Efficacy of 2-Hydroxyflavanone in Rodent Models of Pain and Inflammation: Involvement of Opioidergic and GABAergic Anti-Nociceptive Mechanisms
Abstract
:1. Introduction
2. Results
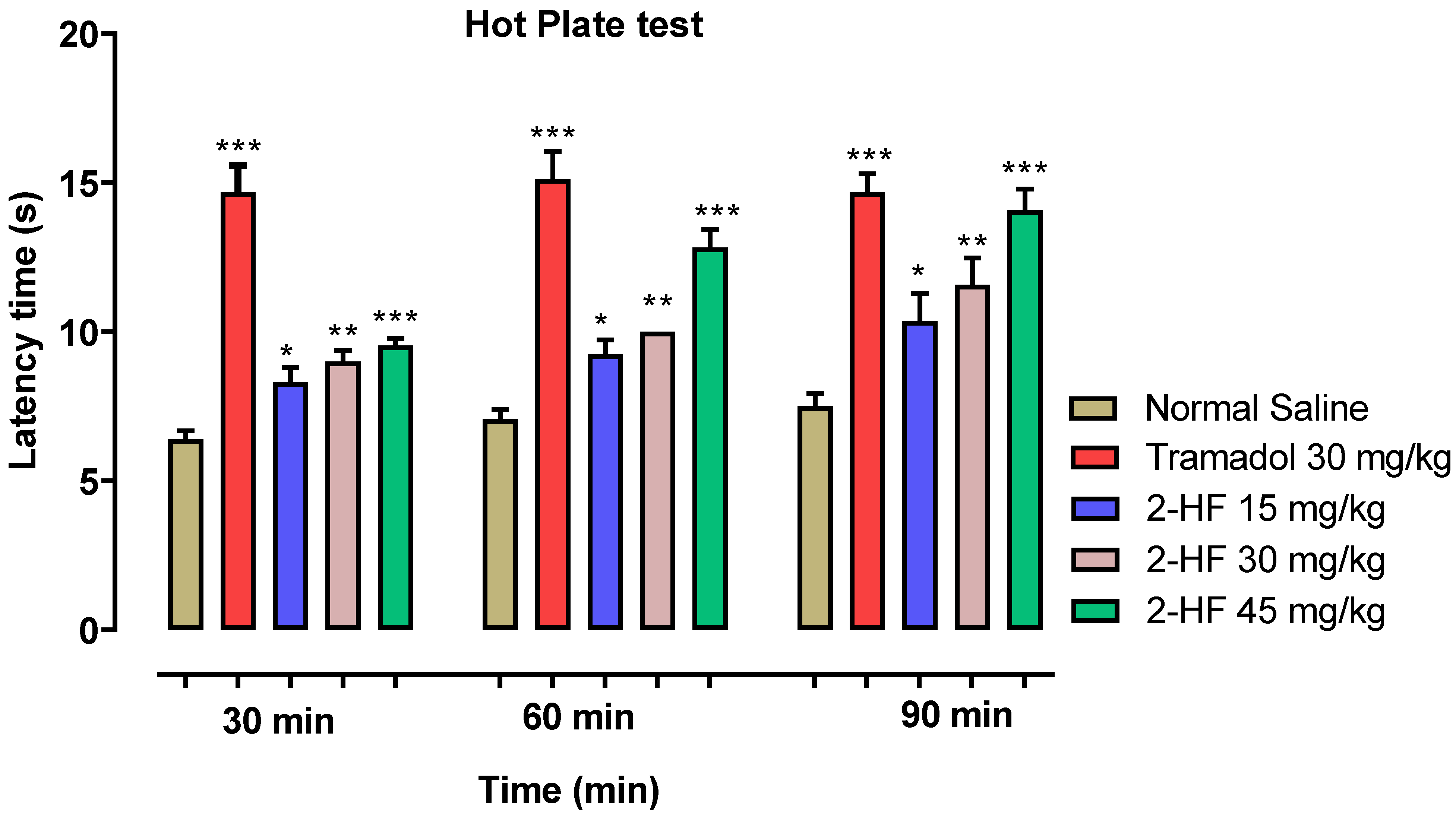
2.1. Effect of 2-HF in Hot Plate Test
2.2. Opioidergic and GABAergic Mechanism of 2-HF
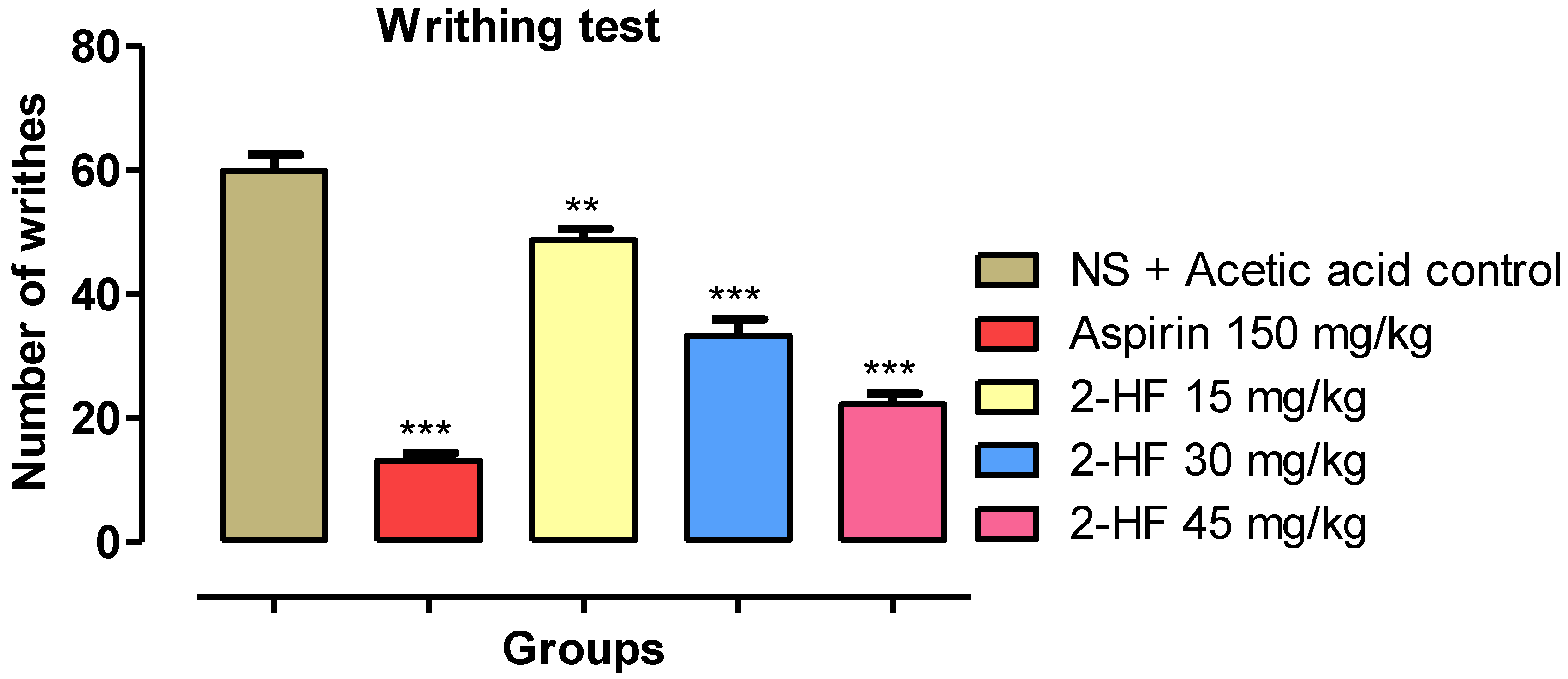
2.3. 2-HF Reduce Acetic Acid-Induced Writhing in Animals
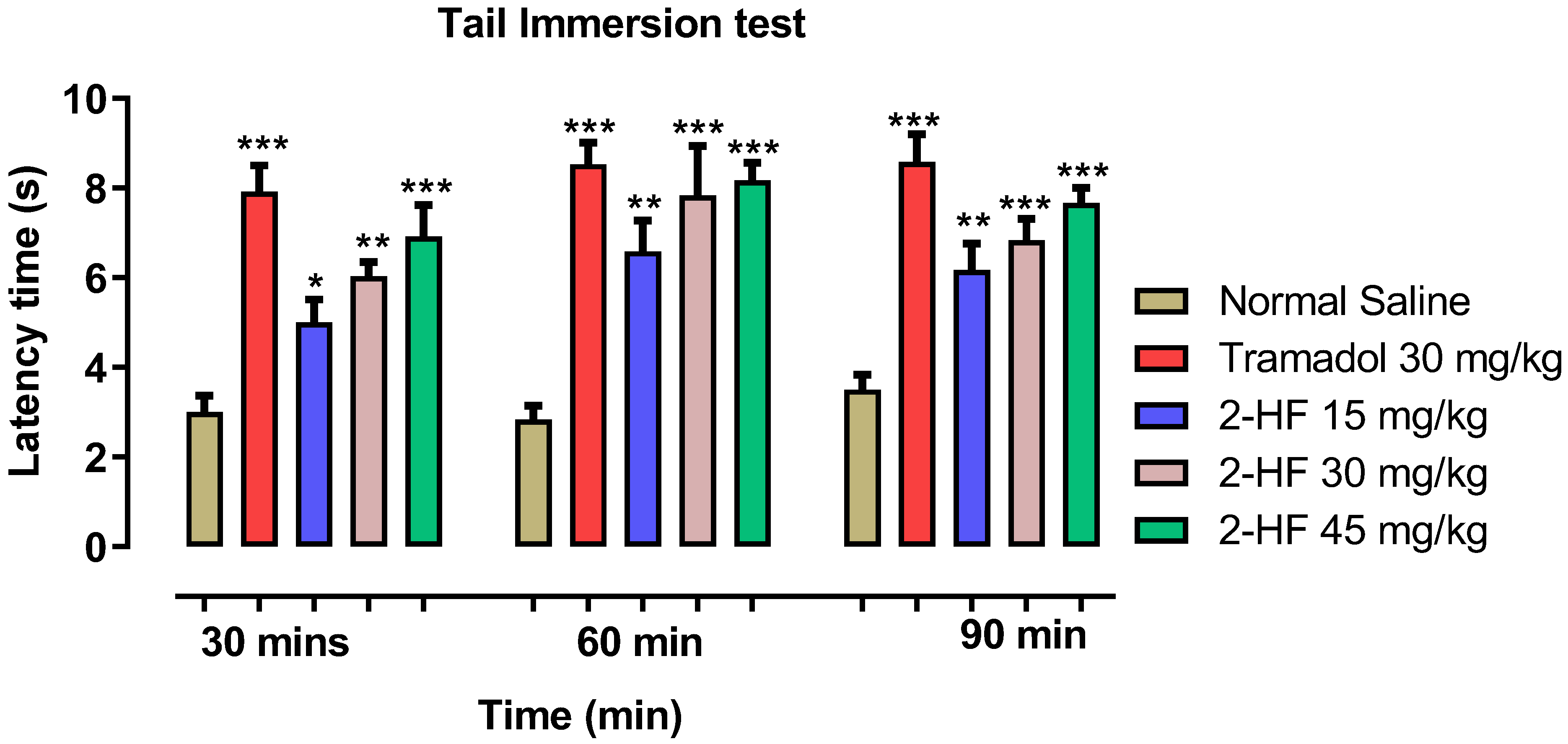
2.4. Analgesic Study Using Tail Immersion Paradigm
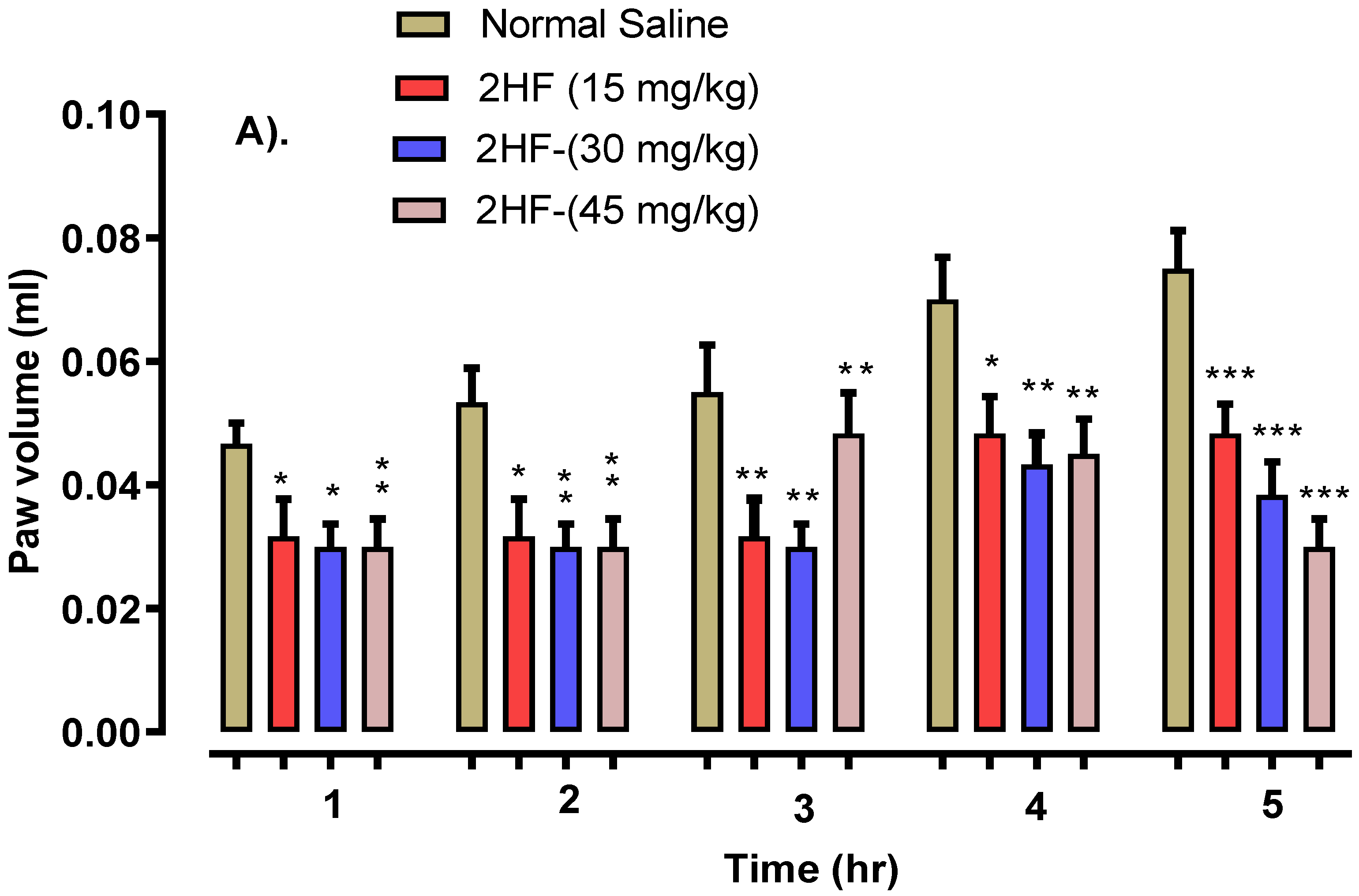
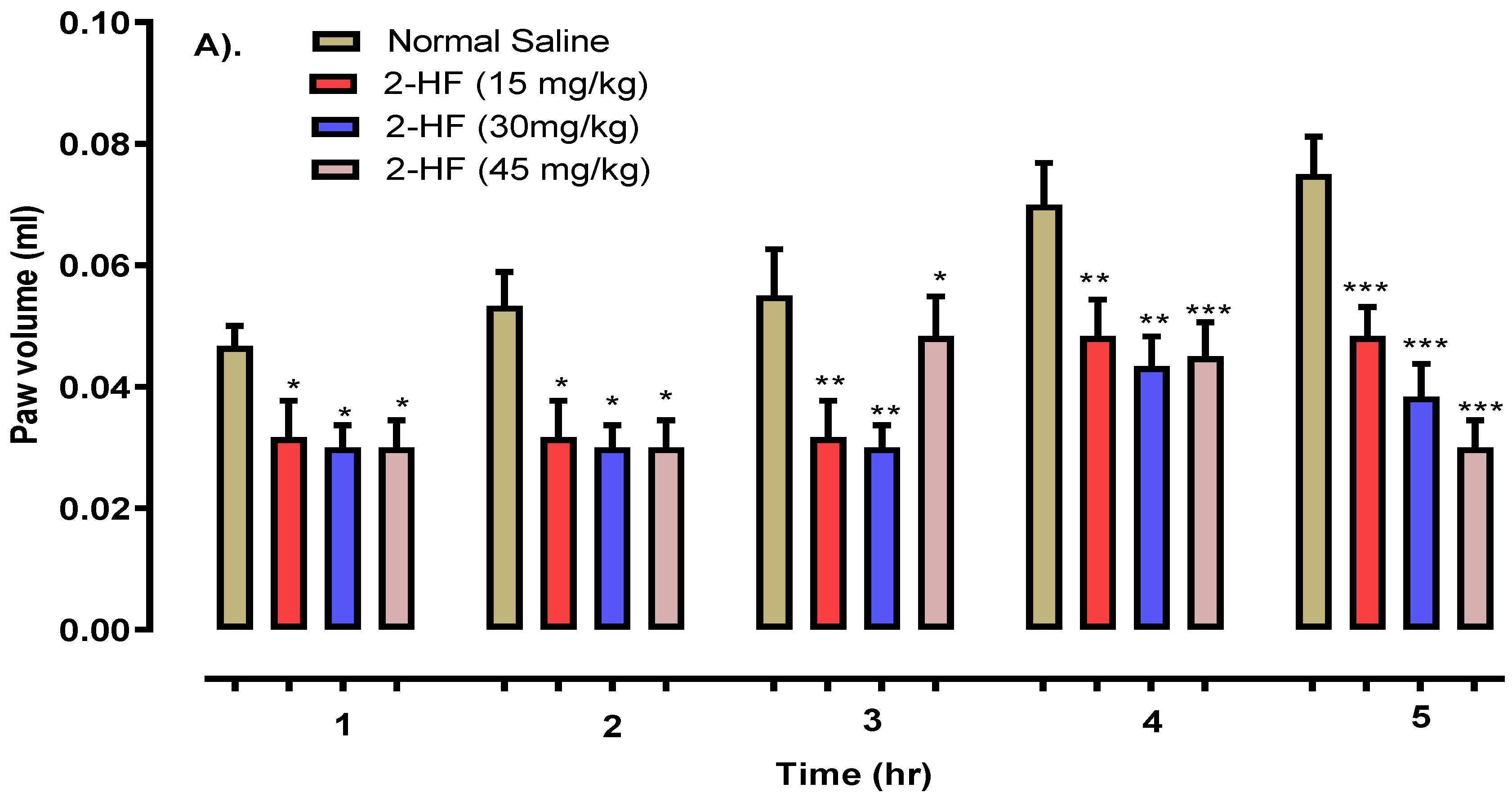
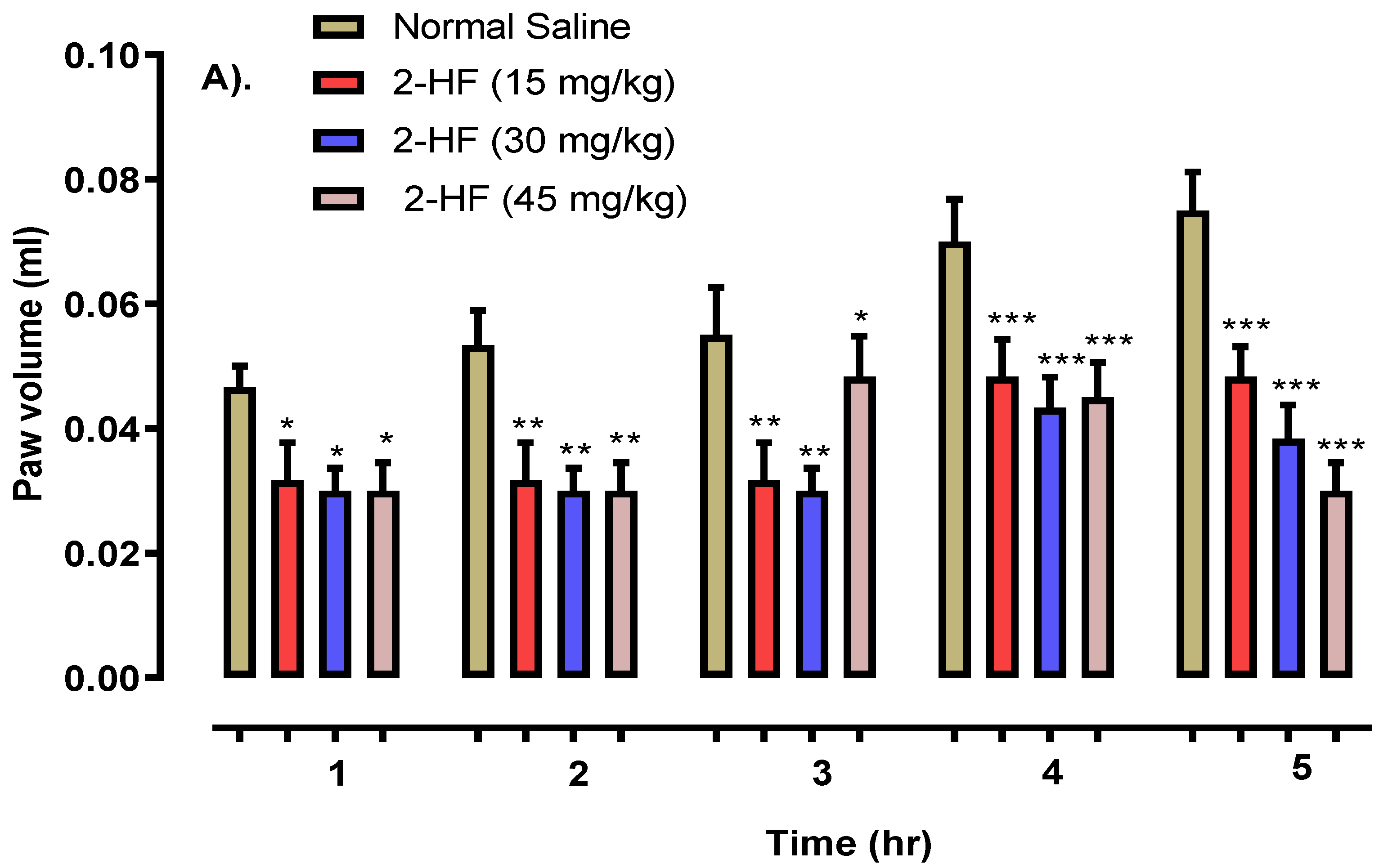
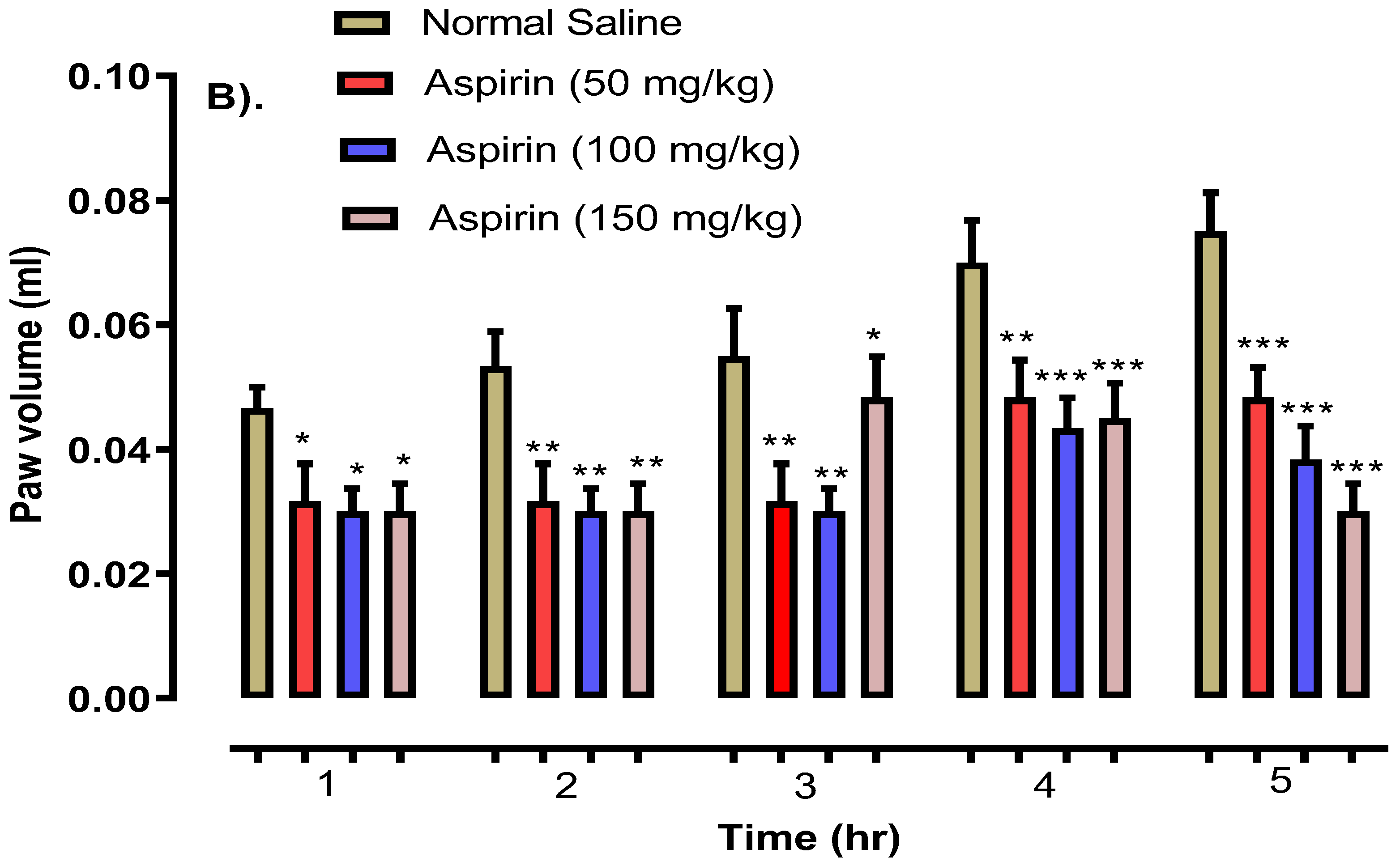
2.5. 2-HF Reduce Carrageenan, Serotonin and Histamine-Induced Inflammation
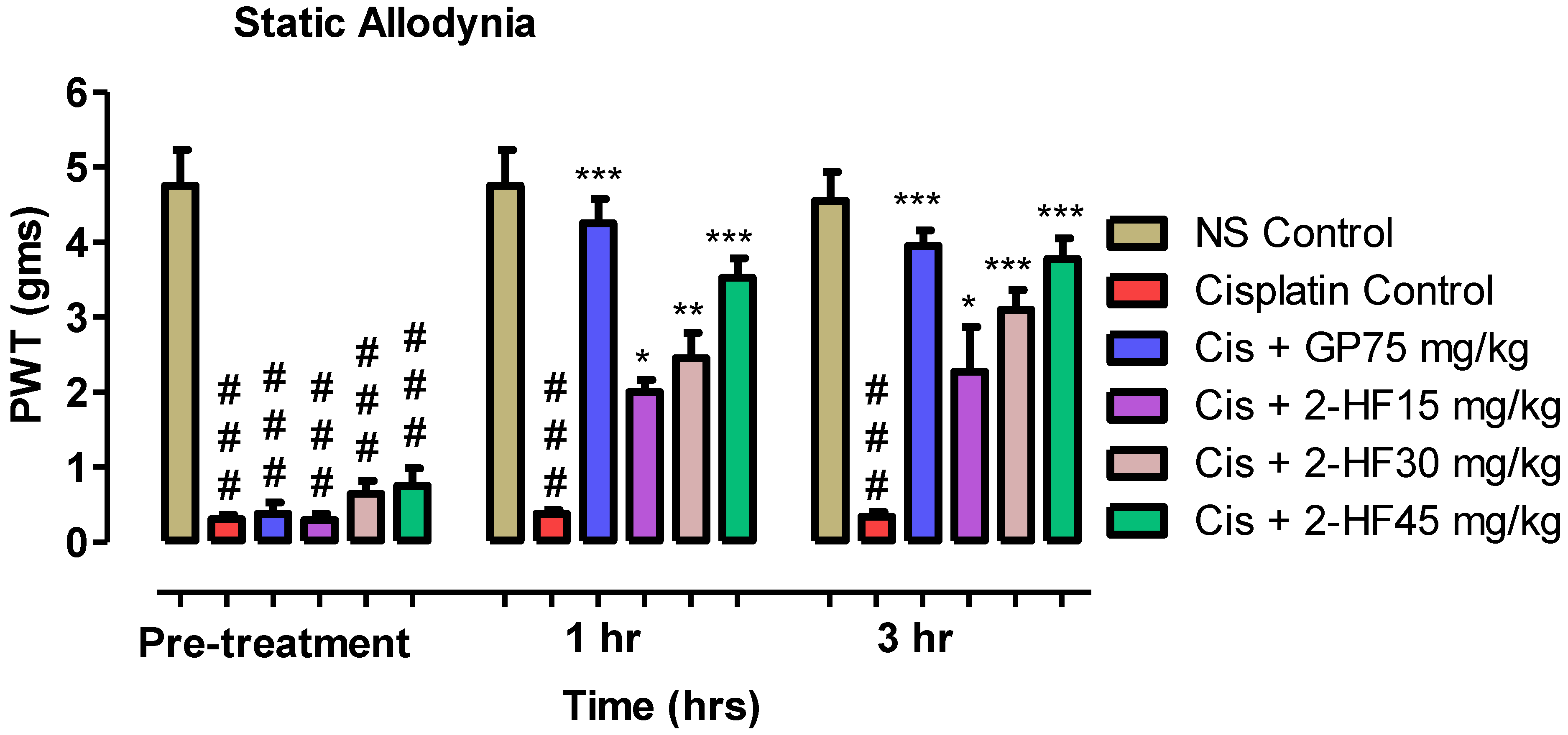
2.6. Effect of 2-HF in Neuropathic Pain Model of Cisplatin
2.6.1. Effect of 2-HF in Static Allodynia
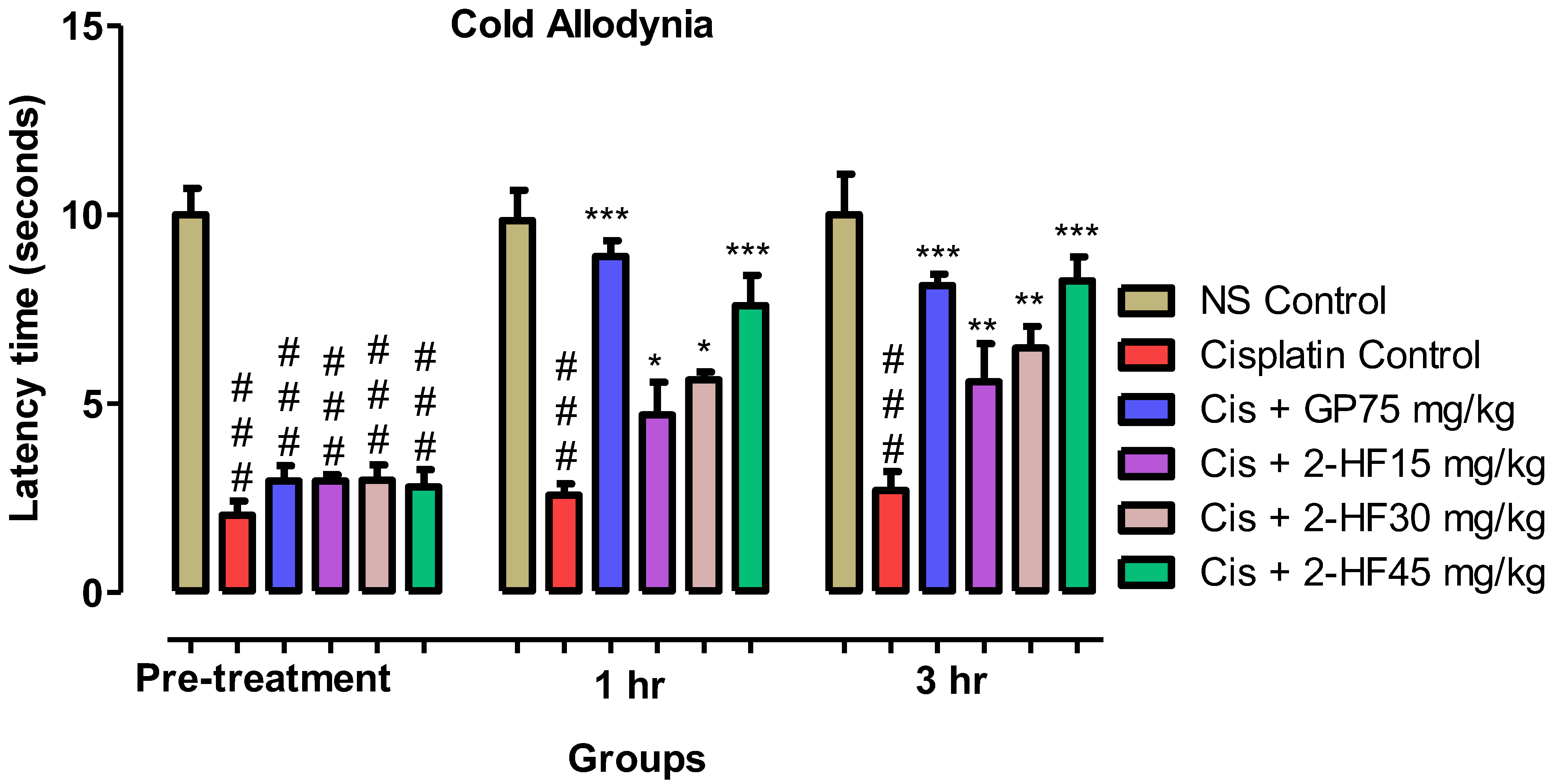
2.6.2. Cold Allodynia
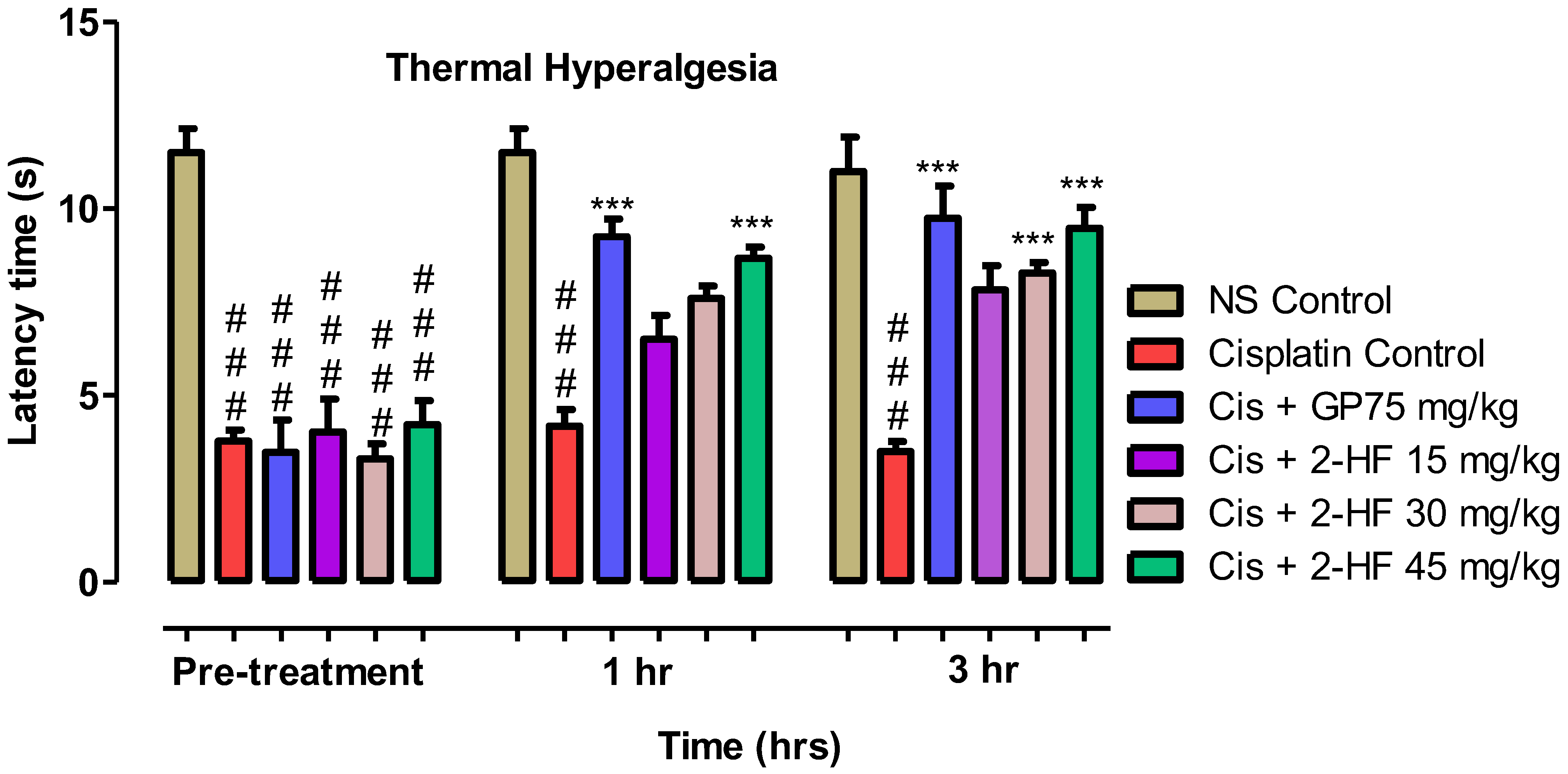
2.6.3. Thermal Hyperalgesia
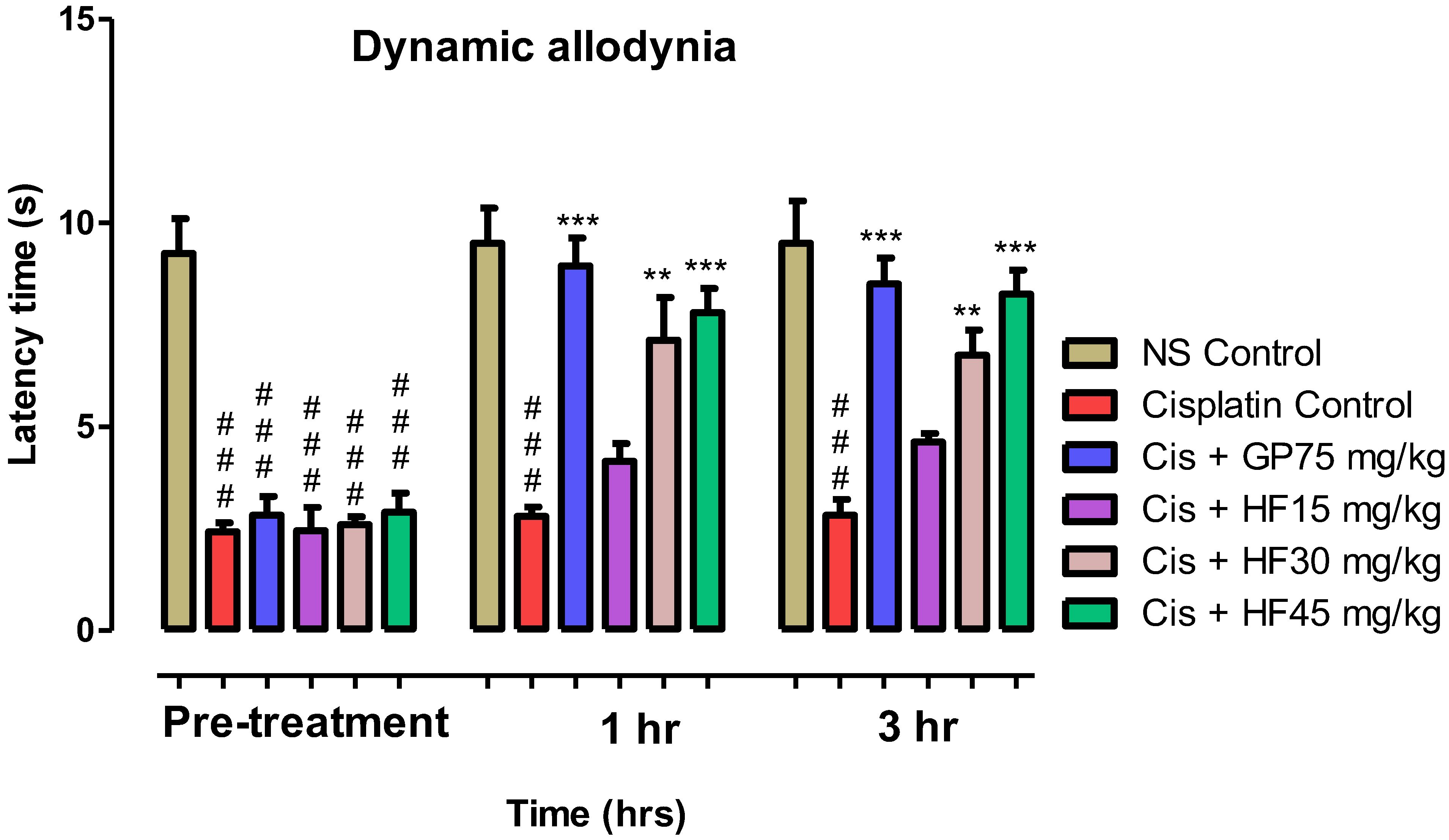
2.6.4. Effect of 2-HF on Dynamic Allodynia
2.7. Acute Toxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Apparatus
4.2. Animals and Ethical Approval
4.3. Anti-Nociceptive Studies
4.3.1. Hot Plate Test
Antagonistic Studies Using Nalaxone and Pentylenetetrazole
4.3.2. Writhing Test
4.3.3. Tail Immersion Model
4.4. Anti-Inflammatory Studies
4.4.1. Carrageenan Aroused Paw Edema Model
4.4.2. Histamine-Induced Edema Model
4.4.3. Serotonin-Induced Paw Edema Model
4.4.4. Xylene-Induced Ear Edema Model
4.5. Efficacy in Neuropathic Pain
4.5.1. Cisplatin-Induced Neuropathy
4.5.2. Induction of Cisplatin-Based Neuropathic Pain
4.5.3. Cold Allodynia
4.5.4. Mechanical Allodynia or Static Allodynia
4.5.5. Dynamic Allodynia
4.5.6. Thermal Hyperalgesia Test
