Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals
Abstract
:1. Introduction
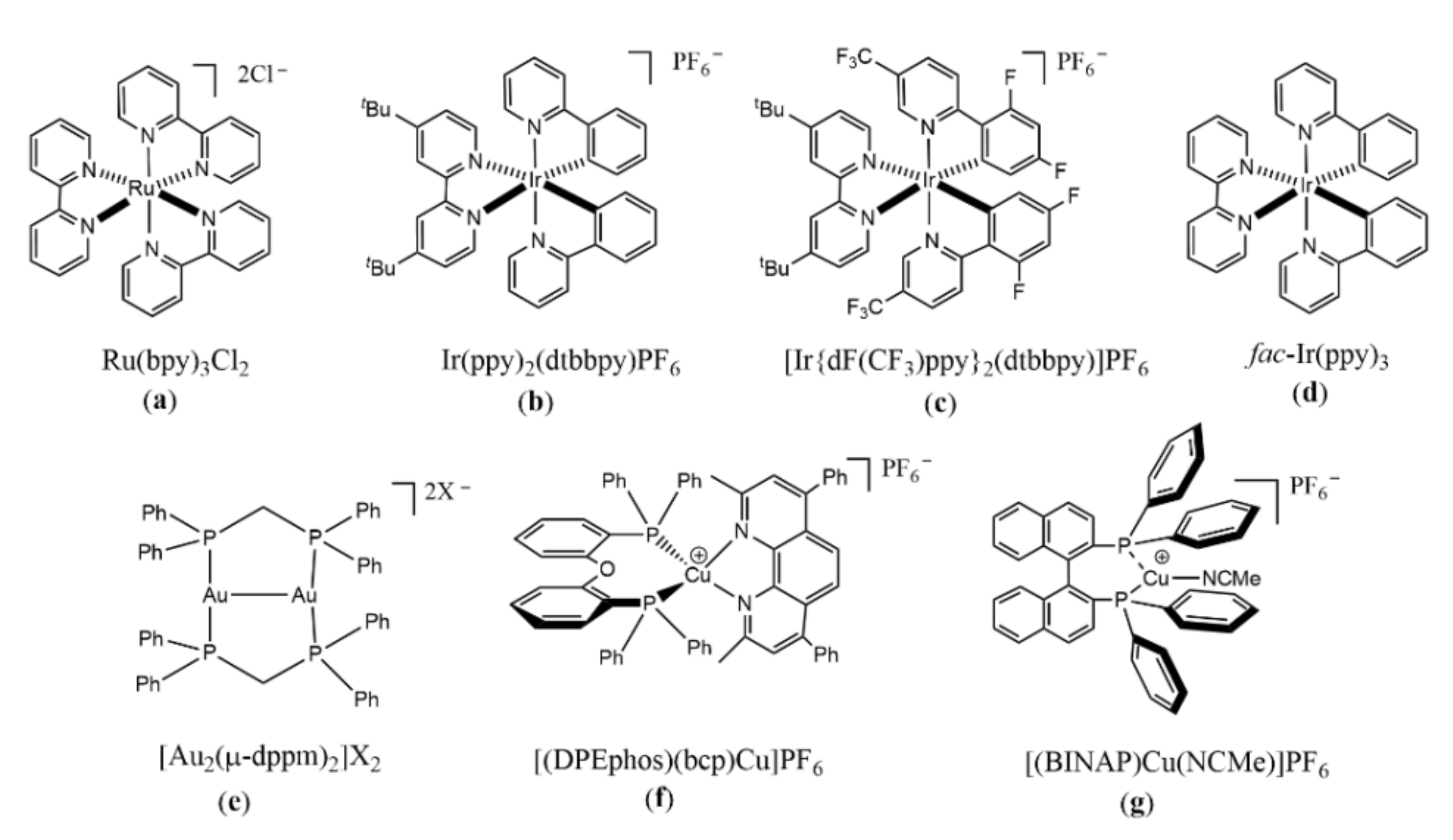
2. Visible-Light-Driven Reduction of Aryl Halides with Metal Complexes as Photocatalysts
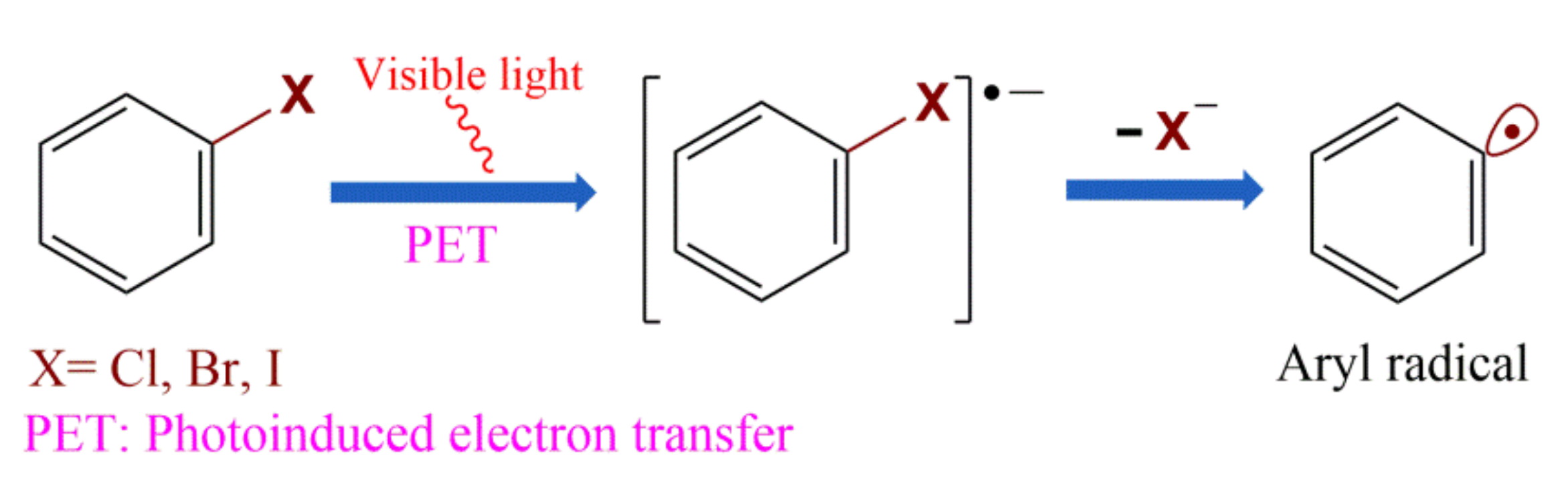
2.1. Conventional Photoinduced Electron Transfer
2.2. Sensitization-Initiated Electron Transfer
3. Visible-Light-Driven Reduction of Aryl Halides with Organic Complexes as Photocatalysts
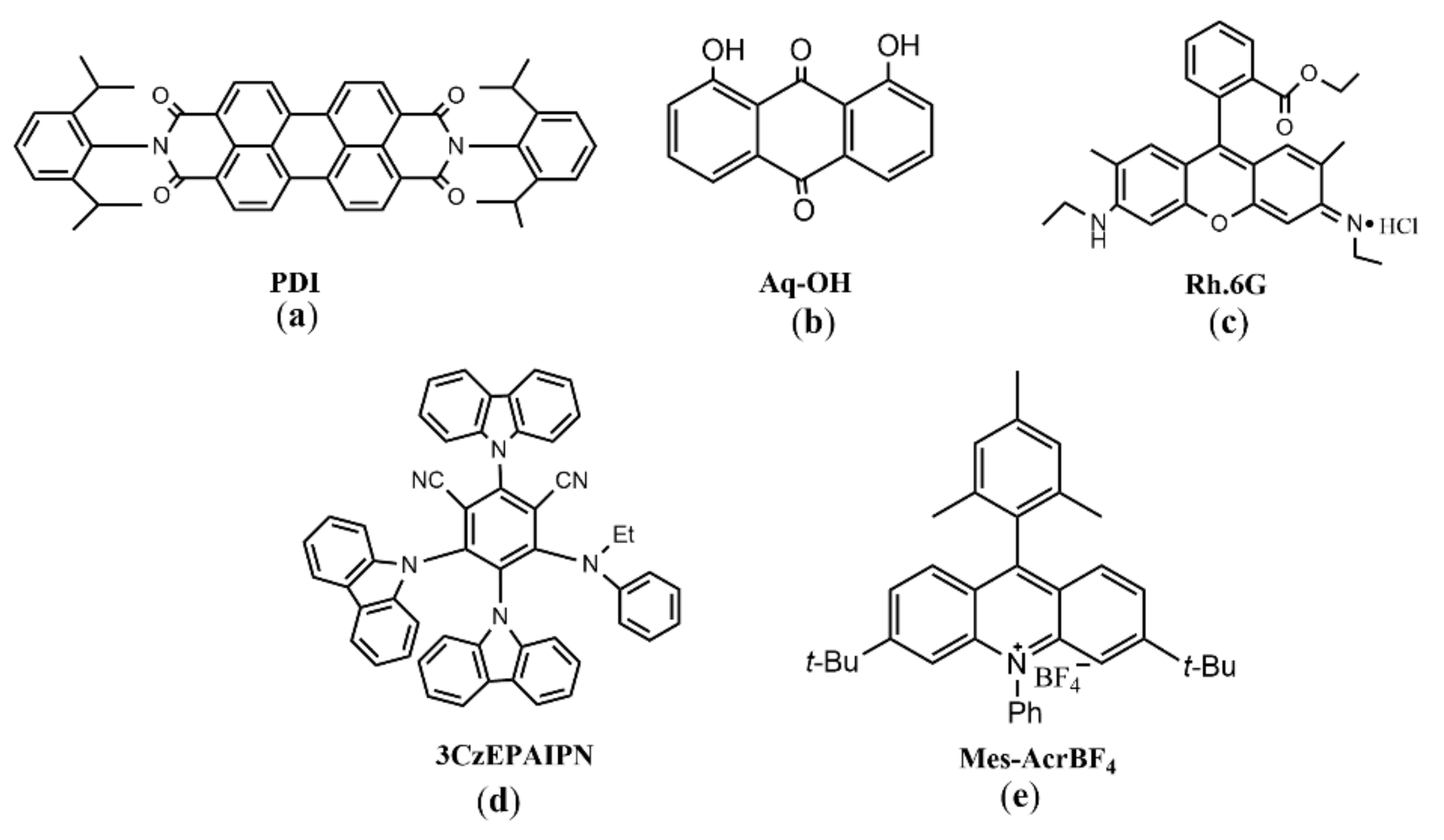
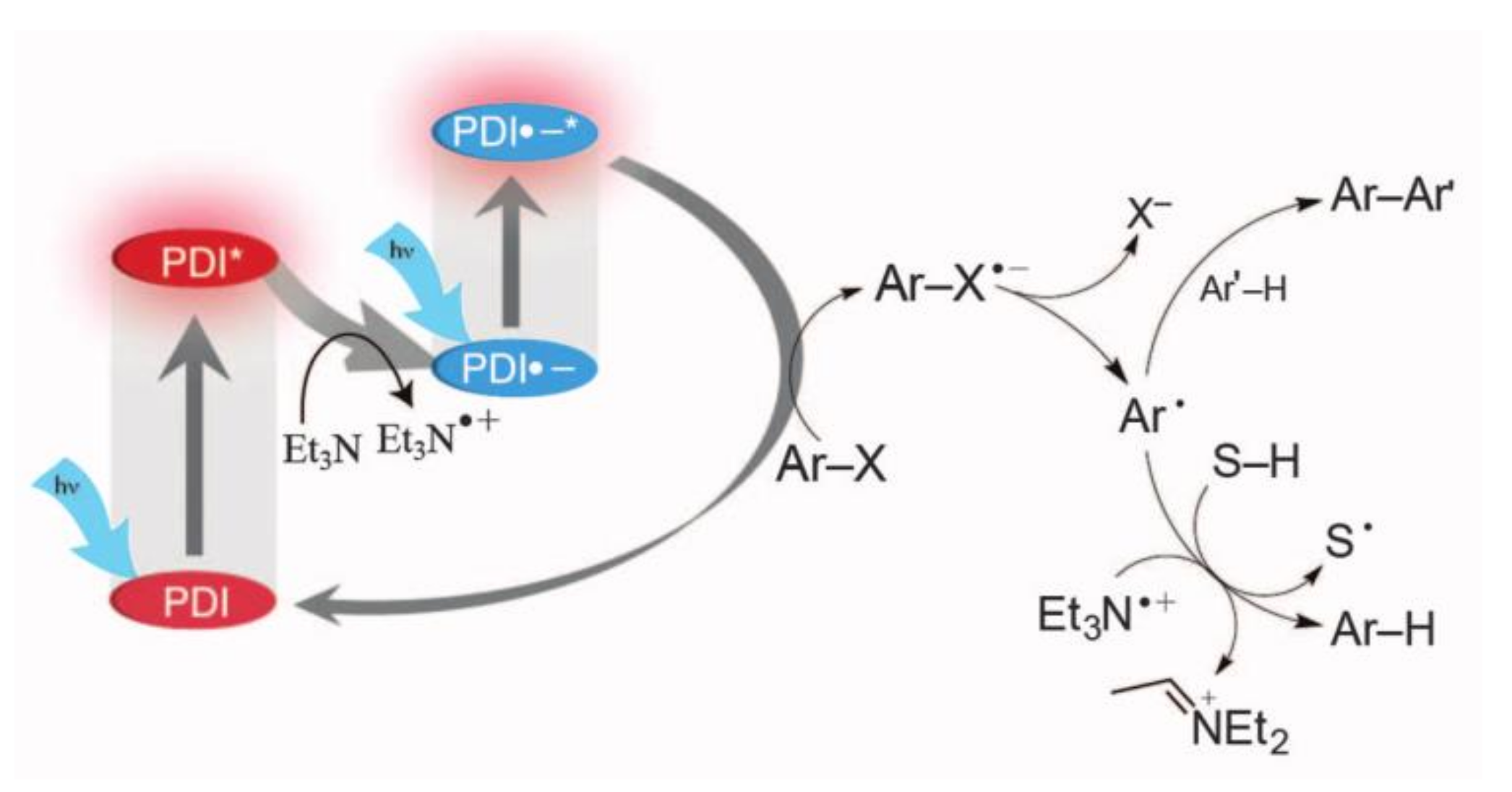
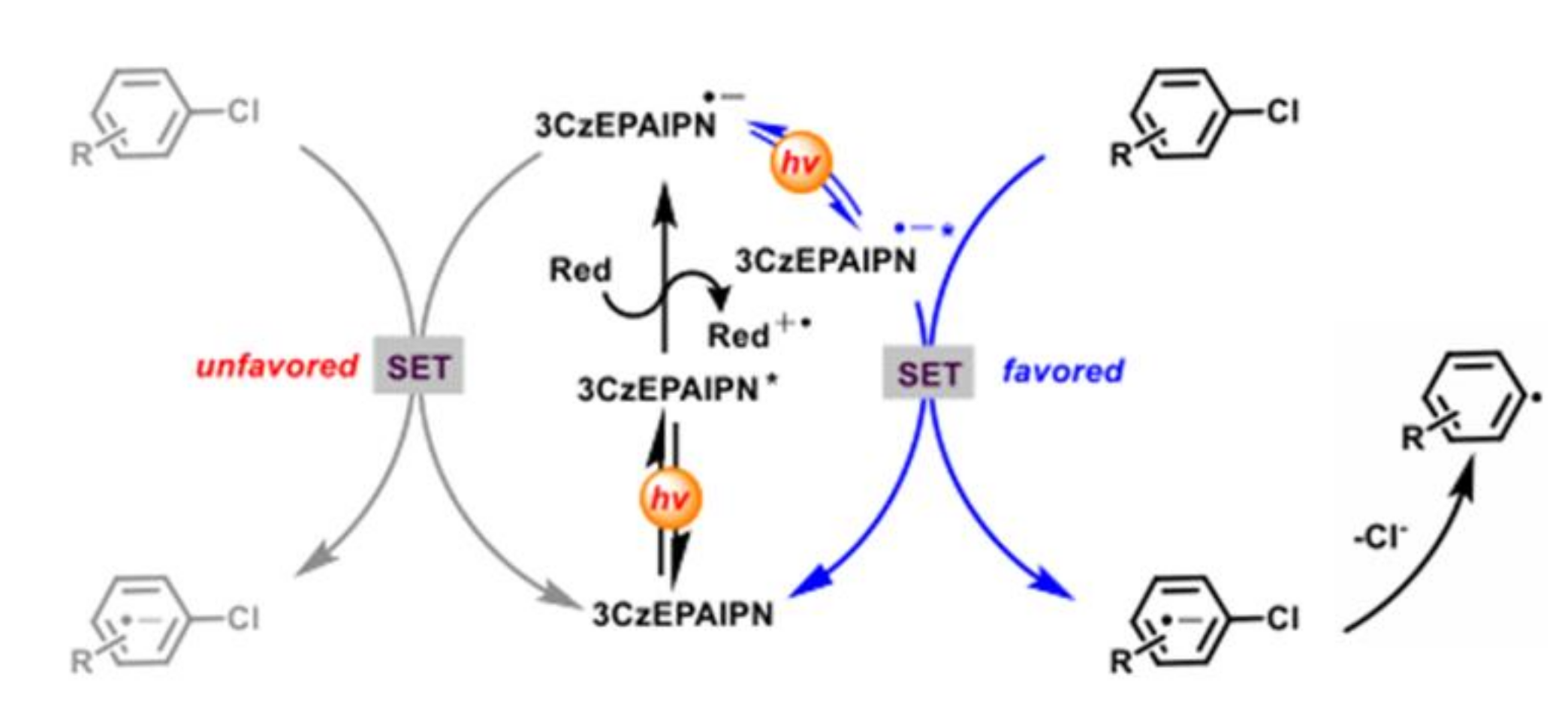
3.1. Single Photon Excitation
3.2. Two-Photon Excitation
4. Others
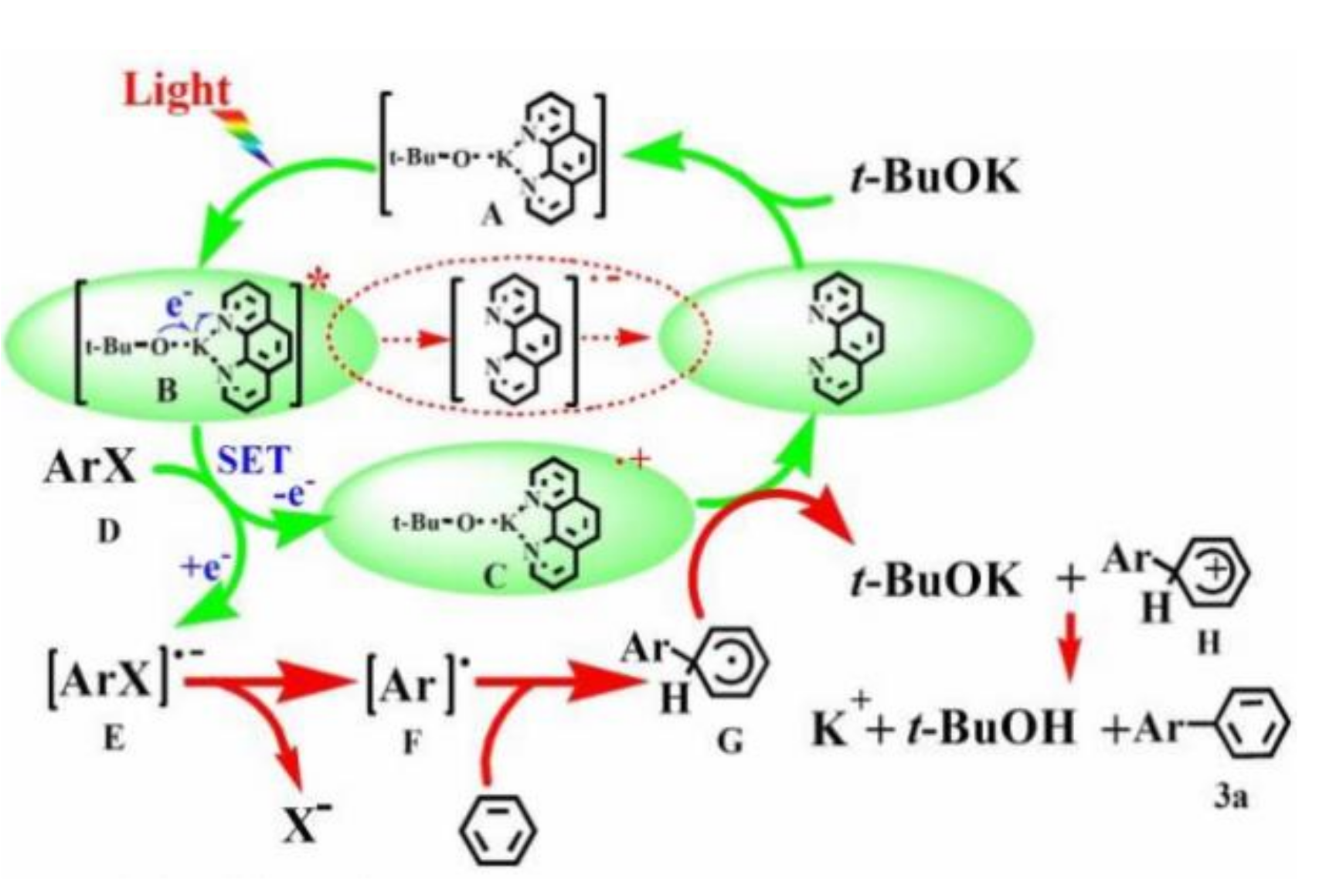
4.1. Visible Light-Induced, Base-Promoted Reduction of Aryl Halides
4.2. Visible-Light-Driven Reduction of Aryl Halides with Semiconductors as Photocatalysts
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinrich, M.; Gansäuer, A. Radicals in Synthesis III; Springer Science & Business Media: Heidelberg, Germany, 2012; Volume 320. [Google Scholar]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Cover Picture: Palladium-catalyzed cross-coupling reactions in total synthesis/metathesis reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4413. [Google Scholar] [CrossRef]
- Okamoto, K.; Zhang, J.; Housekeeper, J.B.; Marder, S.R.; Luscombe, C.K. C-H arylation reaction: Atom efficient and greener syntheses of π-conjugated small molecules and macromolecules for organic electronic materials. Macromolecules 2013, 46, 8059–8078. [Google Scholar] [CrossRef]
- Ackermann, L. (Ed.) Modern Arylation Methods; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Wang, C.S.; Dixneuf, P.H.; Soulé, J.F. Photoredox catalysis for building C-C bonds from C(sp2)–H bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef]
- Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Visible light mediated photoredox catalytic arylation reactions. Acc. Chem. Res. 2016, 49, 1566–1577. [Google Scholar] [CrossRef]
- Qiu, G.; Li, Y.; Wu, J. Recent developments for the photoinduced Ar–X bond dissociation reaction. Org. Chem. Front. 2016, 3, 1011–1027. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, Y.; Li, Y.; Hu, J.; Huang, D.; Bi, Y. Visible-light-mediated functionalization of aryl diazonium salts. Asian J. Org. Chem. 2021, 10, 453–463. [Google Scholar] [CrossRef]
- Kvasovs, N.; Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 2021, 50, 2244–2259. [Google Scholar] [CrossRef]
- Mo, F.; Qiu, D.; Zhang, L.; Wang, J. Recent development of aryl diazonium chemistry for the derivatization of aromatic compounds. Chem. Rev. 2021, 121, 5741–5829. [Google Scholar] [CrossRef]
