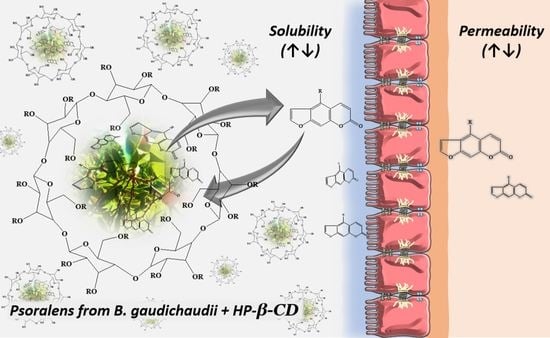
Improvement in Solubility–Permeability Interplay of Psoralens from Brosimum gaudichaudii Plant Extract upon Complexation with Hydroxypropyl-β-cyclodextrin
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase Solubility Studies
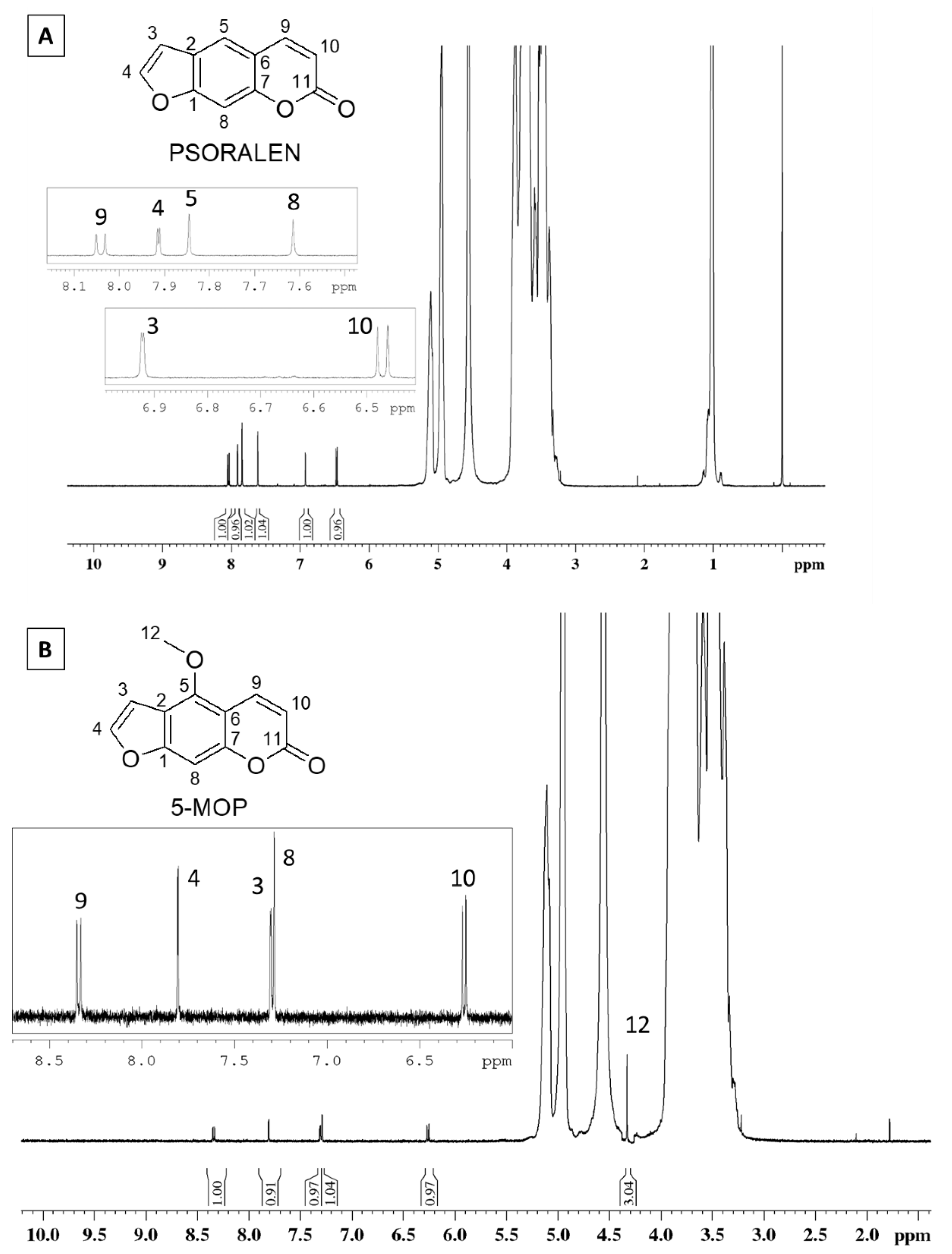
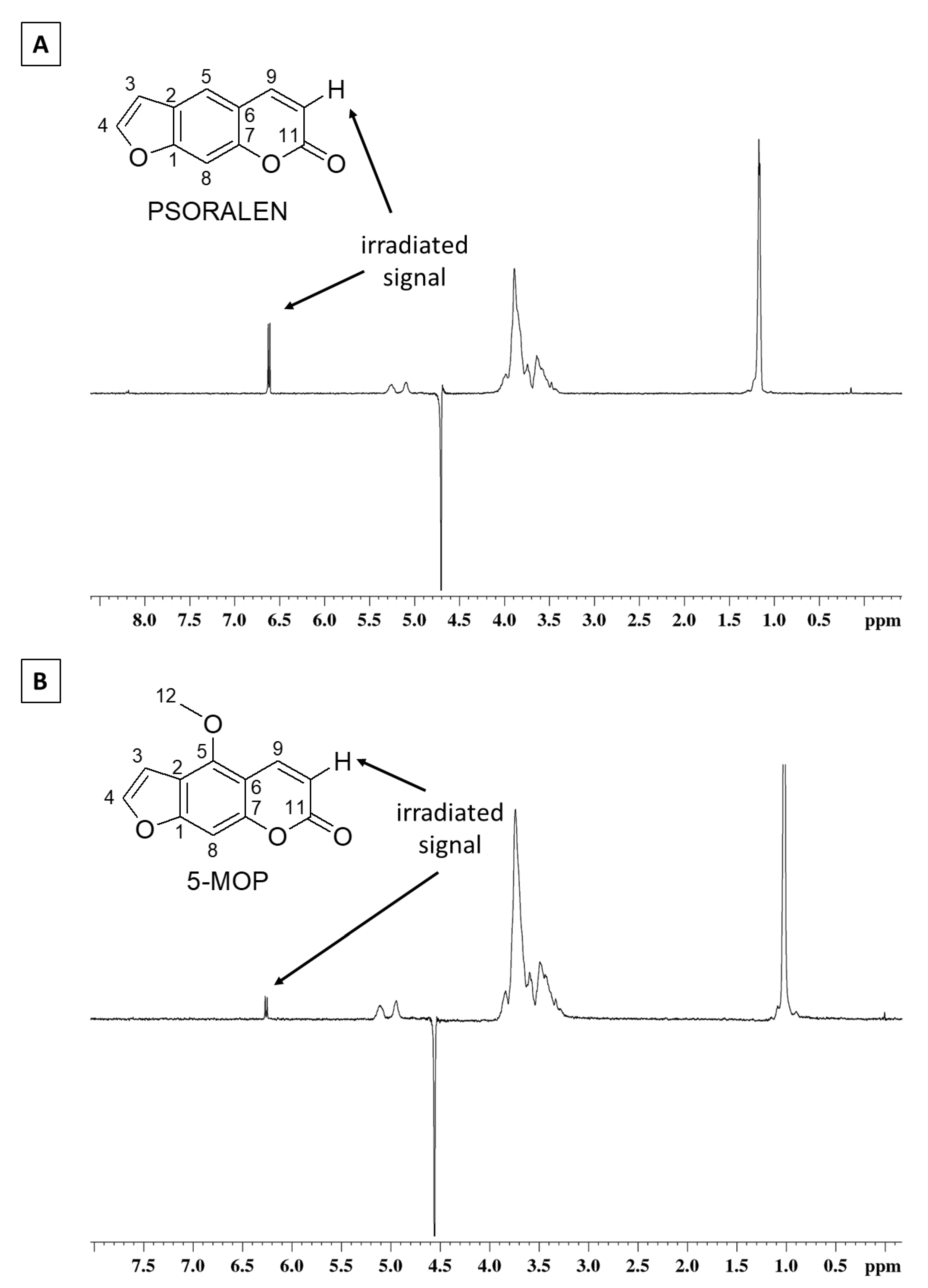
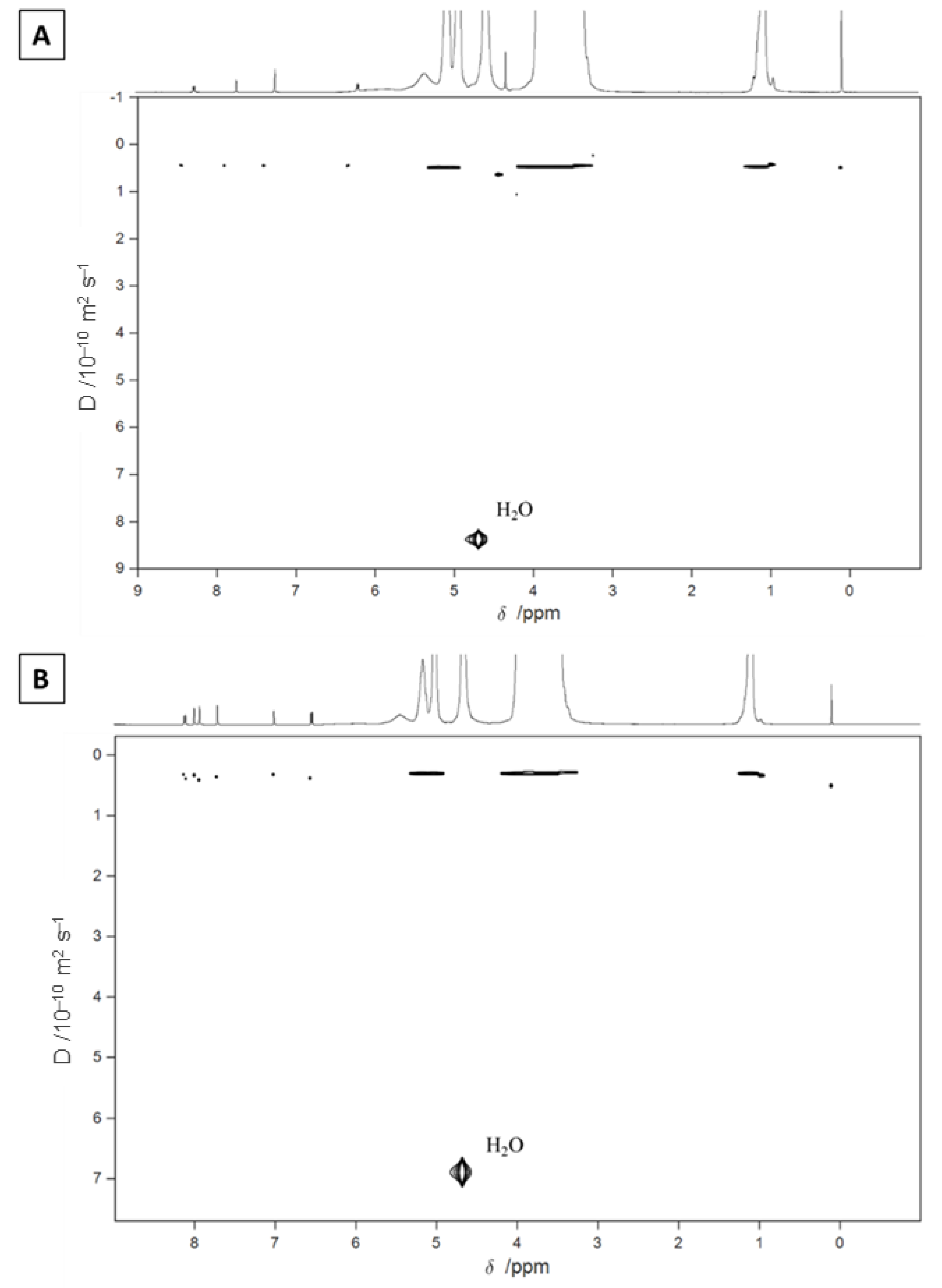
2.2. Nuclear Magnetic Resonance Characterization
2.3. Characterization of the PSO- and 5-MOP-HP-β-CD Inclusion Complex in Solid State
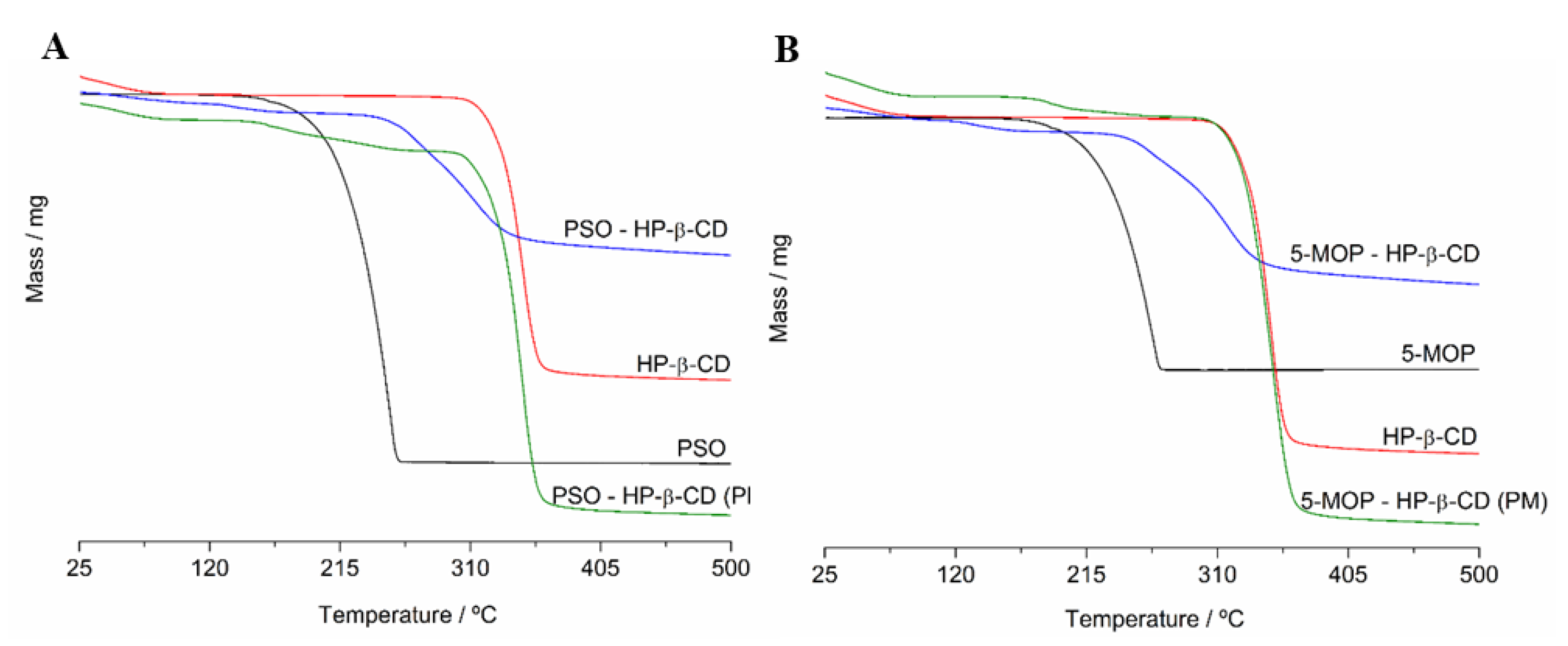
2.3.1. Thermogravimetry (TG)
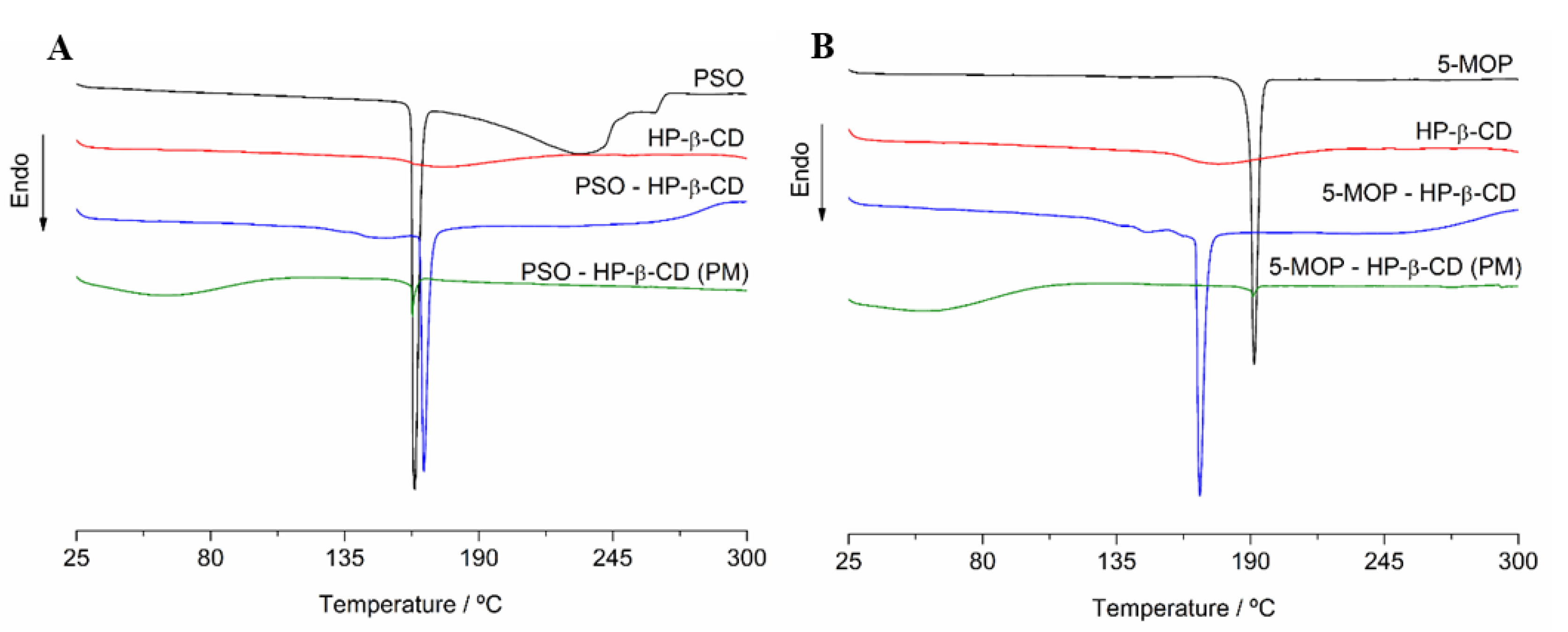
2.3.2. Differential Scanning Calorimetry (DSC)
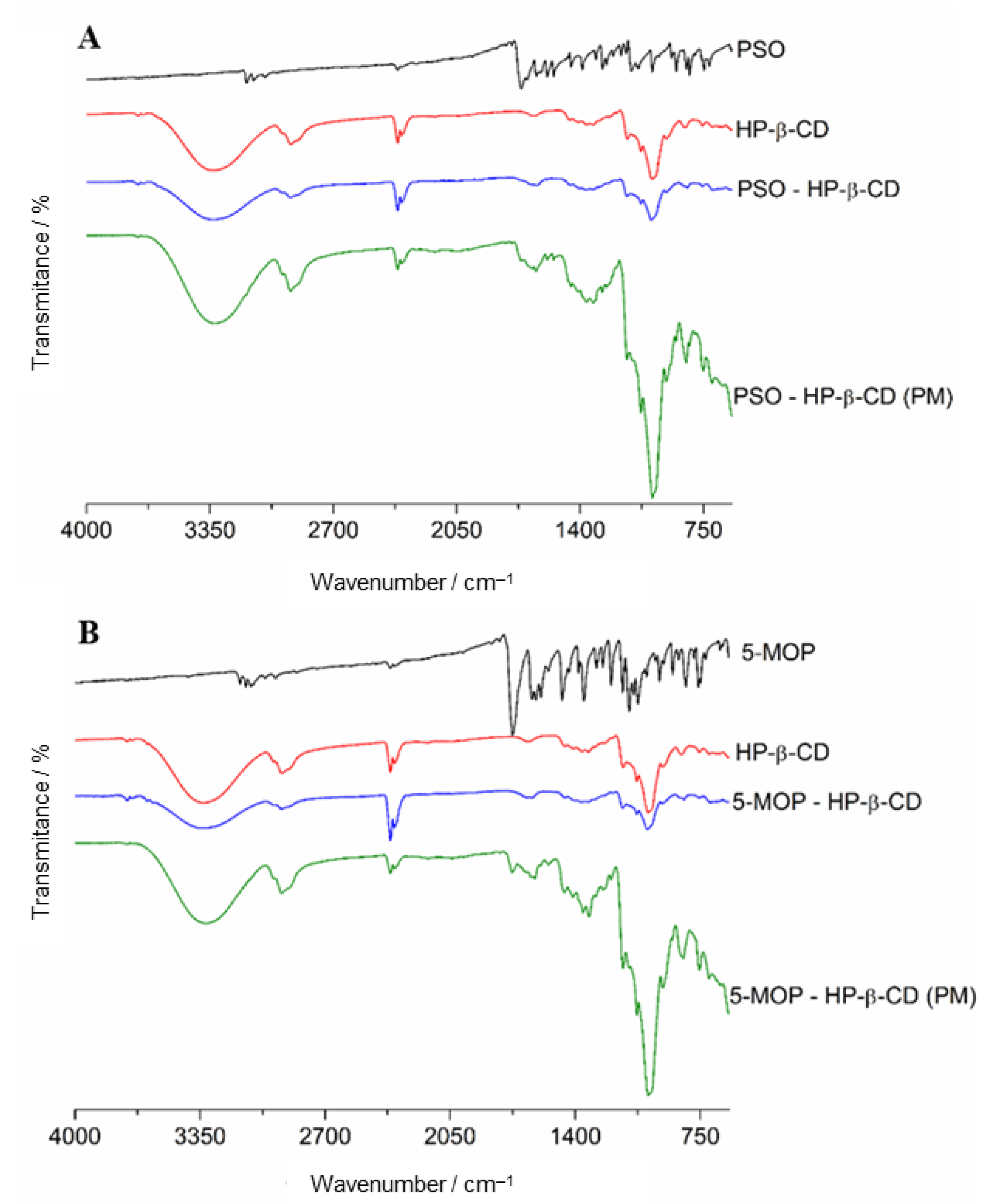
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
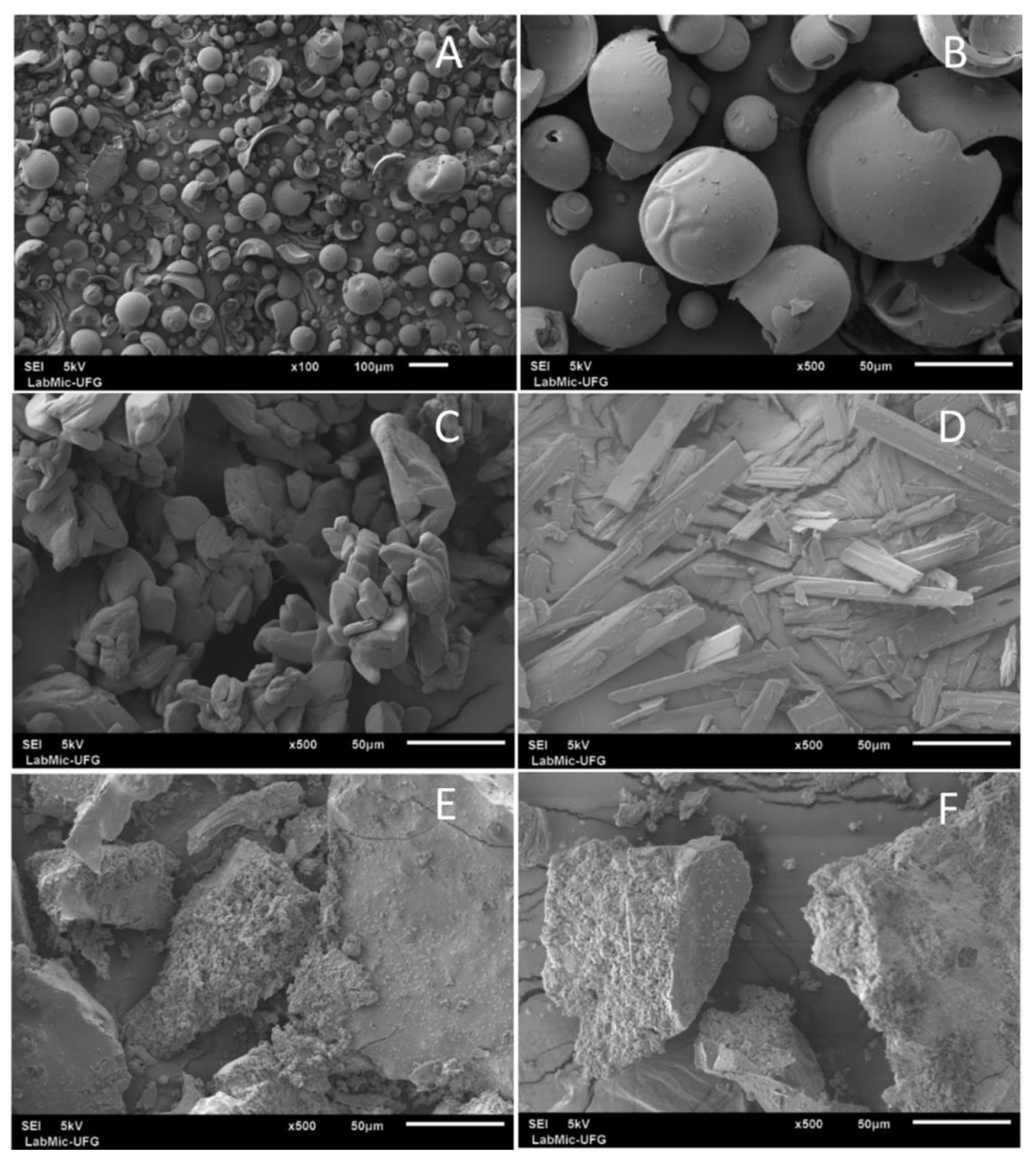
2.3.4. Scanning Electron Microscopy (SEM)
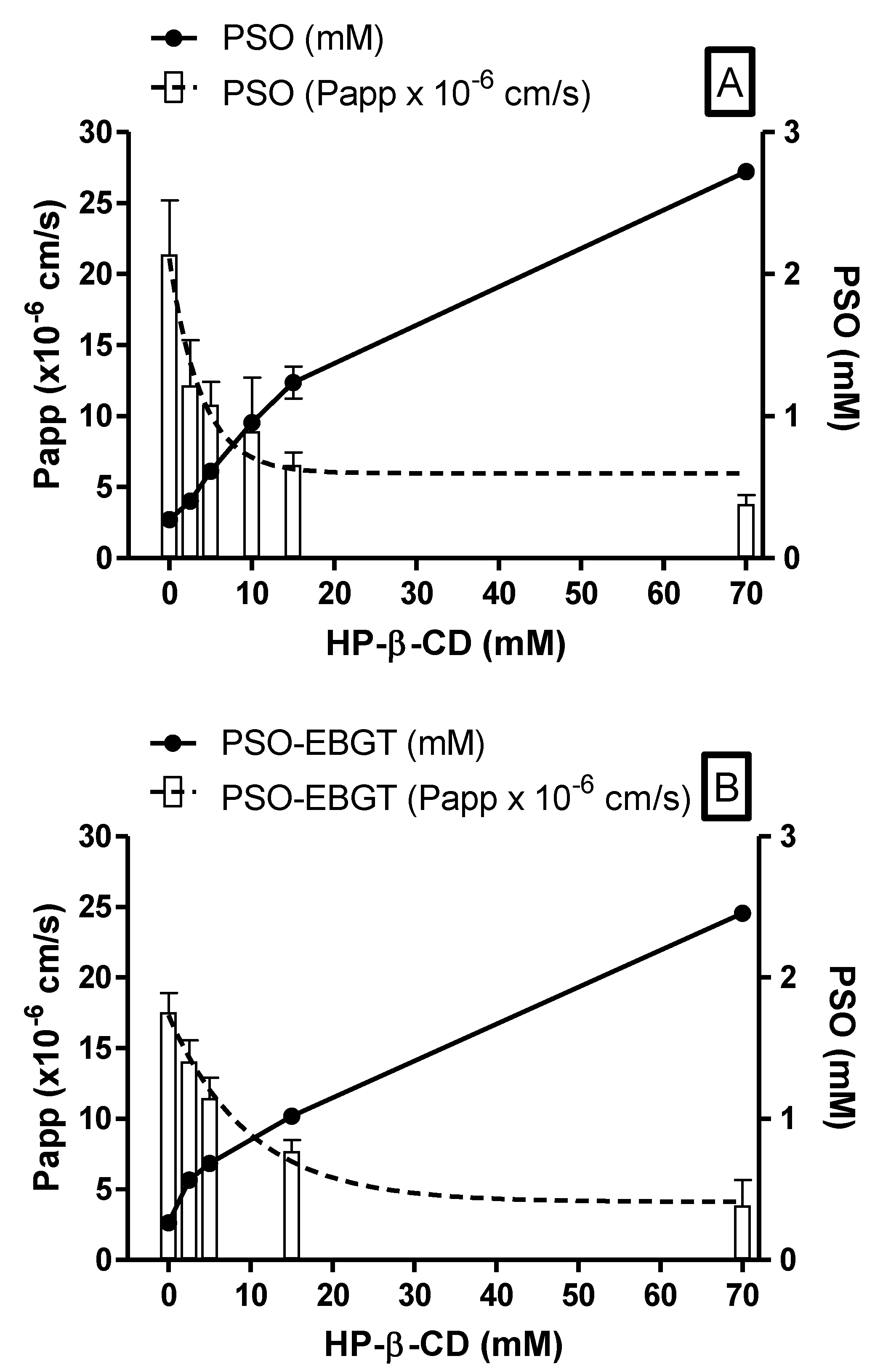
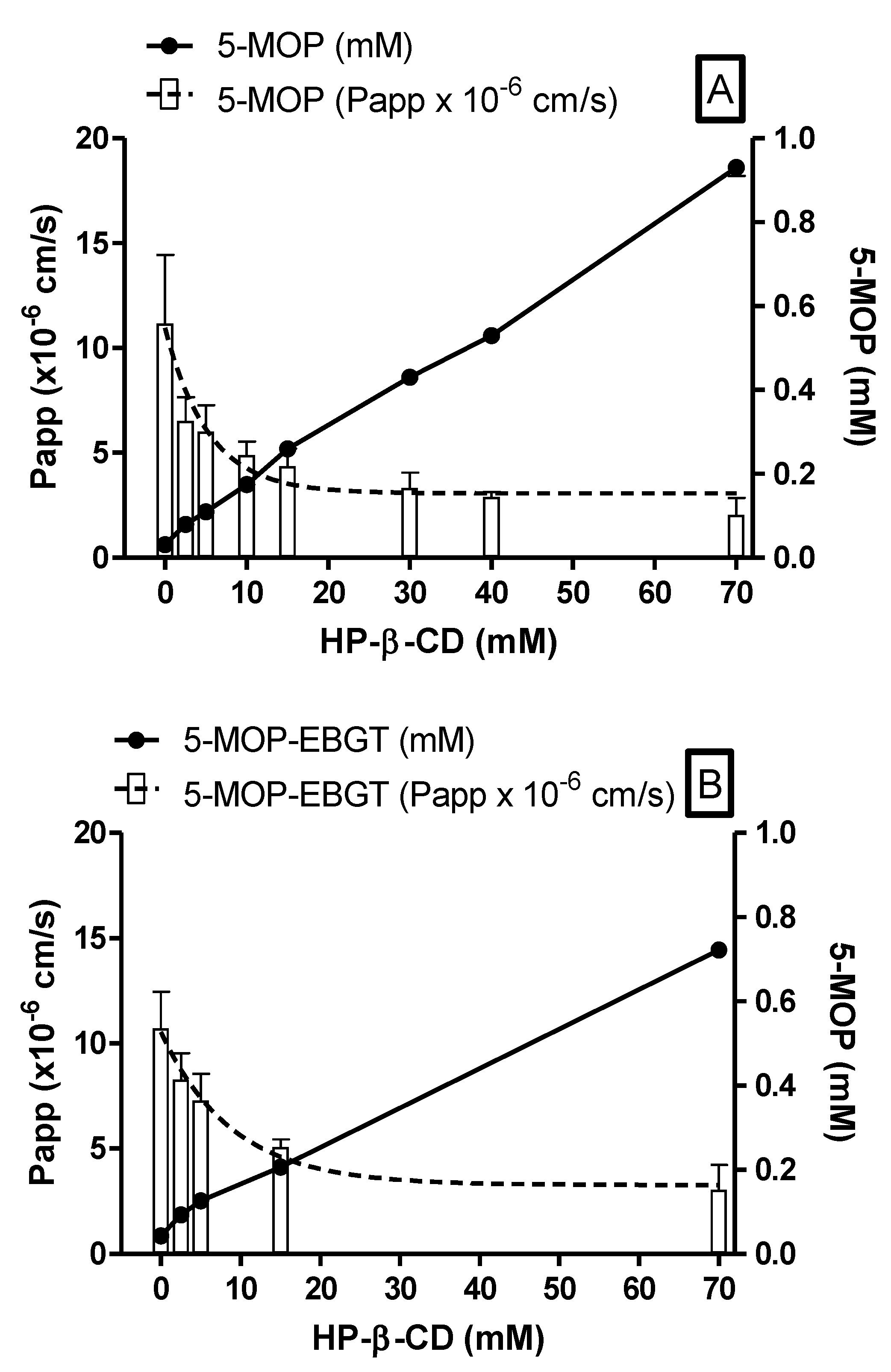
2.4. Ex Vivo Oral Permeability Assessment
3. Materials and Methods
3.1. Solvents and Chemicals
3.2. Plant Material
3.3. Preparation of PSO- and 5-MOP-HP-β-CD Inclusion Complex
3.4. Physical Mixture (PM)
3.5. Characterization of the PSO-and 5-MOP-HP-β-CD Inclusion Complex in Solid State
3.5.1. Differential Scanning Calorimetry (DSC)
3.5.2. Thermogravimetry (TG)
3.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.5.4. Scanning Electron Microscopy (SEM)
3.6. Phase Solubility of the PSO- and 5-MOP-HP-β-CD Inclusion Complex
3.7. Nuclear Magnetic Resonance Characterization (NMR)
3.8. Ex Vivo Permeability
3.9. HPLC-DAD Method
