Recent Advances in Age-Related Macular Degeneration Therapies
Abstract
1. Introduction
2. Pathogenesis, Development, and Characteristics of AMD
2.1. Epidemiology and Diagnosis
2.2. Age-Related Maculopathy (ARM) and Its Progression to AMD Advanced Stages
2.3. AMD Characteristics
2.3.1. Common Characteristics of the AMD Advanced Stages
2.3.2. Clinical Specificities of the Atrophic AMD form (Dry-AMD)
2.3.3. Clinical Specificities of the Exudative AMD form (Wet-AMD)
- Extrafoveal, when neovascularization is located between 200 µm and 2500 µm from the geometric center of the foveal avascular zone,
- Juxtafoveal, when neovascularization is restricted to an area up to 199 µm from the geometric center of the foveal avascular zone (this area may include portions of the foveal avascular zone),
- Subfoveal, when neovascularization is directly beneath the geometric center of the foveal avascular zone.
3. Ongoing Research and Trials in Therapeutic Options against the Atrophic form of AMD (Dry-AMD)
3.1. Visual Cycle
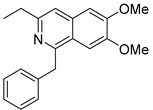
- Emixustat or ACU-4429
- CU239
- Fenretinide
- A1120
- ALK-001
| Drugs | Structure | Target | Clinic or Research | Formulation | Ref. |
|---|---|---|---|---|---|
| Emixustat |  | RPE65 | 1, 2 | Oral (Tablet) | NCT01802866 [61] NCT02130531 [57] NCT01002950 [58] |
| CU239 |  | RPE65 | R | - | [63] |
| Fenretinide |  | RBP4 | 2 | Oral capsules | NCT00429936 [69] |
| A1120 |  | RBP4 | R | - | [67] |
| ALK-001 |  | Vit. A | 1 | Oral capsules | NCT02230228 [74] NCT03845582 [75] |
3.2. β-Amyloid (Aβ)
- RN6G
- GSK933776
3.3. Choriocapillaries (CC) Atrophy
- MC-1101
- Moxaverine
- Sildenafil
- Trimetazidine
3.4. Oxidative Stress
3.4.1. Small Molecules
- OT-551
- Risuteganib
3.4.2. Nutritional Supplements
3.4.3. Neuroprotection
- CNTF
- Brimonidine Tartrate
- Tandospirone
| Drugs | Structure | Target | Clinic or Research | Formulation | Ref. |
|---|---|---|---|---|---|
| CNTF | Protein | Photo and RPE cells | 2 | intravitreal injections (Implant: NT-501) | NCT00447954 [131] |
| Brimonidine |  | Photo and RPE cells | 2 | intravitreal injections (Implant) | NCT00804921 [143] NCT00864838 [144] NCT00658619 [136] NCT02087085 [137] |
| Tandospirone |  | Photo and RPE cells | 3 | Eye drops | NCT00890097 [141] |
3.5. Inflammatory Pathways
3.5.1. Complement Cascade
- Lampalizumab
- Danicopan
- POT-4 and APL-2
- Eculizumab
- Tesidolumab
- CLG561
- Avacincaptad pegol or ARC1905 or Zimura®
- AAV5-VMD2-CR2-fH
- AAVCAGsCD59 or HMR59
- IONIS-FB-LRX
- GT005
- GEM103
3.5.2. Other Inflammatory Targets
- Sirolimus
- Glatiramer acetate
- Fluocinolone acetonide
| Drugs | Structure | Target | Clinic or Research | Formulation | Ref. |
|---|---|---|---|---|---|
| Lampalizumab | antigen-binding (Fab) fragment from monoclonal antibody | Factor D | 3 | Intravitreal injection | NCT01602120 [155] NCT02288559 [152] |
| Danicopan |  | Factor D | 2 | Oral tablet | NCT05019521 [162] |
| POT-4 |  | C3 | 2 | Intravitreal injection | NCT01603043 [164] |
| APL-2 |  | C3 | 2 | Intravitreal injection | NCT02503332 [167] NCT03777332 [169] NCT03525600 [170] |
| Eculizumab | monoclonal antibody | C5 | 2 | Intravenous | NCT00935883 [175] |
| Tesidolumab | fully human IgG1, monoclonal antibody | C5 | 2 | Intravitreal injection | NCT01255462 [178] NCT01527500 [179] NCT02515942 [180] |
| CLG56 | Human antibody | Properdin | 1 2 | Intravitreal injection | NCT01835015 [182] NCT02515942 [180,183] |
| Avacincaptad pegol | PEGylated nucleic acid aptamer | C5 | 1 2 3 | Intravitreal injection | NCT00950638 [185] NCT02686658 [186] NCT04435366 [187] |
| AAV5-VMD2-CR2-fH | ocular gene therapy product | Factor H | R | Intravitreal injection | [188] |
| AAVCAGsCD59 | ocular gene therapy product | MAC | 1 | Intravitreal injection | NCT03144999 [189] |
| IONIS-FB-LRX | ligand-conjugated (LICA) antisense | Factor B | 1 2 | Subcutaneously | ACTRN12616000335493 [191] NCT03446144 [192] NCT03815825 [193] |
| GT005 | AAV2 | Factor I | 1/2 | Subretinal injection | NCT03846193 [194] NCT04437368 [195] NCT04566445 [196] |
| GEM103 | Recombinant protein | Factor H | 1 2 | Intravitreal injection | NCT04246866 [197] NCT04643886 [198] |
| Sirolimus |  | mTOR | 2 | Intravitreal/Subconjunctival injection | NCT01675947 [202] NCT0071249 [203] NCT01445548 [204] |
| Glatiramer acetate |  | Inflammation | 1, 2, 3 | Injection | NCT00466076 [210] NCT00541333 [211] |
| Fluocinolone Acetonide | 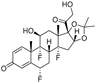 | Inflammation | 2 | Injection | NCT00695318 [215] |
3.6. RPE and Photoreceptors’ Loss: Stem Cells Curative Strategy
- Human-induced pluripotent stem cells (iPSC)
- Human embryonic stem cells (hESC)
- Human central nervous system (HuCNS-SC)
| Drugs | Origin | Goal | Clinic or Research | Formulation | Ref. |
|---|---|---|---|---|---|
| iPSC | Human fibroblast | RPE cells regeneration | - | Transplantation | NCT02464956 [222] |
| hESC | Central nervous | Replace RPE cells | 1/2 | Transplantation | NCT01344993 [226] NCT02463344 [227] NCT02590692 [229] |
| HuCNS-SC | Fertilization | Photoreceptors regeneration | 2 | Transplantation | NCT01632527 [233] NCT02467634 [234] NCT02137915 [236] |
