Abstract
The aromatic hetero-polymer lignin is industrially processed in the paper/pulp and lignocellulose biorefinery, acting as a major energy source. It has been proven to be a natural resource for useful bioproducts; however, its depolymerization and conversion into high-value-added chemicals is the major challenge due to the complicated structure and heterogeneity. Conversely, the various pre-treatments techniques and valorization strategies offers a potential solution for developing a biomass-based biorefinery. Thus, the current review focus on the new isolation techniques for lignin, various pre-treatment approaches and biocatalytic methods for the synthesis of sustainable value-added products. Meanwhile, the challenges and prospective for the green synthesis of various biomolecules via utilizing the complicated hetero-polymer lignin are also discussed.
1. Introduction
The most profuse and renewable energy source on the planet is lignocellulosic biomass derived from plants; it comprises the two carbohydrates polymers, cellulose and hemicellulose, with the phenolic polymer lignin. The lignocellulosic biomass provides an alternative basis to fossil fuels for second-generation biofuels and other biobased chemicals production; however, the lignin biomass delivers excessive recalcitrance [1,2]. Lignin is a non-carbohydrate aromatic hetero-polymer with rich content and a complex structure in nature, present in the walls of the vascular tissue of plants [3,4]. This aromatic polymer is believed to be the second-most-abundant renewable resource, accounting for up to 25% of the total land-based biomass. Lignin does not have a fixed composition in all plant species, as its composition varies in different plant species; variation even exists in different tissues of the same plant. Cork lignin is mostly acetylated on the γ-OH of the side-chain (forty-eight percent acetylation) over the G units, whereas the lignin from the phloem and the xylem are barely acetylated, and this occurs mainly on the S units [5,6,7].
Lignin was considered a waste product in biorefinery during the production of biofuel from lignocellulosic biomass. After extraction, this polymer received attention and was found to have much potential as a natural reservoir of different chemicals and valuable products [8]. Thus, the release of lignin from conventional biomass refineries along with the pulp and paper industry has resulted in urgent requirements and also created the opportunity to increase the transformation of lignin into value-added products dramatically [9,10]. Many chemical and physical methods were explored to acquire the desired compounds from the lignin, but they all have certain limitations. Hence, for obtaining access to polysaccharides, the microbes have to break down or modify the lignin molecular structure [11,12]. The polysaccharide in the cell wall is hydrophilic, while the lignin is hydrophobic (Figure 1A); this combination of hydrophobic lignin and hydrophilic polysaccharides gives plants a significant advantage. The need for energy consumption for the non-specific breaking of these rich energy bonds is highly challenging; thus, the microbial degradation of lignin diverted the researchers’ attention and interest. For the better bioconversion of residual lignin-enriched refinery waste from upstream pretreatment, the most strategic process is to depolymerize macromolecular lignin into small molecular aromatic compounds for further conversion through microorganisms [13]. This conversion is similar to saccharification in cellulose; however, unlike the β-1,4-glucosidic linkage of cellulose, lignin contains diverse aromatic monomers and various types of chemical bonds or interunit linkages such as β-O-4, α-O-4/β-5, β–β, 4-O-5, and 5–5 [10].
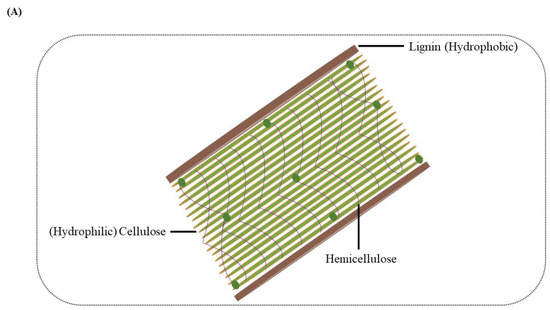
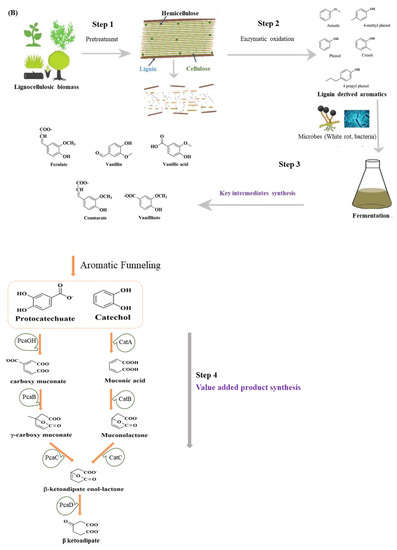
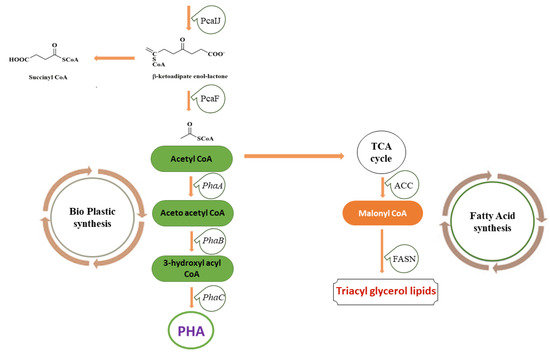
Figure 1.
(A) Composition of the plant cell wall. Arrangement of hydrophobic lignin and hydrophilic cellulose along with other cell wall structure components. (B) Value-added intermediate products synthesis from lignin. In step 1, the pretreatment of the lignocellulosic biomass occurs, separating cellulose, hemicellulose and lignin. The enzyme depolymerization of the lignin takes place in step 2, generating certain lignin-derived aromatics. The aromatics are hydrolyzed in step 3 through the microbial action of lignin-degrading white rot or bacteria, leading to the formation of key intermediates, including protocatechuate and catechol. The protocatechuate and catechol on further conversion and entering the β-ketoadipate pathway lead to value-added products like triglycerides.
Scientists are still working to find economical and reliable methods to degrade lignin for acquiring the desired breakdown products [14,15]. Several enzymes for lignin depolymerization have been discovered, along with the essential auxiliary enzymes involved in valuable compound synthesis. The white and brown-rot fungi contributing to this degradation produce extracellular peroxidase and laccase enzymes, along with the involvement of some peripheral enzymes [15,16,17]. Certain lignin-degrading bacteria are also considered significant for contributing to the bioconversion and biocatalysis of lignin through their enzymes [18,19].
The bioconversion of lignin mainly involves lignin pretreatment, the depolymerization of lignin into small molecular aromatic compounds, their degradation into central metabolites or key intermediates, and, lastly, the value-added product synthesis by microorganisms as represented in Figure 1B. The lignin depolymerization facilitates the conversion of low-molecular-weight lignin into monomers and oligomers, the degradation of monomers/oligomers into archetypal substrates, e.g., protocatechuate (PCA), the formation of acetyl-CoA from PCA through the β-KAP pathway, and the synthesis of lipid/PHA or other important molecules [20,21,22]. The current study focuses on the biodegradation and biodepolymerization of lignin and the challenges of meeting the bioconversion and valorization of lignin to value-added chemicals.
2. Lignin Availability and Structure
Lignin is technically intertwined with cellulose and hemicellulose to form the main structure of plants, providing plants with strength and rigidity. The synthesis of lignin occurs via the free-radical-assisted enzymatic dehydrogenative polymerization of phenylpropanoid precursors, namely p-coumaryl alcohol (H), sinapyl alcohol (S) and coniferyl alcohol (G) [7,23] (Figure 2A). Thus, optically inactive amorphous heteropolymer (lignin) is naturally featured, with a branched and cross-linked network of phenylpropane units (C9). The ratio of lignin subunits differs among different plant species at different growth stages. The lignin monomers are conjugated by different bonds, such as β-O-4, β-5, β-1, 5-5, β-β, α-O-4 and 4-O-5. The β-aryl ethers (β-O-4) are the dominant inter-unit linkages in the native lignin structure, as shown in Figure 2B. The subunits are cross-linked with the polysaccharides present in the xylem and phloem tissue, contributing to recalcitrance by preventing microbial attract from infiltrating into cell walls [24,25]. Achievements have been made to effectively enhance lignocellulosic biomass conversion by increasing the syringl residues ratio. The lignocellulose conversion can also be enhanced by introducing more ester linkages via alternative monolignols expression [24,26]. The composition of depolymerized lignin varies significantly based on the source of the feedstock and how the feedstock is processed. Besides the biomass variations, the lignin isolation methods also have an influential role in defining the structure and nature of lignin. Like the two primary processes involved in the pulp and paper industry, separating lignin from carbohydrates includes kraft and sulfite pulping; thus, the lignin is termed kraft lignin and lignosulfonate [1,27,28]. Similarly, soda lignin involves treatment with soda or alkali, while the lignin formed due to a mixture of organic ethanol and water as solvents from lignocellulose is referred to as organosolv process lignin [29,30]. Additionally, the various other advanced isolation methods can lead to the formation of certain types of lignin, including milled wood lignin, cellulolytic enzyme lignin and enzymatic mild acidolysis lignin [31,32,33]. The types of lignin based either on the different feedstocks or pre-treatment methods along with their monomers’ molecular weights are given in Table 1, while the other advanced methods for lignin isolation, along with the average molecular weights of the extracted lignin, their advantages, and limitations are presented in Table 2.