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Pacifico, A.; Leone, G. Photo(Chemo)Therapy for Vitiligo. Photodermatol. Photoimmunol. Photomed. 2011, 27, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.D.; de Morais, M.C.; da Conceição, E.C.; Vaz, B.G.; Chaves, A.R.; Rezende, K.R. Crude Plant Extract versus Single Compounds for Vitiligo Treatment: Ex Vivo Intestinal Permeability Assessment on Brosimum gaudichaudii Trécul. J. Pharm. Biomed. Anal. 2020, 191, 113593. [Google Scholar] [CrossRef] [PubMed]
- Vilegas, W.; Pozetti, G.L.; Vilegas, J.H.Y. Coumarins from Brosimum gaudichaudii. J. Nat. Prod. 1993, 56, 416–417. [Google Scholar] [CrossRef]
- De Monteiro, V.F.F.; Mathias, L.; Vieira, I.J.C.; Schripsema, J.; Braz-Filho, R. Prenylated Coumarins, Chalcone and New Cinnamic Acid and Dihydrocinnamic Acid Derivatives from Brosimum gaudichaudii. J. Braz. Chem. Soc. 2002, 13, 281–287. [Google Scholar] [CrossRef]
- Jacomassi, E.; Moscheta, I.S.; Machado, S.R. Morfoanatomia e Histoquímica de Brosimum gaudichaudii Trécul (Moraceae). Acta Bot. Bras. 2007, 21, 575–597. [Google Scholar] [CrossRef][Green Version]
- Palhares, D.; de Paula, J.E.; dos Santos Silveira, C.E. Morphology of Stem and Subterranean System of Brosimum gaudichaudii (Moraceae). Acta Bot. Hung. 2006, 48, 89–101. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.d.O. Ethnobotanical Study of Medicinal Plants Used by Ribeirinhos in the North Araguaia Microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Quintão, W.d.S.C.; Alencar-Silva, T.; Borin, M.d.F.; Rezende, K.R.; Albernaz, L.C.; Cunha-Filho, M.; Gratieri, T.; de