- Conclusion on treatments for atrophic AMD
4. Ongoing Research and Clinical Trials for the Treatment of Exudative AMD (Wet-AMD)
4.1. Phototherapies
- Laser photocoagulation
- Photodynamic therapy (PDT)
4.2. Anti-VEGF Drugs
4.2.1. Marketed Drugs
- Pegaptanib sodium (Macugen®)
- Ranibizumab (Lucentis®)
- Aflibercept (Eylea®)
- Bevacizumab (Avastin®)
- Brolucizumab (ESBA 1008, RTH 258, Beovu®)
4.2.2. Future Trends
- Conbercept (Lumitin®)
- Abicipar pegol
- OPT-302
- Small-sized molecules
- Sorafenib
- Pazopanib
- Axitinib
- Acrizanib
- Regorafenib
- SH-11037
- PAN-90806
- Vorolanib
- Lenvatinib
- Brivanib
- Squalamine lactate (Evizon, OHR-102, MSI-1256F)
- siRNA target
- Bevasiranib
- siRNA-027 or AGN211745
- Gene therapies
- OXB-201 or RetinoStat®
- CRISPR-Cas9 ribonucleoproteins (RNPs)
- rAAV.sFlt-1
- AAV.sFLT-01
- ADVM-022
- RGX-314
4.3. Anti-PDGFs
- Pegleranib or E10030 (Fovista®)
- Rinucumab or REGN2176-3
4.4. Angiopoietin 2 Inhibitors
- Faricimab or RG7716
- Nesvacumab or REGN910-3
4.5. Miscellaneous Targets
4.6. Stem Cell Transplant
- Human-induced pluripotent stem cells (iPSC)
- Human embryonic stem cells (hESC)
- Conclusions on treatments for exudative AMD
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gohdes, D.M.; Balamurugan, A.; Larsen, B.A.; Maylahn, C. Age-Related Eye Diseases: An Emerging Challenge for Public Health Professionals. Prev. Chronic Dis. 2005, 2, A17. [Google Scholar] [PubMed]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. Biochemistry and Cell Biology. In The Eye; Elsevier: Amsterdam, The Netherlands, 2016; pp. 157–268.e4. [Google Scholar]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, Physiology and Barriers to Drug Delivery. In Ocular Transporters and Receptors: Their Role in Drug Delivery; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 1–36. ISBN 9781907568862. [Google Scholar]
- Moschos, M.M.; Nitoda, E.; Chatziralli, I.P.; Demopoulos, C.A. Age-Related Macular Degeneration: Pathogenesis, Genetic Background, and the Role of Nutritional Supplements. J. Chem. 2014, 2014, 317536. [Google Scholar] [CrossRef]
- Eye Care, Vision Impairment and Blindness. Available online: https://www.who.int/health-topics/blindness-and-vision-loss#tab=tab_1 (accessed on 25 April 2022).
- Cheng, K.J.; Hsieh, C.M.; Nepali, K.; Liou, J.P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef] [PubMed]
- Prokofyeva, E.; Zrenner, E. Epidemiology of Major Eye Diseases Leading to Blindness in Europe: A Literature Review. Ophthalmic Res. 2012, 47, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk Factors for Progression of Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Schachat, A.P.; Hawkins, B.S.; Keller, J.A.; Alexander, J.; Bloome, M.A.; Elman, M.J.; Margherio, R.R.; Sternberg, P. Laser Photocoagulation for Juxtafoveal Choroidal Neovascularization: Five-Year Results From Randomized Clinical Trials. Arch. Ophthalmol. 1994, 112, 500–509. [Google Scholar] [CrossRef]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global Prevalence of Vision Impairment and Blindness: Magnitude and Temporal Trends, 1990–2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Pascual-Camps, I.; Holz, F.G.; Finger, R.P. Prevalence, Incidence and Future Projection of Diabetic Eye Disease in Europe: A Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2020, 35, 11–23. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Linton, K.L.P. Prevalence of Age-Related Maculopathy: The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Wang, J.J.; Rochtchina, E.; Lee, A.J.; Chia, E.M.; Smith, W.; Cumming, R.G.; Mitchell, P. Ten-Year Incidence and Progression of Age-Related Maculopathy. The Blue Mountains Eye Study. Ophthalmology 2007, 114, 92–98. [Google Scholar] [CrossRef]
- Augood, C.A.; Vingerling, J.R.; De Jong, P.T.V.M.; Chakravarthy, U.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Bentham, G.; Rahu, M.; et al. Prevalence of Age-Related Maculopathy in Older Europeans: The European Eye Study (EUREYE). Arch. Ophthalmol. 2006, 124, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Y.; Zhang, L.; Wu, Q.; Tham, Y.; Rim, T.H.; Kithinji, D.M.; Wu, J.; Cheng, C.; Liang, H.; et al. Global Incidence, Progression, and Risk Factors of Age-Related Macular Degeneration and Projection of Disease Statistics in 30 Years: A Modeling Study. Gerontology 2021, 68, 721–735. [Google Scholar] [CrossRef] [PubMed]
- ETDRS Research Group. Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics: ETDRS Report Number 7. Ophthalmology 1991, 98, 741–756. [Google Scholar] [CrossRef]
- Klein, M.L.; Ferris, F.L.; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. Retinal Precursors and the Development of Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef]
- Pichi, F.; Abboud, E.B.; Ghazi, N.G.; Khan, A.O. Fundus Autofluorescence Imaging in Hereditary Retinal Diseases. Acta Ophthalmol. 2018, 96, e549–e561. [Google Scholar] [CrossRef]
- Prati, F.; Mallus, M.T.; Imola, F.; Albertucci, M. Optical Coherence Tomography (OCT). Catheter. Cardiovasc. Interv. Knowl.-Based Approach 2013, 254, 363–375. [Google Scholar] [CrossRef]
