New Strategies on Green Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Oxide Composites
Abstract
:1. Introduction
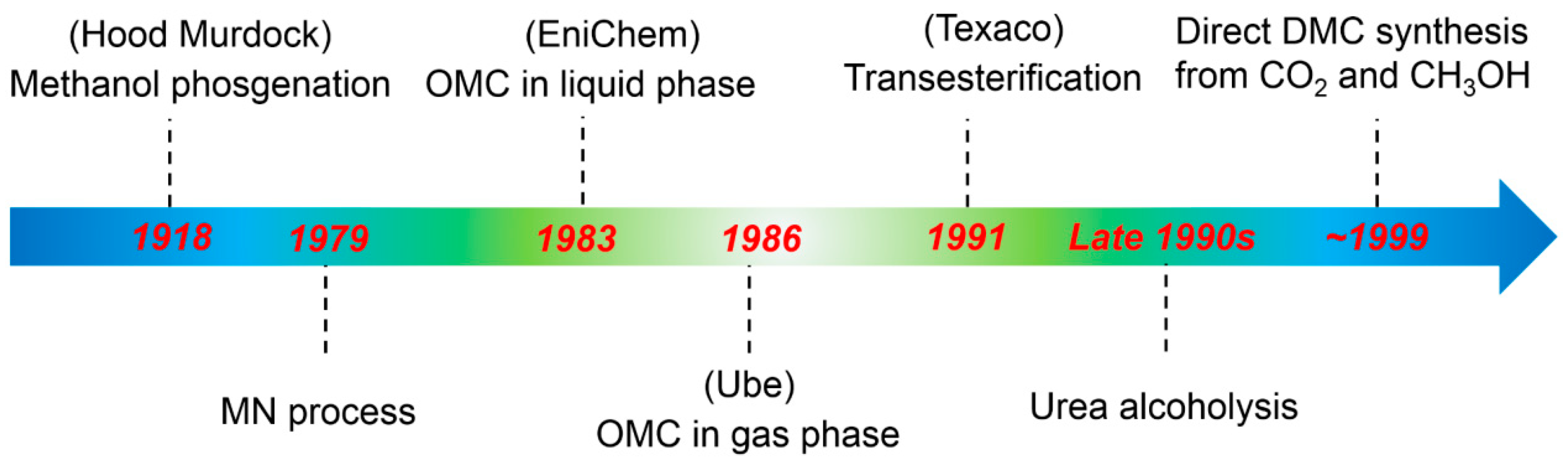
2. Developed Methods for Green DMC Synthesis
2.1. Thermodynamics
2.2. Thermal Catalytic Synthesis
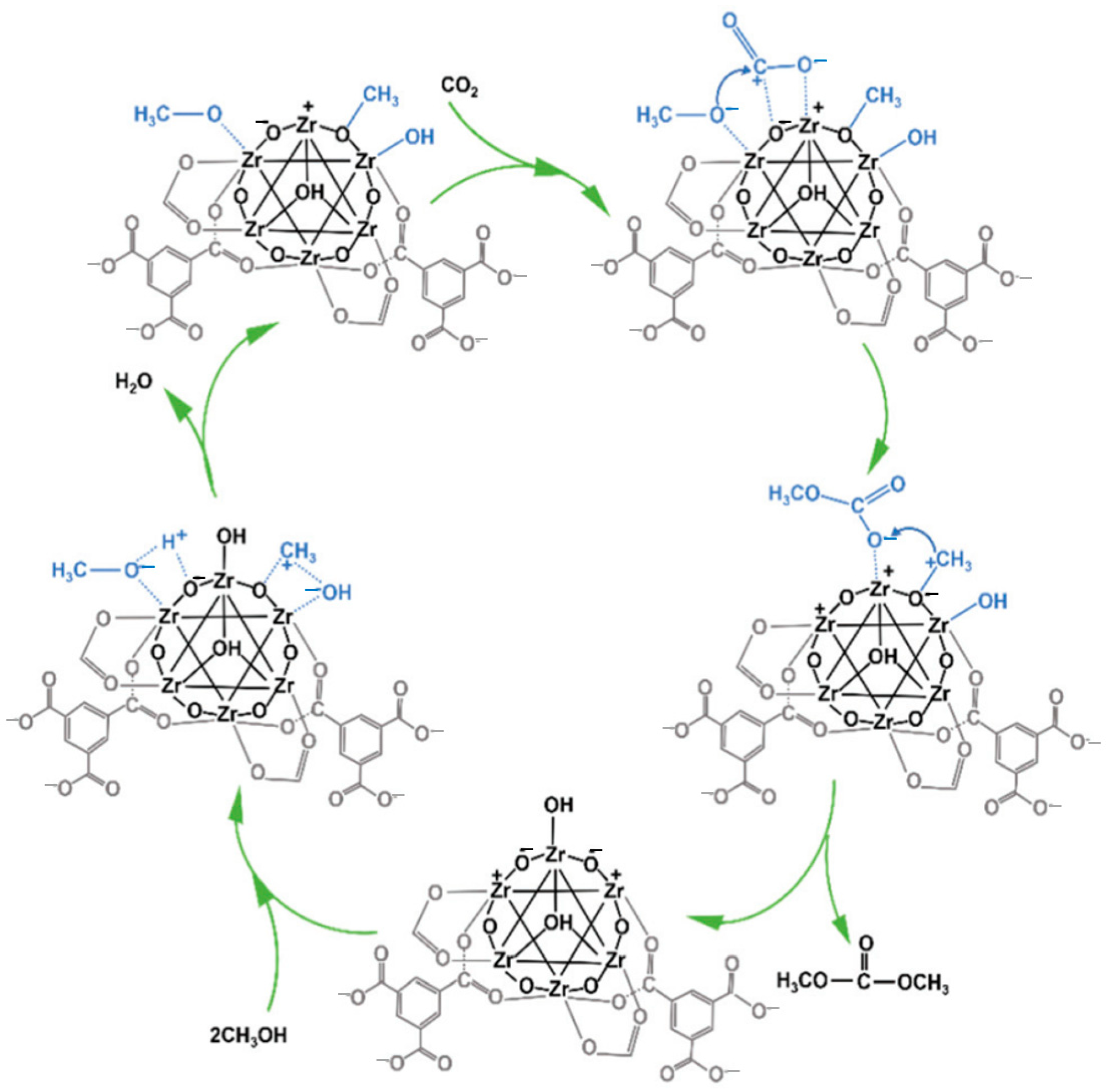
2.3. Zr-Based Catalysts
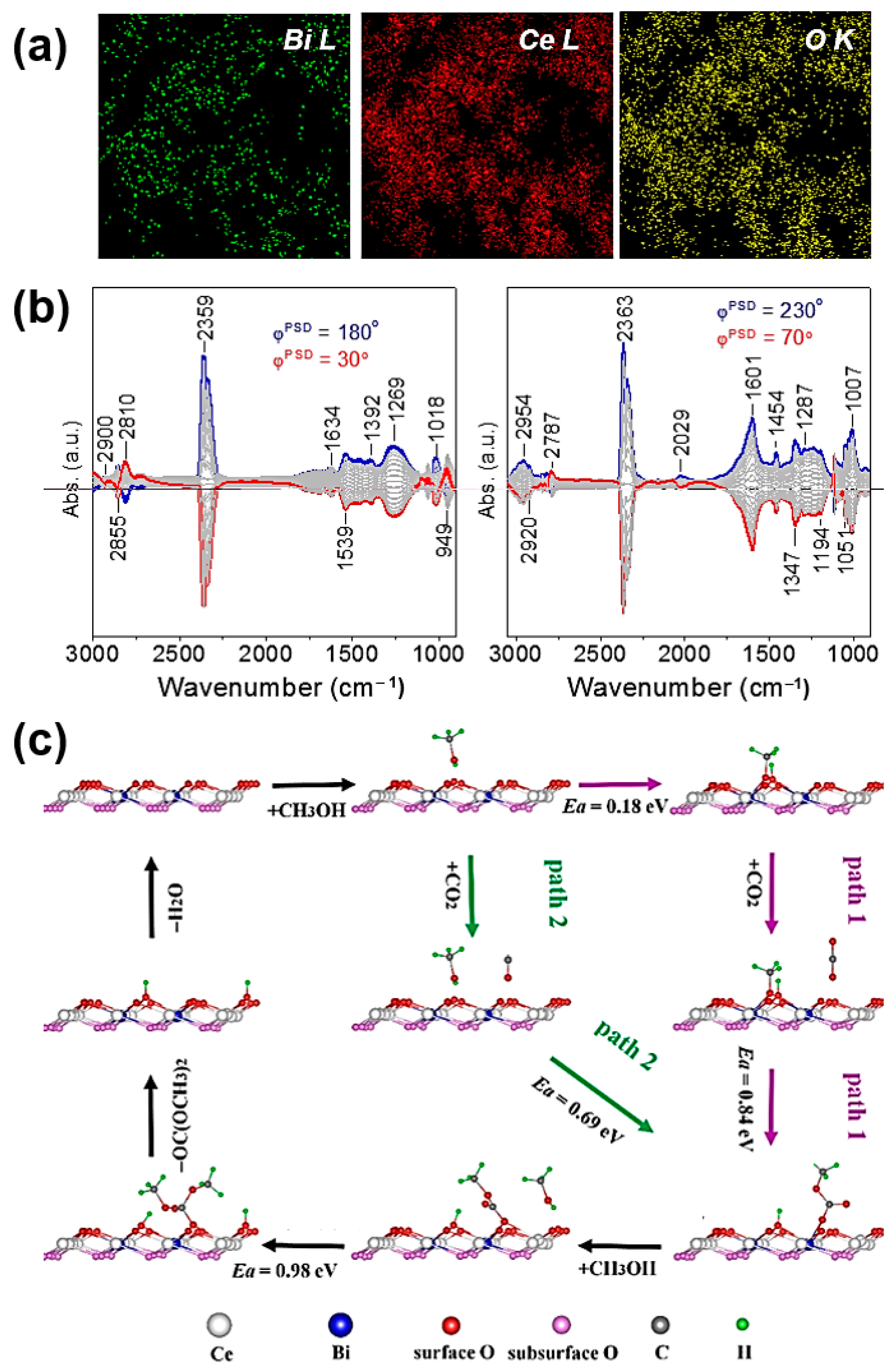
2.4. Ceria-Based Catalysts
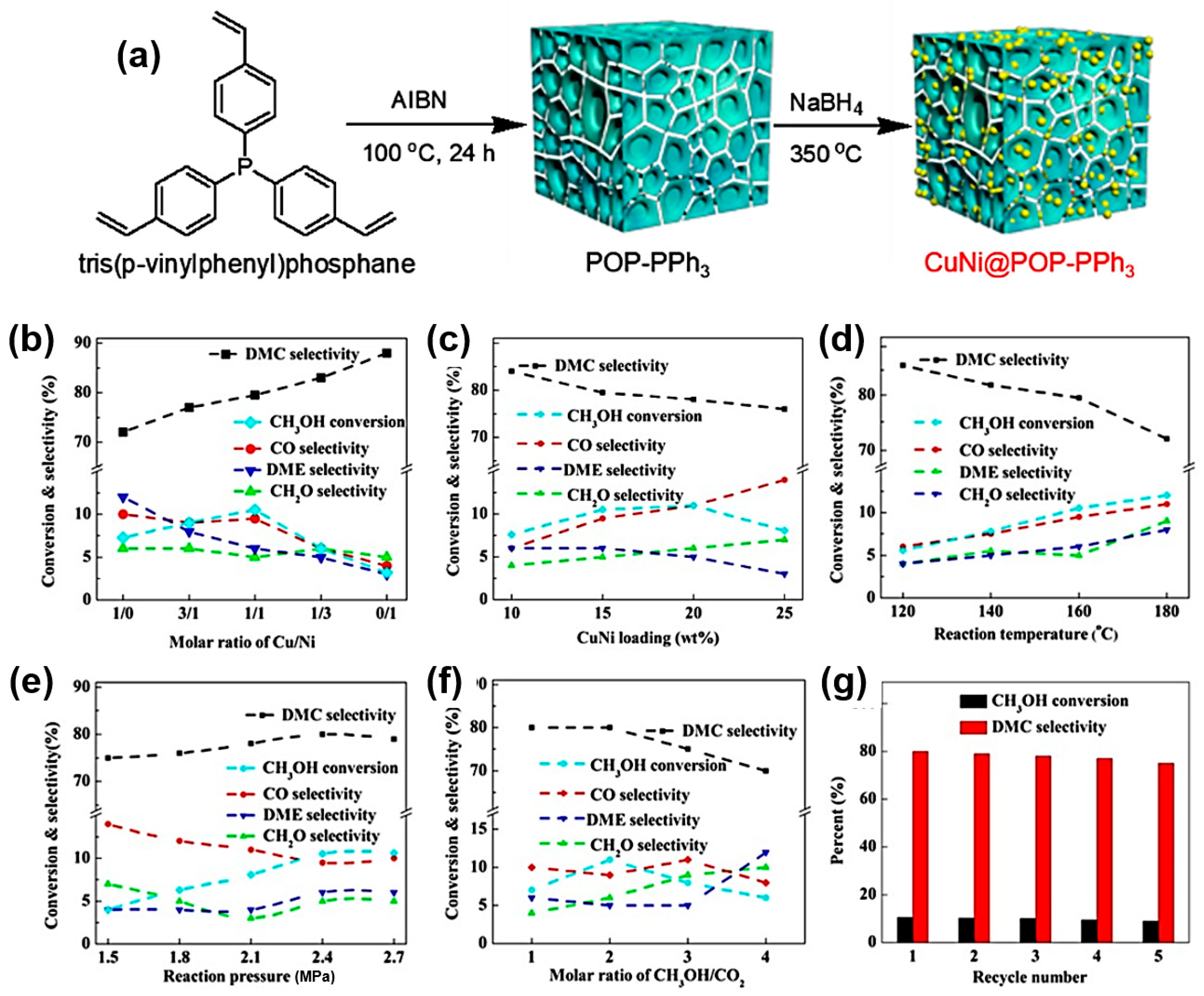
2.5. Cu-Based Nanoparticle Catalysts
3. Conclusions, Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| DMC | dimethyl carbonate |
| TFA | trifluoroacetic acid |
| MTBE | methyl tertbutyl ether |
| TTF | Dutch Title Transfer Facility |
| LTA | Linde Type-A |
| Ov | oxygen vacancies |
| NRs | nanorods |
| BTC | 1, 3,5-benzenetricarboxylic acid |
| NPs | nanoparticles |
| PC | propylene carbonate |
| DME | dimethyl ether |
| EPR | electron paramagnetic resonance |
| TPD | temperature-programmed desorption |
| XPS | X-ray photoelectron spectroscopy |
| CH3Oa | adsorbed methoxy |
| 2-PA | 2-picolinamide |
| CMs | carbon microspheres |
| AC | activated carbon |
| MG | monovacancy graphene |
| GNG | graphitic-N-doped graphene |
| PNG | pyridinic-doped graphene |
| ANG | amino-doped graphene |
| POP | porous organic polymer |
References
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Börner, A. Organic Carbonates as Solvents in Synthesis and Catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef] [PubMed]
- Ballivet-Tkatchenko, D.; Dibenedetto, A. Carbon Dioxide as Chemical Feedstock; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 169–212. [Google Scholar]
- Massey, L.K. (Ed.) Chapter 11—Polycarbonate (PC). In the Effect of Sterilization Methods on Plastics and Elastomers, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 81–109. [Google Scholar]
- Zhao, T.; Hu, X.; Wu, D.; Li, R.; Yang, G.; Wu, Y. Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol at Room Temperature Using Imidazolium Hydrogen Carbonate Ionic Liquid as a Recyclable Catalyst and Dehydrant. ChemSusChem 2017, 10, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Saada, R.; AboElazayem, O.; Kellici, S.; Heil, T.; Morgan, D.; Lampronti, G.; Saha, B. Greener synthesis of dimethyl carbonate using a novel tin-zirconia/graphene nanocomposite catalyst. Appl. Catal. B 2018, 226, 451–462. [Google Scholar] [CrossRef]
- Tan, H.-Z.; Wang, Z.-Q.; Xu, Z.-N.; Sun, J.; Xu, Y.-P.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. Review on the synthesis of dimethyl carbonate. Catal. Today 2018, 316, 2–12. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A. Chapter Two - Synthesis of Organic Carbonates. Adv. Inorg. Chem. 2014, 66, 25–81. [Google Scholar]
- Sun, W.; Zheng, L.; Wang, Y.; Li, D.; Liu, Z.; Wu, L.; Fang, T.; Wu, J. Study of Thermodynamics and Experiment on Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Yttrium Oxide. Ind. Eng. Chem. Res. 2020, 59, 4281–4290. [Google Scholar] [CrossRef]