Carvalho, J.L.; Sá-Barreto, L.C.L.; Gelfuso, G.M. Microemulsions Incorporating Brosimum gaudichaudii Extracts as a Topical Treatment for Vitiligo: In Vitro Stimulation of Melanocyte Migration and Pigmentation. J. Mol. Liq. 2019, 294, 111685. [Google Scholar] [CrossRef]
- Feng, L.; Wang, L.; Jiang, X. Pharmacokinetics, Tissue Distribution and Excretion of Coumarin Components from Psoralea corylifolia L. in Rats. Arch. Pharm. Res. 2010, 33, 225–230. [Google Scholar] [CrossRef]
- Balogh, A.; Merkel, U.; Looks, A.; Vollandt, R.; Wollina, U. Drug Monitoring of Orally Administered 8-Methoxypsoralen in Patients Treated with Extracorporeal Photopheresis. Skin Pharmacol. Physiol. 1998, 11, 258–265. [Google Scholar] [CrossRef]
- Bräutigam, L.; Seegel, M.; Tegeder, I.; Schmidt, H.; Meier, S.; Podda, M.; Kaufmann, R.; Grundmann-Kollmann, M.; Geisslinger, G. Determination of 8-Methoxypsoralen in Human Plasma, and Microdialysates Using Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2003, 798, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kobyletzki, G.; Hoffmann, K.; Kerscher, M.; Altmeyer, P. Plasma Levels of 8-Methoxypsoralen Following PUVA-Bath Photochemotherapy. Photodermatol. Photoimmunol. Photomed. 1998, 14, 136–138. [Google Scholar] [CrossRef]
- Shephard, S.E.; Nestle, F.O.; Panizzon, R. Pharmacokinetics of 8-Methoxypsoralen during Extracorporeal Photopheresis. Photodermatol. Photoimmunol. Photomed. 1999, 15, 64–74. [Google Scholar] [CrossRef]