4.6. Acute Toxicity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meldrum, M.L. A capsule history of pain management. JAMA 2003, 290, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Zhang, W.-D.; Jin, H.-Z.; Basha, S.H.; Priya, S.V.S.S. In-silico anti-inflammatory potential of guaiane dimers from Xylopia vielana targeting COX-2. J. Biomol. Struct. Dyn. 2022, 40, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Subhan, F.; Shahid, M.; Wadood, A.; Shahbaz, N.; Farooq, U.; Ayaz, M.; Raziq, N. 6-Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: A behavioral and molecular simulation study. Eur. J. Pharmacol. 2020, 872, 172972. [Google Scholar] [CrossRef]
- Shams ul Hassan, S.; Ishaq, M.; Zhang, W.; Jin, H.-Z. An overview of the mechanisms of marine fungi-derived antiinflammatory and anti-tumor agents and their novel role in drug targeting. Curr. Pharm. Des. 2021, 27, 2605–2614. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Rus Makovec, M.; Vintar, N.; Makovec, S. Level of Depression, Anxiety and Impairment of Social Relations with Regard to Pain Intensity in a Naturalistic Sample of Patients at the Outpatient Chronic Pain Clinic. Psychiatr. Danub. 2021, 33, 558–564. [Google Scholar]
- Lai, W.-F. Development of Hydrogels with Self-Healing Properties for Delivery of Bioactive Agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Hewitt, D.J.; Hargreaves, R.J.; Curtis, S.P.; Michelson, D. Challenges in analgesic drug development. Clin. Pharmacol. Ther. 2009, 86, 447–450. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, H.; Wang, Z.; Qiu, C.; Peng, Y.B.; Zhu, X.; Li, J.; Ge, X.; Xu, J.; Huang, X.; et al. Piezoelectric ultrasound energy-harvesting device for deep brain stimulation and analgesia applications. Sci. Adv. 2022, 8, eabk0159. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.Z.; Bungau, S. Stress driven discovery of natural products from actinobacteria with anti-oxidant and cytotoxic activities including docking and admet properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar] [CrossRef]