- Tao, N.; Liu, J.; Xu, Y.; Feng, Y.; Wang, Y.; Liu, W.; Wang, H.; Lv, J. Highly selective and stable ZrO2-Al2O3 for synthesis of dimethyl carbonate in reactive distillation. Chem. Pap. 2020, 74, 3503–3515. [Google Scholar] [CrossRef]
- Tan, H.-Z.; Wang, Z.-Q.; Xu, Z.-N.; Sun, J.; Chen, Z.-N.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. Active Pd(ii) complexes: Enhancing catalytic activity by ligand effect for carbonylation of methyl nitrite to dimethyl carbonate. Catal. Sci. Technol. 2017, 7, 3785–3790. [Google Scholar] [CrossRef]
- Fu, Z.; Zhong, Y.; Yu, Y.; Long, L.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. TiO2-Doped CeO2 Nanorod Catalyst for Direct Conversion of CO2 and CH3OH to Dimethyl Carbonate: Catalytic Performance and Kinetic Study. ACS Omega 2018, 3, 198–207. [Google Scholar] [CrossRef]
- Al-Darwish, J.; Senter, M.; Lawson, S.; Rezaei, F.; Rownaghi, A.A. Ceria nanostructured catalysts for conversion of methanol and carbon dioxide to dimethyl carbonate. Catal. Today 2020, 350, 120–126. [Google Scholar] [CrossRef]
- Stoian, D.; Medina, F.; Urakawa, A. Improving the Stability of CeO2 Catalyst by Rare Earth Metal Promotion and Molecular Insights in the Dimethyl Carbonate Synthesis from CO2 and Methanol with 2-Cyanopyridine. ACS Catal. 2018, 8, 3181–3193. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Cheng, H.; Ma, L.; Liu, F.; Liu, Z. Research Progress in the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Res. Catal. Surv. Asia 2012, 16, 138–147. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Liu, D. Dimethyl Carbonate as a Promising Oxygenated Fuel for Combustion: A Review. Energies 2018, 11, 1552. [Google Scholar] [CrossRef]
- Huang, H.; Samsun, R.C.; Peters, R.; Stolten, D. Greener production of dimethyl carbonate by the Power-to-Fuel concept: A comparative techno-economic analysis. Green Chem. 2021, 23, 1734–1747. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Raza, A.; Ikram, M.; Guo, S.; Baiker, A.; Li, G. Green Synthesis of Dimethyl Carbonate from CO2 and Methanol: New Strategies and Industrial Perspective. Adv. Sustain. Syst. 2022, 6, 2200087. [Google Scholar] [CrossRef]
- Choi, J.-C.; Sakakura, T.; Sako, T. Reaction of Dialkyltin Methoxide with Carbon Dioxide Relevant to the Mechanism of Catalytic Carbonate Synthesis. J. Am. Chem. Soc. 1999, 121, 3793–3794. [Google Scholar] [CrossRef]
- Zhao, T.; Han, Y.; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Processing Technol. 2000, 62, 187–194. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent Advances in CO2 Capture and Utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef]
- Hoffman, W.A. A convenient preparation of carbonates from alcohols and carbon dioxide. J. Org. Chem. 1982, 47, 5209–5210. [Google Scholar] [CrossRef]
- Pandey, S.; Srivastava, V.C.; Kumar, V. Comparative thermodynamic analysis of CO2 based dimethyl carbonate synthesis routes. Can. J. Chem. Eng. 2021, 99, 467–478. [Google Scholar] [CrossRef]
