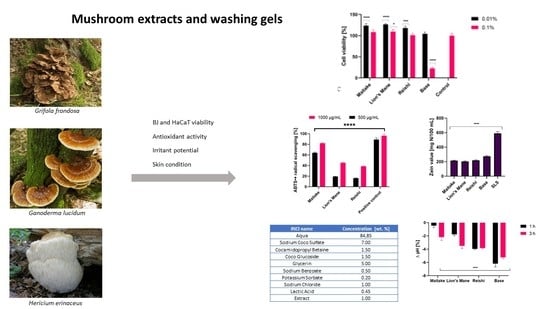
Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Biologically Active Compounds
2.1.1. Determination of Bioactive Compounds by HPLC-ESI/TOF
2.1.2. Total Phenolic Compounds and Flavonoids Content
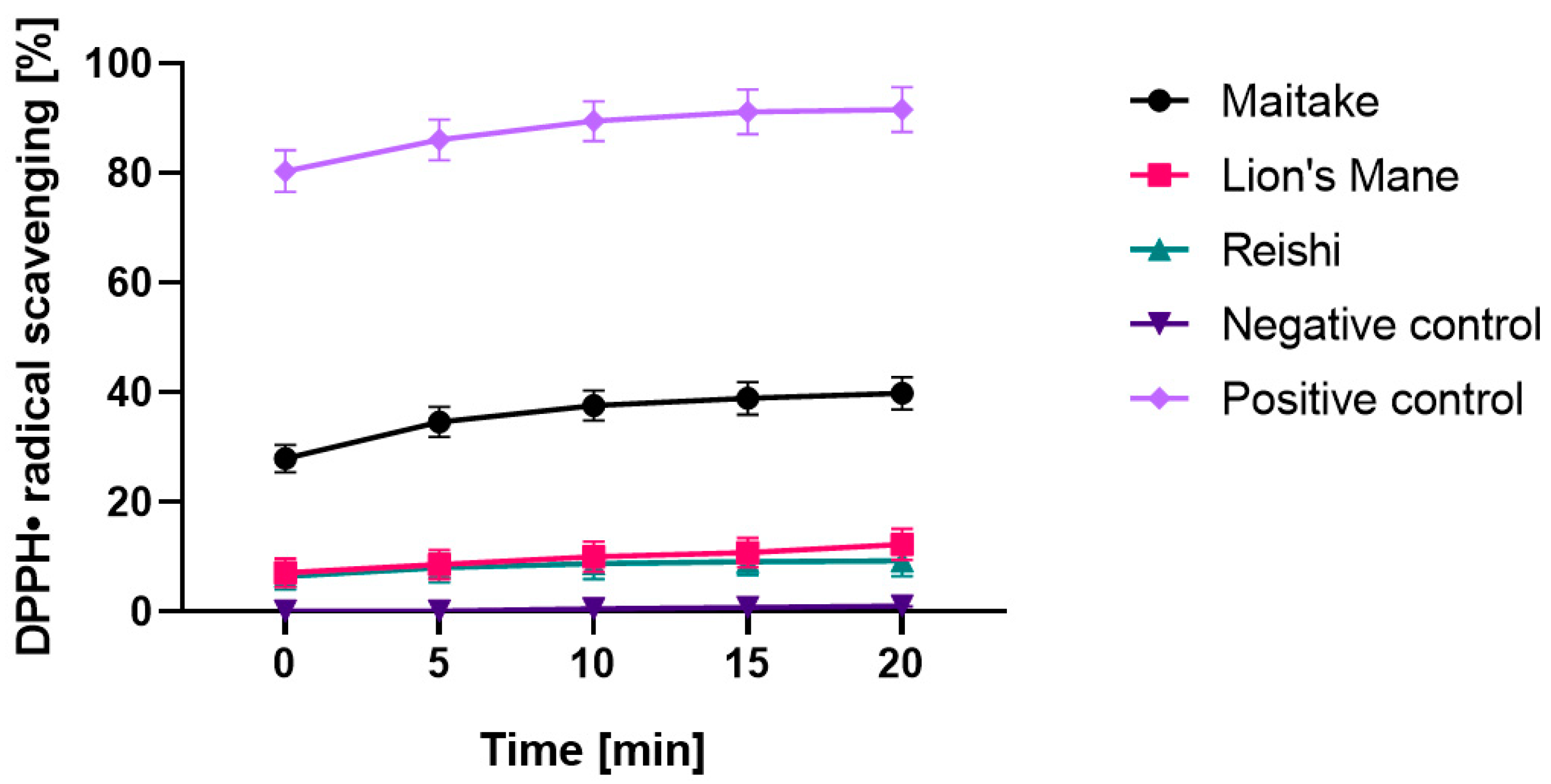
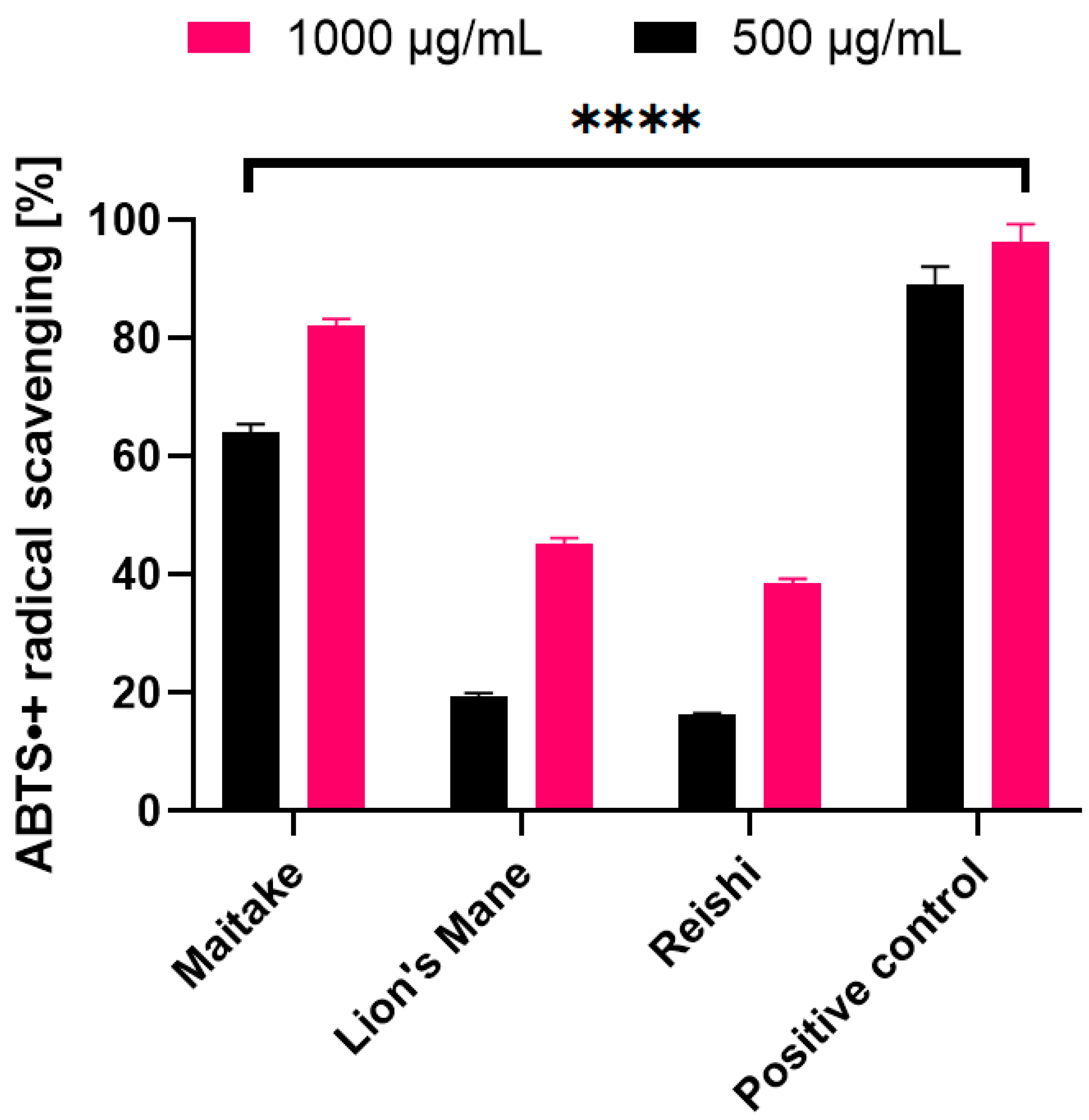
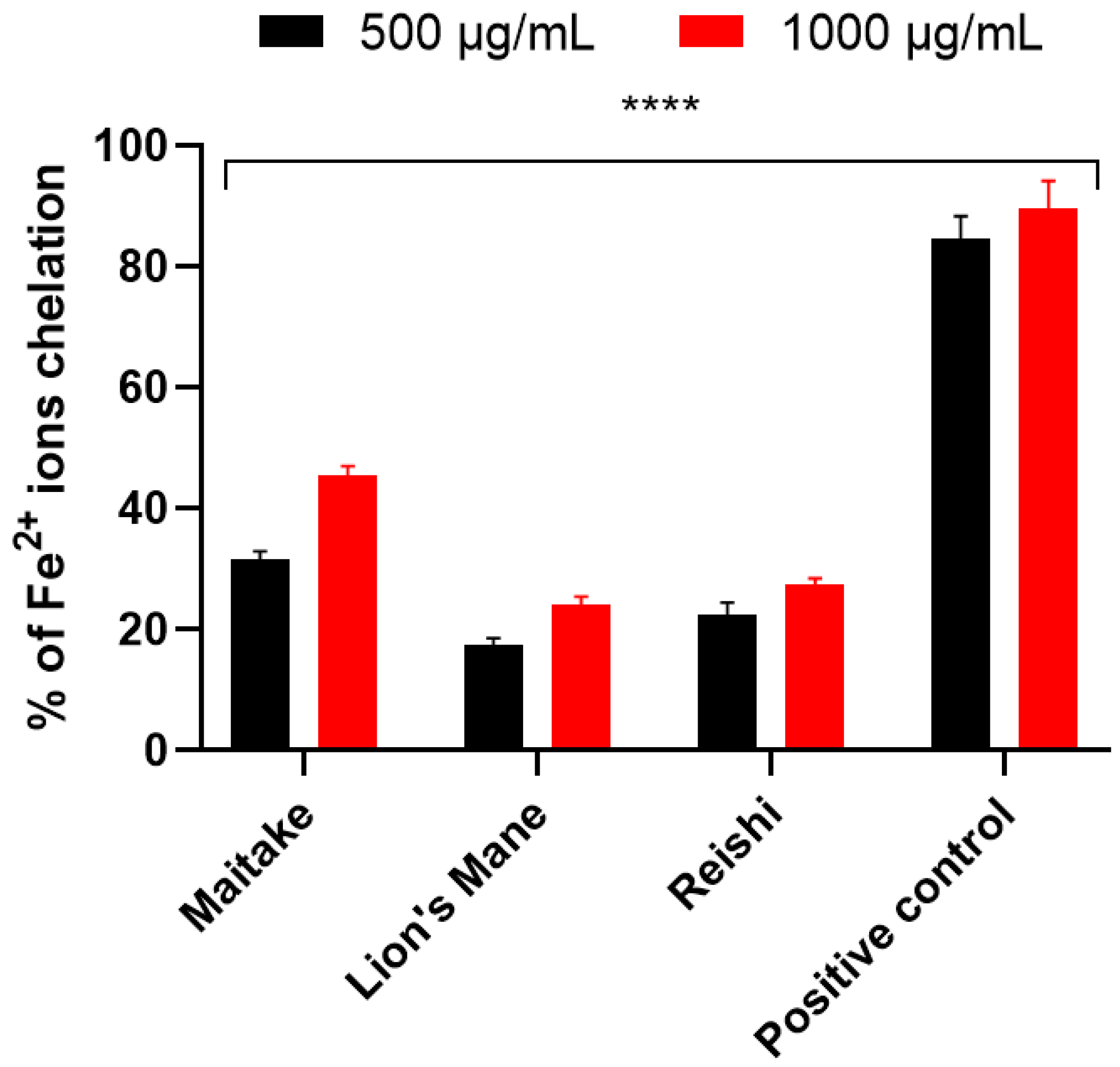
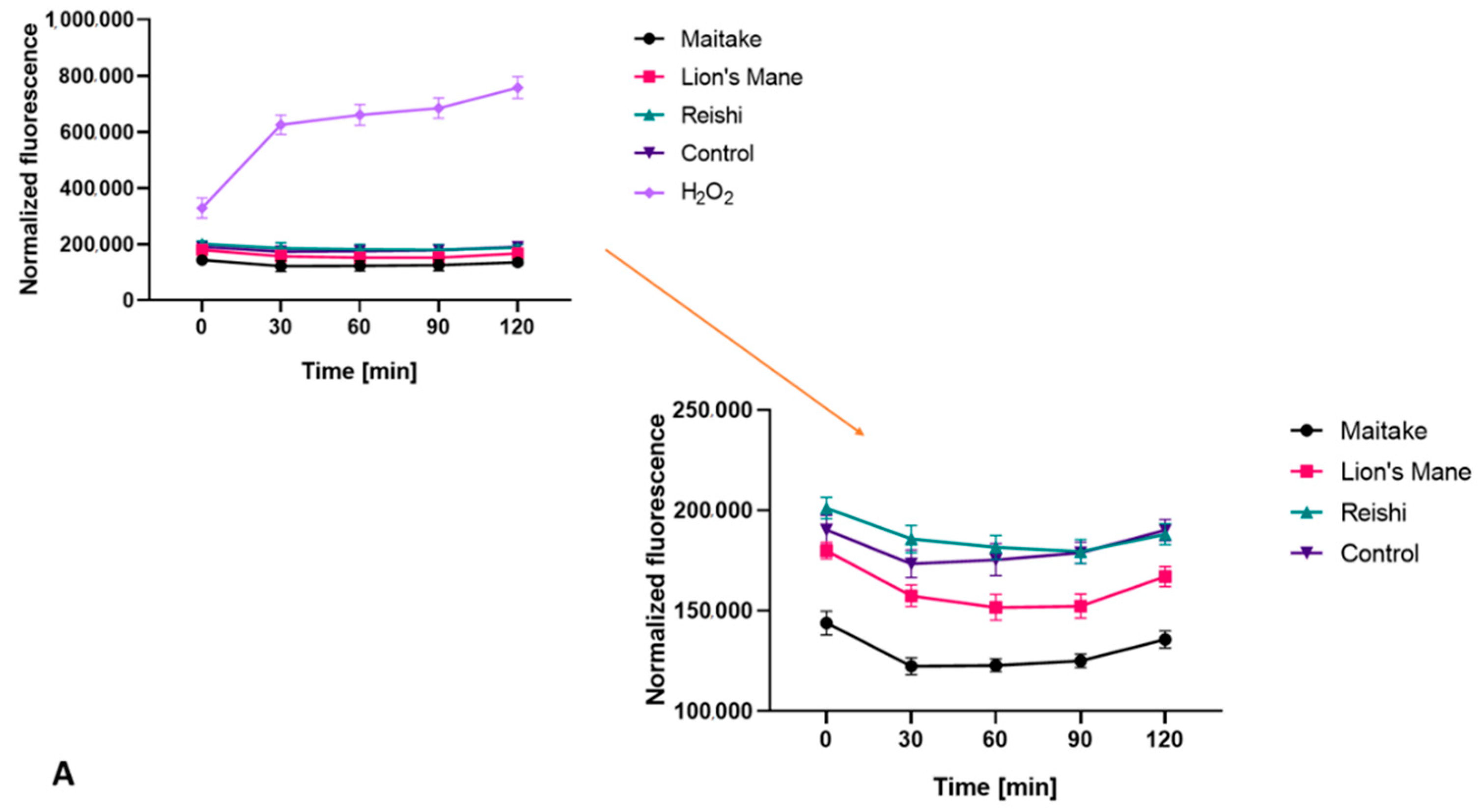
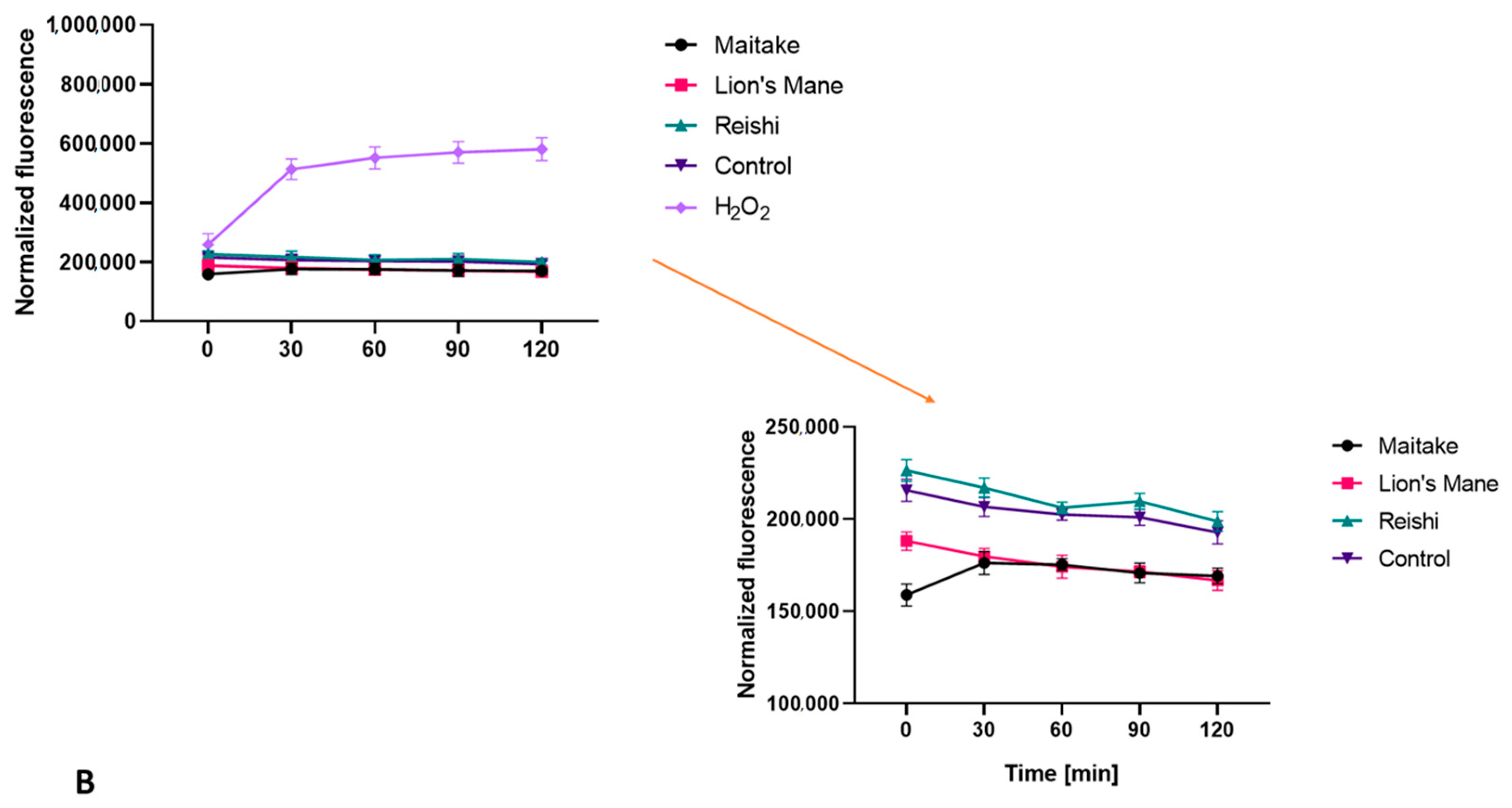
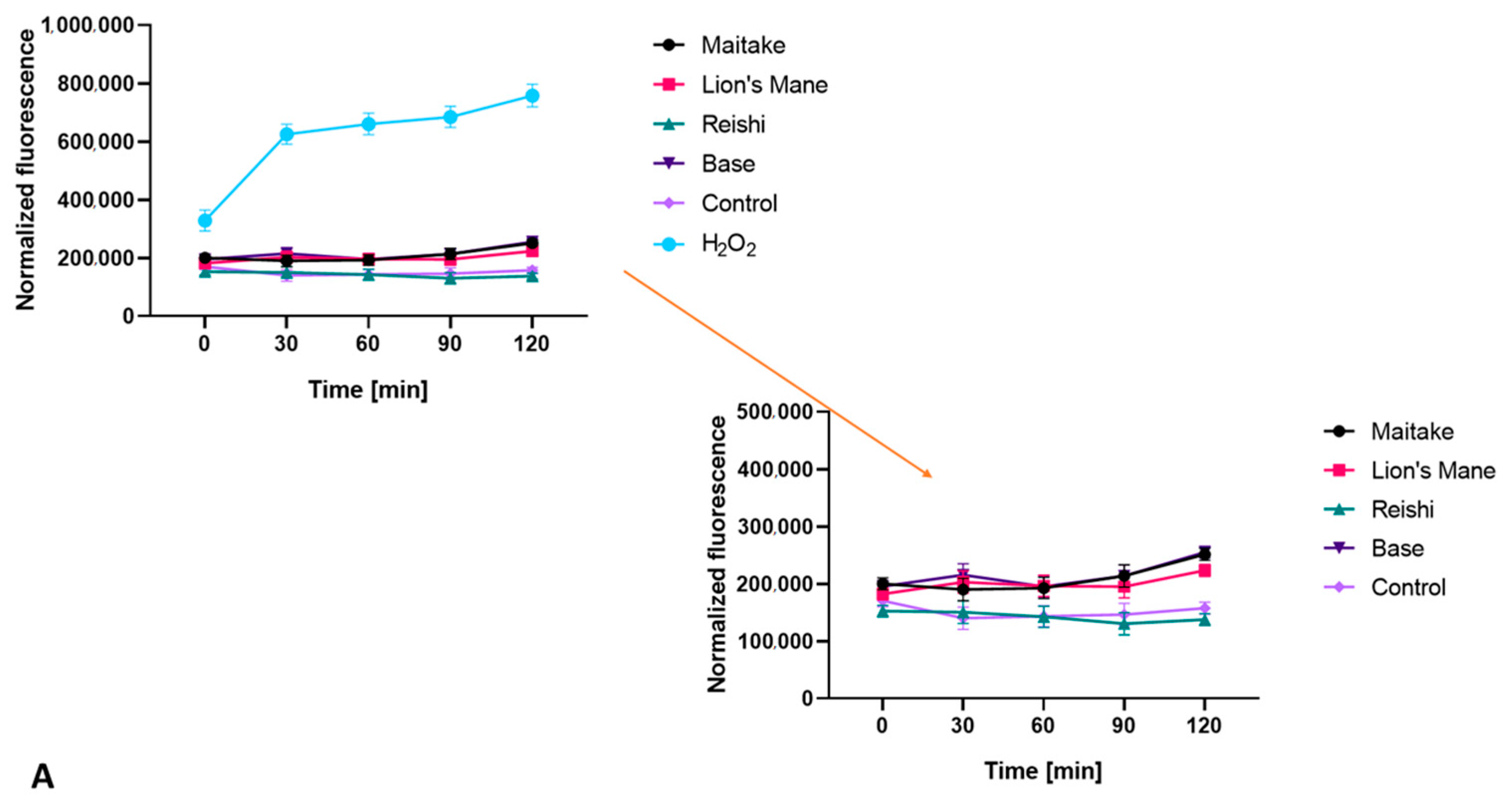
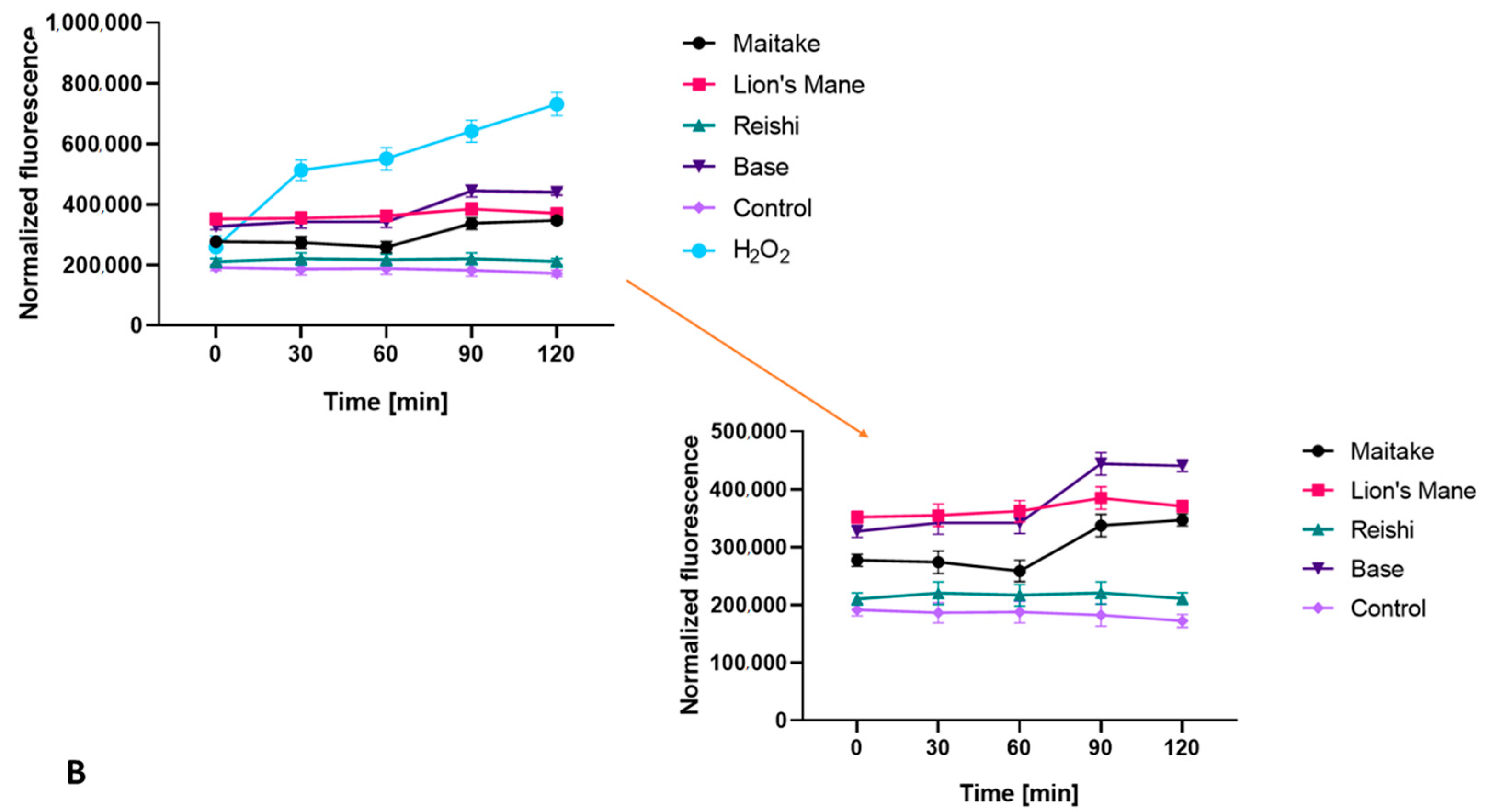
2.2. Determination of Antioxidant Properties
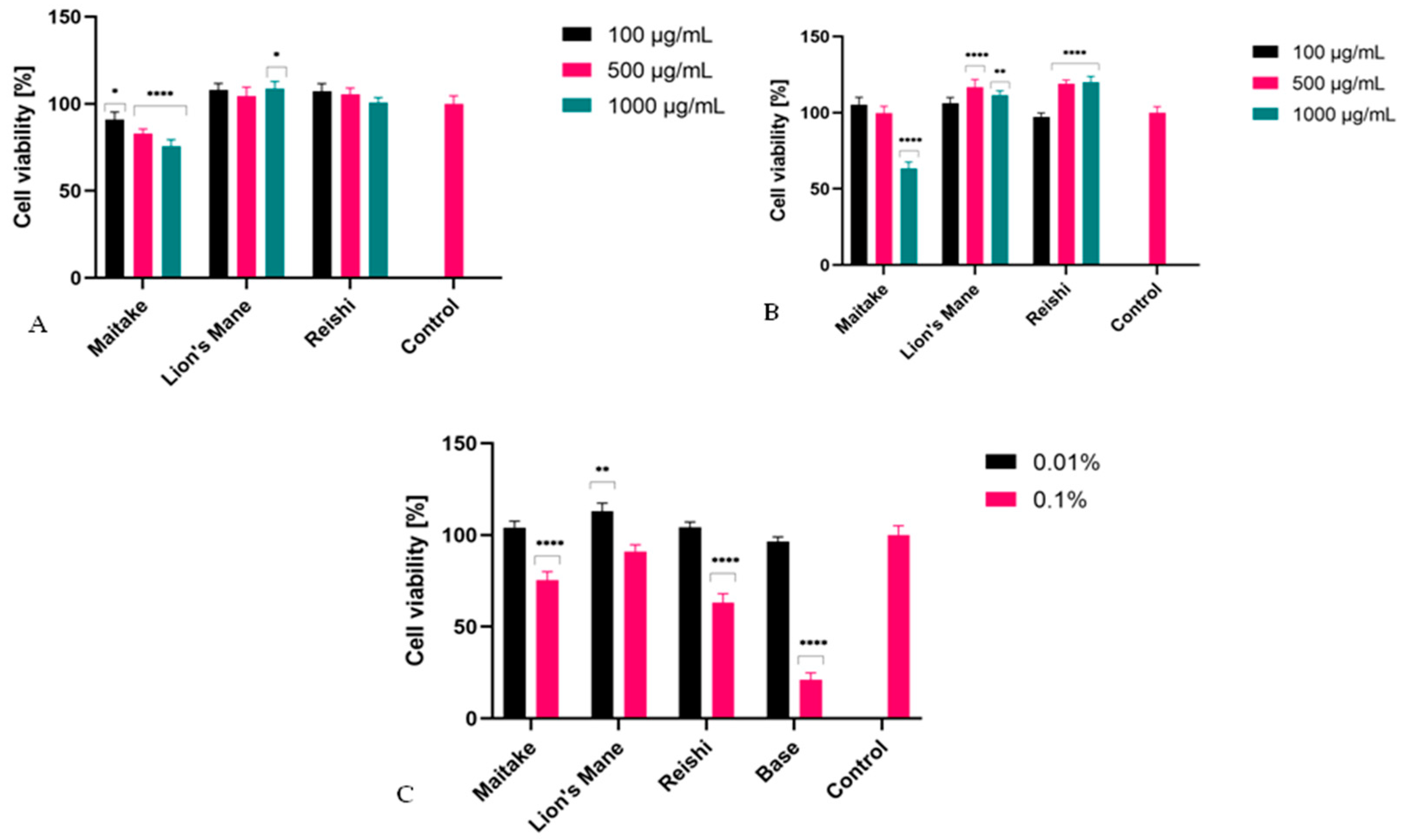
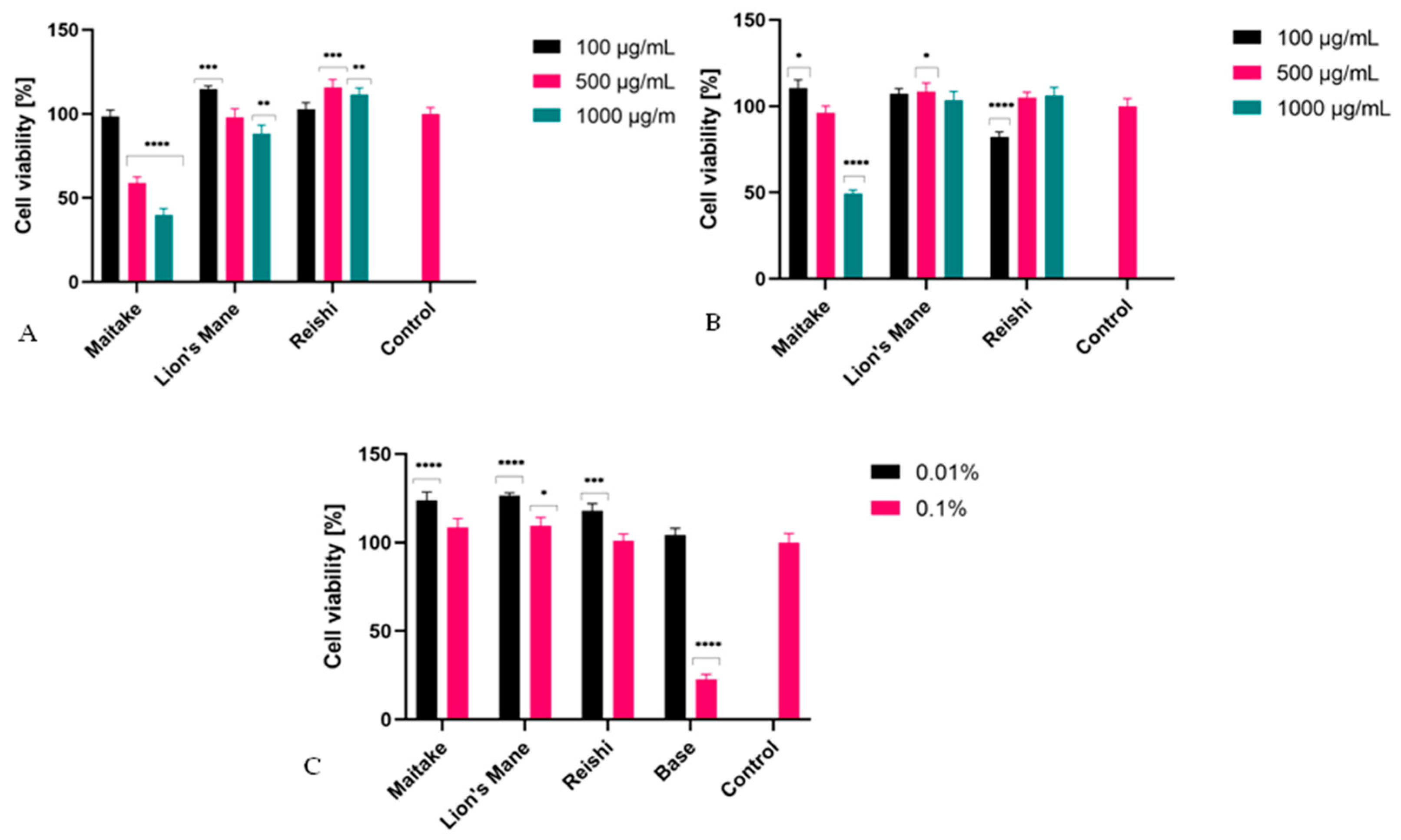
2.3. Cytotoxicity Assessment
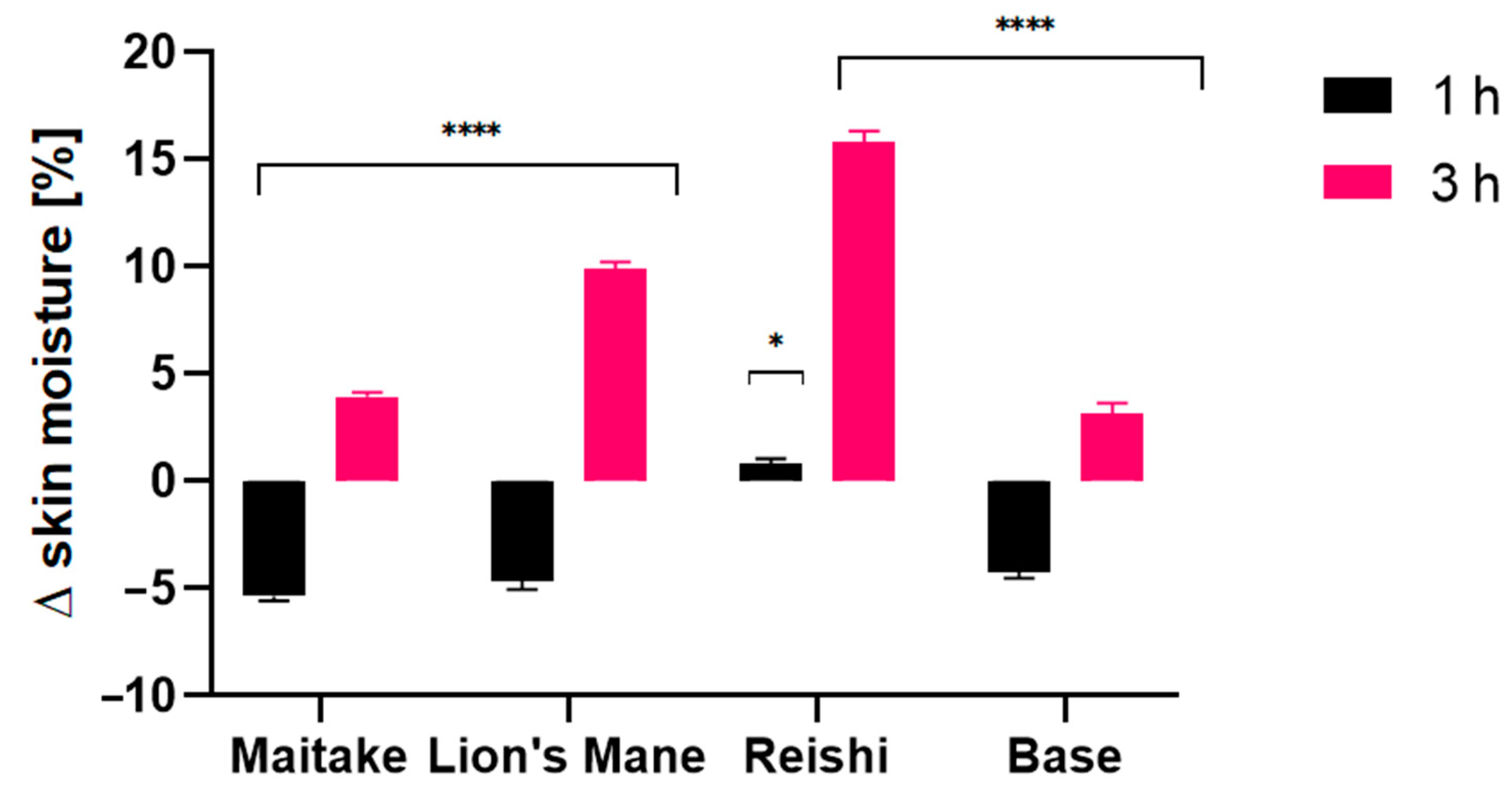
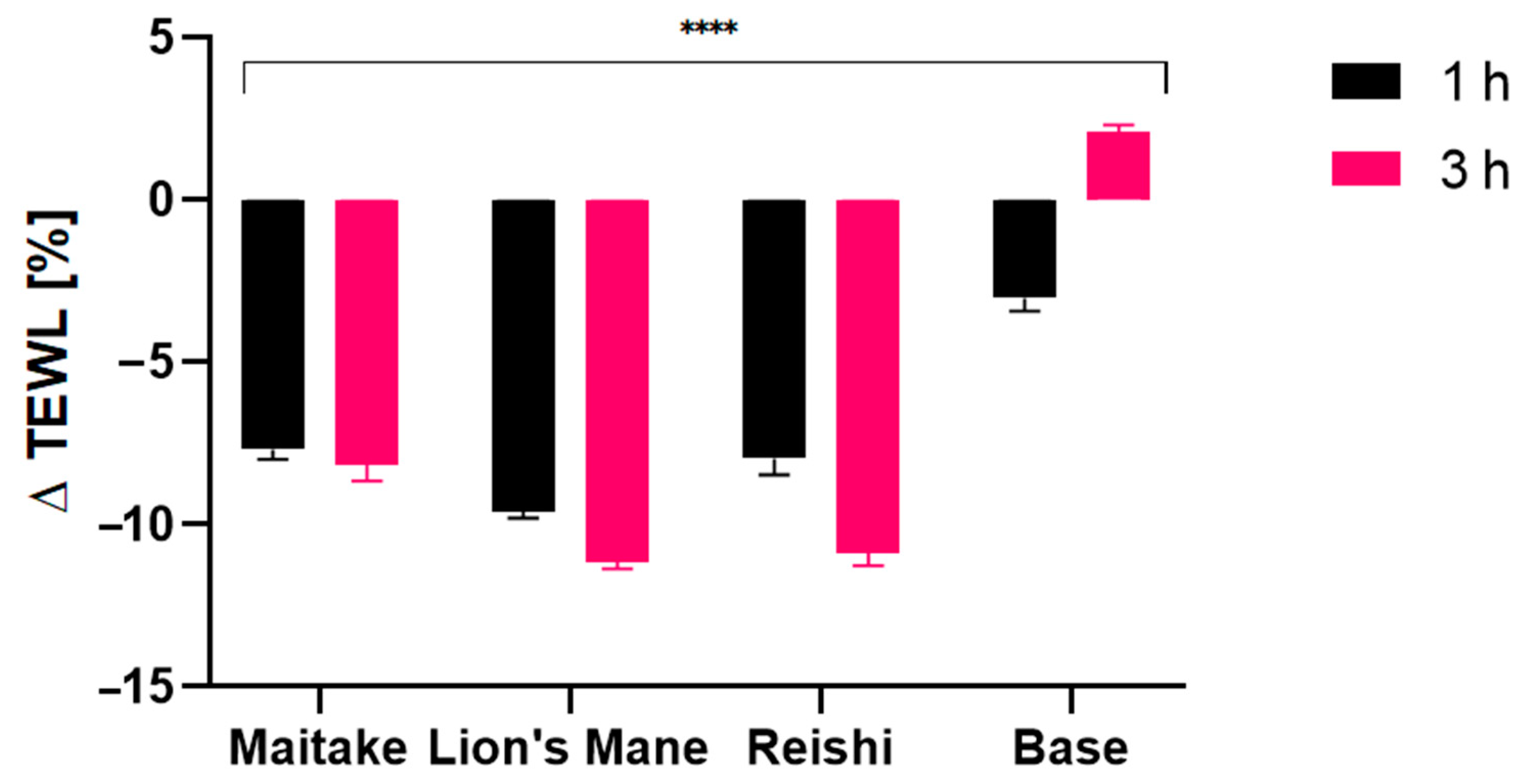
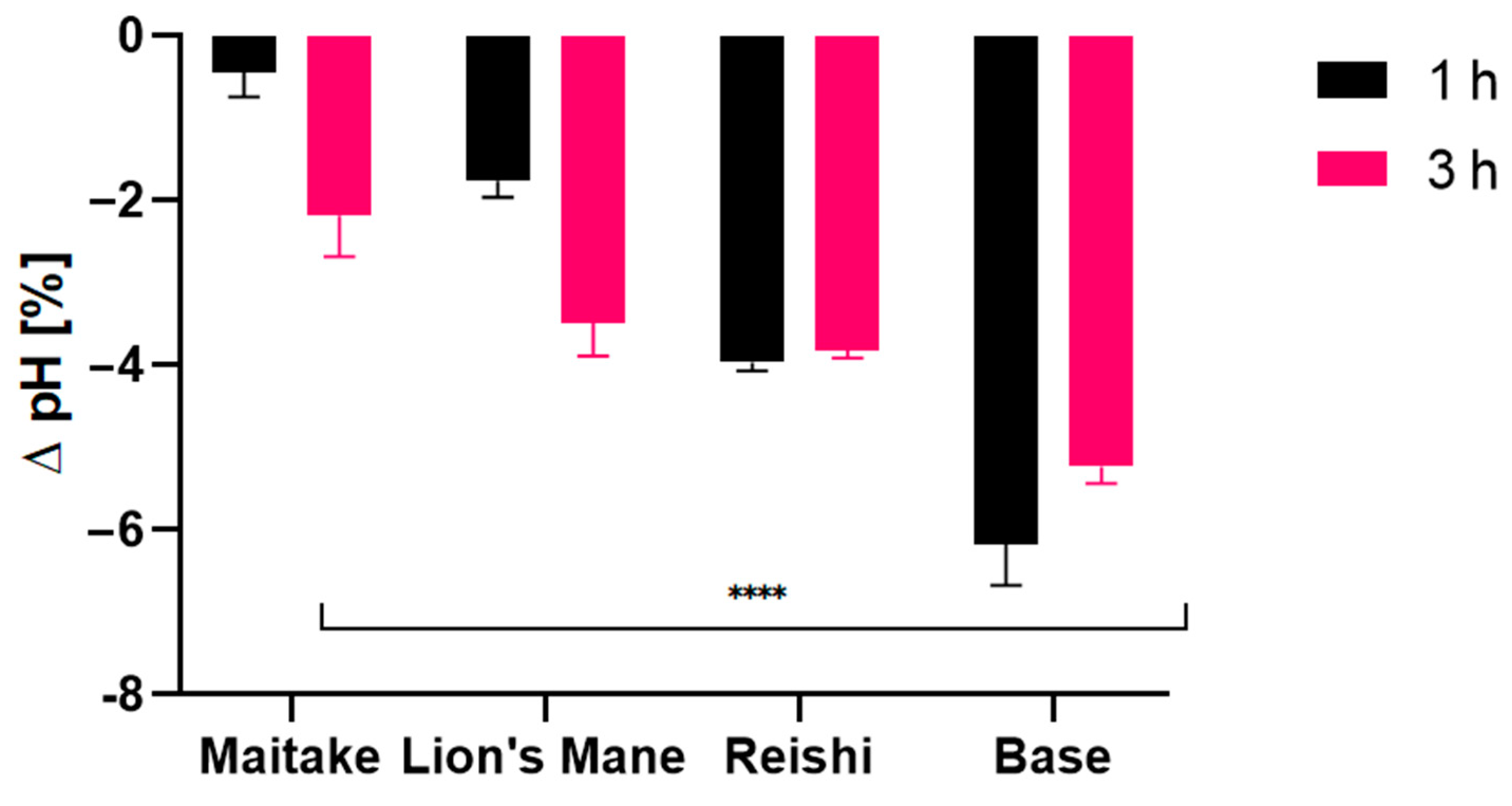
2.4. Transepidermal Water Loss (TEWL), Skin Hydration and Skin pH Measurements
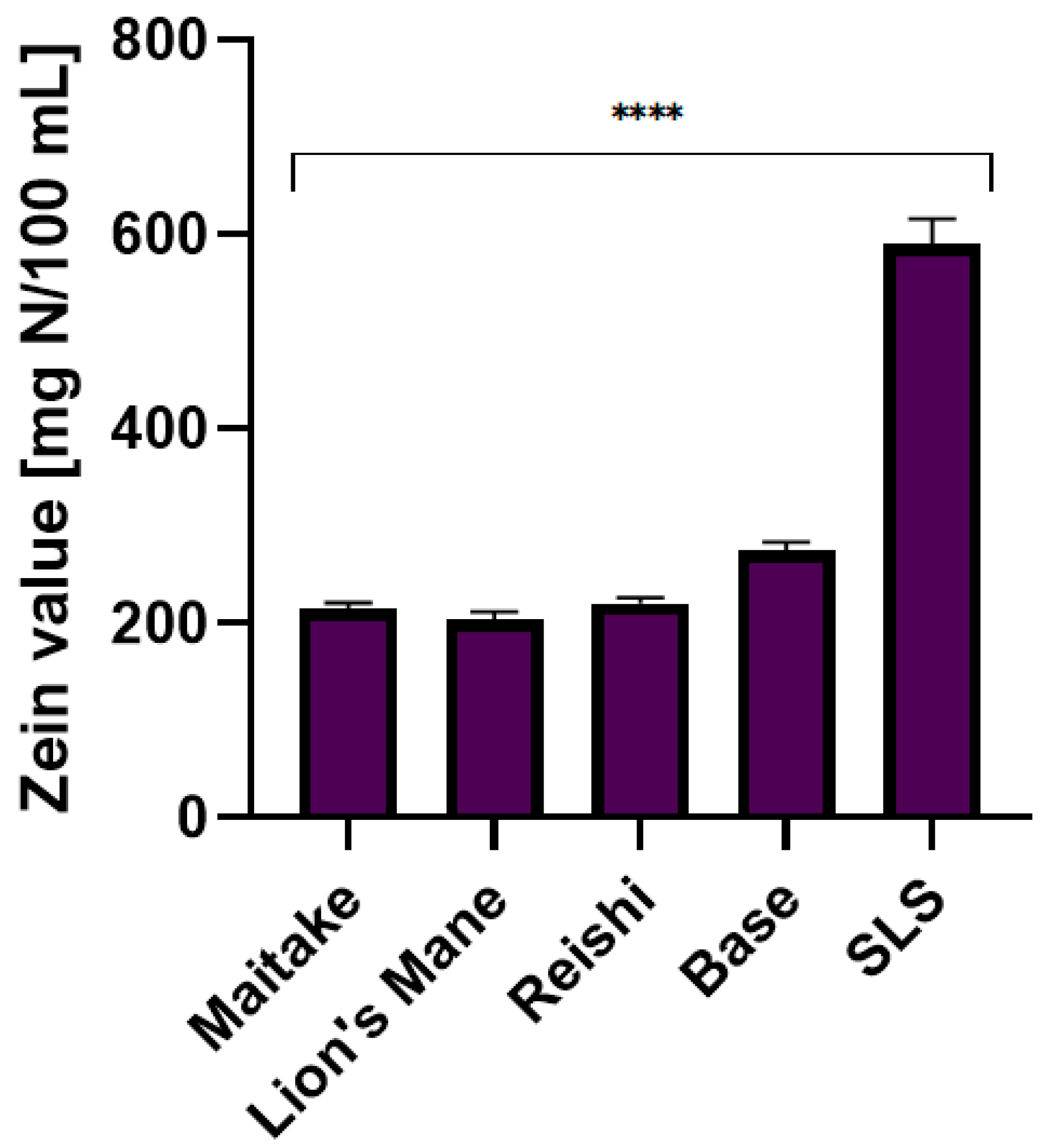
2.5. Irritant Potential of Model Body Wash Gels
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Extracts
3.3. Determination of Biologically Active Compounds
3.3.1. Determination of Bioactive Compounds by HPLC-ESI/TOF
3.3.2. Determination of the Total Phenolic Content (TPC)
3.3.3. Determination of the Total Flavonoids Content (TFC)
3.3.4. Determination of Total Carbohydrate
3.4. Determination of Antioxidant Properties
3.4.1. DPPH Radical Scavenging Assay
3.4.2. ABTS• Scavenging Assay
3.4.3. Fe2+ Chelation Assay
3.4.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4.5. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.5. Cytotoxicity Analysis
3.5.1. Cell Culture
3.5.2. AlamarBlue Assay
3.5.3. Neutral Red Uptake Assay
3.6. Transepidermal Water Loss (TEWL), Skin Hydration and Skin pH Measurements
3.7. Determination of Irritant Potential–Zein Value
3.8. Preparation of the Model Washing Gels
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Nadim, S.D.K.; Jani, J.M. Millenial’sbehaviour and attitude towards natural cosmetics: A case study in university Malysia Terengganu. UMT J. Undergrad. Res. 2021, 3, 63–74. [Google Scholar] [CrossRef]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing Synthetic Ingredients by Sustainable Natural Alternatives: A Case Study Using Topical O/W Emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef] [PubMed]
