, J.M. Reduction of Colletotrichum acutatum infection by a polygalacturonase inhibitor protein extracted from apple. Postharvest Biol. Technol. 2008, 48, 309–313. [Google Scholar] [CrossRef]
- Digrak, M.; Alma, M.H.; Ilçim, A. Antibacterial and Antifungal Activities of Turkish Medicinal Plants. Pharm. Biol. 2001, 39, 346–350. [Google Scholar] [CrossRef]
- Singh, O.; Ali, M.; Akhtar, N. New antifungal xanthones from the seeds of Rhus coriaria L. Z. Nat. C 2011, 66, 17–23. [Google Scholar]
- Ogunyemi, O.; Gyebi, G.; Shaibu, R.; Fabusiwa, M.; Olaiya, C. Antioxidant, Nutritional, and Physicochemical Quality of Yoghurt Produced from a Milk-Based Fermentation Mix Enhanced with Food Spices. Croat. J. Food Sci. Technol. 2021, 13, 201–209. [Google Scholar] [CrossRef]
- Fernandes, R.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.; Munekata, P.E.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Filipčev, B. Chapter 16-The Effects of Aromatic Plants and Their Extracts in Food Products, in Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 279–294. [Google Scholar]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M. Effect of sumach (Rhus coriaria L.) extracts on the oxidative stability of peanut oil. J. Med. Food 2003, 6, 63–66. [Google Scholar] [CrossRef]
- Bozkurt, H. Investigation of the effect of sumac extract and BHT addition on the quality of sucuk (Turkish dry-fermented sausage). J. Sci. Food Agric. 2006, 86, 849–856. [Google Scholar] [CrossRef]
- Dabas, D. Polyphenols as Colorants. Adv. Food Technol. Nutr. Sci. Open J. 2018, SE, S1–S6. [Google Scholar] [CrossRef]
- Gulmez, M.; Oral, N.; Vatansever, L. The Effect of Water Extract of Sumac (Rhus coriaria L.) and Lactic Acid on Decontamination and Shelf Life of Raw Broiler Wings. Poult. Sci. 2006, 85, 1466–1471. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Ashtiani, H.; Ahmadian-Attari, M.M.; Jamalifar, H.; Zaheri, A. Antimicrobial effect of Rhus coriaria L. (Sumac) total extract on skin isolates Staphylococcus epidermidis and Corynebacterium xerosis. J. Med. Plants 2006, 5, 27–31. [Google Scholar]
- Sakhr, K.; El Khatib, S. The Use of Syrian Sumac (Rhus coriaria) as a Meat Tenderizer: Effect on Fat, Protein and Collagen Profiles on Pectoralis superficialis Cut. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1203. [Google Scholar] [CrossRef]