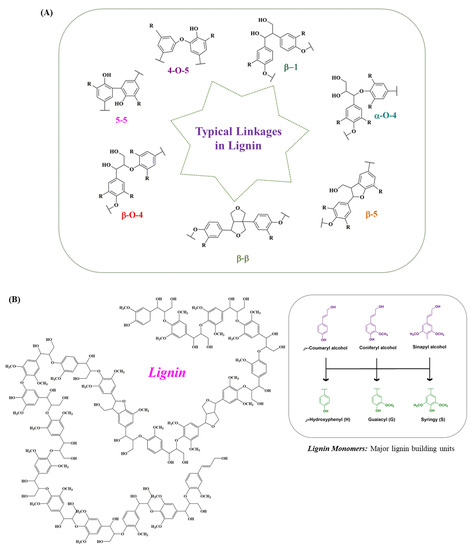
Figure 2.
Typical linkages present in lignin. (A) The hetero-polymer lignin is naturally featured with a branched and cross-linked network of phenylpropane units. These lignin-forming units are conjugated by different linkages, such as β-O-4, α-O-4, β-1, β-5, 5-5, β-β, and 4-O-5. From the lignin structure, it is observed that the dominant inter-unit linkages present in the native lignin structure are the β-aryl ether (β-O-4). (B) Structure of lignin. The combination of different lignin monomers, mainly sinapyl alcohol, coniferyl alcohol and p-coumaryl alcohol, forms a stable lignin structure through energy-rich bonds.

Table 1.
Different types of lignin, their monomer’s molecular weight, lignin content and chemicals.

Table 2.
Comparison of various lignin isolation methods.
3. Overview on Advancements in Lignin Pretreatment and Valorization into Fine Chemicals
In the biorefinery process, a pretreatment step is usually applied to reduce the recalcitrance of lignin and increase solubilizing hemicellulose to expose the crystalline cellulose core to be hydrolyzed by cellulase enzymes for ethanol production. Most lignocellulosic biorefineries use thermochemical pretreatment steps coupled to enzymatic hydrolysis for deconstructing plant polysaccharides, hence yielding lignin-rich streams [44,45]. Lignin can be retained as soluble and fractionated before downstream carbohydrate conversion; it can also be kept as an insoluble residue after extracting most of the carbohydrates by hydrolysis or by pretreatment [46]. It is noteworthy that high-severity pretreatment hydrolysis or deconstruction approaches will chemically modify lignin. The lignin fractionation method alters the chemical bonds and functional groups of lignin, such as cleaving labile C-O linkage and reforming more recalcitrant C-C linkages, which will affect the reactivity and bioconversion efficiency of lignin [47,48,49].
Several efforts have been rationalized to valorize lignin into fuels and valuable chemicals, as shown in Figure 3, which include catalytic pyrolysis, oxidation and hydrotreatment (hydrogenolysis and deoxygenation). Thermochemical treatment (e.g., pyrolysis) is the most-often considered method for rendering a series of heterogeneous mixtures of aromatic species or lignin fragments (i.e., C6–C22) [50,51]. It is reported that the pyrolysis of softwood kraft lignin generates a massive amount of heavy oil and char [52]. However, almost all of the thermochemical strategies result in an arsenal of several monomeric, oligomeric and polymeric compound mixtures, constituting an intricate composition of biooil [53] (Figure 4A,B). Simultaneously, for biofuel generation, an additional expensive and cumbersome hydrodeoxygenation step is required to avoid repolymerization and a self-condensation reaction. The partial hydrodeoxygenation product in a hydroprocessing step is then used to obtain the final desired biofuel [54,55]. Hydrotreatment is an approach with high selectivity, a high lignin conversion rate and the significant reduction of coke content. Shao et al. demonstrated the selective production of liquid hydrocarbons (C7–C9) via the direct hydrodeoxygenation of organosolv lignin over a potential porous catalyst Ru/Nb2O5 in water [56].
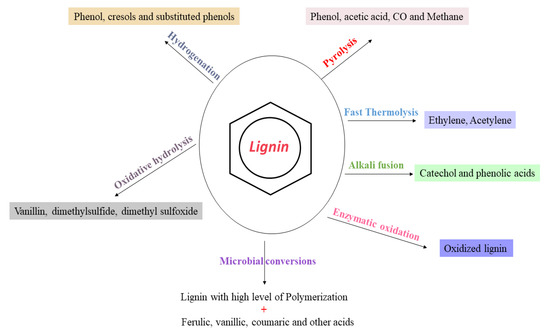
Figure 3.
Value-added chemicals formed from lignin through various treatments.
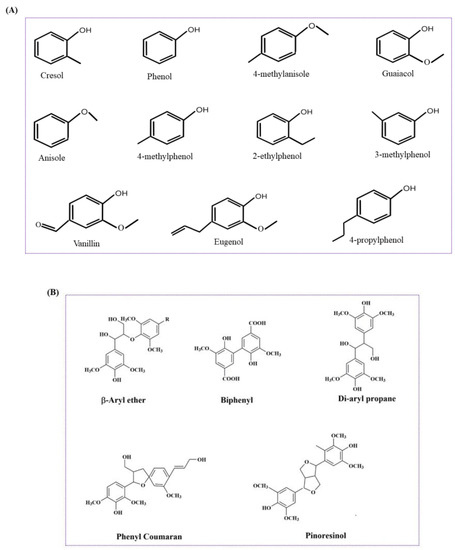
Figure 4.
(A) Lignin-derived monomeric bio-oils and phenolic compounds. (B) Lignin-derived oligomers.
Xu and Liguori used formic acid as a hydrogen source, combined with Pt and Pd as catalysts, to convert lignin into relatively prominent phenolic species [57]. Transition-metal catalysts have been used for lignin hydrogenolysis and hydrodeoxygenation, since most of the catalysts, such as Ni-Mo and Co-Mo/Al2O3 are neither cheap, nor recyclable or robust. The various reactivities of lignin-derived compounds also result in poisons to metal catalysts in biomass-derived streams [58,59]. Several thermochemical or chemical catalytic approaches are carried out to establish potential lignin high-grade platforms; however, industrial utilization is always hindered by its cost, complex compositions, low quality, energy consumption and organic waste treatment [60,61]. Recently, some breakthroughs were achieved by Rahimi et al., who demonstrated a method for the depolymerization of oxidized lignin under a mild condition in aqueous formic acid combined with a metal catalyst that results in more than 60% (wt) aromatic monomer production [62].
Similarly, lignin-consolidated bioprocessing can also plays a prominent role in the valorization of lignin into valuable chemicals. One such example is the development of combinatorial pretreatment to fractionate lignin from corn stover that improved lignin reactivity and increased lipid production. In a previous study, Xie reported that choosing bmr mutants sorghum (sorghum bicolor) with Cunninghamella echinulate FR3 can convert biomass without chemical pretreatment. Similarly, the dilute acid pretreatment of biomass resulted in more weight loss during fungal fermentation than untreated biomass, which showed complete biomass utilization in a consolidated platform without chemical pretreatment [63]. Furthermore, cis,cis-muconate produced with engineered P. putida grown on a biomass-derived lignin-enriched stream demonstrated an integrated strategy towards lignin valorization, forming a vital product [64].
4. Physical, Chemical and Physicochemical Depolymerization
Multiple approaches have emerged for lignin valorization; the variation in these depolymerization methods and the original lignin source results in the formation of variety of products (Figure 5). The lignin isolation from lignocellulose will enable the removal of cellulose/hemicellulose by solubilization, leaving insoluble lignin residues or, in contrast, the removal of lignin and leaving insoluble residues of cellulose/hemicellulose [20,65]. The various types of depolymerization methods, including physical, chemical, physiochemical and biological methods, are reviewed in Table 3 below.
Figure 5.
Important chemicals as byproducts from lignin degradation.

Table 3.
Different pretreatment methods and lignin-degrading strategies along with advantages and limitations.