- Sani, A.; Hassan, D.; Khalil, A.T.; Mughal, A.; El-Mallul, A.; Ayaz, M.; Yessimbekov, Z.; Shinwari, Z.K.; Maaza, M. Floral extracts-mediated green synthesis of NiO nanoparticles and their diverse pharmacological evaluations. J. Biomol. Struct. Dyn. 2021, 39, 4133–4147. [Google Scholar] [CrossRef]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.U.; Haq, I.-U.; Bungau, S. An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef]
- Shams ul Hassan, S.; Qamar Abbas, S.; Ali, F.; Ishaq, M.; Bano, I.; Hassan, M.; Jin, H.-Z.; Bungau, S.G. A Comprehensive In Silico Exploration of Pharmacological Properties, Bioactivities, Molecular Docking, and Anticancer Potential of Vieloplain F from Xylopia vielana Targeting B-Raf Kinase. Molecules 2022, 27, 917. [Google Scholar] [CrossRef]
- Shams ul Hassan, S.; Abbas, S.Q.; Hassan, M.; Jin, H.-Z. Computational Exploration of Anti-Cancer Potential of Guaiane Dimers from Xylopia vielana by Targeting B-Raf Kinase Using Chemo-Informatics, Molecular Docking and MD Simulation Studies. Anti-Cancer Agents Med. Chem. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Hussain, S.; Ullah, F.; Ayaz, M.; Ali Shah, S.A.; Ali Shah, A.-U.-H.; Shah, S.M.; Wadood, A.; Aman, W.; Ullah, R.; Shahat, A.A.; et al. In Silico, Cytotoxic and Antioxidant Potential of Novel Ester, 3-hydroxyoctyl -5-trans-docosenoate Isolated from Anchusa arvensis (L.) M.Bieb. Against HepG-2 Cancer Cells. Drug Des. Dev. Ther. 2019, 13, 4195–4205. [Google Scholar] [CrossRef]