- Guo, W.; Lu, L.Q.; Wang, Y.; Wang, Y.N.; Chen, J.R.; Xiao, W.J. Metal-free, room-temperature, radical alkoxycarbonylation of aryldiazonium salts through visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2015, 127, 2293–2297. [Google Scholar] [CrossRef]
- Zoller, J.; Fabry, D.C.; Rueping, M. Unexpected dual role of titanium dioxide in the visible light heterogeneous catalyzed C-H arylation of heteroarenes. ACS Catal. 2015, 5, 3900–3904. [Google Scholar] [CrossRef]
- Sharma, R.; Yadav, M.R. Recent developments in decarboxylative C(aryl)–X bond formation from (hetero) aryl carboxylic acids. Org. Biomol. Chem. 2021, 19, 5476–5500. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Liu, J. Acid-promoted metal-free protodeboronation of arylboronic acids. RSC Adv. 2017, 7, 34959–34962. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Tang, Y.; Gao, J.; Rao, G.; Mao, Z. Practical transition-metal-free protodeboronation of arylboronic acids in aqueous sodium hypochlorite. Synlett 2020, 31, 2039–2042. [Google Scholar] [CrossRef]
- Nguyen, J.D.; D’Amato, E.M.; Narayanam, J.M.R.; Stephenson, C.R.J. Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions. Nat. Chem. 2012, 4, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Ghosh, T.; Bardagi, J.I.; König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 2014, 346, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-L.; Song, L.; Yan, S.-S.; Ye, J.-H.; Yu, D.-G. Highly reductive photocatalytic systems in organic synthesis. Trends Chem. 2022, 4, 512–527. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Q.; Shao, Q.; Xia, C.; Wu, M. Photocatalytic C-F bond Activation of fluoroarenes, gem-difluoroalkenes and trifluoromethylarenes. Asian J. Org. Chem. 2021, 10, 2454–2472. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Zelewsky, A.V. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Boyington, A.J.; Riu, M.L.Y.; Jui, N.T. Anti-Markovnikov hydroarylation of unactivated olefins via pyridyl radical intermediates. J. Am. Chem. Soc. 2017, 139, 6582–6585. [Google Scholar] [CrossRef] [PubMed]
- Aycock, R.A.; Wang, H.; Jui, N.T. A mild catalytic system for radical conjugate addition of nitrogen heterocycles. Chem. Sci. 2017, 8, 3121–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devery, J.J.; Nguyen, J.D.; Dai, C.; Stephenson, C.R. Light-mediated reductive debromination of unactivated alkyl and aryl bromides. ACS Catal. 2016, 6, 5962–5967. [Google Scholar] [CrossRef]
- Revol, G.; McCallum, T.; Morin, M.; Gagosz, F.; Barriault, L. Photoredox transformations with dimeric gold complexes. Angew. Chem. Int. Ed. 2013, 52, 13342–13345. [Google Scholar] [CrossRef]