5. Enzymatic and Biological Depolymerization
Due to the complex bond types and heterogeneous characteristics in lignin, it cannot be cleaved by hydrolytic enzymes like other natural polymers such as cellulose, starch and protein. However, enzymatic methods often involves a series of special non-specific fungal and bacterial lignin-degrading oxidoreductase enzymes or fenton’s reactions breaking a broad range of chemical linkages within lignin. The oxidoreductases, including laccase, manganese peroxidase (MnP), lignin peroxidase (LiP), versatile peroxidase (VP) and a unique dye-decolorizing peroxidase (DyP) are used in generating reactive radicals to destruct the lignin to a slate of reactive intermediates [15,20,100,116]. Laccase is known to have a low redox potential, which can act on phenolic structural units of lignin and non-phenolic structural units such as p-coumaric acid, 2,2′-azino-di (3-ethylbenzthiazoline-6-sulfonic acid) syringaldehyde and vanillin in the presence of mediators acting as electron shuttles. Previous studies have shown that the synergism of laccase improves the lignin action of microbial strains and increases its biomass, thereby enhancing lignin conversion [117,118,119,120]. LiP have a higher redox potential and attack non-phenolic lignin units by producing intermediate radicals [121]. MnP can chelate and oxidase Mn2+ to Mn3+, acting on both phenolic and non-phenolic lignin structural units, via the lipid peroxidation reaction, to depolymerize natural and synthetic lignin and entire lignocelluloses in vitro. This depolymerization effect could be enhanced by the presence of co-oxidants such as thiols or unsaturated fatty acids and their derivatives [17,122]. For enzymatic depolymerization, it is crucial to avoid the formation of inhibitors for microbial growth and to improve the reaction rate. In addition to the enzyme depolymerization of lignin, a series of free radical reactions mediated by Fenton’s reaction also plays an essential role in lignin depolymerization.
5.1. Microorganisms Involved in Lignin Depolymerization
5.1.1. Fungi
With the rapid development of multi-omics technologies, more and more microorganisms have unveiled their capability to convert lignin into fungible fuels and products [123]. Fungi, including most white-rot and some brown-rot, have been widely applied in lignin deconstruction and the remediation of structurally similar pollutants for many years [124,125]. Several studies have reported that some fungi are responsible for releasing several valuable phenolic precursors, such as syringyl alcohol, ferulic acid, vanillic acid and protocatechuic acid from lignocellulose biomass, but most of these high-value compounds are hardly captured into intermediates and thus are not capable of large-scale fermentation [126,127]. Some of the important fungi efficient in lignin degradation are given in Table 4.

Table 4.
Lignin depolymerization through fungi.
5.1.2. Bacteria
Apart from fungi, the extent of lignin degradation by bacteria is not quite as extensive. However, some well-known lignin degraders, such as Actinobacteria, α-Proteobacteria, and γ-Proteobacteria, collect and transform diverse compounds like coniferyl-alcohol and vanillate to intermediates such as catechol and protocatechuate via peripheral pathways [107,140,141]. These bacteria are capable of secreting enzymes for deposing different origins of lignin or lignin-derived compounds and converting them to precursors for bioproducts. The end-products, such as succinyl-CoA and acetyl-CoA obtained via the β-ketoadipate (β-KAP) pathway, are further converted through the central carbon metabolism to produce polyhydroxyalkanoate (PHA), lipids and other chemicals, as shown in Figure 6. Therefore, these strategies offer a direct and versatile means to funnel the heterogeneous collection of molecules produced from lignin depolymerization to targeted intermediates. The intermediates then form fuels, chemicals, and other materials via a “biological funneling” approach [142,143].
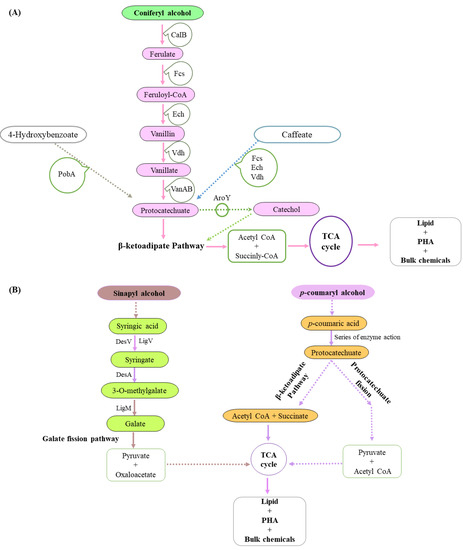
Figure 6.
Aromatics catabolism from the coniferyl, sinapyl and p-coumaryl branch. (A) Conversion of diverse compounds like the coniferyl-alcohol, 4-hydroxybenzoate and caffeate to aromatics protocatechuate and catechol occurs, which in turn are processed through the different enzyme systems involved in the β oxidation pathway. The acetyl CoA and succinyl CoA formed then goes through the TCA cycle, leading to the formation of triglycerols lipids, PHA, and other fine chemicals. (B) The sinapyl alcohol forms pyruvate and oxaloacetate through the Galate fission pathway, which enters to TCA cycle, leading to the formation of fine chemicals. In contrast, the p-coumaryl alcohol forms succinate + acetyl CoA through the β-ketoadipate pathway and pyruvate + acetyl CoA through the protocatechuate fission pathway, which in turn enter the TCA cycle and help in the formation of lipids, PHA or other bulk chemicals.
Certain well-studied bacterial strains, including Cupriavidus Necator H16 and Pseudomonas. putida KT2440, are reported to be excellent candidates for converting lignin-derived aromatic compounds to polyhydroxyalkanoates (PHA) in lignin-enriched biorefinery streams and can even accumulate significant amounts of muconate from lignin-derived aromatics [144,145,146]. Similarly, R. jostii RHA1 and some other Rhodococcus species such as R. opacus DSM1069 and R. opacus PD630 have also exhibited their ability to accumulate triglyceride lipids by converting different lignin sources [142,147,148]. A few recently isolated bacteria such as Pandoraea sp. B-6, some other bacillus species, and a fresh discovery from a thermophilic environment have also shown their potential to convert lignin into a high-value product [149,150,151]. Most of the important lignin-degrading bacteria responsible for the bioconversion of lignin to valuable products like lipids, PHA, and mucanoic acid are shown in Table 5.

Table 5.
Lignin depolymerization through bacteria.
5.2. Metabolic Pathways Involved
Numerous catabolic pathways have been reported to be involved in lignin degradation, including some renowned pathways reviewed by [164]. The biphenyl and β-aryl ether (β-O-4) catabolic pathways are of significant importance, in the sense that both these pathways are highly dominant in lignin degradation. Most importantly, certain value-added chemicals, including vanillin and 4-hydroxybenzoic acid, can be created from lignin through such pathways [165]. Correspondingly, the β-ketoadipate (β-KAP) pathway likewise plays a prominent role in lignin bioconversion; as lignin-derived aromatics can be assembled into high-molecular-weight compounds, mainly polyhydroxyalkanoate (PHA) and lipids, via this pathway [166,167], as shown in Figure 6. This pathway has been discovered and described in numerous prokaryotes, such as Gram-negative bacteria belonging to Sphingomonas paucimobilis SYK-6, Pseudomonas putida, Pseudomonas acidovorans, and Thermophilic geobacillus and Gram-positive bacteria belonging to Corynebacterium glutamicum, Streptomyces viridosporus, Rhodococcus opacus, and Rhodococcus josti. The exact pathway has also been reported in eukaryotes, including the rot fungi Phanerochaete chrysosporium and Trametes versicolor and the filamentous fungi Aspergillus sp., as well as in unicellular yeasts [106,107,164,166,167,168].
Although there is a great diversity in the catabolism of lignin and lignin-derived aromatic compounds by different prokaryotic and eukaryotic microorganisms, all current studies indicated two nodal products (catechol or protocatechuate), that are usually formed in the process of aromatic ring breakdown. This node is typically followed by aromatic ring fission and enzymatic conversion to central metabolites like acetyl-CoA and other constituents of the tricarboxylic acid (TCA) cycle [13,169,170].
6. Challenges and Strategies for Lignin Valorization
Even though some progress has been made in lignin valorization, effectively harnessing the intrinsic capabilities of biology to valorize lignin will require substantial research and developmental efforts. Some of the challenges that are of primary concerns and that need to be considered during the lignin valorization process and the possible strategic solutions to enhance the microbial biotransformation of lignin are discussed below.
6.1. Selection of Lignin Source and Depolymerization Capacity
The first and most challenging step is to depolymerize lignin to monomeric or dimeric/oligomeric compounds for further utilization by microorganisms. Recent literature has reported that bacteria can depolymerize and convert renewable lignin from different sources. However, the depolymerization and utilization efficiency is low due to the lignin macromolecule’s severe recalcitrance. Even though some bacteria can use lignin-derived aromatic compounds, the lignin-depolymerization capacity of most of these bacteria is relatively weak compared to white-rot fungus [62,142,171]. For achieving high product titer and efficient lignin utilization, it is critical to conduct efficient lignin-depolymerization that could degrade heteropolymers into aromatic compounds [172,173]. Lignin sources affect lignin components in the process of depolymerization, so adjusting the source of lignin and lignin manipulation is the first strategy for promoting the efficiency of lignin depolymerization. Previous research indicated that the efficiency of lignin depolymerization could be significantly affected by the origins and types of lignin used. Improvements could be made to approve the associated relationship between lignin characteristic features and their conversion yield, as well as the final production efficiency [174,175].