- Saleem, U.; Akhtar, R.; Anwar, F.; Shah, M.A.; Chaudary, Z.; Ayaz, M.; Ahmad, B. Neuroprotective potential of Malva neglecta is mediated via down-regulation of cholinesterase and modulation of oxidative stress markers. Metab. Brain Dis. 2021, 36, 889–900. [Google Scholar] [CrossRef]
- Ayaz, M.; Nawaz, A.; Ahmad, S.; Mosa, O.F.; Eisa Hamdoon, A.A.; Khalifa, M.A.; Sadiq, A.; Ullah, F.; Wadood, A.; Kabra, A.; et al. Underlying Anticancer Mechanisms and Synergistic Combinations of Phytochemicals with Cancer Chemotherapeutics: Potential Benefits and Risks. J. Food Qual. 2022, 2022, 1189034. [Google Scholar] [CrossRef]
- Akbar, S.; Subhan, F.; Karim, N.; Shahid, M.; Ahmad, N.; Ali, G.; Mahmood, W.; Fawad, K. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 84, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Laktašić Žerjavić, N.; Hrkić, E.; Žagar, I.; Delimar, V.; Kovač Durmiš, K.; Špoljarić Carević, S.; Vukorepa, M.; Matijević, A.; Žura, N.; Perić, P. Local Cryotherapy, Comparison of Cold Air and Ice Massage on Pain and Handgrip Strength in Patients with Rheumatoid Arthritis. Psychiatr. Danub. 2021, 33, 757–761. [Google Scholar] [PubMed]
- Muhammad, I.; Shams ul Hassan, S.; Cheung, S.; Li, X.; Wang, R.; Zhang, W.D.; Yan, S.K.; Zhang, Y.; Jin, H.Z. Phytochemical study of Ligularia subspicata and valuation of its anti-inflammatory activity. Fitoterapia 2021, 148, 104800. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Khan, J.A.; Mahnashi, M.H.; Jan, M.S.; Javed, M.A.; Rashid, U.; Sadiq, A.; Hassan, S.S.U.; Bungau, S. Anti-Inflammatory, Analgesic and Antioxidant Potential of New (2S,3S)-2-(4-isopropylbenzyl)-2-methyl-4-nitro-3-phenylbutanals and Their Corresponding Carboxylic Acids through In Vitro, In Silico and In Vivo Studies. Molecules 2022, 27, 4068. [Google Scholar] [CrossRef] [PubMed]