- Wu, W.; Cui, E.; Zhang, Y.; Zhang, C.; Zhu, F.; Tung, C.-H.; Wang, Y. Involving single-atom silver (0) in selective dehalogenation by AgF under visible-light irradiation. ACS Catal. 2019, 9, 6335–6341. [Google Scholar] [CrossRef]
- Cui, E.; Li, H.; Zhang, C.; Qiao, D.; Gawande, M.B.; Tung, C.-H.; Wang, Y. An advanced plasmonic photocatalyst containing silver(0) single atoms for selective borylation of aryl iodides. Appl. Catal. B Environ. 2021, 299, 120674. [Google Scholar] [CrossRef]
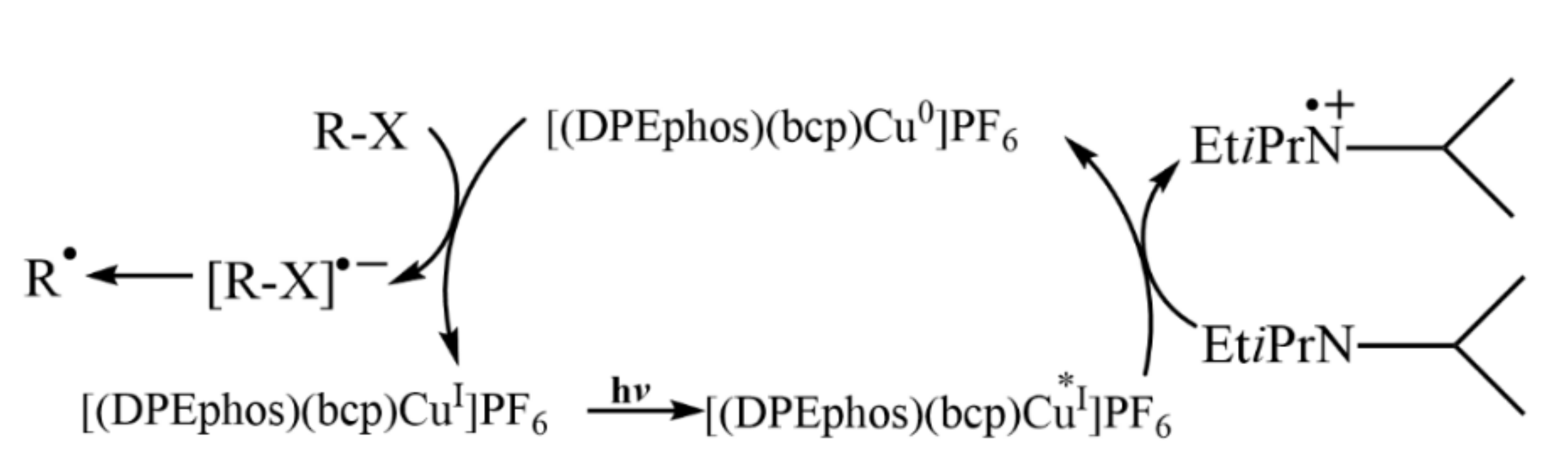
- Michelet, B.; Deldaele, C.; Kajouj, S.; Moucheron, C.; Evano, G. A general copper catalyst for photoredox transformations of organic halides. Org. Lett. 2017, 19, 3576–3579. [Google Scholar] [CrossRef] [Green Version]
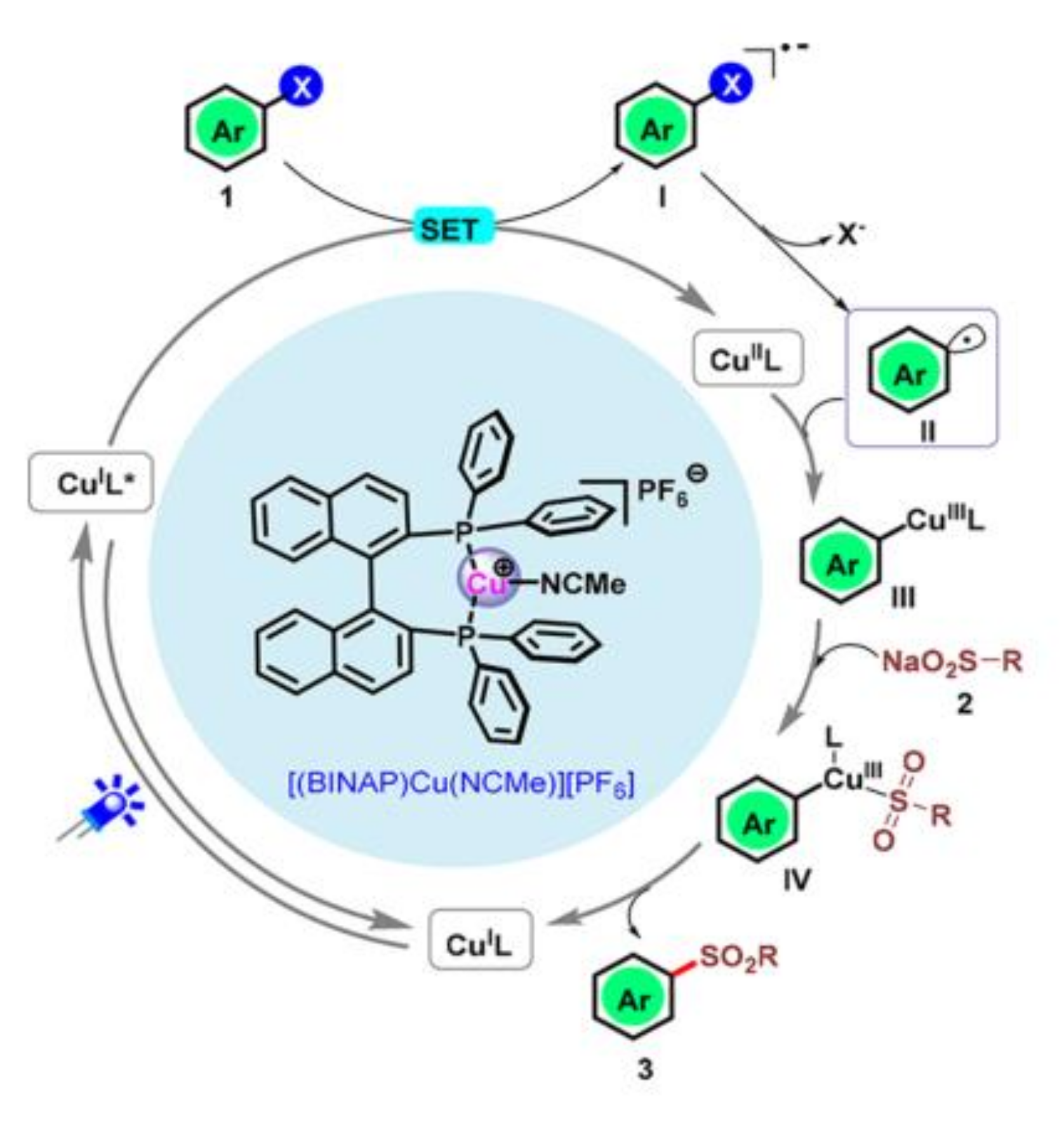
- Yan, Q.; Cui, W.; Song, X.; Xu, G.; Jiang, M.; Sun, K.; Lv, J.; Yang, D. Sulfonylation of aryl halides by visible light/copper catalysis. Org. Lett. 2021, 23, 3663–3668. [Google Scholar] [CrossRef]
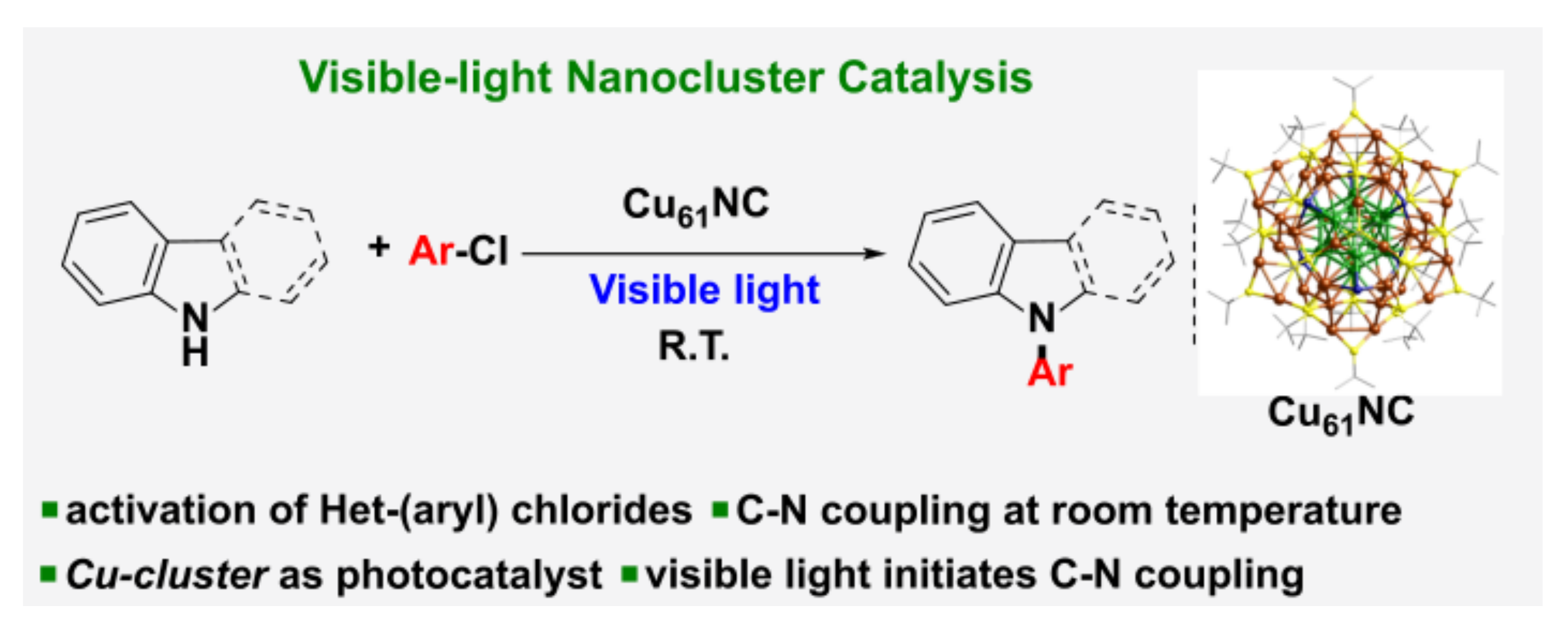
- Sagadevan, A.; Ghosh, A.; Maity, P.; Mohammed, O.F.; Bakr, O.M.; Rueping, M. Visible-light copper nanocluster catalysis for the C-N coupling of aryl chlorides at room temperature. J. Am. Chem. Soc. 2022, 144, 12052–12061. [Google Scholar] [CrossRef]
- Cybularczyk-Cecotka, M.; Szczepanik, J.; Giedyk, M. Photocatalytic strategies for the activation of organic chlorides. Nat. Catal. 2020, 3, 872–886. [Google Scholar] [CrossRef]
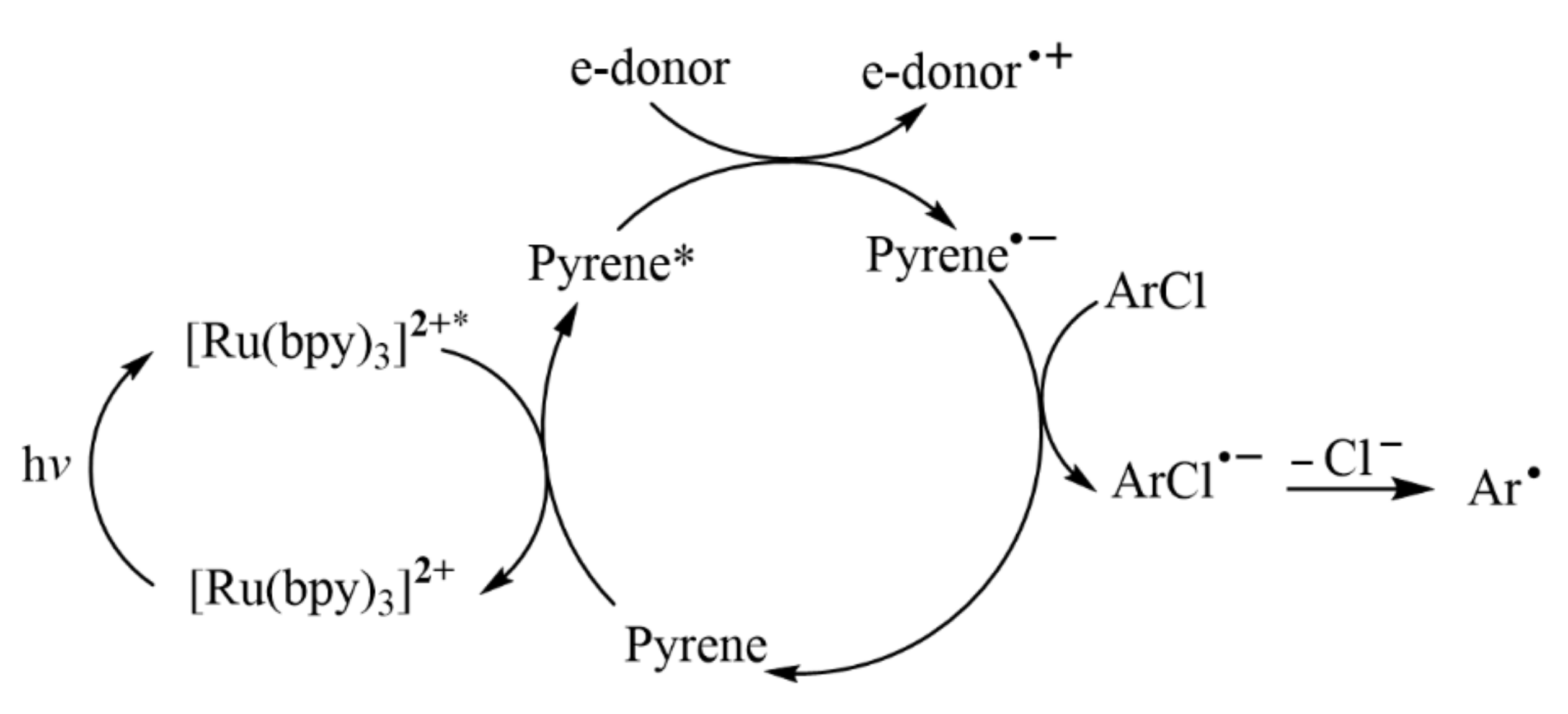
- Shiozuka, A.; Sekine, K.; Kuninobu, Y. Photoinduced organic reactions by employing pyrene catalysts. Synthesis 2022, 54, 2330–2339. [Google Scholar]
- Ghosh, I. Excited radical anions and excited anions in visible light photoredox catalysis. Phys. Sci. Rev. 2019, 4, 20170185. [Google Scholar] [CrossRef]
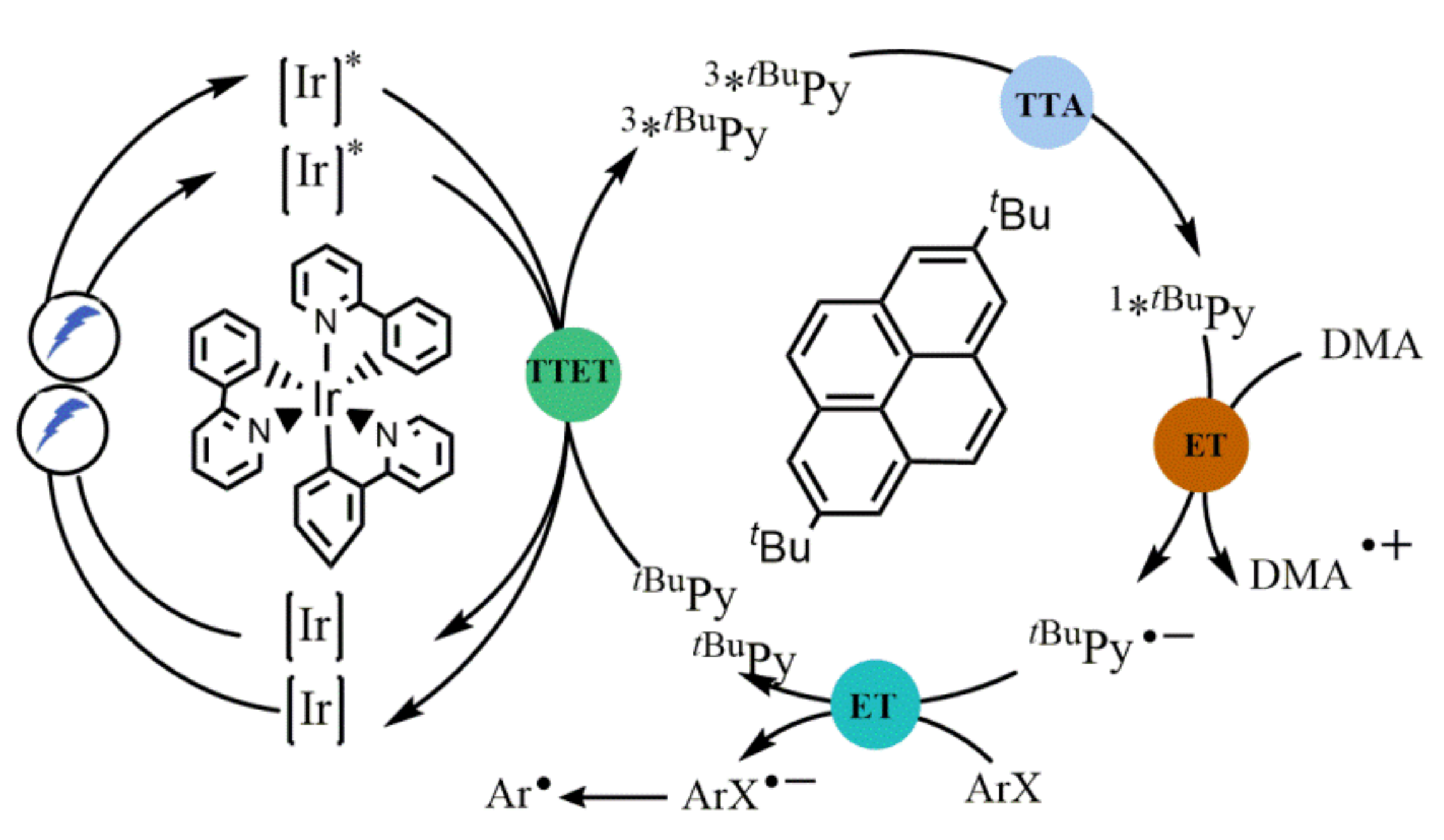
- Ghosh, I.; Shaikh, R.S.; König, B. Sensitization-initiated electron transfer for photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 8584. [Google Scholar] [CrossRef]
- Marchini, M.; Bergamini, G.; Cozzi, P.G.; Ceroni, P.; Balzani, V. Photoredox catalysis: The need to elucidate the photochemical mechanism. Angew. Chem. Int. Ed. 2017, 56, 12820–12821. [Google Scholar] [CrossRef]
- Coles, M.S.; Quach, G.; Beves, J.E.; Moore, E.G. A photophysical study of sensitization-initiated electron transfer: Insights into the mechanism of photoredox activity. Angew. Chem. Int. Ed. 2020, 59, 9522–9526. [Google Scholar] [CrossRef]