- Thomas, S.E.; O’Sullivan, J.; Balac, N. Plasma Levels of 8-Methoxypsoralen Following Oral or Bath-Water Treatment. Br. J. Dermatol. 1991, 125, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of Phytochemicals with Cyclodextrin Derivatives—An Insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest Developments in the Application of Cyclodextrin Host-Guest Complexes in Beverage Technology Processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.R.; Giordano, F.; Vilakazi, S.L. X-Ray Structure and Thermal Properties of a 1:1 Inclusion Complex Between Permethylated β-Cyclodextrin and Psoralen. Supramol. Chem. 2004, 16, 389–393. [Google Scholar] [CrossRef]
- Pathak, K.; Kumari, S. Cavamax W7 Composite Psoralen Ethosomal Gel versus Cavamax W7 Psoralen Solid Complex Gel for Topical Delivery: A Comparative Evaluation. Int. J. Pharm. Investig. 2013, 3, 171. [Google Scholar] [CrossRef]
- Vincieri, F.F.; Mazzi, G.; Mulinacci, N.; Bambagiotti-Alberti, M.; Dall’ Acqua, F.; Vedaldi, D. Improvement of Dissolution Characteristics of Psoralen by Cyclodextrins Complexation. Farmaco 1995, 50, 543–547. [Google Scholar]
- Loftsson, T.; Brewster, M.E.; Másson, M. Role of Cyclodextrins in Improving Oral Drug Delivery. Am. J. Drug Deliv. 2004, 2, 261–275. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the Cytotoxicity of β-Cyclodextrin Derivatives: Evidence for the Role of Cholesterol Extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations II: Solubilization, Binding Constant, and Complexation Efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the Unstirred Water Layer on Permeability Enhancement by Hydrophilic Cyclodextrins. Int. J. Pharm. 