- Dediol, I.; Situm, M.; Bulat, V.; Stugnetic, T.; Juras, J.; Tomljenovic, D.; Buljan, M. Painful and Itchy Dermatoses Carry the Highest Psychological Burden for Dermatovenereological Patients. Psychiatr. Danub. 2021, 33, 613–621. [Google Scholar]
- Li, H.; Wang, F. Core-shell chitosan microsphere with antimicrobial and vascularized functions for promoting skin wound healing. Mater. Des. 2021, 204, 109683. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Alqahl, S.A.; Ullah, F.; Sadiq, A.; Zeb, A.; Ghufran, M.; Kuraev, A.; et al. HPLC-DAD phenolics analysis, α-glucosidase, α-amylase inhibitory, molecular docking and nutritional profiles of Persicaria hydropiper L. BMC Complement. Med. Ther. 2022, 22, 26. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.P.; Fialip, J.; Eschalier, A.; Coudore, F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp. Neurol. 2003, 182, 12–20. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Liang, H.; Wu, Z.; Li, J.; Ou-Yang, J.; Yang, X.; Peng, Y.B.; Zhu, B. Transcranial Focused Ultrasound Stimulation of Periaqueductal Gray for Analgesia. IEEE Trans. Bio-Med. Eng. 2022. [Google Scholar] [CrossRef]
- Nakazato-Imasato, E.; Tanimoto-Mori, S.; Kurebayashi, Y. Effect of mexiletine on dynamic allodynia induced by chronic constriction injury of the sciatic nerve in rats. J. Vet. Med. Sci. 2009, 71, 991–994. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. ROS-Generating Amine-Functionalized Magnetic Nanoparticles Coupled with Carboxymethyl Chitosan for pH-Responsive Release of Doxorubicin. Int. J. Nanomed. 2022, 17, 589–601. [Google Scholar] [CrossRef]
- Smith, G.D.; Harrison, S.M.; Birch, P.J.; Elliott, P.J.; Malcangio, M.; Bowery, N.G. Increased sensitivity to the antinociceptive activity of (+/−)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology 1994, 33, 1103–1108. [Google Scholar] [CrossRef]