- Glaser, F.; Kerzig, C.; Wenger, O.S. Sensitization-initiated electron transfer via upconversion: Mechanism and photocatalytic applications. Chem. Sci. 2021, 12, 9922–9933. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Du, Y.; Pearson, R.M.; Lim, C.-H.; Sartor, S.M.; Ryan, M.D.; Yang, H.; Damrauer, N.H.; Miyake, G.M. Strongly reducing, visible-light organic photoredox catalysts as sustainable alternatives to precious metals. Chem.-Eur. J. 2017, 23, 10962–10968. [Google Scholar] [CrossRef]
- Speckmeier, E.; Fischer, T.G.; Zeitler, K. A toolbox approach to construct broadly applicable metal-free catalysts for photoredox chemistry: Deliberate tuning of redox potentials and importance of halogens in donor–acceptor cyanoarenes. J. Am. Chem. Soc. 2018, 140, 15353–15365. [Google Scholar] [CrossRef]
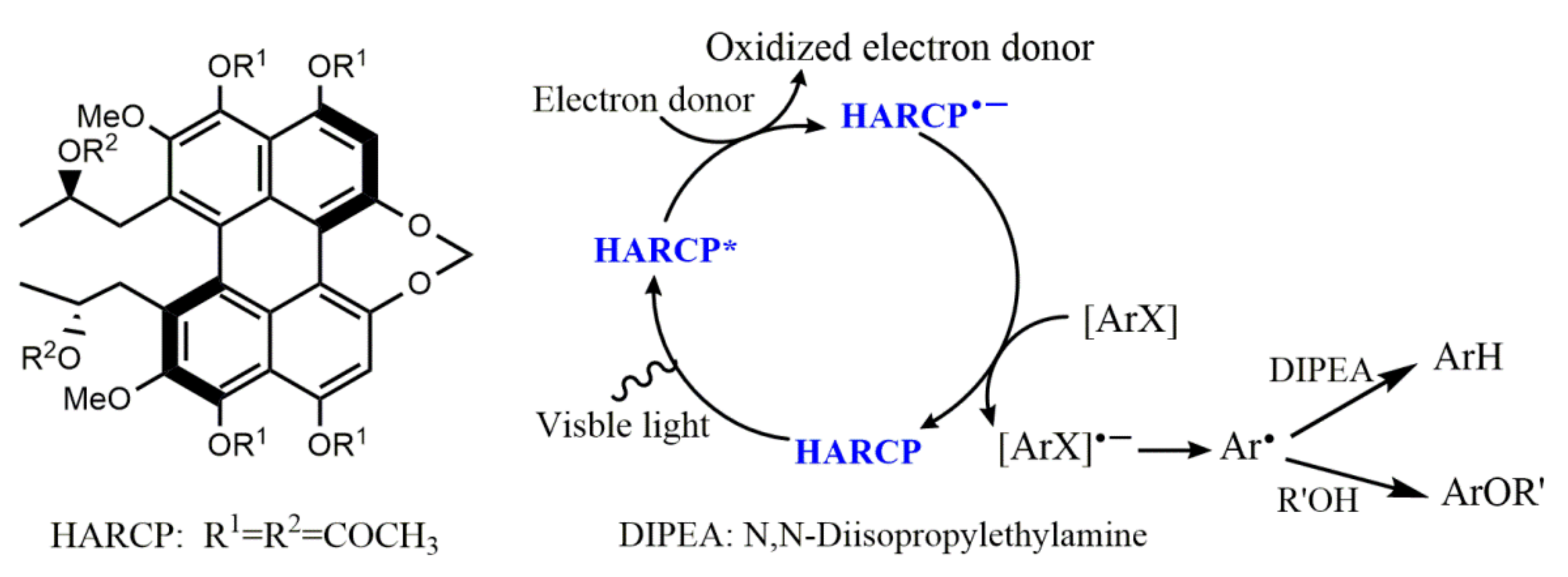
- Li, M.; Li, J.; Guo, B.; Liu, X.; Yuan, Z.; Wu, Y.; Yin, H.; Huang, S.; Zhang, Y.; Rao, Y. Discovery and characterization of a novel perylenephotoreductant for the activation of aryl halides. J. Catal. 2021, 399, 111–120. [Google Scholar] [CrossRef]
- Ou, W.; Zou, R.; Han, M.; Su, C. Tailorable carbazolyl cyanobenzene-based photocatalysts for visible light-induced reduction of aryl halides. Chin. Chem. Lett. 2020, 31, 1899–1902. [Google Scholar] [CrossRef]
- Chen, K.-Q.; Zhang, B.-B.; Wang, Z.-X.; Chen, X.-Y. N-heterocyclic nitreniums can be employed as photoredox catalysts for the single-electron reduction of aryl halides. Org. Lett. 2022, 24, 4598–4602. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Li, T.; Li, N.; Zhang, Y.; Shen, L.; Ma, Z.; Xia, C. Redox-neutral photochemical Heck-type arylation of vinylphenols activated by visible light. Chem. Sci. 2020, 11, 2130–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyington, A.J.; Seath, C.P.; Zearfoss, A.M.; Xu, Z.; Jui, N.T. Catalytic strategy for regioselective arylethylamine synthesis. J. Am. Chem. Soc. 2019, 141, 4147–4153. [Google Scholar] [CrossRef]
- Schmalzbauer, M.; Ghosh, I.; König, B. Utilising excited state organic anions for photoredox catalysis: Activation of (hetero) aryl chlorides by visible light-absorbing 9-anthrolate anions. Faraday Discuss. 2019, 215, 364–378. [Google Scholar] [CrossRef]
- Schmalzbauer, M.; Svejstrup, T.D.; Fricke, F.; Brandt, P.; Johansson, M.J.; Bergonzini, G.; König, B. Redox-neutral photocatalytic C-H carboxylation of arenes and styrenes with CO2. Chem 2020, 6, 2658–2672. [Google Scholar] [CrossRef]
- Broggi, J.; Terme, T.; Vanelle, P. Organic electron donors as powerful single-electron reducing agents in organic synthesis. Angew. Chem. Int. Ed. 2014, 53, 384–413. [Google Scholar] [CrossRef]
- Discekici, E.H.; Treat, N.J.; Poelma, S.O.; Mattson, K.M.; Hudson, Z.M.; Luo, Y.; Hawker, C.J.; Alaniz, J.R. A highly reducing metal-free photoredox catalyst: Design and application in radical dehalogenations. Chem. Commun. 2015, 51, 11705–11708. [Google Scholar] [CrossRef] [Green Version]