- Eta, V.; Mäki-Arvela, P.; Leino, A.-R.; Kordás, K.; Salmi, T.; Murzin, D.Y.; Mikkola, J.-P. Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide: Circumventing Thermodynamic Limitations. Ind. Eng. Chem. Res. 2010, 49, 9609–9617. [Google Scholar] [CrossRef]
- Li, C.-F.; Zhong, S.-H. Study on application of membrane reactor in direct synthesis DMC from CO2 and CH3OH over Cu–KF/MgSiO catalyst. Catal. Today 2003, 82, 83–90. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Huang, A.; Caro, J. Hydrophilic SOD and LTA membranes for membrane-supported methanol, dimethylether and dimethylcarbonate synthesis. Micro. Meso. Mater. 2015, 207, 33–38. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, H.; Kang, X.; Chen, L.; Yuan, X.; Qi, Z. Analysis of direct synthesis of dimethyl carbonate from methanol and CO2 intensified by in-situ hydration-assisted reactive distillation with side reactor. Chem. Eng. Processing 2018, 129, 109–117. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Jiang, Q.; Guo, S.; Huang, J.; Xu, L.; Wang, Y.; Li, G. Facile Synthesis of Cobalt Clusters-CoNx Composites: Synergistic Effect Boosts up Electrochemical Oxygen Reduction. J. Mater. Chem. A 2022, 10, 16920–16927. [Google Scholar] [CrossRef]
- Shi, Q.; Raza, A.; Xu, L.; Li, G. Bismuth oxyhalide quantum dots modified titanate-necklaces with exceptional population of oxygen vacancies and photocatalytic activity. J. Colloid. Interface Sci. 2022, 625, 750–760. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Liu, X.; Xu, L.; Liu, B.; Zhang, J.; Xu, H.; Han, Z.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 195, 401–409. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Ionic liquids modified oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters on carbon-based materials as an efficient electrocatalyst for electrochemical water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Wei, X.; Akbar, M.U.; Raza, A.; Li, G. A Review Based on Bismuth Oxyhalides Materials for Photocatalysis. Nanoscale Adv. 2021, 3, 3353–3372. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, S.; Han, W.; Li, Z.; Xu, W.W.; Zhang, J.; Li, G. Tailoring optical and photocatalytic properties by single-Ag-atom exchange in Au13Ag12(PPh3)10Cl8 nanoclusters. Nano Res. 2022, 15, 2971–2976. [Google Scholar] [CrossRef]
- Jung, K.T.; Bell, A.T. An in Situ Infrared Study of Dimethyl Carbonate Synthesis from Carbon Dioxide and Methanol over Zirconia. J. Catalysis 2001, 204, 339. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Z.; Wei, X.; Wu, Z.; Liu, N.; Xu, J.; Xue, B.; Li, G. Pt/Ce-La Nanocomposite for Hydrogenation Promoted by a Synergistic Effect of Support with Redox and Basic Property. Catal. Lett. 2022, in press. [Google Scholar] [CrossRef]
- Waheed, A.; Cao, C.; Zhang, Y.; Zheng, K.; Li, G. Insight into Au/ZnO Catalyzed Aerobic Benzyl Alcohol Oxidation by Modulation-Excitation Attenuated Total Reflection IR Spectroscopy. New J. Chem. 2022, 46, 5361–5367. [Google Scholar] [CrossRef]