- Kaličanin, B.; Velimirović, D. A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health. Biol. Trace Elem. Res. 2016, 170, 476–484. [Google Scholar] [CrossRef]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; Giorgi, M.; Guido, M.; Congedo, M.; Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Grabowska, K.; Apola, A.; Kryczyk-Poprawa, A.; Muszyńska, B. Mycelial culture extracts of selected wood-decay mushrooms as a source of skin-protecting factors. Biotechnol. Lett. 2021, 43, 1051–1061. [Google Scholar] [CrossRef]
- Rijal, R.; Rana, M.; Srivastava, S. Biochemical Composition, Nutritional Values and Medicinal Properties of Some Edible Mushrooms: A Review. Environ. Ecol. 2021, 39, 358–369. [Google Scholar]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Bielecka, J.; Mielcarek, K.; Grabia, M.; Socha, K. Treasures from the forest: Evaluation of mushroom extracts as anti-cancer agents. Biomed. Pharmacother. 2021, 143, 112106. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 15, 373–381. [Google Scholar] [CrossRef]
- Elsayed, E.A.; ElEnshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2016, 24, 13–22. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran. J. Pharm. Res. 2012, 11, 1095–1102. [Google Scholar]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Shin, Y.J.; Lee, S.C. Antioxidant activity and β-glucan contents of hydrothermal extracts from maitake (Grifolafrondosa). Food Sci. Biotechnol. 2014, 23, 277–282. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Polysaccharides in Grifolafrondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericiumerinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Elnahas, M.O.; Thomas, P.W.; Daba, G.M. To heal or not to heal? Medicinal mushrooms wound healing capacities. ARC J. Pharm. Sci. 2019, 5, 28–35. [Google Scholar]
- Kim, J.W.; Kim, H.I.; Kim, J.H.; Kwon, O.C.; Son, E.S.; Lee, C.S.; Park, Y.J. Effects of ganodermanondiol, a new melanogenesis inhibitor from the medicinal mushroom Ganoderma lucidum. Int. J. Mol. Sci. 2016, 17, 1798. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef]
- Li, L.D.; Mao, P.W.; Shao, K.D.; Bai, X.H.; Zhou, X.W. Ganoderma proteins and their potential applications in cosmetics. Appl. Microbiol. Biotechnol. 2019, 103, 9239–9250. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Coll. Int. Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Pezron, I.; Galet, L.; Clausse, D. Surface interaction between a protein monolayer and surfactants and its correlation with skin irritation by surfactants. J. Colloid Interface Sci. 1996, 180, 285–289. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2006. [Google Scholar]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Role of the Surfactant Polar Head Structure in Protein-Surfactant Complexation: Zein Protein Solubilization by SDS and by SDS/C12En Surfactant Solutions. Langmuir 2003, 19, 1009–1016. [Google Scholar] [CrossRef]