- Arias-Rotondo, D.M.; McCuske, J.K. The photophysics of photoredox catalysis: A roadmap for catalyst design. Chem. Soc. Rev. 2016, 45, 5803–5820. [Google Scholar] [CrossRef]
- McCarthy, B.G.; Pearson, R.M.; Lim, C.H.; Sartor, S.M.; Damrauer, N.H.; Miyake, G.M. Structure–property relationships for tailoring phenoxazines as reducing photoredox catalysts. J. Am. Chem. Soc. 2018, 140, 5088–5101. [Google Scholar] [CrossRef]
- Büldt, L.A.; Wenger, O.S. Chromium(0), molybdenum(0), and tungsten(0) isocyanide complexes as luminophores and photosensitizers with long-lived excited states. Angew. Chem. Int. Ed. 2017, 56, 5676–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkins, S.B.; Peters, J.C. A highly emissive Cu2N2 diamond core complex supported by a [PNP]-ligand. J. Am. Chem. Soc. 2005, 127, 2030–2031. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lambert, T.H.; Lin, S. Reductive electrophotocatalysis: Merging electricity and light to achieve extreme reduction potentials. J. Am. Chem. Soc. 2020, 142, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Cowper, N.G.; Chernowsky, C.P.; Williams, O.P.; Wickens, Z.K. Potent reductants via electron-primed photoredox catalysis: Unlocking aryl chlorides for radical coupling. J. Am. Chem. Soc. 2020, 142, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Glaser, F.; Larsen, C.B.; Kerzig, C.; Wenger, O.S. Aryl dechlorination and defluorination with an organic super-photoreductant. Photochem. Photobiol. Sci. 2020, 19, 1035–1041. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, L. Visible-light-induced organocatalytic borylation of aryl chlorides. J. Am. Chem. Soc. 2019, 141, 9124–9128. [Google Scholar] [CrossRef]
- Yin, H.; Jin, Y.; Hertzog, J.E.; Mullane, K.C.; Carroll, P.J.; Manor, B.C.; Anna, J.M.; Schelter, E.J. The hexachlorocerate(III) anion: A potent, benchtop stable, and readily available ultraviolet a photosensitizer for aryl chlorides. J. Am. Chem. Soc. 2016, 138, 16266–16273. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Ravelli, D.; Protti, S.; Fagnoni, M.; Albini, A. Photochemical synthesis: Using light to build C-C bonds under mild conditions. Comptes Rendus Chim. 2017, 20, 261–271. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J.; Chen, Z.; Chen, J.; Yan, M.; Zhang, X. A convenient synthesis of sulfones via light promoted coupling of sodium sulfinates and aryl halides. Adv. Synth. Catal. 2019, 361, 956–960. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, T.; He, C.; Shi, D.; Zhang, F.; Duan, C. Organized aggregation makes insoluble perylene diimide efficient for the reduction of aryl halides via consecutive visible light-induced electron-transfer processes. J. Am. Chem. Soc. 2016, 138, 3958–3961. [Google Scholar] [CrossRef]