- Song, Z.; Subramaniam, B.; Chaudhari, R.V. Transesterification of Propylene Carbonate with Methanol Using Fe–Mn Double Metal Cyanide Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 5698–5710. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.; Li, F.; Li, A.; Luo, J.; Li, L.; Wang, F.; Zhao, N.; Xiao, F. Direct synthesis of dimethyl carbonate from CO2 and methanol over trifluoroacetic acid modulated UiO-66. J. CO2 Util. 2018, 27, 272–282. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.; Li, F.; Luo, J.; Zhao, N.; Xiao, F. Metal-organic frameworks MOF-808-X as highly efficient catalysts for direct synthesis of dimethyl carbonate from CO2 and methanol. Chin. J. Catal. 2019, 40, 553–566. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Abroshan, H.; Ge, Q.; Li, G.; Jin, R. Dual Effects of Water Vapor over Ceria-Supported Gold Clusters. Nanoscale 2018, 10, 6558–6565. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, X.; Shi, Q.; Li, J.; Ping, G.; Xu, H.; Ding, H.; Li, G. Synergistic Effects of PtFe/CeO2 Catalyst afford high Catalytic Performance in selective hydrogenation of cinnamaldehyde. J. Rare Earths 2022, 40, in press. [Google Scholar] [CrossRef]
- Raza, A.; Qin, Z.; Ahmad, S.O.A.; Ikram, M.; Li, G. Recent Advances in Structural Tailoring in BiOX-Based 2D Composites for Solar Energy Harvesting. J. Environ. Chem. Eng. 2021, 9, 106569. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent progress in heterogeneous catalysis over atomically and structurally precise metal nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, G.; Han, Z.; Zhang, S.; Sarker, D.; Xu, W.; Pan, X.; Li, G.; Baiker, A. Synergistic Effects of Ternary PdO-CeO2-OMS-2 Catalyst afford high Catalytic Performance and Stability in the Reduction of NO with CO. ACS Appl. Mater. Interfaces 2021, 13, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, Y.; Guo, S.; Han, Z.; Ta, N.; Li, G.; Baiker, A. NO reduction with CO over CuOx/CeO2 Nanocomposites: Influence of Oxygen Vacancies and Lattice Strain. Catal. Sci. Technol. 2021, 11, 6543–6552. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Shi, Q.; Gong, X.; Xu, H.; Li, G. Morphology effect of ceria supports on gold nanocluster catalyzed CO oxidation. Nanoscale Adv. 2021, 3, 7002–7006. [Google Scholar] [CrossRef]
- Sato, S.; Sato, F.; Gotoh, H.; Yamada, Y. Selective Dehydration of Alkanediols into Unsaturated Alcohols over Rare Earth Oxide Catalysts. ACS Catal. 2013, 3, 721–734. [Google Scholar] [CrossRef]
- Wu, X.L.; Xiao, M.; Meng, Y.Z.; Lu, Y.X. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mole. Catal. A 2005, 238, 158–162. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Q.; Ye, Z.; Li, Y.; Yang, Y.; Pu, H.; Li, G. Monolithic ZnxCe1−xO2 catalysts for catalytic synthesis of dimethyl carbonate from CO2 and methanol. New J. Chem. 2020, 44, 12522–12530. [Google Scholar] [CrossRef]