- SalİH, Y.K.; Gürbüz, Y. Sumac (Rhus coriaria L.) and Ginger (Zingiber officinale) as Feed Additive in Poultry Nutrition. Kahramanmaraş Sütçü İmam Üniversitesi Doğa Bilimleri Dergisi 2015, 18, 44. [Google Scholar] [CrossRef]
- Sharbati, A.; Daneshyar, M.; Aghazadeh, A.; Aliakbarlu, J.; Hamian, F. Effects of Rhus coriaria on nutrient composition, thiobarbituric acid reactive substances and colour of thigh meat in heat-stressed broilers. S. Afr. J. Anim. Sci. 2015, 45, 49. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Using sumac (Rhus coriaria L.), as a miraculous spice with outstanding pharmacological activities. Not. Sci. Biol. 2022, 14, 11118. [Google Scholar] [CrossRef]
- Aguilar-Ortigoza, C.J.; Sosa, V.; Aguilar-Ortigoza, M. Toxic Phenols in various Anacardiaceae species. Econ. Bot. 2003, 57, 354. [Google Scholar] [CrossRef]
| Nutritional Components | Fresh | Dried |
|---|---|---|
| Moisture (%) | 10.60 | 2.43 |
| Oil (%) | 7.40 | 18.74 |
| Protein (%) | 2.60 | 4.69 |
| Crude fibre (%) | 14.60 | NDM |
| Carbohydrate (%) | NDM | 71.21 |
| Crude energy (kcal/100 g) | 147.8 | NDM |
| Ash (%) | 1.80 | 2.93 |
| Water soluble extract | 63.80 | NDM |
| Acidity (%) | 4.60 | NDM |
| pH | 3.70 | 3.02 |
| Nutrient | Quanttity |
|---|---|
| Oleic Acid (%) | 37.70 |
| Linoleic Acid (%) | 27.40 |
| Palmitic Acid (%) | 21.10 |
| Stearic Acid (%) | 4.70 |
| Other Fatty acids (%) | 9.10 |
| Vitamin B6 (ppm) | 69.83 |
| Vitamin C (ppm) | 38.91 |
| Vitamin B1(ppm) | 30.65 |
| Vitamin B2 (ppm) | 24.68 |
| Nicotinamide (ppm) | 17.95 |
| Potassium (ppm) | 7963.35 |
| Calcium (ppm) | 3661.57 |
| Phosphorus (ppm) | 1238.74 |
| Magnessium (ppm) | 855.95 |
| Iron (ppm) | 144.53 |
| Classes of Compounds | Key Bioactive Compounds | Plant Parts | References |
|---|---|---|---|
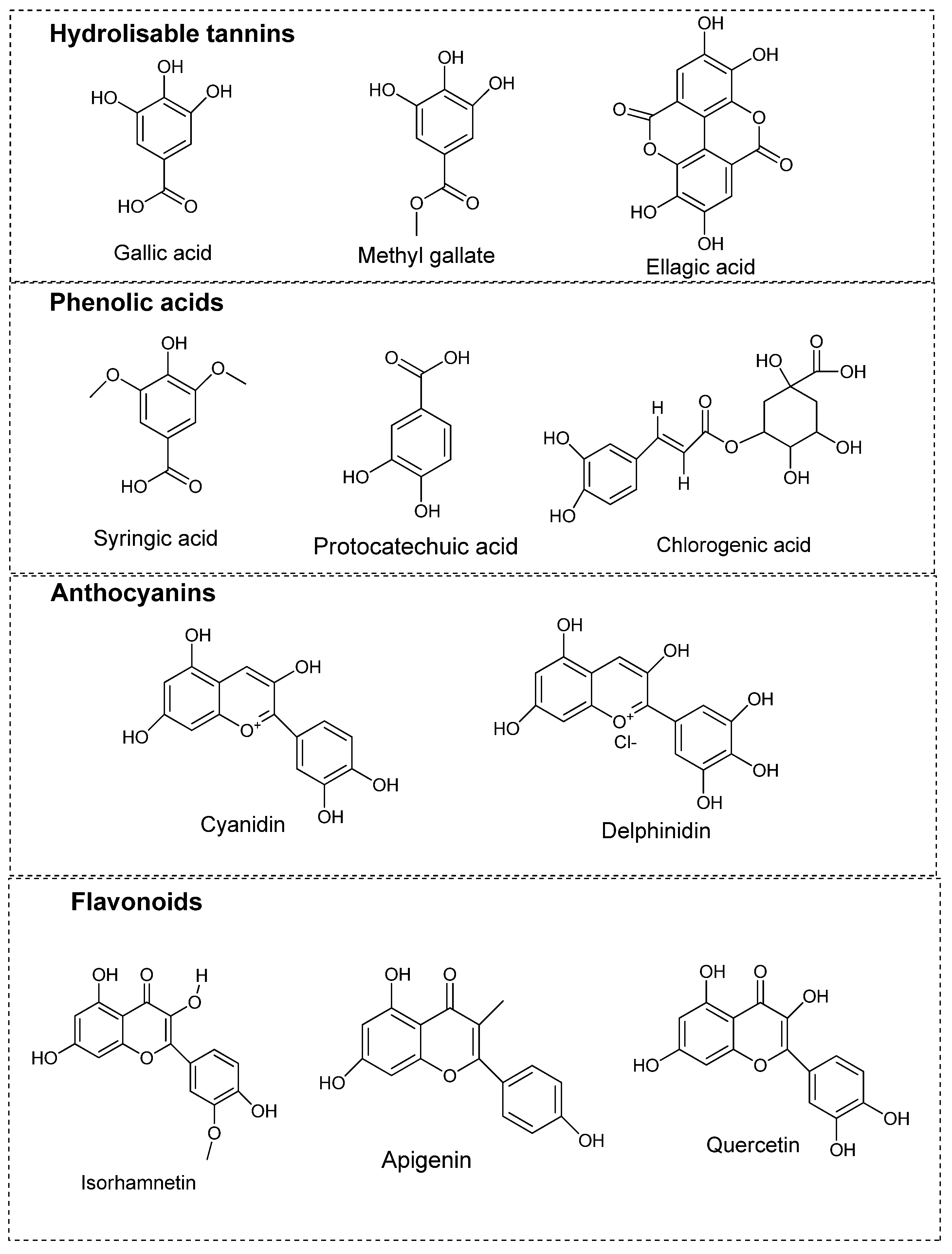
| Hydrolysable tannins | Gallic acid, methyl gallate, digallic acid, tri-gallic acid, ellagic acid, galloylhexose, O-galloylmorbergenin, O-galloyl arbutin | Fruits, leaves, seeds | [24,27] |
| Phenolic acids | Protocatechuic acid, syringic acid, coumaryl-hexoside, caffeoylquinic acid, p-benzoic acid, vanilic acid | Fruits | [9,24] |
| Conjugated phenolic acid | Galloyl-hexose-malic acid, digalloyl-hexose malic acid, keampferol-hexose malic acid, quercetin-hexose malic acid, Isorhamnetin hexose malic acid | Fruits | [24,28] |