- Neumeier, M.; Sampedro, D.; Májek, M.; de la Peña O’Shea, V.A.; Jacobi von Wangelin, A.; Pérez-Ruiz, R. Dichromatic photocatalytic substitutions of aryl halides with a small organic dye. Chem.-Eur. J. 2018, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Bardagi, J.I.; Ghosh, I.; Schmalzbauer, M.; Ghosh, T.; König, B. Anthraquinones as photoredox catalysts for the reductive activation of aryl halides. Eur. J. Org. Chem. 2018, 2018, 34–40. [Google Scholar] [CrossRef]
- Shaikh, R.S.; Düsel, S.J.S.; König, B. Visible-light photo-arbuzov reaction of aryl bromides and trialkyl phosphites yielding aryl phosphonates. ACS Catal. 2016, 6, 8410–8414. [Google Scholar] [CrossRef]
- Xu, J.; Cao, J.; Wu, X.; Wang, H.; Yang, X.; Tang, X.; Toh, R.W.; Zhou, R.; Yeow, E.K.L.; Wu, J. Unveiling extreme photoreduction potentials of donor–acceptor cyanoarenes to access aryl radicals from aryl chlorides. J. Am. Chem. Soc. 2021, 143, 13266–13273. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.A.; Wang, L.; Onuska, N.P.R.; Williams, O.F.; Begam, K.; Moran, A.M.; Dunietz, B.D.; Nicewicz, D.A. Discovery and characterization of an acridine radical photoreductant. Nature 2020, 580, 76–80. [Google Scholar] [CrossRef]
- Hammarström, L. Accumulative charge separation for solar fuels production: Coupling light-induced single electron transfer to multielectron catalysis. Acc. Chem. Res. 2015, 48, 840–850. [Google Scholar] [CrossRef]
- Budén, M.E.; Guastavino, J.F.; Rossi, R.A. Room-temperature photoinduced direct C-H-arylation via base-promoted homolytic aromatic substitution. Org. Lett. 2013, 15, 1174–1177. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, L.; Wang, L.; Gong, M.; Wang, W.; Yuan, R. Visible light photoredox catalyzed biaryl synthesis using nitrogen heterocycles as promoter. ACS Catal. 2015, 5, 45–50. [Google Scholar] [CrossRef]
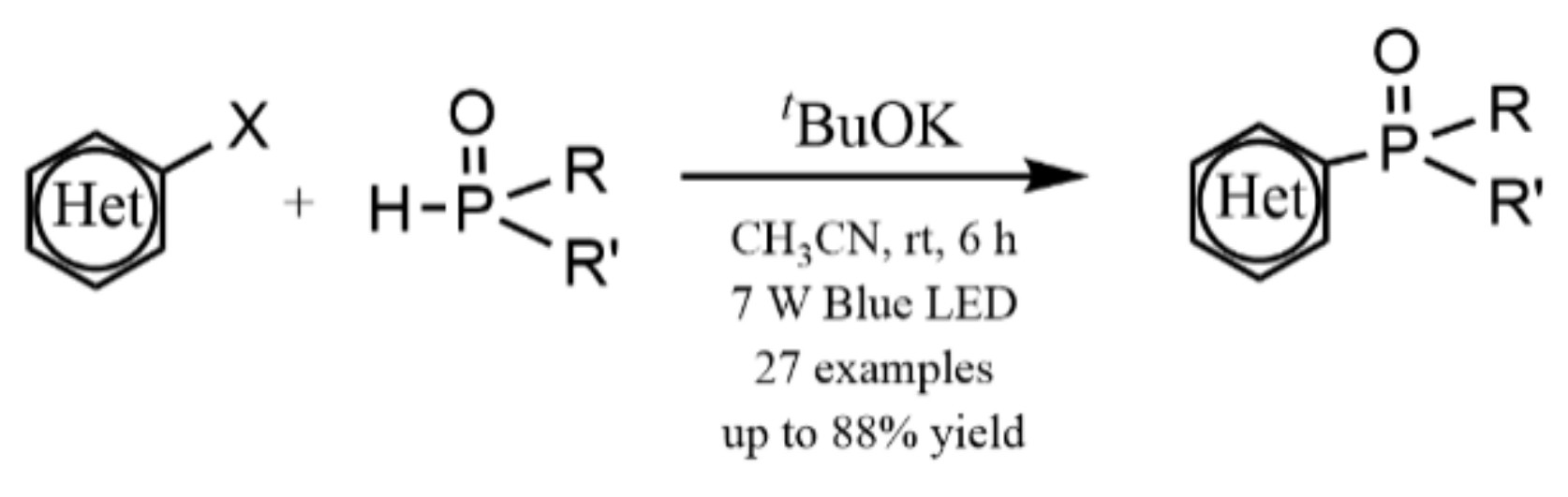
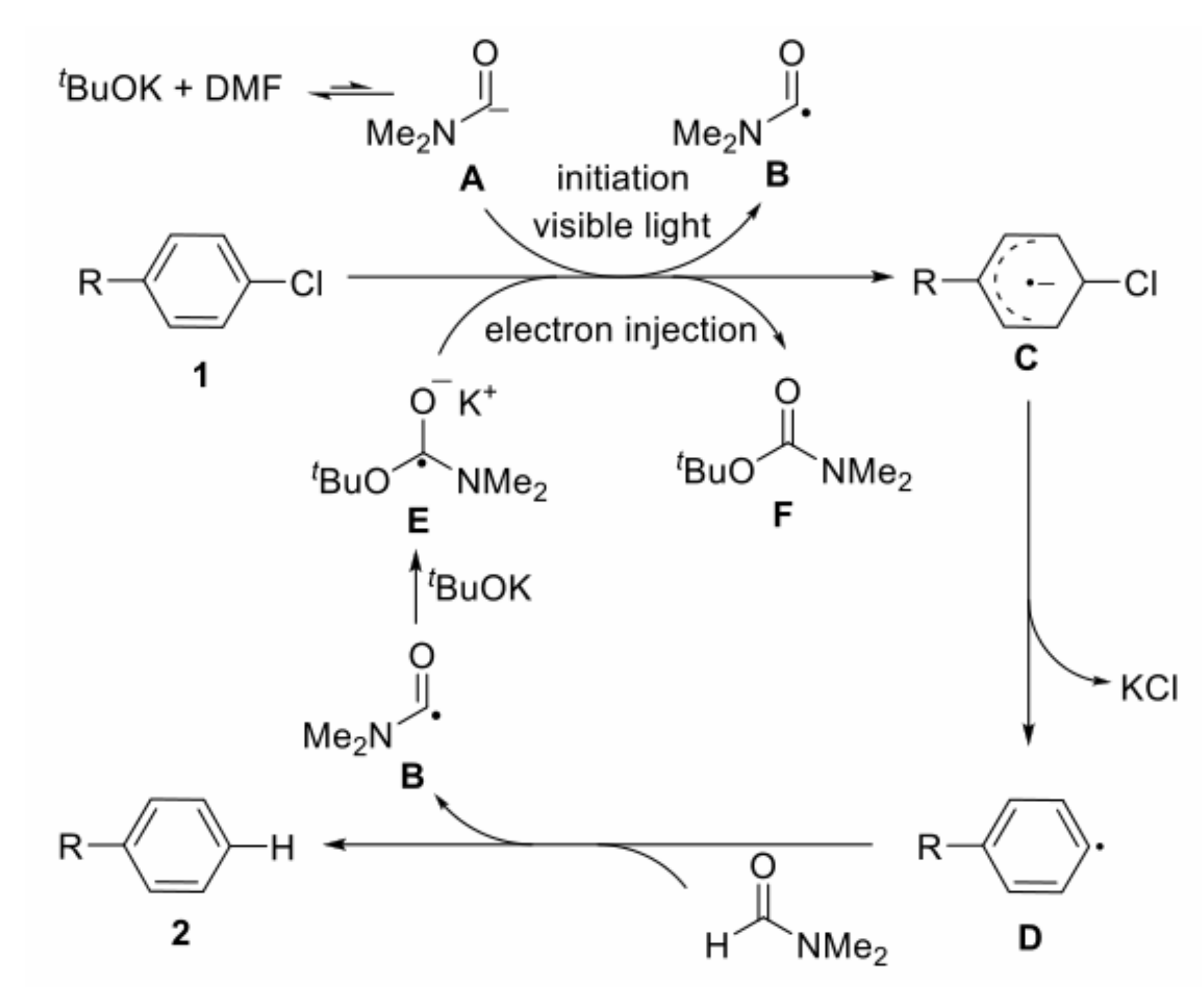
- Yuan, J.; To, W.-P.; Zhang, Z.-Y.; Yue, C.-D.; Meng, S.; Chen, J.; Liu, Y.; Yu, G.-A.; Chen, C.-M. Visible-light-promoted transition-metal-free phosphinylation of heteroaryl halides in the presence of potassium tert-butoxide. Org. Lett. 2018, 20, 7816–7820. [Google Scholar] [CrossRef]
- Sun, C.L.; Shi, Z.J. Transition-metal-free coupling reactions. Chem. Rev. 2014, 114, 9219–9280. [Google Scholar] [CrossRef]