- Ly, A.; Nivison-Smith, L.; Assaad, N.; Kalloniatis, M. Infrared Reflectance Imaging in Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2016, 36, 303–316. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry Age-Related Macular Degeneration: Mechanisms, Therapeutic Targets, and Imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Mehta, S. Age-Related Macular Degeneration. Prim. Care 2015, 42, 377–391. [Google Scholar] [CrossRef]
- De Jong, P.T.V.M. Elusive Drusen and Changing Terminology of AMD Review-Article. Eye 2018, 32, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.M.; Akhtar, S.; Currie, Z. Ageing Changes in the Eye. Postgrad. Med. J. 2006, 82, 581–587. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, S.; Rajapakse, S.; Matsubara, J.A. Understanding AMD by Analogy: Systematic Review of Lipid-Related Common Pathogenic Mechanisms in AMD, AD, AS and GN. Lipids Health Dis. 2018, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Souka, A.A.R. Aflibercept in Age-Related Macular Degeneration: Evaluating Its Role as a Primary Therapeutic Option. Eye 2017, 31, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Hill, J.M.; Clement, C.; Sambamurti, K.; Dua, P.; Lukiw, W.J. Beta-Amyloid Precursor Protein (ΒAPP) Processing in Alzheimer’s Disease (AD) and Age-Related Macular Degeneration (AMD). Mol. Neurobiol. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Velez-montoya, R.; Oliver, S.C.N.; Olson, J.L.; Fine, S.L.; Mandava, N.; Quiroz-mercado, H. Current Knowledge and Trends in Age-Related Macular Degeneration. Retin. J. Retin. Vitr. Dis. 2013, 33, 1487–1502. [Google Scholar] [CrossRef]
- Cheung, L.K.; Eaton, A. Age-Related Macular Degeneration. Pharmacotherapy 2013, 33, 838–855. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy, Exosomes and Drusen Formation in Age-Related Macular Degeneration. Autophagy 2009, 5, 563–564. [Google Scholar] [CrossRef]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant Lipid and Protein Components of Drusen. PLoS ONE 2010, 5, e10329. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Clark, M.E.; Chimento, M.F.; Li, C.M.; Medeiros, N.E.; Curcio, C.A. Prevalence and Morphology of Druse Types in the Macula and Periphery of Eyes with Age-Related Maculopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Van Lookeren Campagne, M.; Lecouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of Age-Related Macular Degeneration and Therapeutic Opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Farazdaghi, M.; Ebrahimi, K. Role of the Choroid in Age-Related Macular Degeneration: A Current Review. J. Ophthalmic Vis. Res. 2019, 14, 78. [Google Scholar] [CrossRef]
- Arya, M.; Sabrosa, A.S.; Duker, J.S.; Waheed, N.K. Choriocapillaris Changes in Dry Age-Related Macular Degeneration and Geographic Atrophy: A Review. Eye Vis. 2018, 5, 22. [Google Scholar] [CrossRef]
- Leung, E.; Landa, G. Update on Current and Future Novel Therapies for Dry Age-Related Macular Degeneration. Expert Rev. Clin. Pharmacol. 2013, 6, 565–579. [Google Scholar] [CrossRef]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris Breakdown Precedes Retinal Degeneration in Age-Related Macular Degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef]
- Lengyel, I.; Tufail, A.; Al Hosaini, H.; Luthert, P.; Bird, A.C.; Jeffery, G. Association of Drusen Deposition with Choroidal Intercapillary Pillars in the Aging Human Eye. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2886–2892. [Google Scholar] [CrossRef]
- Guyer, D.R.; Fine, S.L.; Maguire, M.G.; Hawkins, B.S.; Owens, S.L.; Murphy, R.P. Subfoveal Choroidal Neovascular Membranes in Age-Related Macular Degeneration: Visual Prognosis in Eyes with Relatively Good Initial Visual Acuity. Arch. Ophthalmol. 1986, 104, 702–705. [Google Scholar] [CrossRef]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The Natural History and Prognosis of Neovascular Age-Related Macular Degeneration. A Systematic Review of the Literature and Meta-Analysis. Ophthalmology 2008, 115, 116–127. [Google Scholar] [CrossRef]
- Masse, H.; Wolff, B.; Bonnabel, A.; Bourhis, A.; Cornut, P.L.; De Bats, F.; Gualino, V.; Halfon, J.; Koehrer, P.; Souteyrand, G.; et al. Vue d’ensemble Des Pratiques Médicales Dans La DMLA Exsudative En France. J. Fr. Ophtalmol. 2016, 39, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, H.; Kase, S.; Shirasawa, M.; Otsuka, H.; Hisatomi, T.; Sonoda, S. TNF- a Decreases VEGF Secretion in Highly Polarized RPE Cells but Increases It in Non-Polarized RPE Cells Related to Crosstalk between JNK and NF-k B Pathways. PLoS ONE 2013, 8, e69994. [Google Scholar] [CrossRef] [PubMed]
- Blaauwgeers, H.G.T.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.E.; Kijlstra, A.; Van Hinsbergh, V.W.M.; et al. Polarized Vascular Endothelial Growth Factor Secretion by Human Retinal Pigment Epithelium and Localization of Vascular Endothelial Growth Factor Receptors on the Inner Choriocapillaris: Evidence for a Trophic Paracrine Relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef]