- Chen, Z.; Du, S.; Zhang, J.; Wu, X.-F. From ‘Gift’ to gift: Producing organic solvents from CO2. Green Chem. 2020, 22, 8169–8182. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Wang, H.; Li, Z.; Zheng, K.; Li, S.; Li, G. Transition-Metal-Mediated Catalytic Properties of CeO2-Supported Gold Clusters in Aerobic Alcohol Oxidation. Nano Res. 2018, 11, 2139–2148. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.W.; Han, Z.-k.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous Dimethyl Carbonate Synthesis from CO2 and Methanol over BixCe1-xOδ Monoliths: Effect of Bismuth Doping on Population of Oxygen Vacancies, Activity, and Reaction Pathway. Nano Res. 2022, 15, 1366–1374. [Google Scholar] [CrossRef]
- Darbha, S.J. Direct synthesis of dimethyl carbonate from CO2 and methanol over CeO2 catalysts of different morphologies. Chem. Sci. 2016, 128, 957–965. [Google Scholar]
- Moura, J.S.; Fonseca, J.d.S.L.; Bion, N.; Epron, F.; Silva, T.d.F.; Maciel, C.G.; Assaf, J.M.; Rangel, M.d.C. Effect of lanthanum on the properties of copper, cerium and zirconium catalysts for preferential oxidation of carbon monoxide. Catal. Today 2014, 228, 40–50. [Google Scholar] [CrossRef]
- Reddy, B.M.; Katta, L.; Thrimurthulu, G. Novel Nanocrystalline Ce1−xLaxO2−δ (x = 0.2) Solid Solutions: Structural Characteristics and Catalytic Performance. Chem. Mater. 2010, 22, 467–475. [Google Scholar] [CrossRef]
- Cho, B.K. Chemical modification of catalyst support for enhancement of transient catalytic activity: Nitric oxide reduction by carbon monoxide over rhodium. J. Catal. 1991, 131, 74–87. [Google Scholar] [CrossRef]
- Lee, J.G.; Park, J.H.; Shul, Y.G. Tailoring gadolinium-doped ceria-based solid oxide fuel cells to achieve 2 W cm−2 at 550 °C. Nat. Commun. 2014, 5, 4045. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Hungría, A.B.; Fernández-García, M.; Iglesias-Juez, A.; Soria, J.; Conesa, J.C.; Anderson, J.A.; Munuera, G. Operando DRIFTS study of the redox and catalytic properties of CuO/Ce1−xTbxO2−δ (x = 0–0.5) catalysts: Evidence of an induction step during CO oxidation. Phys. Chem. Chem. Phys. 2012, 14, 2144–2151. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thrimurthulu, G.; Katta, L. Design of Efficient CexM1−xO2−δ (M = Zr, Hf, Tb and Pr) Nanosized Model Solid Solutions for CO Oxidation. Catal. Lett. 2011, 141, 572–581. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Cui, L.; Ouyang, C.; Shi, S.; Tang, W.; Li, H.; Lee, J.-S.; Chen, L. First-principles investigation on redox properties of M-doped CeO2 (M=Mn, Pr, Sn, Zr). Phys. Rev. B 2010, 82, 125104. [Google Scholar] [CrossRef]
- Stoian, D.; Bansode, A.; Medina, F.; Urakawa, A. Catalysis under microscope: Unraveling the mechanism of catalyst de- and re-activation in the continuous dimethyl carbonate synthesis from CO2 and methanol in the presence of a dehydrating agent. Catal. Today 2017, 283, 2–10. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Ma, Z.; Zhang, X. Selective catalytic reduction of NO with NH3 over CuOX-carbonaceous materials. Catal. Commun. 2012, 17, 8–12. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Jiang, Q.; Xu, L.; Han, Z.; Guo, S.; Li, G.; Baiker, A. Effect of Configuration of Copper Oxide-Ceria Catalysts in NO Reduction with CO: Superior Performance of Copper-Ceria Solid Solution. ACS Appl. Mater. Interfaces 2021, 13, 61078–61087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jiang, Q.; Guo, S.; Baiker, A.; Li, G. Ternary CuCrCeOx Solid Solution Enhances N2-Selectivity in the NO Reduction with CO in the Presence of Water and Oxygen. ChemCatChem 2022, 14, e202200203. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Waheed, A.; Gao, Y.; Xu, H.; Abroshan, H.; Li, G. Oxygen Vacancy Engineering: An Approach to Promote Photocatalytic Conversion of Methanol to Methyl Formate over CuO/TiO2-Spindle. Nano Res. 2020, 13, 939–946. [Google Scholar] [CrossRef]
- Shi, Q.; Ping, G.; Wang, X.; Xu, H.; Li, J.; Cui, J.; Abroshan, H.; Ding, H.; Li, G. CuO/TiO2 Heterojunction Composite: An Efficient Photocatalyst for Selective Oxidation of Methanol to Methyl Formate. J. Mater. Chem. A 2019, 7, 2253–2260. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Shi, Q.; Ping, G.; Xu, H.; Waleed, A.; Li, G. CuO Nanoparticle-Decorated TiO2-Nanotube Heterojunctions for Direct Synthesis of Methyl Formate via Photo-Oxidation of Methanol. ACS Omega 2020, 5, 15942–15948. [Google Scholar] [CrossRef]
- Bell, A.T. The Impact of Nanoscience on Heterogeneous Catalysis. Science 2003, 299, 1688. [Google Scholar] [CrossRef]
- Ren, M.; Ren, J.; Hao, P.; Yang, J.; Wang, D.; Pei, Y.; Lin, J.-Y.; Li, Z. Influence of Microwave Irradiation on the Structural Properties of Carbon-Supported Hollow Copper Nanoparticles and Their Effect on the Synthesis of Dimethyl Carbonate. ChemCatChem 2016, 8, 861–871. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Abroshan, H.; Ge, Q.; Yang, X.; Xu, H.; Li, G. Catalytic CO Oxidation Using Bimetallic MxAu25-x Clusters: A Combined Experimental and Computational Study on Doping Effects. J. Phys. Chem. C 2016, 120, 10261–10267. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Zheng, H.; Fu, T.; Ju, Y.; Wang, Y. Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol. Appl. Catal. B 2015, 179, 95–105. [Google Scholar] [CrossRef]
- Li, G.; Abroshan, H.; Chen, Y.; Jin, R.; Kim, H.J. Experimental and mechanistic understanding of aldehyde hydrogenation using Au25 nanoclusters with Lewis-acids: Unique sites for catalytic reactions. J. Am. Chem. Soc. 2015, 137, 14295–14304. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R. Atomic level tuning of the catalytic properties: Doping effects of 25-atom bimetallic nanoclusters on styrene oxidation. Catal. Today 2016, 278, 187–191. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, R.; Quan, Y.; Liu, J.; Wang, J.; Pei, Y.; Wang, X.; Li, Z.; Ren, J. Highly efficient synthesis of dimethyl carbonate over copper catalysts supported on resin-derived carbon microspheres. Chem. Eng. Sci. 2019, 207, 1060–1071. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Tian, S.; Ye, Z.; Tang, Q.; Ye, L.; Li, G. Highly Effective Synthesis of Dimethyl Carbonate over CuNi Alloy Nanoparticles @Porous Organic Polymers Composite. Appl. Catal. A 2019, 587, 117275. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.; Wang, X.; Lu, Y.; Meng, Y. Highly effective synthesis of dimethyl carbonate from methanol and carbon dioxide using a novel copper–nickel/graphite bimetallic nanocomposite catalyst. Chem. Eng. J. 2009, 147, 287–296. [Google Scholar] [CrossRef]