- Rathore, V.; Sattar, M.; Kumar, R.; Kumar, S. Synthesis of unsymmetrical diaryl acetamides, benzofurans, benzophenones, and xanthenes by transition-metal-free oxidative cross-coupling of sp3 and sp2 C-H Bonds. J. Org. Chem. 2016, 81, 9206–9218. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, S.; Zhang, J.; Oswald, V.F.; Amassian, A.; Marder, S.R.; Blakey, S.B. KOtBu-initiated aryl C-H iodination: A powerful tool for the synthesis of high electron affinity compounds. J. Am. Chem. Soc. 2016, 138, 3946–3949. [Google Scholar] [CrossRef] [PubMed]
- Cismesia, M.A.; Yoon, T.P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 2015, 6, 5426–5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barham, J.P.; Coulthard, G.; Emery, K.J.; Doni, E.; Cumine, F.; Nocera, G.; John, M.P.; Berlouis, L.E.A.; McGuire, T.; Tuttle, T.; et al. KOtBu: A privileged reagent for electron transfer reactions? J. Am. Chem. Soc. 2016, 138, 7402–7410. [Google Scholar] [CrossRef] [Green Version]
- Buden, M.E.; Bardagí, J.I.; Puiatti, M.; Rossi, R.A. Initiation in photoredox C-H functionalization reactions. Is dimsyl anion a key ingredient? J. Org. Chem. 2017, 82, 8325–8333. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.-H.; Qu, J.-P.; Kang, Y.-B. Visible-light-induced, base-promoted transition-metal-free dehalogenation of aryl fluorides, chlorides, bromides, and iodides. Org. Lett. 2020, 22, 3084–3088. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor photocatalysis—Mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, I.; Sapra, S.; König, B. Quantum dots in visible-light photoredox catalysis: Reductive dehalogenations and C-H arylation reactions using aryl bromides. Chem. Mater. 2017, 29, 5225–5231. [Google Scholar] [CrossRef]
- Han, G.; Sun, Y. Visible-light-driven organic transformations on semiconductors. Mater. Today Phys. 2021, 16, 100297. [Google Scholar] [CrossRef]
- Petroff II, J.T.; Nguyen, A.H.; Porter, A.J.; Morales, F.D.; Kennedy, M.P.; Weinstein, D.; Nazer, H.E.; McCulla, R.D. Enhanced photocatalytic dehalogenation of aryl halides by combined poly-p-phenylene (PPP) and TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2017, 335, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Magano, A.; Salaverri, N.; Marzo, L.; Mas-Ballesté, R.; Alemán, J. Synergistic Effect on Covalent Triazine Framework Tailored for Heter Ogeneous Photocatalytic Metal-Free C-Br and C-Cl Activation. Available online: https://ssrn.com/abstract=4137490 (accessed on 3 August 2022).