- Constable, I.; Shen, W.Y.; Rakoczy, E. Emerging Biological Therapies for Age-Related Macula Degeneration. Expert Opin. Biol. Ther. 2005, 5, 1373–1385. [Google Scholar] [CrossRef]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete Response to Anti-VEGF Therapy in Neovascular AMD: Exploring Disease Mechanisms and Therapeutic Opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C. Vitamin a Metabolism in Rod and Cone Visual Cycles. Annu. Rev. Nutr. 2012, 32, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Gregori, N.Z.; Ciulla, T.A.; Lam, B.L.; Hussain, R.M.; Gregori, N.Z.; Ciulla, T.A.; Lam, B.L. Pharmacotherapy of Retinal Disease with Visual Cycle Modulators. Expert Opin. Pharmacother. 2018, 19, 471–481. [Google Scholar] [CrossRef]
- Waugh, N.; Loveman, E.; Colquitt, J.; Royle, P.; Yeong, J.L.; Hoad, G.; Lois, N. Treatments for Dry Age-Related Macular Degeneration and Stargardt Disease: A Systematic Review. Health Technol. Assess. 2018, 22, 1–196. [Google Scholar] [CrossRef]
- Saad, L.; Washington, I. Can Vitamin A Be Improved to Prevent Blindness Due to Age-Related Macular Degeneration, Stargardt Disease and Other Retinal Dystrophies? Adv. Exp. Med. Biol. 2016, 854, 355–361. [Google Scholar] [CrossRef]
- Kubota, R.; Boman, N.L.; David, R.; Mallikaarjun, S.; Patil, S.; Birch, D. Safety and Effect on Rod Function of ACU-4429, a Novel Small-Molecule Visual Cycle Modulator. Retina 2012, 32, 183–188. [Google Scholar] [CrossRef]
- Kubota, R.Y.O.; Al-fayoumi, S.; Mallikaarjun, S. Phase 1, Dose-Ranging Study of Emixustat Hydrochloride (ACU-4429), a Novel Visual Cycle Modulator, in Healthy Volunteers. Retina 2014, 34, 603–609. [Google Scholar] [CrossRef]
- Bavik, C.; Henry, S.H.; Zhang, Y.; Mitts, K.; McGinn, T.; Budzynski, E.; Pashko, A.; Lieu, K.L.; Zhong, S.; Blumberg, B.; et al. Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity. PLoS ONE 2015, 10, e0124940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular Pharmacodynamics of Emixustat in Protection against Retinal Degeneration. J. Clin. Investig. 2015, 125, 2781–2794. [Google Scholar] [CrossRef] [PubMed]
- Pharmacokinetic and Pharmacodynamic Study of Emixustat in Subjects with Geographic Atrophy Associated with Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02130531?term=NCT02130531&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Study of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of ACU-4429 in Subjects with Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT01002950?term=NCT01002950&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Dugel, P.U.; Novack, R.L.; Csaky, K.G.; Richmond, P.P.; Birch, D.G.; Kubota, R. Phase II, Randomized, Placebo-Controlled, 90-Day Study of Emixustat Hydrochloride in Geographic Atrophy Associated with Dry Age-Related Macular Degeneration. Retina 2015, 35, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ciulla, T.A.; Berrocal, A.M.; Gregori, N.Z.; Flynn, H.W.; Lam, B.L. Stargardt Macular Dystrophy and Evolving Therapies. Expert Opin. Biol. Ther. 2018, 18, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy Assessment Treatment Trials of Emixustat Hydrochloride. Available online: https://clinicaltrials.gov/ct2/show/NCT01802866?term=NCT01802866&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Rosenfeld, P.J.; Dugel, P.U.; Holz, F.G.; Heier, J.S.; Pearlman, J.A.; Novack, R.L.; Csaky, K.G.; Koester, J.M.; Gregory, J.K.; Kubota, R. Emixustat Hydrochloride for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Clinical Trial. Ophthalmology 2018, 125, 1556–1567. [Google Scholar] [CrossRef]
- Shin, Y.; Moiseyev, G.; Petrukhin, K.; Cioffi, C.L.; Muthuraman, P.; Takahashi, Y.; Ma, J.X. A Novel RPE65 Inhibitor CU239 Suppresses Visual Cycle and Prevents Retinal Degeneration. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2420–2429. [Google Scholar] [CrossRef]
- Berni, R.; Formelli, F. In Vitro Interaction of Fenretinide with Plasma Retinol-Binding Protein and Its Functional Consequences. FEBS Lett. 1992, 308, 43–45. [Google Scholar] [CrossRef]
- Malpeli, G.; Folli, C.; Berni, R. Retinoid Binding to Retinol-Binding Protein and the Interference with the Interaction with Transthyretin. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1996, 1294, 48–54. [Google Scholar] [CrossRef]
- Mata, N.L.; Lichter, J.B.; Vogel, R.; Han, Y.; Bui, T.V.; Singerman, L.J. Investigation of Oral Fenretinide for Treatment of Geographic Atrophy in Age-Related Macular Degeneration. Retina 2013, 33, 498–507. [Google Scholar] [CrossRef]
- Dobri, N.; Qin, Q.; Kong, J.; Yamamoto, K.; Liu, Z.; Moiseyev, G.; Ma, J.X.; Allikmets, R.; Sparrow, J.R.; Petrukhin, K. A1120, a Nonretinoid RBP4 Antagonist, Inhibits Formation of Cytotoxic Bisretinoids in the Animal Model of Enhanced Retinal Lipofuscinogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 85–95. [Google Scholar] [CrossRef]
- Edgar, M.; Lichter, J.; Mata, N.L. Compositions and Methods for Treating Ophthalmic Conditions. Patent WO2012/078525 A2, 5 April 2018. [Google Scholar]
- Study of Fenretinide in the Treatment of Geographic Atrophy Associated with Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00429936?term=Fenretinide&cond=AMD&draw=2&rank=1 (accessed on 10 June 2020).