- Han, X.; Sun, W.; Zhao, C.; Shi, R.; Wang, X.; Liu, S.; Li, Z.; Ren, J. Synthesis of dimethyl carbonate on single Cu atom embedded in N-doped graphene: Effect of nitrogen species. Mol. Catal. 2017, 443, 1–13. [Google Scholar] [CrossRef]
- Shi, K.; Huang, S.-Y.; Zhang, Z.-Y.; Wang, S.-P.; Ma, X.-B. Novel fabrication of copper oxides on AC and its enhanced catalytic performance on oxidative carbonylation of methanol. Chin. Chem. Lett. 2017, 28, 70–74. [Google Scholar] [CrossRef]
- Hao, P.; Ren, J.; Yang, L.; Qin, Z.; Lin, J.; Li, Z. Direct and generalized synthesis of carbon-based yolk–shell nanocomposites from metal-oleate precursor. Chem. Eng. J. 2016, 283, 1295–1304. [Google Scholar] [CrossRef]
- Sun, W.; Shi, R.; Wang, X.; Liu, S.; Han, X.; Zhao, C.; Li, Z.; Ren, J. Density-functional theory study of dimethyl carbonate synthesis by methanol oxidative carbonylation on single-atom Cu1/graphene catalyst. Appl. Surf. Sci. 2017, 425, 291–300. [Google Scholar] [CrossRef]
- Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Li, G. Recent Advances in Carbonaceous Sustainable Nanomaterials for Wastewater Treatments. Sustain. Mater. Technol. 2022, 32, e00406. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Yu, C.; Zhang, J.; Pan, X.; Li, G. Ionic liquid-assisted one-step preparation of ultrafine amorphous metallic hydroxide nanoparticles for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 15767–15773. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, X.; Ali, S.; Cao, C.; Rafi, A.A.; Li, G. Photoelectrochemical Energy Conversion over 2D Materials. Photochem 2022, 2, 272–298. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Z.; Cui, C.; Luo, Z.; Yang, B.; Jiang, Y.; Lai, C.; Wang, Z.; Wang, X.; Fang, X.; et al. In-Situ Generation and Global Property Profiling of Metal nanoclusters by Ultraviolet Laser Dissociation-Mass Spectrometry. Sci. China Chem. 2022, 65, 1196–1203. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Li, Z.; Yang, X.; Meng, F.; Liang, H.; Lei, Y.; Wu, H.; Zhang, J.; Li, G.; et al. Surface Isolation of Single Metal complexes or Clusters by a Coating Sieving Layer via Atomic Layer Deposition. Cell Rep. Phys. Sci. 2022, 3, 100787. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef]
- Zhang, C.; Selch, D.; Xie, Z.; Roberts, C.; Cooper, H.; Chen, G. Object-based benthic habitat mapping in the Florida Keys from hyperspectral imagery. Coastal Shelf Sci. 2013, 134, 88–97. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tatsumi, T.; Zhao, Y. Anionic polymer as a quasi-neutral medium for low-cost synthesis of titanosilicate molecular sieves in the presence of high-concentration alkali metal ions. J. Catal. 2016, 338, 321–328. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Liang, X.-Q.; Song, W.-G.; Wu, Z.-Y. Identification of the nitrogen species on N-doped graphene layers and Pt/NG composite catalyst for direct methanol fuel cell. Phys. Chem. Chem. Phys. 2010, 12, 12055–12059. [Google Scholar] [CrossRef] [PubMed]