6.2. Selection of Ideal Microorganisms
Depolymerization approaches will result in many heterogeneous aromatic compounds. Many of these products are likely to be detrimental to the growth of bacteria, such as phenol, vanillin, 4-hydroxybenzoic acid, and coumaric acid [176,177,178]. One of the challenges is reducing or avoiding the influence of lignin-derived phenolic monomers and tannin derivatives on microbial growth. To address this challenge, it is important to screen and select appropriate microorganisms for utilizing and tolerating a broad range of lignin-derived aromatic compounds. The ideal microorganisms need not only be capable of utilizing these heterogeneous compounds but also should adapt to the potentially unfavorable compounds derived from lignin depolymerization and must be domesticated for use in industrial bioreactors [179].
Oceanimonas doudoroffii was recognized as a functional strain that was isolated from a contaminated marine environment and revealed the capacity of consuming lignin and its byproducts as the solitary carbon source for the synthesis of PHA [159]. Similarly, another vigorous bacterium, Cupriavidus basilensis B-8, screened from the steeping fluid of the erosive bamboo slips has been reported for its high capacity for lignin-derived aromatic compound catabolism [179].
6.3. Enzyme–Microbe and Microbe–Microbe Synergy
Commonly, depolymerizing lignin into solely monomeric or dimeric aromatic compounds is challenging. Despite the unveiling of fungal and bacterial enzymatic machinery for lignin depolymerization, the genetic manipulation to engineer lignin bioprocess streams is still not much developed. Hence, the efficient depolymerization and conversion of lignin always requires the synergistic effects of different enzymes [180]. A recent report demonstrated that combining the commercial laccase from Trametes versicolor with an oleaginous bacterium, Rhodococcus opacus PD630, can selectively degrade different chemical linkages to synergize lignin depolymerization. Thus, the development of a simultaneous depolymerization and fermentation process results in the fast growth of cells and higher lipid yields on kraft lignin [181]. Some other work has reported that MnP can accelerate lignin depolymerization when applied in the anaerobic digestion of municipal solid waste [182]. Besides laccase and MnP, some fungi could produce a hydroxyl radical via Fenton’s reaction; thus, the lignin structure will be more accessible for the lignin-degrading enzymes through this chemical oxidation generated by Fenton’s reaction [183]. Meanwhile, some other lignin-degradation enzymes, such as lignin peroxidase, versatile peroxidase and the novel dye-decolorizing peroxidase, could be explored for further potential development to synergize lignin degradation. Another significant provision of lignin-degrading enzymes is engineering microorganisms to secrete the main heterologous enzymes in the target microbe to enable efficient lignin depolymerization.
A biological team reported up to 20% lignin decomposition from some basidiomycetes, including Coriolus versicolor, Trametes gallica, lignin-degrading Microbacterium sp., and Streptomyces sp. for the generation of valuable chemicals due to potential synergy [181,184]. Similarly, lignin-degrading enzymes like laccase and MnP have been successfully expressed in Escherichia coli and yeast. However, the value of ligninolytic enzymes and its optimal mixture to degrade lignin synergistically for the synthesis of value-added products is currently unclear [15,185]. Therefore, advanced study is required to gain knowledge of potential interaction, which will help in the development of a lignin-degrading enzymes cocktail for lignin conversion to useful target products. Moreover, the application of biological omics-based techniques and advanced chemical analytics will not only provide an efficient tool to search for new enzymes but also new clues about how natural systems utilize these complex substrates.
6.4. Optimization of Catabolic Pathways and Yield of Desire Product
The second essential and challenging step is to catabolize lignin-derived aromatic compounds into other value-added intermediates by microorganisms. Numerous researchers have proven the possibility of the biological valorization of lignin; however, a common problem is still the low yield of the final chemical products [106,186]. Despite lignin-degraders being continuously explored, there is still a lack of particular traits responsible for producing target products from lignin at a predicted level. Thus, the mechanism of their catabolic pathways suggests a possible approach to tackle the low yield of target products [107]. To obtain a higher yield of natural intermediates in the upper pathways of lignin catabolism, one effort could be made to block the advance metabolism of a desire product [187]. Several studies have unveiled opportunities for optimizing the yield of the final product via microbial pathway engineering; however, research on aromatic catabolism is solely concentrated on previously reported strains, such as P. putida KT2240, R. jostii RHA1 and Cupriavidus Necator H16. Therefore, it is necessary to explore those microorganisms that are efficient t changing lignin into value-added products and that eventually may reveal new pathways to deliver novel value-added products. Finally, a fundamental understanding of the lignin conversion process could optimize the conversion process of lignin derived from biorefinery into value-added products.
7. Conclusions and Outlook
Lignin depolymerization is a hot topic of research due to its cost effectiveness and due to it acting as a renewable energy source for value-added biochemical synthesis. Therefore, the current study has reviewed the depolymerization and bioconversion of lignin to pave a way towards the synthesis of green and sustainable bioproducts. Preliminary investigations showed that nature includes many bio catalytic processes that can act synergistically with the various physical and chemical approaches to isolate and utilize the challenging lignin from lignocellulose biomass and convert it into energy-storage materials. Overall, the studies point to the fantastic potential and feasibility of lignin conversion into valuable bioproducts using catalytic approaches. However, fundamental understanding and extra studies are required to give insights into the potential interactions for adapting lignin-degrading enzyme cocktails. Moreover, further research on lignin pretreatments and catabolic pathway manipulation to develop an efficient bioconversion system is crucial for solving all of the bottlenecks in the process.
Author Contributions
Conceptualization, S.X. and S.S.; writing—original draft preparation, M.U.; software, P.L.; writing—review and editing, M.U., P.L., S.S. and S.X.; supervision, S.X. and S.S.; funding acquisition, S.S.; project administration, S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the youth science fund project of the National Natural Science Foundation of China (Grant Number 31900081).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Battashi, H.S.; Annamalai, N.; Sivakumar, N.; Al-Bahry, S.; Tripathi, B.N.; Nguyen, Q.D.; Gupta, V.K. Lignocellulosic Biomass (LCB): A Potential Alternative Biorefinery Feedstock for Polyhydroxyalkanoates Production. Rev. Environ. Sci. Bio/Technol. 2019, 18, 183–205. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 1; pp. 1–15. ISBN 978-0-12-815936-1. [Google Scholar]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, S.; Gan, L. Structure and Characteristics of Lignin. In Lignin Chemistry and Applications; Huang, J., Fu, S., Gan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2; pp. 25–50. ISBN 978-0-12-813941-7. [Google Scholar]
- Lourenço, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Lignin Composition and Structure Differs between Xylem, Phloem and Phellem in Quercus Suber L. Front. Plant Sci. 2016, 7, 1612. [Google Scholar] [CrossRef] [PubMed]