- Cioffi, C.L.; Dobri, N.; Freeman, E.E.; Conlon, M.P.; Chen, P.; Stafford, D.G.; Schwarz, D.M.C.; Golden, K.C.; Zhu, L.; Kitchen, D.B.; et al. Design, Synthesis, and Evaluation of Nonretinoid Retinol Binding Protein 4 Antagonists for the Potential Treatment of Atrophic Age-Related Macular Degeneration and Stargardt Disease. J. Med. Chem. 2014, 57, 7731–7757. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.L.; Racz, B.; Freeman, E.E.; Conlon, M.P.; Chen, P.; Stafford, D.G.; Schwarz, D.M.C.; Zhu, L.; Kitchen, D.B.; Barnes, K.D.; et al. Bicyclic [3.3.0]-Octahydrocyclopenta[c]Pyrrolo Antagonists of Retinol Binding Protein 4: Potential Treatment of Atrophic Age-Related Macular Degeneration and Stargardt Disease. J. Med. Chem. 2015, 58, 5863–5888. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.L.; Muthuraman, P.; Raja, A.; Varadi, A.; Racz, B.; Petrukhin, K. Discovery of Bispecific Antagonists of Retinol Binding Protein 4 That Stabilize Transthyretin Tetrame. J. Med. Chem. Chem. 2020, 63, 11054–11084. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; Van Lookeren Campagne, M. Geographic Atrophy: Clinical Features and Potential Therapeutic Approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Phase 1 Safety Study of ALK-001 in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT02230228?term=ALK-001&cond=AMD&draw=2&rank=4 (accessed on 10 June 2020).
- Phase 3 Study of ALK-001 in Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT03845582 (accessed on 20 July 2020).
- Ding, J.D.; Johnson, L.V.; Herrmann, R.; Farsiu, S.; Smith, S.G.; Groelle, M.; Mace, B.E.; Sullivan, P.; Jamison, J.A.; Kelly, U.; et al. Anti-Amyloid Therapy Protects against Retinal Pigmented Epithelium Damage and Vision Loss in a Model of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 279–287. [Google Scholar] [CrossRef]
- Lashkari, K.; Teague, G.; Chen, H.; Lin, Y.; Kumar, S.; Mclaughlin, M.M.; Lo, F.J. A Monoclonal Antibody Targeting Amyloid β (A β) Restores Complement Factor I Bioactivity: Potential Implications in Age-Related Macular Degeneration and Alzheimer’s Disease. PLoS ONE 2018, 13, e0195751. [Google Scholar] [CrossRef]
- Damico, F.M.; Gasparin, F.; Scolari, M.R.; Pedral, L.S.; Takahashi, B.S. New Approaches and Potential Treatments for Dry Age-Related Macular Degeneration. Arq. Bras. Oftalmol. 2012, 75, 71–76. [Google Scholar] [CrossRef]
- Safety and Tolerability Study of RN6G in Subjects with Advanced Dry, Age-Related Macular Degeneration including Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT01003691?term=RN6G&cond=AMD&draw=2&rank=2 (accessed on 24 June 2020).
- Safety and Tolerability Study of RN6G in Patients with Dry, Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00877032?term=RN6G&cond=AMD&draw=2&rank=3 (accessed on 24 June 2020).
- Efficacy, Safety and Tolerability Study of RN6G in Subjects with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01577381?term=RN6G&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Andreasen, N.; Simeoni, M.; Ostlund, H.; Lisjo, P.I.; Fladby, T.; Loercher, A.E.; Byrne, G.J.; Murray, F.; Scott-Stevens, P.T.; Wallin, A.; et al. First Administration of the Fc-Attenuated Anti-β Amyloid Antibody GSK933776 to Patients with Mild Alzheimer’s Disease: A Randomized, Placebo-Controlled Study. PLoS ONE 2015, 10, e0098153. [Google Scholar] [CrossRef]
- Pharmacokinetic (PK) Study of GSK933776 in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT02033668?term=GSK933776&cond=AMD&draw=2&rank=2 (accessed on 15 June 2020).
- Clinical Study to Investigate Safety and Efficacy of GSK933776 in Adult Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01342926?term=GSK933776&cond=AMD&draw=2&rank=1 (accessed on 15 June 2020).
- Rosenfeld, P.J.; Berger, B.; Reichel, E.; Danis, R.P.; Gress, A.; Ye, L.; Magee, M.; Parham, L.R.; McLaughlin, M.M. A Randomized Phase 2 Study of an Anti–Amyloid β Monoclonal Antibody in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmol. Retin. 2018, 2, 1028–1040. [Google Scholar] [CrossRef]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The Dynamic Nature of Bruch’s Membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.A.; Woller, W.H. Therapeutic Formulation and Methods of. Treatment. Patent WO2013/188217 A1, 9 February 2016. [Google Scholar]
- U.S. Food and Drug Administration. Apresoline Hydrochloride; Ciba-Geigy Corporation Pharmaceuticals Division: Summit, NJ, USA, 1997. [Google Scholar]
- Chiou, B.G. Is Dry AMD Treatable? Retin. Today 2012, 1101, 69–71. [Google Scholar]
- A Pilot Study of the Safety of MC-1101 in both Normal Volunteers and Patients with Early Dry AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01013376?term=MC-1101&cond=AMD&draw=2&rank=1 (accessed on 18 June 2020).
- Safety Study of a Topical Treatment for Dry Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01922128?term=MC-1101&cond=AMD&draw=2&rank=4 (accessed on 18 June 2020).
- Efficacy and Safety Study of MC-1101 1% TID in the Treatment of Nonexudative Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01601483?term=MC-1101&cond=AMD&draw=2&rank=3 (accessed on 18 June 2020).