- Yuan, T.; Zheng, M.; Antonietti, M.; Wang, X. Ceramic boron carbonitrides for unlocking organic halides with visible light. Chem. Sci. 2021, 12, 6323–6332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, L. Silver nanoparticles doped TiO2 catalyzed Suzuki-coupling of bromoaryl with phenylboronic acid under visible light. J. Photochem. Photobiol. B Biol. 2020, 205, 111807. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Sarvari, M.; Bazyar, Z. Visible light driven photocatalytic cross-coupling reactions on nano Pd/ZnO photocatalyst at room-temperature. ChemistrySelect 2018, 3, 1898–1907. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Pitre, S.P.; Ismaili, H.; Scaiano, J.C. Heterogeneous light-mediated reductive dehalogenations and cyclizations utilizing platinum nanoparticles on titania (PtNP@TiO2). Adv. Synth. Catal. 2014, 356, 2819–2824. [Google Scholar] [CrossRef]
- Kuivila, H.G. Organotin hydrides and organic free radicals. Acc. Chem. Res. 1968, 1, 299–305. [Google Scholar] [CrossRef]
- Rosa, A.M.; Lobo, A.M.; Branco, P.S.; Prabhakar, S. The chemistry and reactivity of aryl radicals-the C-C bond formation from o-bromobenzylphenylethers with tin hydride and azobisisobutyronitrile. Tetrahedron 1997, 53, 285–298. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Song, W.; Chen, C.; Zhao, J. Photocatalytic hydrodehalogenation for the removal of halogenated aromatic contaminants. ChemCatChem 2019, 11, 258–268. [Google Scholar] [CrossRef]
- Marina, N.; Lanterna, A.E.; Scaiano, J.C. Expanding the color space in the two-color heterogeneous photocatalysis of ullmann C-C coupling reactions. ACS Catal. 2018, 8, 7593–7597. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Bo, A.; Jia, J.; Liu, H.; Arnold, D.P.; Huang, Y.; Wu, H.; Zhu, H. Visible light-driven cross-coupling reactions at lower temperatures using a photocatalyst of palladium and gold alloy nanoparticles. ACS Catal. 2014, 4, 1725–1734. [Google Scholar] [CrossRef]
- Vijeta, A.; Casadevall, C.; Reisner, E. An integrated carbon nitride-nickel photocatalyst for the amination of aryl halides using sodium azide. Angew. Chem. Int. Ed. 2022, 61, e202203176. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Chen, R.; Duo, F.; Hu, M.; Lu, X. Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals. Molecules 2022, 27, 5364. https://doi.org/10.3390/molecules27175364
Lan J, Chen R, Duo F, Hu M, Lu X. Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals. Molecules. 2022; 27(17):5364. https://doi.org/10.3390/molecules27175364
Chicago/Turabian StyleLan, Jihong, Rongxiang Chen, Fangfang Duo, Menghui Hu, and Xiaoyan Lu. 2022. "Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals" Molecules 27, no. 17: 5364. https://doi.org/10.3390/molecules27175364
APA StyleLan, J., Chen, R., Duo, F., Hu, M., & Lu, X. (2022). Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals. Molecules, 27(17), 5364. https://doi.org/10.3390/molecules27175364