- Kakee, A.; Takanaga, H.; Terasaki, T.; Naito, M.; Tsuruo, T.; Sugiyama, Y. Efflux of a suppressive neurotransmitter, GABA, across the blood-brain barrier. J. Neurochem. 2001, 79, 110–118. [Google Scholar] [CrossRef]
- Thirugnanasambantham, P.; Viswanathan, S.; Mythirayee, C.; Krishnamurty, V.; Ramachandran, S.; Kameswaran, L. Analgesic activity of certain flavone derivatives: A structure-activity study. J. Ethnopharmacol. 1990, 28, 207–214. [Google Scholar] [CrossRef]
- Laughlin, T.M.; Tram, K.V.; Wilcox, G.L.; Birnbaum, A.K. Comparison of antiepileptic drugs tiagabine, lamotrigine, and gabapentin in mouse models of acute, prolonged, and chronic nociception. J. Pharmacol. Exp. Ther. 2002, 302, 1168–1175. [Google Scholar] [CrossRef]
- Tang, W.; Wan, S.; Yang, Z.; Teschendorff, A.E.; Zou, Q. Tumor origin detection with tissue-specific miRNA and DNA methylation markers. Bioinformatics 2018, 34, 398–406. [Google Scholar] [CrossRef]
- Dias, J.M.; de Brito, T.V.; de Aguiar Magalhães, D.; da Silva Santos, P.W.; Batista, J.A.; do Nascimento Dias, E.G.; de Barros Fernandes, H.; Damasceno, S.R.B.; Silva, R.O.; Aragão, K.S.; et al. Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation 2014, 37, 1826–1836. [Google Scholar] [CrossRef]
- Patel, N.K.; Bairwa, K.; Gangwal, R.; Jaiswal, G.; Jachak, S.M.; Sangamwar, A.T.; Bhutani, K.K. 2′-Hydroxy flavanone derivatives as an inhibitors of pro-inflammatory mediators: Experimental and molecular docking studies. Bioorganic. Med. Chem. Lett. 2015, 25, 1952–1955. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Sangeetha, K.S.S. Anti-Inflammatory Effect of Selected Dihydroxyflavones. J. Clin. Diagn. Res. JCDR 2015, 9, FF05–FF07. [Google Scholar] [CrossRef]