| Flavonoids | Quercetin, isoquercitrin, quercitrin, rutin, keampferol, myricetin, apigenin, isorhamnetin, isovitexin, rhamnetin, ampelopsin, glycitein-O-glucoside, oxoglycyrrhetinic acid, amenthoflavone, agathisflavone, hinokiflavone and sumaflavone | Leaves, fruits, seeds | [9,24,29] |
| Anthocyanins | Cyanidin, peonidin, pelargonidin, petunidin, coumarates, delphinidin, myrtillin, crysanthemin | Leaves, fruits, seeds | [24] |
| Organic acids | Malic acid, citric acid, tartaric acid, linoleic acid, linoleic acid, oleic acid, linolenic acid, palmitic acid, stearic acid | Fruits, seeds | [24,30] |
| Coumarins | Umbelliferon | Fruits | [24] |
| Xanthones | 2,3-dihydroxy-7-methylxanthone, 2,3,6-trihydroxy7-hydroxymethylene, xanthone-1-carboxylic acid, 2-methoxy-4-hydroxy-7-methyl-3-O-beta-D-glucopyranosyl xanthone-1,8-dicarboxylic acid | Leaves, fruits | [9,24] |
| Terpenoids | Betunolic acid, alpha-tocopherol, tocopherol mannoside, farnesylacetate, pentadecanal, hexadecanal, deacetylforskolin, oxoglycyrrhetinic acid | Leaves, fruits | [4,24,25] |
| Steroids | Beta-sitosterol | Fruits, seeds | [24] |
| Essential oils | (E)-caryophyllene, n-nonanal, cembrene, alpha-nonoic acid, (2E)-decenal, p-anisaldhehyde, (Z)-caryophyllene oxide | fruits | [4,9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batiha, G.E.-S.; Ogunyemi, O.M.; Shaheen, H.M.; Kutu, F.R.; Olaiya, C.O.; Sabatier, J.-M.; De Waard, M. Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols. Molecules 2022, 27, 5179. https://doi.org/10.3390/molecules27165179
Batiha GE-S, Ogunyemi OM, Shaheen HM, Kutu FR, Olaiya CO, Sabatier J-M, De Waard M. Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols. Molecules. 2022; 27(16):5179. https://doi.org/10.3390/molecules27165179
Chicago/Turabian StyleBatiha, Gaber El-Saber, Oludare M. Ogunyemi, Hazem M. Shaheen, Funso R. Kutu, Charles O. Olaiya, Jean-Marc Sabatier, and Michel De Waard. 2022. "Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols" Molecules 27, no. 16: 5179. https://doi.org/10.3390/molecules27165179
APA StyleBatiha, G. E.-S., Ogunyemi, O. M., Shaheen, H. M., Kutu, F. R., Olaiya, C. O., Sabatier, J.-M., & De Waard, M. (2022). Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols. Molecules, 27(16), 5179. https://doi.org/10.3390/molecules27165179