- Neutelings, G. Lignin Variability in Plant Cell Walls: Contribution of New Models. Plant Sci. 2011, 181, 379–386. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Its Integration into Metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic Biomass Based Biorefinery: A Successful Platform towards Circular Bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Integrated Biorefinery Processes for Conversion of Lignocellulosic Biomass to Value Added Materials: Paving a Path towards Circular Economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic Oxidation of Biorefinery Lignin to Value-Added Chemicals to Support Sustainable Biofuel Production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Silva, J.P.; Ticona, A.R.P.; Hamann, P.R.V.; Quirino, B.F.; Noronha, E.F. Deconstruction of Lignin: From Enzymes to Microorganisms. Molecules 2021, 26, 2299. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Brink, D.P.; Ravi, K.; Lidén, G.; Gorwa-Grauslund, M.F. Mapping the Diversity of Microbial Lignin Catabolism: Experiences from the ELignin Database. Appl. Microbiol. Biotechnol. 2019, 103, 3979–4002. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona Alzate, C.A. The Potential Use of Lignin as a Platform Product in Biorefineries: A Review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Cagide, C.; Castro-Sowinski, S. Technological and Biochemical Features of Lignin-Degrading Enzymes: A Brief Review. Environ. Sustain. 2020, 3, 371–389. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Williamson, J.J.; Rashid, G.M.M. Bacterial Enzymes for Lignin Depolymerisation: New Biocatalysts for Generation of Renewable Chemicals from Biomass. Curr. Opin. Chem. Biol. 2020, 55, 26–33. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial Enzymes Involved in Lignin Degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- De Vries, L.; Guevara-Rozo, S.; Cho, M.; Liu, L.-Y.; Renneckar, S.; Mansfield, S.D. Tailoring Renewable Materials via Plant Biotechnology. Biotechnol. Biofuels 2021, 14, 167. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer Lignins: Harnessing the Plasticity of Lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current Advancement on the Isolation, Characterization and Application of Lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Mahon, E.L.; Mansfield, S.D. Tailor-Made Trees: Engineering Lignin for Ease of Processing and Tomorrow’s Bioeconomy. Curr. Opin. Biotechnol. 2019, 56, 147–155. [Google Scholar] [CrossRef]
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Graglia, M.; Kanna, N.; Esposito, D. Lignin Refinery: Towards the Preparation of Renewable Aromatic Building Blocks. ChemBioEng Rev. 2015, 2, 377–392. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Gordobil, O.; González Alriols, M.; Labidi, J. Direct Lignin Depolymerization Process from Sulfur-Free Black Liquors. Fuel Process. Technol. 2020, 197, 106201. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Lazaridis, P.A.; Mach-Aigner, A.; Matis, K.A.; Triantafyllidis, K.S. Enhancing Lignocellulosic Biomass Hydrolysis by Hydrothermal Pretreatment, Extraction of Surface Lignin, Wet Milling and Production of Cellulolytic Enzymes. ChemSusChem 2019, 12, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, C.; Dai, L.; Xu, C.; Zhong, Y.; Yu, F.; Si, C. Biomass Fractionation and Lignin Fractionation towards Lignin Valorization. ChemSusChem 2020, 13, 4284–4295. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem. 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Başakçılardan Kabakcı, S.; Tanış, M.H. Pretreatment of Lignocellulosic Biomass at Atmospheric Conditions by Using Different Organosolv Liquors: A Comparison of Lignins. Biomass Convers. Biorefinery 2021, 11, 2869–2880. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.-J.; Zhong, C.; Li, B.-Z.; Yuan, Y.-J. Alkali-Based Pretreatment-Facilitated Lignin Valorization: A Review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Crestini, C.; Melone, F.; Sette, M.; Saladino, R. Milled Wood Lignin: A Linear Oligomer. Biomacromolecules 2011, 12, 3928–3935. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Saquing, C.; Baumberger, S.; Argyropoulos, D.S. Toward a Better Understanding of the Lignin Isolation Process from Wood. J. Agric. Food Chem. 2006, 54, 5939–5947. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, F.; Sun, R.-C.; Ralph, J. Isolation of Cellulolytic Enzyme Lignin from Wood Preswollen/Dissolved in Dimethyl Sulfoxide/N-Methylimidazole. J. Agric. Food Chem. 2010, 58, 3446–3450. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Zhang, H.; Wan, C. Insights into Structural Changes of Lignin toward Tailored Properties during Deep Eutectic Solvent Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Xiao, L.-P.; Song, G. Unraveling the Structural Transformation of Wood Lignin during Deep Eutectic Solvent Treatment. Front. Energy Res. 2020, 8, 48. [Google Scholar] [CrossRef]
- Kothari, N.; Bhagia, S.; Pu, Y.; Yoo, C.G.; Li, M.; Venketachalam, S.; Pattathil, S.; Kumar, R.; Cai, C.M.; Hahn, M.G.; et al. The Effect of Switchgrass Plant Cell Wall Properties on Its Deconstruction by Thermochemical Pretreatments Coupled with Fungal Enzymatic Hydrolysis or Clostridium Thermocellum Consolidated Bioprocessing. Green Chem. 2020, 22, 7924–7945. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Chi, N.T.L. Current Challenges and Innovative Developments in Pretreatment of Lignocellulosic Residues for Biofuel Production: A Review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-Enzyme Interaction: A Roadblock for Efficient Enzymatic Hydrolysis of Lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Gomez-Monedero, B.; Faria, J.; Bimbela, F.; Ruiz, M.P. Catalytic Hydroprocessing of Lignin β-O-4 Ether Bond Model Compound Phenethyl Phenyl Ether over Ruthenium Catalysts. Biomass Convers. Biorefinery 2017, 7, 385–398. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent Progress in the Thermal and Catalytic Conversion of Lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y. Bio-Oil from Fast Pyrolysis of Lignin: Effects of Process and Upgrading Parameters. Bioresour. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.-W.; Tsang, D.C.W. Advances in Lignin Valorization towards Bio-Based Chemicals and Fuels: Lignin Biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective Production of Arenes via Direct Lignin Upgrading over a Niobium-Based Catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Liguori, L.; Barth, T. Palladium-Nafion SAC-13 Catalysed Depolymerisation of Lignin to Phenols in Formic Acid and Water. J. Anal. Appl. Pyrolysis 2011, 92, 477–484. [Google Scholar] [CrossRef]
- Xu, W.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Depolymerization and Hydrodeoxygenation of Switchgrass Lignin with Formic Acid. ChemSusChem 2012, 5, 667–675. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Heteronuclear Single-Quantum Correlation–Nuclear Magnetic Resonance (HSQC–NMR) Fingerprint Analysis of Pyrolysis Oils. Energy Fuels 2011, 25, 5791–5801. [Google Scholar] [CrossRef]
- Kawale, H.D.; Kishore, N. Thermochemical Putrefaction of Delonix Regia Biomass and Tube Waste to Produce High-Quality Pyrolytic Bio-Oil. J. Therm. Anal. Calorim. 2022, 147, 2969–2983. [Google Scholar] [CrossRef]
- Pang, S. Advances in Thermochemical Conversion of Woody Biomass to Energy, Fuels and Chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-Acid-Induced Depolymerization of Oxidized Lignin to Aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Xie, S.; Qin, X.; Cheng, Y.; Laskar, D.; Qiao, W.; Sun, S.; Reyes, L.H.; Wang, X.; Dai, S.Y.; Sattler, S.E. Simultaneous Conversion of All Cell Wall Components by an Oleaginous Fungus without Chemi-Physical Pretreatment. Green Chem. 2015, 17, 1657–1667. [Google Scholar] [CrossRef]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic Acid Production from Lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Periyasamy, S.; Karthik, V.; Senthil Kumar, P.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, Physical and Biological Methods to Convert Lignocellulosic Waste into Value-Added Products. A Review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Vreugdenhil, B.J.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of Lignin-Rich Residues for the Production of Biofuels via Syngas Fermentation: Comparison of Gasification Technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Han, T.; Yang, W.; Jönsson, P.G. Pyrolysis and Subsequent Steam Gasification of Metal Dry Impregnated Lignin for the Production of H2-Rich Syngas and Magnetic Activated Carbon. Chem. Eng. J. 2020, 394, 124902. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and Chemical Pretreatment of Lignocellulosic Biomass. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 6; pp. 143–196. ISBN 978-0-12-815162-4. [Google Scholar] [CrossRef]