- Dasilva, S.C.; Sahu, R.P.; Konger, R.L.; Perkins, S.M.; Kaplan, M.H.; Travers, J.B. Increased skin barrier disruption by sodium lauryl sulfate in mice expressing a constitutively active STAT6 in T cells. Arch. Dermatol. Res. 2012, 304, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 2003, 54, 29–46. [Google Scholar] [PubMed]
- Nielsen, G.D.; Nielsen, J.B.; Andersen, K.E.; Grandjean, P. Effects of industrial detergents on the barrier function of human skin. Int. J. Occup. Environ. Health 2000, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Faucher, J.A.; Goddard, E.D. Interaction of keratinous substrates with sodium lauryl sulfate: I. Sorption. J. Soc. Cosmet. Chem. 1978, 29, 323–337. [Google Scholar]
- Hall-Manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Skin irritation potential of mixed surfactant systems. Food Chem. Toxicol. 1998, 36, 233–238. [Google Scholar] [CrossRef]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B 2015, 135, 497–503. [Google Scholar] [CrossRef]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Effect of molecular weight of polyvinylpyrrolidone on the skin irritation potential and properties of body wash cosmetics in the coacervate form. Pure Appl. Chem. 2019, 91, 1521–1532. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Ziemlewska, A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Biol. Macromol. 2020, 15, 973–979. [Google Scholar] [CrossRef]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericiumerinaceum. Tetrahedron Lett. 1996, 37, 7399–7402. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Shinba, K.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S.; et al. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry 1992, 32, 175–178. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Mizuno, T. Hericenone A and B as cytotoxic principles from the mushroom Hericiumerinaceum. Tetrahedron Lett. 1990, 31, 373–376. [Google Scholar] [CrossRef]