- Deerattrakul, V.; Panitprasert, A.; Puengampholsrisook, P.; Kongkachuichay, P. Enhancing the Dispersion of Cu-Ni Metals on the Graphene Aerogel Support for Use as a Catalyst in the Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol. ACS Omega 2020, 5, 12391–12397. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, A.H.; Chaugule, A.A.; Gosavi, S.W.; Kim, H. CexZr1−xO2 solid solutions for catalytic synthesis of dimethyl carbonate from CO2: Reaction mechanism and the effect of catalyst morphology on catalytic activity. Fuel 2018, 216, 245–254. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, M.; Wang, S.; Han, D.; Lu, Y.; Meng, Y. Cerium oxide-based catalysts made by template-precipitation for the dimethyl carbonate synthesis from Carbon dioxide and methanol. J. Clean. Prod. 2015, 103, 847–853. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Qin, Z.; Tian, S.; Ye, Z.; Ye, L.; Abroshan, H.; Li, G. TixCe1-xO2 Nanocomposites: A Monolithic Catalyst for Direct Conversion of Carbon Dioxide and Methanol to Dimethyl Carbonate. Green Chem. 2019, 21, 4642–4649. [Google Scholar] [CrossRef]


| Entry | Catalyst | T (°C) | P (bar) | MeOH Conv. (%) | DMC Sel. (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ti0.04Ce0.96O2 | 140 | 22 | 5.4 | 83.1 | [11] |
| 2 | Cu-KF/MgSiOx | 140 | 4 | 9.2 | 96.0 | [25] |
| 3 | Zn0.10Ce0.90O2 | 160 | 24 | 20.5 | 82.1 | [50] |
| 4 | Bi0.12Ce0.88Oδ | 140 | 24 | 20.6 | 85.1 | [53] |
| 5 | CeO2-spindles | 150 | 50 | 63 | 97 | [54] |
| 6 | CuNi@POP-PPh3 | 160 | 24 | 10.5 | 80 | [77] |
| 7 | CuNi/graphite | 100 | 12 | 10.1 | 88.0 | [78] |
| 8 | Zr0.10Ce0.90O2 | 140 | 75 | 11.2 | 9.6 | [95] |
| 9 | CeO2-4A | 120 | 6 | 4.0 | 81.4 | [96] |
| 10 | Ti0.10Ce0.90O2 | 140 | 24 | 24.3 | 78.5 | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Khalid, M.S.; Wang, M.; Li, G. New Strategies on Green Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Oxide Composites. Molecules 2022, 27, 5417. https://doi.org/10.3390/molecules27175417
Zhang Y, Khalid MS, Wang M, Li G. New Strategies on Green Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Oxide Composites. Molecules. 2022; 27(17):5417. https://doi.org/10.3390/molecules27175417
Chicago/Turabian StyleZhang, Yifei, Muhammad Shoaib Khalid, Meng Wang, and Gao Li. 2022. "New Strategies on Green Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Oxide Composites" Molecules 27, no. 17: 5417. https://doi.org/10.3390/molecules27175417
APA StyleZhang, Y., Khalid, M. S., Wang, M., & Li, G. (2022). New Strategies on Green Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over Oxide Composites. Molecules, 27(17), 5417. https://doi.org/10.3390/molecules27175417