- Pemp, B.; Garhofer, G.; Lasta, M.; Schmidl, D.; Wolzt, M.; Schmetterer, L. The Effects of Moxaverine on Ocular Blood Flow in Patients with Age-Related Macular Degeneration or Primary Open Angle Glaucoma and in Healthy Control Subjects. Acta Ophthalmol. 2012, 90, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Resch, H.; Weigert, G.; Karl, K.; Pemp, B.; Garhofer, G.; Schmetterer, L. Effect of Systemic Moxaverine on Ocular Blood Flow in Humans. Acta Ophthalmol. 2009, 87, 731–735. [Google Scholar] [CrossRef] [PubMed]
- An Open Study Comparing the Effects of Moxaverine on Ocular Blood Flow in Patients with Age-Related Macular Degeneration, Primary Open Angle Glaucoma and Healthy Control Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT00709449?term=Moxaverine&cond=AMD&draw=2&rank=1 (accessed on 22 June 2020).
- Metelitsina, T.I.; Grunwald, J.E.; DuPont, J.C.; Ying, G.S.; Liu, C. Effect of Viagra on Retinal Vein Diameter in AMD Patients. Exp. Eye Res. 2006, 83, 128–132. [Google Scholar] [CrossRef]
- Effects of Sildenafil on Choroidal Thickness in AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01830790?term=NCT01830790&draw=2&rank=1 (accessed on 17 July 2020).
- Vuong, V.S.; Tran, S.; Migacz, J.V.; Gorczynska, I.; Cunefare, D.; Farsiu, S.; Yiu, G. Effect of Sildenafil Citrate on Choroidal Thickness in Age-Related Macular Degeneration. IOVS ARVO J. 2017, 58, 13. [Google Scholar]
- Yiu, G.; Vuong, V.S.; Tran, S.; Migacz, J.; Cunefare, D.; Farsiu, S.; Khandelwal, N.; Agrawal, R.; Cheung, C.M.G. Vascular Response to Sildenafil Citrate in Aging and Age-Related Macular Degeneration. Sci. Rep. 2019, 9, 5049. [Google Scholar] [CrossRef]
- Kaszuba-Bartkowiak, K.; Nowak, M.S.; Jurowski, P.; Goś, R. The Role of Trimetazidine in the Protection of the Retina. Arch. Med. Sci. 2007, 2007, 66. [Google Scholar]
- Villa, R.F.; Benzi, G.; Curti, D. The Effect of Ischemia and Pharmacological Treatment Evaluated on Synaptosomes and Purified Mitochondria from Rat Cerebral Cortex. Biochem. Pharmacol. 1981, 30, 2399–2408. [Google Scholar] [CrossRef]
- Nowak, M.S.; Wybór, K.; Goś, R.; Zeman-Miecznik, A.; Waszczykowska, A.; Pastuszka, M.; Kłysik, A.; Gajdowska, A. Protective Effect on Visual Functions of Long-Term Use of Trimetazidine in Treatment of Primary Open Angle Glaucoma and Degenerative Myopia. Arch. Med. Sci. 2007, 3, 152–156. [Google Scholar]
- Cohen, S.Y.; Bourgeois, H.; Corbe, C.; Chaine, G.; Espinasse-Berrod, M.A.; Garcia-Sanchez, J.; Gaudric, A.; Hullo, A.; Leys, A.; Soubrane, G.; et al. Randomized Clinical Trial France DMLA2: Effect of Trimetazidine on Exudative and Nonexudative Age-Related Macular Degeneration. Retina 2012, 32, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Klettner, A.; Kauppinen, A.; Blasiak, J.; Roider, J.; Salminen, A.; Kaarniranta, K. Cellular and Molecular Mechanisms of Age-Related Macular Degeneration: From Impaired Autophagy to Neovascularization. Int. J. Biochem. Cell Biol. 2013, 45, 1457–1467. [Google Scholar] [CrossRef]
- Lipecz, A.; Miller, L.; Kovacs, I.; Czakó, C.; Csipo, T.; Baffi, J.; Csiszar, A.; Tarantini, S.; Ungvari, Z.; Yabluchanskiy, A.; et al. Microvascular Contributions to Age-Related Macular Degeneration (AMD): From Mechanisms of Choriocapillaris Aging to Novel Interventions. GeroScience 2019, 41, 813–845. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium (RPE) Led to Metabolic Dysfunction in Both the RPE and Retinal Photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; Bindewald, A.; Holz, F.G. Keypathophysiologic Pathways in Age-Related Macular Disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 710–716. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-Neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–2018. [Google Scholar] [CrossRef]
- Woodell, A.; Rohrer, B. A Mechanistic Review of Cigarette Smoke and Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2014, 801, 301–307. [Google Scholar] [CrossRef]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide Pharmaceutical Development for Age-Related Degeneration and Disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef]
- OT-551 Antioxidant Eye Drops to Treat Geographic Atrophy in Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00306488?term=OT-551&cond=AMD&draw=2&rank=1 (accessed on 23 June 2020).
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of Geographic Atrophy by the Topical Administration of OT-551: Results of a Phase II Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef]
- The OMEGA Study: Use of Eye Drops to Treat Geographic Atrophy Associated with Age-Related Macular Degeneration (Dry AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00485394?term=NCT00485394&cond=AMD&draw=2&rank=1 (accessed on 23 June 2020).
- Sternberg, P.; Rosenfeld, P.J.; Slakter, J.S.; Koester, J.M.; Reaves, A. Topical OT-551 for Treating Geographic Atrophy: Phase II Results. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6416. [Google Scholar]
- A Clinical Trial Designed to Evaluate the Safety and Exploratory Efficacy of 1.0 Mg Luminate® (Alg-1001) As a Treatment for Non-Exudative Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03626636?term=Risuteganib&cond=AMD&draw=2&rank=1 (accessed on 23 June 2020).