- Mori, K.; Kikuchi, H.; Obara, Y.; Iwashita, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Oshima, Y.; Nakahata, N. Inhibitory effect of hericenone B from Hericiumerinaceus on collagen-induced platelet aggregation. Phytomedicine 2010, 17, 1082–1085. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tamanoi, H.; Hasegawa, Y.; Segawa, Y.; Masuyama, A. Divergent synthesis of bioactive resorcinols isolated from the fruiting bodies of Hericiumerinaceum: Total syntheses of hericenones A, B, and I, hericenols B–D, and erinacerins A and B. J. Org. Chem. 2014, 79, 5227–5238. [Google Scholar] [CrossRef]
- Yang, F.; Wang, H.; Feng, G.; Zhang, S.; Wang, J.; Cui, L. Rapid identification of chemical constituents in Hericiumerinaceus based on LC-MS/MS metabolomics. J. Food Qual. 2021, 2021, 5560626. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericiumerinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, T.W.; Lin, J.Y.; Chen, Y.W.; Li, T.J.; Chen, C.C. Identification of Common Liver Metabolites of the Natural Bioactive Compound Erinacine A, Purified from Hericiumerinaceus Mycelium. Appl. Sci. 2022, 12, 1201. [Google Scholar] [CrossRef]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.L.D.; Soković, M.; Ferreira, I.C.F.R. A detailed comparative study between chemical and bioactive properties of Ganodermalucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Hennicke, F.; Cheikh-Ali, Z.; Liebisch, T.; Maciá-Vicente, J.G.; Bode, H.B.; Piepenbring, M. Distinguishing commercially grown Ganoderma lucidum from Ganoderma lingzhi from Europe and East Asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry 2016, 127, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Wang, X.; Guan, S.; Xia, J.; Sun, J.; Guo, H.; Guo, D.A. Analysis of triterpenoids in Ganoderma lucidum using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Hanachi, P.; Golkho, S.H. Using HPLC to determination the composition and antioxidant activity of Berberis vulgaris. Eur. J. Sci. Res. 2009, 29, 47–54. [Google Scholar]
- Khan, I.; Ullah, S.; Oh, D.H. Chitosan grafted monomethyl fumaric acid as a potential food preservative. Carbohydr. Polym. 2016, 152, 87–96. [Google Scholar] [CrossRef]
- Özer, Z.; Kılıç, T.; Çarıkçı, S.; Yılmaz, H. Investigation of phenolic compounds and antioxidant activity of Teucrium polium L. decoction and infusion. Balıkesir Üniversitesi Fen Bilimleri Enstitüsü Dergisi 2018, 20, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Hraš, A.R.; Hadolin, M.; Knez, Ž.; Bauman, D. Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000, 71, 229–233. [Google Scholar] [CrossRef]