- Mir, N.T.; Saleem, U.; Anwar, F.; Ahmad, B.; Ullah, I.; Hira, S.; Ismail, T.; Ali, T.; Ayaz, M. Lawsonia Inermis Markedly Improves Cognitive Functions in Animal Models and Modulate Oxidative Stress Markers in the Brain. Medicina 2019, 55, 192. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ullah, F.; Wadood, A.; Sadiq, A.; Shareef, A.; Ayaz, M. Cytotoxicity, anti-angiogenic, anti-tumor and molecular docking studies on phytochemicals isolated from Polygonum hydropiper L. BMC Complement. Med. Ther. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, W.; Baneux, P.; Boot, R.; Decelle, T.; Deeny, A.A.; Fumanelli, M.; Illgen-Wilcke, B. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 2002, 36, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Gomathi, P.; Rajeshwar, Y.; Kakoti, B.B.; Selven, V.T. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J. Ethnopharmacol. 2005, 98, 267–273. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef]
- Xie, Y.G.; Zhao, X.C.; ul Hassan, S.S.; Zhen, X.Y.; Muhammad, I.; Yan, S.; Yuan, X.; Li, H.L.; Jin, H.Z. One new sesquiterpene and one new iridoid derivative from Valeriana amurensis. Phytochem. Lett. 2019, 32, 6–9. [Google Scholar] [CrossRef]
- Muhammad, I.; Luo, W.; Shoaib, R.M.; Li, G.; Shams ul Hassan, S.; Yang, Z.H.; Xiao, X.; Tu, G.L.; Yan, S.K.; Ma, X.P.; et al. Guaiane-type sesquiterpenoids from Cinnamomum migao H. W. Li: And their anti-inflammatory activities. Phytochemistry 2021, 190, 112850. [Google Scholar] [CrossRef]
- Majid, M.; Nasir, B.; Zahra, S.S.; Khan, M.R.; Mirza, B.; Haq, I.-U. Ipomoea batatas L. Lam. ameliorates acute and chronic inflammations by suppressing inflammatory mediators, a comprehensive exploration using in vitro and in vivo models. BMC Complement. Altern. Med. 2018, 18, 216. [Google Scholar] [CrossRef]
- Naji-Esfahani, H.; Vaseghi, G.; Safaeian, L.; Pilehvarian, A.-A.; Abed, A.; Rafieian-Kopaei, M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab. Anim. 2016, 50, 15–20. [Google Scholar] [CrossRef]