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib—a Novel Integrin Inhibitor for the Treatment of Non-Exudative (Dry) Age-Related Macular Degeneration and Diabetic Macular Edema. Expert Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, L.; Ristau, T.; Lechanteur, Y.T.; Hahn, M.; Hoyng, C.B.; Kirchhof, B.; Den Hollander, A.I.; Fauser, S. Nutritional Risk Factors for Age-Related Macular Degeneration. Biomed Res. Int. 2014, 2014, 413150. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss. Arch. Ophthalmol. 2001, 119, 1417–1436. [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L.; Group, A.R. The Age-Related Eye Disease Study 2 (AREDS2): Study Design and Baseline Characteristics (AREDS2 Report Number 1). Ophthalmology 2013, 119, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.A.; Fellow, O.; Eye, C. Age Related Macular Degeneration Should Your Patients Be Taking Additional. Clin. Pract. 2007, 36, 1026–1028. [Google Scholar]
- Cangemi, F.E. TOZAL Study: An Open Case Control Study of an Oral Antioxidant and Omega-3 Supplement for Dry AMD. BMC Ophthalmol. 2007, 7, 3. [Google Scholar] [CrossRef]
- Mandal, N.A.; Patlolla, J.M.R.; Zheng, L.; Agbaga, M.; Tran, J.A.; Wicker, L.; Kasus-jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin Protects Retinal Cells from Light-and Oxidant Stress-Induced Cell Death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, W.; Yuan, S.; Chen, Z.; Yang, Q.; Yuan, D.; Wang, F. Suppression of Experimental Choroidal Neovascularization by Curcumin in Mice. PLoS ONE 2012, 7, e53329. [Google Scholar] [CrossRef]
- Meng, Y.; Tao, J. Effect of Curcumin on Aging Retinal Pigment Epithelial Cells. Drug Des. Devel. Ther. 2015, 9, 5337–5344. [Google Scholar] [CrossRef][Green Version]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Evans, J. Antioxidant Supplements to Prevent or Slow down the Progression of AMD: A Systematic Review and Meta-Analysis. Eye 2008, 22, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, W.; Luo, L.; Huang, D.; Kauper, K.; Stabila, P.; Matthew, M.; Laties, A.M.; Wen, R. CNTF Induces Regeneration of Cone Outer Segments in a Rat Model of Retinal Degeneration. PLoS ONE 2010, 5, e9495. [Google Scholar] [CrossRef] [PubMed]
- Do Rhee, K.; Nusinowitz, S.; Chao, K.; Yu, F.; Bok, D.; Yang, X. CNTF-Mediated Protection of Photoreceptors Requires Initial Activation of the Cytokine Receptor Gp130 in Müller Glial Cells. Proc. Natl. Acad. Sci. USA 2013, 110, E4520–E4529. [Google Scholar] [CrossRef]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-Year Intraocular Delivery of Ciliary Neurotrophic Factor by Encapsulated Cell Technology Implants in Patients with Chronic Retinal Degenerative Diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef] [PubMed]
- A Study of an Encapsulated Cell Technology (ECT) Implant for Patients with Atrophic Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00447954?term=NT-501&cond=AMD&draw=2&rank=1 (accessed on 13 June 2020).
- Cantor, L.B. The Evolving Pharmacotherapeutic Profile of Brimonidine, an A2-Adrenergic Agonist, after Four Years of Continuous Use. Expert Opin. Pharmacother. 2000, 1, 815–834. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Alphagan; Allergan: Irvine, CA, USA, 2016. [Google Scholar]
- Walters, T.R. Development and Use of Brimonidine in Treating Acute and Chronic Elevations of Intraocular Pressure: A Review of Safety, Efficacy, Dose Response, and Dosing Studies. Surv. Ophthalmol. 1996, 41, S19–S26. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Biodegradable Intraocular Therapies for Retinal Disorders: Progress to Date. Drugs Aging 2010, 27, 117–134. [Google Scholar] [CrossRef]
- Safety and Efficacy of Brimonidine Intravitreal Implant in Patients with Geographic Atrophy Due to Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00658619?term=Brimonidine&cond=AMD&draw=2&rank=1 (accessed on 13 June 2020).
- A Safety and Efficacy Study of Brimonidine Intravitreal Implant in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02087085?term=Brimonidine&cond=AMD&draw=1&rank=2 (accessed on 13 June 2020).
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; López, F.J. Phase 2 Study of the Safety and Efficacy of Brimonidine Drug Delivery System (Brimo DDS) Generation 1 in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 2021, 41, 144–155. [Google Scholar] [CrossRef]
- Freeman, W.R. Brimonidine Drug Delivery System for Geographic Atrophy. Available online: https://www.retinalphysician.com/issues/2019/november-2019/brimonidine-drug-delivery-system-for-geographic-at (accessed on 15 July 2020).
- Collier, R.J.; Wang, Y.; Smith, S.S.; Martin, E.; Ornberg, R. Complement Deposition and Microglial Activation in the Outer Retina in Light-Induced Retinopathy: Inhibition by a 5-HT 1A Agonist. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8108–8116. [Google Scholar] [CrossRef]
- Geographic Atrophy Treatment Evaluation. Available online: https://clinicaltrials.gov/ct2/show/NCT00890097?term=NCT00890097&draw=2&rank=1 (accessed on 11 June 2020).
- Jaffe, G.J.; Schmitz-Valckenberg, S.; Boyer, D.; Heier, J.; Wolf-Schnurrbusch, U.; Staurenghi, G.; Schmidt-Erfurth, U.; Holz, F.G. Randomized Trial to Evaluate Tandospirone in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The GATE Study. Am. J. Ophthalmol. 2015, 160, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Effectiveness of Oral Acetazolamide, Brimonidine Tartarate, and Anterior Chamber Paracentesis in Intraocular Pressure (IOP) After Bevacizumab. Available online: https://clinicaltrials.gov/ct2/show/NCT00804921?term=Brimonidine&cond=AMD&draw=2&rank=4 (accessed on 13 June 2020).