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericiumerinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ren, A.; Mu, D.; Zhao, M. Current progress in the study on biosynthesis and regulation of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 88, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Pathak, P.; Chaudhary, N.; Rathi, A.; Vyas, D. Ganoderma lucidum: Cultivation and Production of Ganoderic and Lucidenic Acid. In Recent Trends in Mushroom Biology; Global books Organisation: Delhi, India, 2021. [Google Scholar]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, B. Distribution of antioxidant activities and total phenolic contents in acetone, ethanol, water and hot water extracts from 20 edible mushrooms via sequential extraction. Austin J. Nutr. Food Sci. 2014, 2, 5. [Google Scholar]
- Yeh, J.Y.; Hsieh, L.H.; Wu, K.T.; Tsai, C.F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifolafrondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Al Masud, A.; Lira, N.Y.; Shakil, S. Proximate analysis, phtochemical screening and antioxidant activity of different strains of Ganoderma lucidum (Reishi mushroom). Open J. Biol. Sci. 2020, 5, 024–027. [Google Scholar] [CrossRef]
- Dickson, F.M.; Lawrence, J.N.; Benford, D.J. Surfactant-induced cytotoxicity in cultures of human keratinocytes and a commercially available cell line (3T3). Toxicol. Vitr. 1993, 7, 381–384. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Complexes of ectoine with the anionic surfactants as active ingredients of cleansing cosmetics with reduced irritating potential. Molecules 2020, 25, 1433. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive ingredients and medicinal values of Grifolafrondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifolafrondosa) D-Fraction on the activation of NK cells in cancer patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef]
- Hong, L.; Weiyu, W.; Qin, W.; Shuzhen, G.; Lebin, W. Antioxidant and immunomodulatory effects of a α-glucan from fruit body of maitake (Grifolafrondosa). Food Agric. Immunol. 2013, 24, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.C.; Yang, J.H. Dual effects of alpha-hydroxy acids on the skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.T.; Sim, G.S.; Lee, D.H.; Lee, B.C.; Pyo, H.B.; Choe, T.B.; Yun, J.W. Production of exopolysaccharide from mycelial culture of Grifolafrondosa and its inhibitory effect on matrix metalloproteinase-1 expression in UV-irradiated human dermal fibroblasts. FEMS Microbiol. Lett. 2005, 251, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.C.; Yang, H.