- Khan, M.F.S.; Akbar, M.; Xu, Z.; Wang, H. A Review on the Role of Pretreatment Technologies in the Hydrolysis of Lignocellulosic Biomass of Corn Stover. Biomass Bioenergy 2021, 155, 106276. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A.; Mohee, R. Ultrasound-Assisted Biological Conversion of Biomass and Waste Materials to Biofuels: A Review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of Ultrasound Pretreatment on Wood Physiochemical Structure. Ultrason. Sonochem. 2017, 34, 136–141. [Google Scholar] [CrossRef]
- Rana, M.; Nshizirungu, T.; Park, J.-H. Effect of Simultaneous Use of Microwave and Ultrasound Irradiation on the Sulfuric Acid Hydrolysis Lignin (SAHL) Depolymerization. Sustain. Energy Fuels 2022, 6, 861–878. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave Irradiation—A Green and Efficient Way to Pretreat Biomass. Bioresour. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romani, A.; Rodriguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave Heating Processing as Alternative of Pretreatment in Second-Generation Biorefinery: An Overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a Pretreatment for Lignocellulosic Biomass: Fundamentals and Applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Han, S.-Y.; Park, C.-W.; Endo, T.; Febrianto, F.; Kim, N.-H.; Lee, S.-H. Extrusion Process to Enhance the Pretreatment Effect of Ionic Liquid for Improving Enzymatic Hydrolysis of Lignocellulosic Biomass. Wood Sci. Technol. 2020, 54, 599–613. [Google Scholar] [CrossRef]
- Novakovic, J.; Kontogianni, N.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Towards Upscaling the Valorization of Wheat Straw Residues: Alkaline Pretreatment Using Sodium Hydroxide, Enzymatic Hydrolysis and Biogas Production. Environ. Sci. Pollut. Res. 2021, 28, 24486–24498. [Google Scholar] [CrossRef]
- Sheng, Y.; Xu, Y. Nuclear Magnetic Resonance Analysis of Ascorbic Acid Assisted Lignocellulose Decomposition in Dilute Acid Pretreatment and Its Stimulation on Enzymatic Hydrolysis. Bioresour. Technol. 2022, 343, 126147. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of Dilute Acid Pretreatment of Wild Rice Grass (Zizania Latifolia) from Loktak Lake for Enzymatic Hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Tu, W.-C.; Hallett, J.P. Recent Advances in the Pretreatment of Lignocellulosic Biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Shahbazi, G.; Zhang, B. Dilute and Concentrated Acid Hydrolysis of Lignocellulosic Biomass. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2010; pp. 143–158. ISBN 978-1-84569-510-1. [Google Scholar] [CrossRef]
- Kärcher, M.A.; Iqbal, Y.; Lewandowski, I.; Senn, T. Comparing the Performance of Miscanthus x Giganteus and Wheat Straw Biomass in Sulfuric Acid Based Pretreatment. Bioresour. Technol. 2015, 180, 360–364. [Google Scholar] [CrossRef]
- Badiei, M.; Asim, N.; Jahim, J.M.; Sopian, K. Comparison of Chemical Pretreatment Methods for Cellulosic Biomass. APCBEE Procedia 2014, 9, 170–174. [Google Scholar] [CrossRef]
- Travaini, R.; Martin, J.; Lorenzo Hernando, A.; Bolado, S. Ozonolysis: An Advantageous Pretreatment for Lignocellulosic Biomass Revisited. Bioresour. Technol. 2015, 199, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Kianmehr, M.; Arabhosseini, A.; Sarlaki, E.; Assadi-Alamouti, A.; Sadeghi, R. Ozonolysis: A Novel and Effective Oxidation Technique for Lignocellulosic Biomass Pretreatment. In Proceedings of the 12th National Congress on Biosystems Engineering and Agricultural Mechanization, Ahvaz, Iran, 5–7 February 2020. [Google Scholar]
- Osorio-González, C.S.; Hegde, K.; Brar, S.K.; Vezina, P.; Gilbert, D.; Avalos-Ramírez, A. Pulsed-Ozonolysis Assisted Oxidative Treatment of Forestry Biomass for Lignin Fractionation. Bioresour. Technol. 2020, 313, 123638. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Aita, G.M.; Walker, M.S. Effect of Ionic Liquid Pretreatment on the Chemical Composition, Structure and Enzymatic Hydrolysis of Energy Cane Bagasse. Bioresour. Technol. 2012, 117, 251–256. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; Silva, A.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; Barros, R.R.O.; Ferrara, M.A.; Bon, E.P.S. Biomass Pretreatment: A Critical Choice for Biomass Utilization via Biotechnological Routes. BMC Proc. 2014, 8, O34. [Google Scholar] [CrossRef]
- Bhatt, S.M.; Shilpa. Lignocellulosic Feedstock Conversion, Inhibitor Detoxification and Cellulosic Hydrolysis—A Review. Biofuels 2014, 5, 633–649. [Google Scholar] [CrossRef]
- Borand, M.; Karaosmanoğlu, F. Effects of Organosolv Pretreatment Conditions for Lignocellulosic Biomass in Biorefinery Applications: A Review. J. Renew. Sustain. Energy 2018, 10, 33104. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.-F.; Wang, Z.; Zheng, T.; Yao, J. Deep Eutectic Solvent with Bifunctional Brønsted-Lewis Acids for Highly Efficient Lignocellulose Fractionation. Bioresour. Technol. 2022, 347, 126723. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Application of Deep Eutectic Solvents in Biomass Pretreatment and Conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Zhang, H.; Han, L.; Dong, H. An Insight to Pretreatment, Enzyme Adsorption and Enzymatic Hydrolysis of Lignocellulosic Biomass: Experimental and Modeling Studies. Renew. Sustain. Energy Rev. 2021, 140, 110758. [Google Scholar] [CrossRef]
- Weiss, R.; Guebitz, G.M.; Pellis, A.; Nyanhongo, G.S. Harnessing the Power of Enzymes for Tailoring and Valorizing Lignin. Trends Biotechnol. 2020, 38, 1215–1231. [Google Scholar] [CrossRef]
- Hermosilla, E.; Rubilar, O.; Schalchli, H.; da Silva, A.S.; Ferreira-Leitao, V.; Diez, M.C. Sequential White-Rot and Brown-Rot Fungal Pretreatment of Wheat Straw as a Promising Alternative for Complementary Mild Treatments. Waste Manag. 2018, 79, 240–250. [Google Scholar] [CrossRef]
- Giri, R.; Sharma, R.K. Fungal Pretreatment of Lignocellulosic Biomass for the Production of Plant Hormone by Pichia Fermentans under Submerged Conditions. Bioresour. Bioprocess. 2020, 7, 30. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, R.; Srivastava, M.; Syed, A.; Bahadur Pal, D.; Bahkali, A.H.; Mishra, P.K.; Gupta, V.K. Impact of Mixed Lignocellulosic Substrate and Fungal Consortia to Enhance Cellulase Production and Its Application in NiFe2O4 Nanoparticles Mediated Enzymatic Hydrolysis of Wheat Straw. Bioresour. Technol. 2022, 345, 126560. [Google Scholar] [CrossRef]
- Nurika, I.; Shabrina, E.N.; Azizah, N.; Suhartini, S.; Bugg, T.D.H.; Barker, G.C. Application of Ligninolytic Bacteria to the Enhancement of Lignocellulose Breakdown and Methane Production from Oil Palm Empty Fruit Bunches (OPEFB). Bioresour. Technol. Rep. 2022, 17, 100951. [Google Scholar] [CrossRef]
- Hemati, A.; Aliasgharzad, N.; Khakvar, R.; Delangiz, N.; Asgari Lajayer, B.; van Hullebusch, E.D. Bioaugmentation of Thermophilic Lignocellulose Degrading Bacteria Accelerate the Composting Process of Lignocellulosic Materials. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent Advances in Lignin Valorization with Bacterial Cultures: Microorganisms, Metabolic Pathways, and Bio-Products. Biotechnol. Biofuels 2019, 12, 32. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Bae, J.-H.; Sohn, J.-H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209. [Google Scholar] [CrossRef]
- Vitrone, F.; Ramos, D.; Vitagliano, V.; Ferrando, F.; Salvadó, J. All-Lignocellulosic Fiberboards from Giant Reed (Arundo Donax L.): Effect of Steam Explosion Pre-Treatment on Physical and Mechanical Properties. Constr. Build. Mater. 2022, 319, 126064. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the Effectiveness of Liquid Hot Water and Steam Explosion Pretreatments of Fast-Growing Poplar (Populus Trichocarpa) Wood. Wood Sci. Technol. 2022, 56, 87–109. [Google Scholar] [CrossRef]
- Zabihi, S.; Sharafi, A.; Motamedi, H.; Esmaeilzadeh, F.; Doherty, W.O.S. Environmentally Friendly Acetic Acid/Steam Explosion/Supercritical Carbon Dioxide System for the Pre-Treatment of Wheat Straw. Environ. Sci. Pollut. Res. 2021, 28, 37867–37881. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, J.M.C.; Verlinden, R.A.J.; van der Meer, P.C.; van der Wielen, L.A.M.; Straathof, A.J.J. Liquid Hot Water Pretreatment of Lignocellulosic Biomass at Lab and Pilot Scale. Processes 2021, 9, 1518. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Dias, M.; Daza-Serna, L.; de Carvalho, C.C.C.R.; Friedl, A. Integral Analysis of Liquid-Hot-Water Pretreatment of Wheat Straw: Evaluation of the Production of Sugars, Degradation Products, and Lignin. Sustainability 2022, 14, 362. [Google Scholar] [CrossRef]