2007, 342, 250–253. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Hoffman, A.; Amidon, G.E.; Amidon, G.L. The Solubility-Permeability Interplay in Using Cyclodextrins as Pharmaceutical Solubilizers: Mechanistic Modeling and Application to Progesterone. J. Pharm. Sci. 2010, 99, 2739–2749. [Google Scholar] [CrossRef]
- Takahashi, A.I.; Veiga, F.J.B.; Ferraz, H.G. Literature Review of Cyclodextrins Inclusion Complexes Characterization—Part I: Phase Solubility Diagram, Dissolution and Scanning Electron Microscopy. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 531–539. [Google Scholar]
- Kurkov, S.V.; Ukhatskaya, E.V.; Loftsson, T. Drug/Cyclodextrin: Beyond Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 297–301. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of Cyclodextrin Solubilization of Drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as Solubilizers: Formation of Complex Aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Jaski, J.M.; Barão, C.E.; Morais Lião, L.; Silva Pinto, V.; Zanoelo, E.F.; Cardozo-Filho, L. β-Cyclodextrin Complexation of Extracts of Olive Leaves Obtained by Pressurized Liquid Extraction. Ind. Crop. Prod. 2019, 129, 662–672. [Google Scholar] [CrossRef]
- Takahashi, A.I.; Veiga, F.J.B.; Ferraz, H.G. A Literature Review of Cyclodextrin Inclusion Complexes Characterization—Part III: Differential Scanning Calorimetry and Thermogravimetry. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 16–20. [Google Scholar]