-L.; Pan, J.-H.; Korivi, M.; Pan, J.-Y.; Hsieh, M.-C.; Chao, P.-M.; Huang, P.-J.; Tsai, C.-T.; Hseu, Y.-C. Hericium erinaceus inhibits TNF-α-induced angiogenesis and ROS generation through suppression of MMP-9/NF-κB signaling and activation of Nrf2-mediated antioxidant genes in human EA. hy926 endothelial cells. Oxidative Med. Cell. Longev. 2015, 2016, 8257238. [Google Scholar] [CrossRef] [Green Version]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Abate, M.; Pepe, G.; Randino, R.; Pisanti, S.; Basilicata, M.G.; Covelli, V.; Bifulco, M.; Cabri, W.; D’Ursi, A.M.; Campiglia, P.; et al. Ganoderma lucidum ethanol extracts enhance re-epithelialization and prevent keratinocytes from free-radical injury. Pharmaceuticals 2020, 13, 224. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Matejić, J.; Džamić, A.; Mihajilov-Krstev, T.; Ranđelović, V.; Krivošej, Z.; Marin, P. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. taxa. Open Life Sci. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Sawangwan, T.; Wansanit, W.; Pattani, L.; Noysang, C. Study of prebiotic properties from edible mushroom extraction. Agric. Nat. Resour. 2018, 52, 519–524. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Tomasz, B.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Evangelista-Vargas, S.; Santiani, A. Detection of intracellular reactive oxygen species (superoxide anion and hydrogen peroxide) and lipid peroxidation during cryopreservation of alpaca spermatozoa. Reprod. Domest. Anim. 2017, 52, 819–824. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee Kombucha ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Deo, N.; Jockusch, S.; Turro, N.J.; Somasundaran, P. Surfactant interactions with zein protein. Langmuir 2003, 19, 5083–5088. [Google Scholar] [CrossRef]
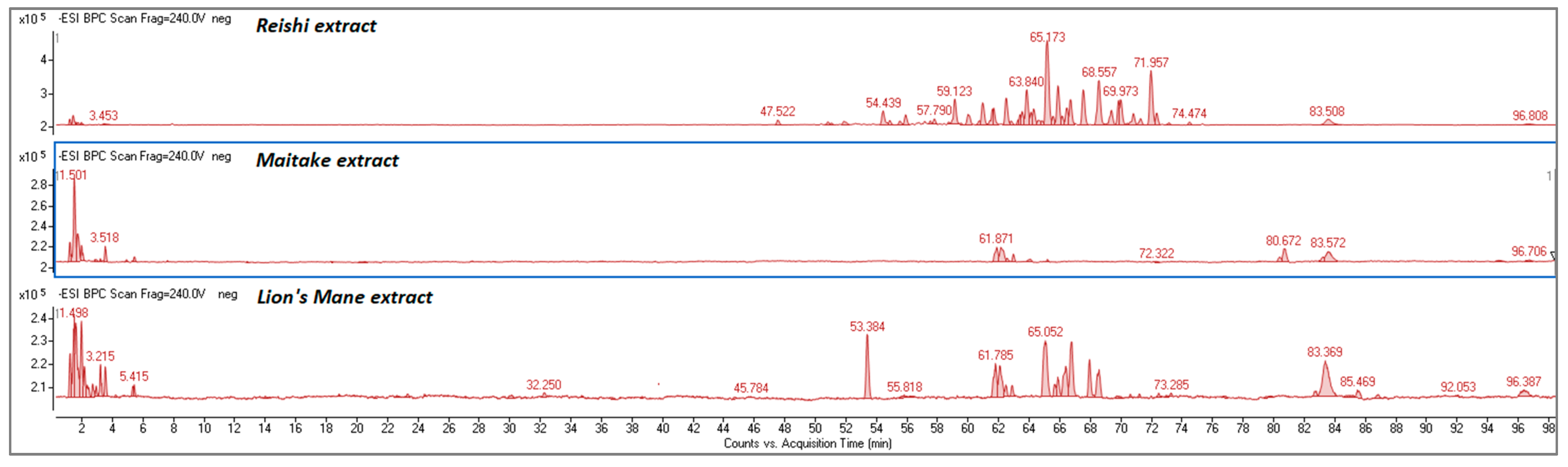
| Rt (min.) | m/z-H | Error (ppm) | Molecular Formula | Compound | Identification |
|---|---|---|---|---|---|
| 1.90 | 133.0142 | −0.35 | C4H6O5 | Malic acid | [41] |
| 1.96 | 479.1275 | 3.93 | C15H28O17 | Unknown | |
| 2.09 | 115.0042 | 4.46 | C4H4O4 | Fumaric acid | [41] |
| 2.15 | 523.0952 | 2.15 | C19H24O17 | Unknown | |
| 3.32 | 128.0350 | −2.45 | C5H7NO3 | Pyroglutamic acid | [41] |
| 5.41 | 474.1567 | −4.88 | C17H31O15 | Unknown | |