- Nieder-Heitmann, M.; Haigh, K.; Louw, J.; Görgens, J.F. Economic Evaluation and Comparison of Succinic Acid and Electricity Co-Production from Sugarcane Bagasse and Trash Lignocelluloses in a Biorefinery, Using Different Pretreatment Methods: Dilute Acid (H2SO4), Alkaline (NaOH), Organosolv, Ammonia Fibre Expansion (AFEXTM), Steam Explosion (STEX), and Wet Oxidation. Biofuels Bioprod. Biorefining 2020, 14, 55–77. [Google Scholar] [CrossRef]
- Lee, J.M.; Jameel, H.; Venditti, R.A. A Comparison of the Autohydrolysis and Ammonia Fiber Explosion (AFEX) Pretreatments on the Subsequent Enzymatic Hydrolysis of Coastal Bermuda Grass. Bioresour. Technol. 2010, 101, 5449–5458. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent Advances on Ammonia-Based Pretreatments of Lignocellulosic Biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and Conversion of Lignin to Value-Added Bioproducts by Microbial and Enzymatic Catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Xie, S.; Lin, F.; Jin, M.; Yuan, J.S. Combinatorial Pretreatment and Fermentation Optimization Enabled a Record Yield on Lignin Bioconversion. Biotechnol. Biofuels 2018, 11, 21. [Google Scholar] [CrossRef]
- Govil, T.; Vaughn, M.; Salem, D.R.; Sani, R.K. Biorefining of Lignin Wastes: Modularized Production of Value-Added Compounds. In Microbial Biotechnology for Renewable and Sustainable Energy; Saini, J.K., Sani, R.K., Eds.; Springer Nature: Singapore, 2022; pp. 135–163. ISBN 978-981-16-3852-7. [Google Scholar] [CrossRef]
- Brenelli, L.; Squina, F.M.; Felby, C.; Cannella, D. Laccase-Derived Lignin Compounds Boost Cellulose Oxidative Enzymes AA9. Biotechnol. Biofuels 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lin, L.; Sui, H.; Wang, H.; Zhang, Z.; Jiao, N.; Zhou, J. Efficient Extracellular Laccase Secretion via Bio-Designed Secretory Apparatuses to Enhance Bacterial Utilization of Recalcitrant Lignin. Green Chem. 2021, 23, 2079–2094. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Z.; Shi, J.; Yang, C.; Fang, Y.; Chen, G.; Chen, H.; Tian, C. Enzymatic Hydrolysis of Corn Stover Lignin by Laccase, Lignin Peroxidase, and Manganese Peroxidase. Bioresour. Technol. 2022, 361, 127699. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Arora, P.K. Biotechnological Applications of Manganese Peroxidases for Sustainable Management. Front. Environ. Sci. 2022, 365. [Google Scholar] [CrossRef]
- Floudas, D.; Bentzer, J.; Ahrén, D.; Johansson, T.; Persson, P.; Tunlid, A. Uncovering the Hidden Diversity of Litter-Decomposition Mechanisms in Mushroom-Forming Fungi. ISME J. 2020, 14, 2046–2059. [Google Scholar] [CrossRef]
- Goodell, B. Fungi Involved in the Biodeterioration and Bioconversion of Lignocellulose Substrates. In Genetics and Biotechnology; Benz, J.P., Schipper, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 369–397. ISBN 978-3-030-49924-2. [Google Scholar]
- Bai, Z.; Ma, Q.; Dai, Y.; Yuan, H.; Ye, J.; Yu, W. Spatial Heterogeneity of SOM Concentrations Associated with White-Rot Versus Brown-Rot Wood Decay. Sci. Rep. 2017, 7, 13758. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal Biodegradation and Enzymatic Modification of Lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar]
- Pham, L.T.M.; Kim, Y.H. Discovery and Characterization of New O-Methyltransferase from the Genome of the Lignin-Degrading Fungus Phanerochaete Chrysosporium for Enhanced Lignin Degradation. Enzym. Microb. Technol. 2016, 82, 66–73. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Ryu, S.-H.; Jeong, H.; Lee, S.-S.; Kim, M.; Choi, I.-G. Phanerochaete Chrysosporium Multienzyme Catabolic System for in Vivo Modification of Synthetic Lignin to Succinic Acid. ACS Chem. Biol. 2017, 12, 1749–1759. [Google Scholar] [CrossRef]
- Ruhong, L.; Liao, Q.; Xia, A.; Deng, Z.; Huang, Y.; Zhu, X.; Zhu, X. Synergistic Treatment of Alkali Lignin via Fungal Coculture for Biofuel Production: Comparison of Physicochemical Properties and Adsorption of Enzymes Used as Catalysts. Front. Energy Res. 2020, 8, 231. [Google Scholar] [CrossRef]
- Kong, W.; Fu, X.; Wang, L.; Alhujaily, A.; Zhang, J.; Ma, F.; Zhang, X.; Yu, H. A Novel and Efficient Fungal Delignification Strategy Based on Versatile Peroxidase for Lignocellulose Bioconversion. Biotechnol. Biofuels 2017, 10, 218. [Google Scholar] [CrossRef]
- Nguyen, H.; Kondo, K.; Yagi, Y.; Iseki, Y.; Okuoka, N.; Watanabe, T.; Mikami, B.; Nagata, T.; Katahira, M. Functional and Structural Characterizations of Lytic Polysaccharide Monooxygenase, Which Cooperates Synergistically with Cellulases, from Ceriporiopsis Subvermispora. ACS Sustain. Chem. Eng. 2022, 10, 923–934. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Microbial Pretreatment of Corn Stover with Ceriporiopsis Subvermispora for Enzymatic Hydrolysis and Ethanol Production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef]
- Van Erven, G.; Wang, J.; Sun, P.; de Waard, P.; van der Putten, J.; Frissen, G.E.; Gosselink, R.J.A.; Zinovyev, G.; Potthast, A.; van Berkel, W.J.H.; et al. Structural Motifs of Wheat Straw Lignin Differ in Susceptibility to Degradation by the White-Rot Fungus Ceriporiopsis Subvermispora. ACS Sustain. Chem. Eng. 2019, 7, 20032–20042. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; Miki, Y.; Martínez, M.J.; Hammel, K.E.; Martínez, A.T. Lignin-Degrading Peroxidases from Genome of Selective Ligninolytic Fungus Ceriporiopsis Subvermispora. J. Biol. Chem. 2012, 287, 16903–16916. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; Martínez, M.J.; Romero, A.; Hammel, K.E.; Medrano, F.J.; Martínez, A.T. Ligninolytic Peroxidase Genes in the Oyster Mushroom Genome: Heterologous Expression, Molecular Structure, Catalytic and Stability Properties, and Lignin-Degrading Ability. Biotechnol. Biofuels 2014, 7, 2. [Google Scholar] [CrossRef]
- Alexieva, Z.; Yemendzhiev, H.; Zlateva, P. Cresols Utilization by Trametes Versicolor and Substrate Interactions in the Mixture with Phenol. Biodegradation 2010, 21, 625–635. [Google Scholar] [CrossRef]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase Production by Trametes Versicolor in Solid-State Fermentation Using Tea Residues as Substrate and Its Application in Dye Decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Pizzul, L.; del Pilar Castillo, M.; Cuevas, R.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by the Chilean White-Rot Fungus Anthracophyllum Discolor. J. Hazard. Mater. 2011, 185, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Kumar, M.; Singh, S.S.; Thakur, I.S. Genomic Analysis of Burkholderia Sp. ISTR5 for Biofunneling of Lignin-Derived Compounds. Biotechnol. Biofuels 2019, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Singh, R. The Emerging Role for Bacteria in Lignin Degradation and Bio-Product Formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Han, H.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X. Lignin Depolymerization and Utilization by Bacteria. Bioresour. Technol. 2018, 269, 557–566. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Li, H.; Giannis, A.; He, C. Recent Biotechnology Advances in Bio-Conversion of Lignin to Lipids by Bacterial Cultures. Front. Chem. 2022, 10, 894593. [Google Scholar] [CrossRef]
- Ravi, K.; García-Hidalgo, J.; Gorwa-Grauslund, M.F.; Lidén, G. Conversion of Lignin Model Compounds by Pseudomonas Putida KT2440 and Isolates from Compost. Appl. Microbiol. Biotechnol. 2017, 101, 5059–5070. [Google Scholar] [CrossRef]
- Tomizawa, S.; Chuah, J.A.; Matsumoto, K.; Doi, Y.; Numata, K. Understanding the Limitations in the Biosynthesis of Polyhydroxyalkanoate (PHA) from Lignin Derivatives. ACS Sustain. Chem. Eng. 2014, 2, 1106–1113. [Google Scholar] [CrossRef]
- Kuatsjah, E.; Johnson, C.W.; Salvachúa, D.; Werner, A.Z.; Zahn, M.; Szostkiewicz, C.J.; Singer, C.A.; Dominick, G.; Okekeogbu, I.; Haugen, S.J.; et al. Debottlenecking 4-Hydroxybenzoate Hydroxylation in Pseudomonas Putida KT2440 Improves Muconate Productivity from p-Coumarate. Metab. Eng. 2022, 70, 31–42. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Hernández, M.A.; Lanfranconi, M.P.; Silva, R.A.; Villalba, M.S. Rhodococcus as Biofactories for Microbial Oil Production. Molecules 2021, 26, 4871. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Williamson, J.J.; Alberti, F. Microbial Hosts for Metabolic Engineering of Lignin Bioconversion to Renewable Chemicals. Renew. Sustain. Energy Rev. 2021, 152, 111674. [Google Scholar] [CrossRef]
- Liu, D.; Yan, X.; Si, M.; Deng, X.; Min, X.; Shi, Y.; Chai, L. Bioconversion of Lignin into Bioplastics by Pandoraea Sp. B-6: Molecular Mechanism. Environ. Sci. Pollut. Res. 2019, 26, 2761–2770. [Google Scholar] [CrossRef]