- Giordano, F.; Novak, C.; Moyano, J.R. Thermal Analysis of Cyclodextrins and Their Inclusion Compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Jafar, M.; Khalid, M.S.; Alghamdi, H.; Amir, M.; Al Makki, S.A.; Alotaibi, O.S.; Al Rmais, A.A.; Imam, S.S.; Alshehri, S.; Gilani, S.J. Formulation of Apigenin-Cyclodextrin-Chitosan Ternary Complex: Physicochemical Characterization, In Vitro and In Vivo Studies. AAPS PharmSciTech 2022, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Kachrimanis, K.; Djurić, Z.; Ibrić, S. Influence of Hydrophilic Polymers on the Complexation of Carbamazepine with Hydroxypropyl-β-Cyclodextrin. Eur. J. Pharm. Sci. 2015, 78, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alkholifi, F.K.; Alharbi, K.S.; Mostafa, E.M.; Alanazi, A.S.; Gilani, S.J.; Musa, A.; et al. Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics 2021, 13, 1997. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Da Silva, T.L.; Antunes, A.H.; Rezende, K.R. A Sensitive Medium-Throughput Method to Predict Intestinal Absorption in Humans Using Rat Intestinal Tissue Segments. J. Pharm. Sci. 2015, 104, 2807–2812. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Sá Couto, A.R. Self-Association of Cyclodextrins and Cyclodextrin Complexes in Aqueous Solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Pawar, B.M.; Rahman, S.N.R.; Pawde, D.M.; Goswami, A.; Shunmugaperumal, T. Orally Administered Drug Solubility-Enhancing Formulations: Lesson Learnt from Optimum Solubility-Permeability Balance. AAPS PharmSciTech 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Porat, D.; Dahan, A. Active