| 32.25 | 209.0445 | −4.98 | C10H10O5 | Unknown | |
| 53.38 | 526.1752 | 0.26 | C17H35O18 | Unknown | |
| 61.78 | 329.2340 | 1.98 | C18H34O5 | (15z)-9,12,13-Trihydroxy-15-octadecenoic acid | [41] |
| 62.05 | 329.1389 | −1.66 | C19H22O5 | Hericenone A | [42] |
| 62.50 | 329.2331 | −0.75 | C18H34O5 | Octadecenoic acid derivatives | [41] |
| 62.90 | 329.2342 | 2.58 | C18H34O5 | Octadecenoic acid derivatives | [41] |
| 65.05 | 431.2445 | 1.36 | C25H36O6 | Erinacine A | [43] |
| 67.70 | 473.1812 | −1.07 | C25H30O9 | Unknown | |
| 66.39 | 473.1803 | −2.97 | C25H30O9 | Unknown | |
| 66.77 | 477.2868 | 2.14 | C27H42O7 | Erinacine D | [42] |
| 67.65 | 432.2188 | 1.77 | C27H31NO4 | Hericenone B | [42] |
| 68.57 | 473.1821 | 0.83 | C25H30O9 | Unknown | |
| 83.37 | 265.1471 | −3.02 | C12H26O4S | Dodecyl sulfate | [41] |
| 96.725 | 309.2790 | −2.91 | C20H38O2 | Ethyl oleate | [41] |
| Rt (min.) | m/z-H | Error (ppm) | Molecular Formula | Compound | Identification |
|---|---|---|---|---|---|
| 1.95 | 133.0148 | 4.13 | C4H6O5 | Malic acid | [44] |
| 3.17 | 128.0355 | 1.42 | C5H7NO3 | Pyroglutamic acid | [44] |
| 47.52 | 533.3141 | 3.95 | C30H46O8 | Unknown | |
| 54.39 | 531.2972 | 1.63 | C30H44O8 | Ganoderic acid G | [45] |
| 55.92 | 531.2969 | 1.05 | C30H44O8 | Ganoderic acid G | [45] |
| 57.240 | 529.2821 | 2.66 | C30H42O8 | 12-Hydroxy-Ganoderic acid D | [45] |
| 57.906 | 515.3029 | 2.85 | C30H44O7 | Ganoderic acid A/B | [45] |
| 59.223 | 517.2815 | 1.56 | C29H42O8 | Lucidenic acid P | [46] |
| 60.96 | 513.2856 | −0.34 | C30H42O7 | Unknown | |
| 61.69 | 513.2867 | 1.79 | C30H42O7 | Unknown | |
| 62.49 | 515.3030 | 3.05 | C30H44O7 | Ganoderic acid A/B | [45] |
| 63.84 | 513.2862 | 0.82 | C30H42O7 | Unknown | |
| 65.140 | 515.3006 | −1.6 | C30H44O7 | Ganoderic acid A/B | [45] |
| 65.87 | 571.2921 | 1.47 | C32H44O9 | Ganoderic acid K | [45] |
| 66.72 | 457.2654 | −0.08 | C20H42O11 | Unknown | |
| 67.657 | 511.2715 | 2.68 | C30H40O7 | Ganoderic acid D | [45] |
| 68.56 | 513.2868 | 1.99 | C30H42O7 | Ganoderic acid AM1 | [45] |
| 69.97 | 511.2720 | 3.66 | C30H40O7 | Ganoderic acid D | [45] |
| 71.967 | 569.2768 | 2.09 | C32H42O9 | 12-Acetoxy-ganoderic acid F | [45] |
| 83.647 | 265.1483 | 1.49 | C12H26O4S | Dodecyl sulfate | [41] |
| 96.725 | 309.2810 | 3.53 | C20H38O2 | Ethyl oleate | [41] |
| Rt (min.) | m/z-H | Error (ppm) | Molecular Formula | Compound | Identification |
|---|---|---|---|---|---|