- Shahid, M.; Subhan, F.; Ahmad, N.; Ali, G.; Akbar, S.; Fawad, K.; Sewell, R.D.E. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur. J. Pain 2017, 21, 668–680. [Google Scholar] [CrossRef]
- Ahmad, N.; Subhan, F.; Islam, N.U.; Shahid, M.; Rahman, F.U.; Fawad, K. A Novel Pregabalin Functionalized Salicylaldehyde Derivative Afforded Prospective Pain, Inflammation, and Pyrexia Alleviating Propensities. Arch. Der Pharm. 2017, 350. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.A.; Ali, G.; Rahman, K.; Khan, Y.; Ayaz, M.; Mosa, O.F.; Nawaz, A.; Hassan, S.S.u.; Bungau, S. Efficacy of 2-Hydroxyflavanone in Rodent Models of Pain and Inflammation: Involvement of Opioidergic and GABAergic Anti-Nociceptive Mechanisms. Molecules 2022, 27, 5431. https://doi.org/10.3390/molecules27175431
Khan FA, Ali G, Rahman K, Khan Y, Ayaz M, Mosa OF, Nawaz A, Hassan SSu, Bungau S. Efficacy of 2-Hydroxyflavanone in Rodent Models of Pain and Inflammation: Involvement of Opioidergic and GABAergic Anti-Nociceptive Mechanisms. Molecules. 2022; 27(17):5431. https://doi.org/10.3390/molecules27175431
Chicago/Turabian StyleKhan, Faiz Ali, Gowhar Ali, Khista Rahman, Yahya Khan, Muhammad Ayaz, Osama F. Mosa, Asif Nawaz, Syed Shams ul Hassan, and Simona Bungau. 2022. "Efficacy of 2-Hydroxyflavanone in Rodent Models of Pain and Inflammation: Involvement of Opioidergic and GABAergic Anti-Nociceptive Mechanisms" Molecules 27, no. 17: 5431. https://doi.org/10.3390/molecules27175431
APA StyleKhan, F. A., Ali, G., Rahman, K., Khan, Y., Ayaz, M., Mosa, O. F., Nawaz, A., Hassan, S. S. u., & Bungau, S. (2022). Efficacy of 2-Hydroxyflavanone in Rodent Models of Pain and Inflammation: Involvement of Opioidergic and GABAergic Anti-Nociceptive Mechanisms. Molecules, 27(17), 5431. https://doi.org/10.3390/molecules27175431