Intestinal Drug Absorption and the Solubility-Permeability Interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Fine-Shamir, N.; Dahan, A. Methacrylate-Copolymer Eudragit EPO as a Solubility-Enabling Excipient for Anionic Drugs: Investigation of Drug Solubility, Intestinal Permeability, and Their Interplay. Mol. Pharm. 2019, 16, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.C.; de Almeida, P.H.G.; Ferreira, N.L.O.; Arruda, R.L.; Borges, L.L.; de Freitas, O.; da Conceição, E.C. Validation of a Photostability Indicating Method for Quantification of Furanocoumarins from Brosimum gaudichaudii Soft Extract. Rev. Bras. Farmacogn. 2018, 28, 118–123. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Stott, K.; Stonehouse, J.; Keeler, J.; Hwang, T.-L.; Shaka, A.J. Excitation Sculpting in High-Resolution Nuclear Magnetic Resonance Spectroscopy: Application to Selective NOE Experiments. J. Am. Chem. Soc. 1995, 117, 4199–4200. [Google Scholar] [CrossRef]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of Epithelial Cell Culture in Studies of Drug Transport. In Epithelial Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2002; Volume 188, pp. 233–272. [Google Scholar]
- Oltra-Noguera, D.; Mangas-Sanjuan, V.; Centelles-Sangüesa, A.; Gonzalez-Garcia, I.; Sanchez-Castaño, G.; Gonzalez-Alvarez, M.; Casabo, V.-G.; Merino, V.; Gonzalez-Alvarez, I.; Bermejo, M. Variability of Permeability Estimation from Different Protocols of Subculture and Transport Experiments in Cell Monolayers. J. Pharmacol. Toxicol. Methods 2015, 71, 21–32. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, R.D.; Silva, J.C.G.; Silva, L.A.D.; Oliveira, G.d.A.R.; Lião, L.M.; Lima, E.M.; de Morais, M.C.; da Conceição, E.C.; Rezende, K.R. Improvement in Solubility–Permeability Interplay of Psoralens from Brosimum gaudichaudii Plant Extract upon Complexation with Hydroxypropyl-β-cyclodextrin. Molecules 2022, 27, 4580. https://doi.org/10.3390/molecules27144580
Machado RD, Silva JCG, Silva LAD, Oliveira GdAR, Lião LM, Lima EM, de Morais MC, da Conceição EC, Rezende KR. Improvement in Solubility–Permeability Interplay of Psoralens from Brosimum gaudichaudii Plant Extract upon Complexation with Hydroxypropyl-β-cyclodextrin. Molecules. 2022; 27(14):4580. https://doi.org/10.3390/molecules27144580
Chicago/Turabian StyleMachado, Rúbia Darc, Júlio C. G. Silva, Luís A. D. Silva, Gerlon de A. R. Oliveira, Luciano M. Lião, Eliana M. Lima, Mariana C. de Morais, Edemilson C. da Conceição, and Kênnia R. Rezende. 2022. "Improvement in Solubility–Permeability Interplay of Psoralens from Brosimum gaudichaudii Plant Extract upon Complexation with Hydroxypropyl-β-cyclodextrin" Molecules 27, no. 14: 4580. https://doi.org/10.3390/molecules27144580
APA StyleMachado, R. D., Silva, J. C. G., Silva, L. A. D., Oliveira, G. d. A. R., Lião, L. M., Lima, E. M., de Morais, M. C., da Conceição, E. C., & Rezende, K. R. (2022). Improvement in Solubility–Permeability Interplay of Psoralens from Brosimum gaudichaudii Plant Extract upon Complexation with Hydroxypropyl-β-cyclodextrin. Molecules, 27(14), 4580. https://doi.org/10.3390/molecules27144580