| 1.84 | 133.0144 | 1.14 | C4H6O5 | Malic acid | [41] |
| 2.11 | 115.0039 | 1.88 | C4H4O4 | Fumaric acid | [41] |
| 2.34 | 191.0194 | −1.7 | C6H8O7 | Citric acid | [41] |
| 3.518 | 382.128 | 2.79 | C18H23O9 | Unknown | |
| 61.870 | 329.2349 | 4.70 | C18H34O5 | (15z)-9,12,13-Trihydroxy-15-octadecenoic acid | [41] |
| 62.17 | 329.2340 | 1.98 | C18H34O5 | Octadecenoic acid derivatives | [41] |
| 80.67 | 313.2400 | 4.99 | C18H34O4 | Unknown | |
| 83.647 | 265.1466 | −4.9 | C12H26O4S | Dodecyl sulfate | [41] |
| 96.725 | 309.2792 | −2.27 | C20H38O2 | Ethyl oleate | [41] |
| Maitake | Lion’s Mane | Reishi |
|---|---|---|
| Total phenolic compounds content [µg GAE/g DW] | ||
| 183.75 ± 0.21 | 59.70 ± 0.14 | 13.23 ± 0.07 |
| Total flavonoids content [µg QE/g DW] | ||
| 38.38 ± 0.07 | 13.68 ± 0.21 | 3.43 ± 0.03 |
| Mushroom Extract Type | μmol Trolox/g Dry Weight |
|---|---|
| Maitake | 21.1 ± 3.2 |
| Lion’s Mane | 7.6 ± 1.1 |
| Reishi | 9.8 ± 2.4 |
| INCI Name | Concentration [wt%] |
|---|---|
| Aqua | 84.85 |
| Sodium coco sulfate | 7.00 |
| Cocamidopropyl betaine | 1.50 |
| Coco glucoside | 1.50 |
| Glycerin | 5.00 |
| Sodium benzoate | 0.50 |
| Potassium sorbate | 0.20 |
| Sodium chloride | 1.00 |
| Lactic acid | 0.45 |
| Extract | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemlewska, A.; Wójciak, M.; Mroziak-Lal, K.; Zagórska-Dziok, M.; Bujak, T.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Sowa, I. Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts. Molecules 2022, 27, 5090. https://doi.org/10.3390/molecules27165090
Ziemlewska A, Wójciak M, Mroziak-Lal K, Zagórska-Dziok M, Bujak T, Nizioł-Łukaszewska Z, Szczepanek D, Sowa I. Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts. Molecules. 2022; 27(16):5090. https://doi.org/10.3390/molecules27165090
Chicago/Turabian StyleZiemlewska, Aleksandra, Magdalena Wójciak, Kamila Mroziak-Lal, Martyna Zagórska-Dziok, Tomasz Bujak, Zofia Nizioł-Łukaszewska, Dariusz Szczepanek, and Ireneusz Sowa. 2022. "Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts" Molecules 27, no. 16: 5090. https://doi.org/10.3390/molecules27165090
APA StyleZiemlewska, A., Wójciak, M., Mroziak-Lal, K., Zagórska-Dziok, M., Bujak, T., Nizioł-Łukaszewska, Z., Szczepanek, D., & Sowa, I. (2022). Assessment of Cosmetic Properties and Safety of Use of Model Washing Gels with Reishi, Maitake and Lion’s Mane Extracts. Molecules, 27(16), 5090. https://doi.org/10.3390/molecules27165090