- Salvachúa, D.; Karp, E.M.; Nimlos, C.T.; Vardon, D.R.; Beckham, G.T. Towards Lignin Consolidated Bioprocessing: Simultaneous Lignin Depolymerization and Product Generation by Bacteria. Green Chem. 2015, 17, 4951–4967. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Navas, L.E.; Fetherolf, M.M.; Liu, L.-Y.; Dalhuisen, T.; Renneckar, S.; Eltis, L.D.; Mohn, W.W. Discovery of Lignin-Transforming Bacteria and Enzymes in Thermophilic Environments Using Stable Isotope Probing. ISME J. 2022, 16, 1944–1956. [Google Scholar] [CrossRef]
- Shields-Menard, S.A.; AmirSadeghi, M.; Green, M.; Womack, E.; Sparks, D.L.; Blake, J.; Edelmann, M.; Ding, X.; Sukhbaatar, B.; Hernandez, R.; et al. The Effects of Model Aromatic Lignin Compounds on Growth and Lipid Accumulation of Rhodococcus Rhodochrous. Int. Biodeterior. Biodegrad. 2017, 121, 79–90. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhang, L.; Xu, Z.; Ben, H.; Gaffrey, M.J.; Yang, Y.; Yang, S.; Yuan, J.S.; Qian, W.-J.; et al. Discovery of Potential Pathways for Biological Conversion of Poplar Wood into Lipids by Co-Fermentation of Rhodococci Strains. Biotechnol. Biofuels 2019, 12, 60. [Google Scholar] [CrossRef]
- Kosa, M.; Ragauskas, A.J. Bioconversion of Lignin Model Compounds with Oleaginous Rhodococci. Appl. Microbiol. Biotechnol. 2012, 93, 891–900. [Google Scholar] [CrossRef]
- Kumar, M.; Singhal, A.; Verma, P.K.; Thakur, I.S. Production and Characterization of Polyhydroxyalkanoate from Lignin Derivatives by Pandoraea Sp. ISTKB. ACS Omega 2017, 2, 9156–9163. [Google Scholar] [CrossRef]
- Salvachúa, D.; Rydzak, T.; Auwae, R.; De Capite, A.; Black, B.A.; Bouvier, J.T.; Cleveland, N.S.; Elmore, J.R.; Furches, A.; Huenemann, J.D.; et al. Metabolic Engineering of Pseudomonas Putida for Increased Polyhydroxyalkanoate Production from Lignin. Microb. Biotechnol. 2020, 13, 290–298. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Characterization of Poly-3-Hydroxybutyrate (PHB) Produced from Ralstonia Eutropha Using an Alkali-Pretreated Biomass Feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, X.; Li, Q.; Wang, X.; Xie, S.; Chai, L.; Yuan, J. Directed Bioconversion of Kraft Lignin to Polyhydroxyalkanoate by Cupriavidus Basilensis B-8 without Any Pretreatment. Process Biochem. 2017, 52, 238–242. [Google Scholar] [CrossRef]
- Numata, K.; Morisaki, K. Screening of Marine Bacteria to Synthesize Polyhydroxyalkanoate from Lignin: Contribution of Lignin Derivatives to Biosynthesis by Oceanimonas Doudoroffii. ACS Sustain. Chem. Eng. 2015, 3, 569–573. [Google Scholar] [CrossRef]
- Becker, J.; Kuhl, M.; Kohlstedt, M.; Starck, S.; Wittmann, C. Metabolic Engineering of Corynebacterium Glutamicum for the Production of Cis, Cis-Muconic Acid from Lignin. Microb. Cell Fact. 2018, 17, 115. [Google Scholar] [CrossRef]
- Barton, N.; Horbal, L.; Starck, S.; Kohlstedt, M.; Luzhetskyy, A.; Wittmann, C. Enabling the Valorization of Guaiacol-Based Lignin: Integrated Chemical and Biochemical Production of Cis,Cis-Muconic Acid Using Metabolically Engineered Amycolatopsis Sp ATCC 39116. Metab. Eng. 2018, 45, 200–210. [Google Scholar] [CrossRef]
- Johnson, C.W.; Abraham, P.E.; Linger, J.G.; Khanna, P.; Hettich, R.L.; Beckham, G.T. Eliminating a Global Regulator of Carbon Catabolite Repression Enhances the Conversion of Aromatic Lignin Monomers to Muconate in Pseudomonas Putida KT2440. Metab. Eng. Commun. 2017, 5, 19–25. [Google Scholar] [CrossRef]
- Sonoki, T.; Takahashi, K.; Sugita, H.; Hatamura, M.; Azuma, Y.; Sato, T.; Suzuki, S.; Kamimura, N.; Masai, E. Glucose-Free Cis,Cis-Muconic Acid Production via New Metabolic Designs Corresponding to the Heterogeneity of Lignin. ACS Sustain. Chem. Eng. 2018, 6, 1256–1264. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for Degradation of Lignin in Bacteria and Fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef]
- Gonçalves, C.C.; Bruce, T.; de Oliveira Gorgulho Silva, C.; Fillho, E.X.F.; Noronha, E.F.; Carlquist, M.; Parachin, N.S. Bioprospecting Microbial Diversity for Lignin Valorization: Dry and Wet Screening Methods. Front. Microbiol. 2020, 11, 1081. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Le, R.K.; Kosa, M.; Yang, B.; Yuan, J.; Ragauskas, A.J. Identifying and Creating Pathways to Improve Biological Lignin Valorization. Renew. Sustain. Energy Rev. 2019, 105, 349–362. [Google Scholar] [CrossRef]
- Wells, T., Jr.; Ragauskas, A.J. Biotechnological Opportunities with the β-Ketoadipate Pathway. Trends Biotechnol. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Granja-Travez, R.S.; Persinoti, G.F.; Squina, F.M.; Bugg, T.D.H. Functional Genomic Analysis of Bacterial Lignin Degraders: Diversity in Mechanisms of Lignin Oxidation and Metabolism. Appl. Microbiol. Biotechnol. 2020, 104, 3305–3320. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.; Bleem, A.; Kuatsjah, E.; Werner, A.Z.; DuBois, J.L.; McGeehan, J.E.; Eltis, L.D.; Beckham, G.T. Critical Enzyme Reactions in Aromatic Catabolism for Microbial Lignin Conversion. Nat. Catal. 2022, 5, 86–98. [Google Scholar] [CrossRef]
- Weiland, F.; Kohlstedt, M.; Wittmann, C. Guiding Stars to the Field of Dreams: Metabolically Engineered Pathways and Microbial Platforms for a Sustainable Lignin-Based Industry. Metab. Eng. 2022, 71, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Barnhart-Dailey, M.C.; Ye, D.; Hayes, D.C.; Maes, D.; Simoes, C.T.; Appelhans, L.; Carroll-Portillo, A.; Kent, M.S.; Timlin, J.A. Internalization and Accumulation of Model Lignin Breakdown Products in Bacteria and Fungi. Biotechnol. Biofuels 2019, 12, 175. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Microbial Delignification and Hydrolysis of Lignocellulosic Biomass to Enhance Biofuel Production: An Overview and Future Prospect. Bull. Natl. Res. Cent. 2019, 43, 51. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Evstigneyev, E.I.; Shevchenko, S.M. Structure, Chemical Reactivity and Solubility of Lignin: A Fresh Look. Wood Sci. Technol. 2019, 53, 7–47. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Pineda, A.; Lee, A.F. Heterogeneously Catalyzed Lignin Depolymerization. Appl. Petrochem. Res. 2016, 6, 243–256. [Google Scholar] [CrossRef]
- Kurosawa, K.; Laser, J.; Sinskey, A.J. Tolerance and Adaptive Evolution of Triacylglycerol-Producing Rhodococcus Opacus to Lignocellulose-Derived Inhibitors. Biotechnol. Biofuels 2015, 8, 76. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Hunsinger, G.B.; Pienkos, P.T.; Johnson, D.K. Connecting Lignin-Degradation Pathway with Pre-Treatment Inhibitor Sensitivity of Cupriavidus Necator. Front. Microbiol. 2014, 5, 247. [Google Scholar] [CrossRef]
- Moraes, E.C.; Alvarez, T.M.; Persinoti, G.F.; Tomazetto, G.; Brenelli, L.B.; Paixão, D.A.A.; Ematsu, G.C.; Aricetti, J.A.; Caldana, C.; Dixon, N. Lignolytic-Consortium Omics Analyses Reveal Novel Genomes and Pathways Involved in Lignin Modification and Valorization. Biotechnol. Biofuels 2018, 11, 75. [Google Scholar] [CrossRef]
- Hou, L.; Ji, D.; Dong, W.; Yuan, L.; Zhang, F.; Li, Y.; Zang, L. The Synergistic Action of Electro-Fenton and White-Rot Fungi in the Degradation of Lignin. Front. Bioeng. Biotechnol. 2020, 8, 99. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic Enzymatic and Microbial Lignin Conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Jayasinghe, P.A.; Hettiaratchi, J.P.A.; Mehrotra, A.K.; Kumar, S. Effect of Enzyme Additions on Methane Production and Lignin Degradation of Landfilled Sample of Municipal Solid Waste. Bioresour. Technol. 2011, 102, 4633–4637. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Brzonova, I.; Kozliak, E.; Kubátová, A.; Chebeir, M.; Qin, W.; Christopher, L.; Ji, Y. Kenaf Biomass Biodecomposition by Basidiomycetes and Actinobacteria in Submerged Fermentation for Production of Carbohydrates and Phenolic Compounds. Bioresour. Technol. 2014, 173, 352–360. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and Its Potential Industrial Applications. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 82, pp. 1–28. [Google Scholar] [CrossRef]
- Xie, S.; Ragauskas, A.J.; Yuan, J.S. Lignin Conversion: Opportunities and Challenges for the Integrated Biorefinery. Ind. Biotechnol. 2016, 12, 161–167. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Biotransformation of Lignin: Mechanisms, Applications and Future Work. Biotechnol. Prog. 2020, 36, e2922. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).