- Oral Acetazolamide, Brimonidine Tartarate, and Anterior Chamber Paracentesis for Ocular Hypertension Control after Intravitreal Bevacizumab. Available online: https://clinicaltrials.gov/ct2/show/NCT00864838?term=Brimonidine&cond=AMD&draw=2&rank=3 (accessed on 13 June 2020).
- Whitcup, S.M.; Sodhi, A.; Atkinson, J.P.; Holers, V.M.; Sinha, D.; Rohrer, B.; Dick, A.D. The Role of the Immune Response in Age-Related Macular Degeneration. Int. J. Inflam. 2013, 2013, 348092. [Google Scholar] [CrossRef]
- Wakefield, D.; Lloyd, A. The Role of Cytokines in the Pathogenesis of Inflammatory Eye Disease. Cytokine 1992, 4, 1–5. [Google Scholar] [CrossRef]
- Davey, M.P.; Rosenbaum, J.T. The Human Leukocyte Antigen Complex and Chronic Ocular Inflammatory Disorders. Am. J. Ophthalmol. 2000, 129, 235–243. [Google Scholar] [CrossRef]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and Its Role in Age-Related Macular Degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Schmidt-Erfurth, U.; Van Lookeren Campagne, M.; Henry, E.C.; Brittain, C. The Pathophysiology of Geographic Atrophy Secondary to Age-Related Macular Degeneration and the Complement Pathway as a Therapeutic Target. Retina 2017, 37, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.O.; Ritter, R.; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef]
- A Study of Safety, Tolerability, and Evidence of Activity of FCFD4514S Administered Monthly or Every Other Month to Patients with Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT01229215?term=NCT01229215&cond=AMD&draw=2&rank=1 (accessed on 16 July 2020).
- A Study of Lampalizumab Intravitreal Injections Administered Every Two Weeks or Every Four Weeks to Participants with Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT02288559?term=Lampalizumab&draw=2&rank=2 (accessed on 30 April 2020).
- Yaspan, B.L.; Williams, D.F.; Holz, F.G.; Regillo, C.D.; Li, Z.; Dressen, A.; Van Lookeren Campagne, M.; Le, K.N.; Graham, R.R.; Beres, T.; et al. Targeting Factor D of the Alternative Complement Pathway Reduces Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration. Sci. Transl. Med. 2017, 9, eaaf1443. [Google Scholar] [CrossRef]
- Hariri, A.; Nittala, M.G.; Sadda, S.R. Outer Retinal Tubulation as a Predictor of the Enlargement Amount of Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology 2015, 122, 407–413. [Google Scholar] [CrossRef]
- An Extension Study to Evaluate the Long-Term Safety of Lampalizumab in Participants with Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT01602120?term=Lampalizumab&draw=2&rank=3 (accessed on 30 April 2020).
- A Study Investigating the Efficacy and Safety of Lampalizumab Intravitreal Injections in Participants with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02247479?term=Lampalizumab&cond=AMD&draw=2&rank=3 (accessed on 16 July 2020).
- A Study Investigating the Safety and Efficacy of Lampalizumab Intravitreal Injections in Participants with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02247531?term=Lampalizumab&cond=AMD&draw=2&rank=2 (accessed on 16 July 2020).
- Long-Term Safety of Lampalizumab Intravitreal (ITV) Injections in Participants with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration (OMASPECT). Available online: https://clinicaltrials.gov/ct2/show/NCT02745119?term=Lampalizumab&cond=AMD&draw=2&rank=1 (accessed on 16 July 2020).
- Roche Roche Provides Update on First Lampalizumab Phase III Study for Geographic Atrophy, an Advanced Form of Age-Related Macular Degeneration. Available online: https://www.roche.com/media/releases/med-cor-2017-09-08b.htm (accessed on 20 July 2020).
- Yuan, X.; Gavriilaki, E.; Thanassi, J.A.; Yang, G.; Baines, A.C.; Podos, S.D.; Huang, Y.; Huang, M.; Brodsky, R.A. Small-Molecule Factor D Inhibitors Selectively Block the Alternative Pathway of Complement in Paroxysmal Nocturnal Hemoglobinuria and Atypical Hemolytic Uremic Syndrome. Haematologica 2017, 102, 466–475. [Google Scholar] [CrossRef]
- Risitano, A.M.; Kulasekararaj, A.G.; Lee, J.W.; Maciejewski, J.P.; Notaro, R.; Brodsky, R.; Huang, M.; Geffner, M.; Browett, P. Danicopan: An Oral Complement Factor D Inhibitor for Paroxysmal Nocturnal Hemoglobinuria. Haematologica 2021, 106, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- A Study of Danicopan in Participants with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT05019521?term=Danicopan&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 11 April 2022).
- Narayanan, R.; Kuppermann, B.D. Corticosteroids and Anti-Complement Therapy in Retinal Diseases. Pharmacol. Ther. Ocul. Dis. 2016, 242, 309–320. [Google Scholar] [CrossRef]
- A Multicenter, Proof-of-Concept Study of Intravitreal AL-78898A in Patients with Geographic Atrophy (GA) Associated with Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01603043?term=AL-78898A&cond=AMD&draw=2&rank=2 (accessed on 8 July 2020).
- Park, D.H.; Connor, K.M.; Lambris, J.D.; Clark, S.J. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007. [Google Scholar] [CrossRef]
- Kassa, E.; Ciulla, T.A.; Hussain, R.M.; Dugel, P.U. Complement Inhibition as a Therapeutic Strategy in Retinal Disorders. Expert Opin. Biol. Ther. 2019, 19, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Study of APL-2 Therapy in Patients Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT02503332?term=APL-2&cond=AMD&draw=2&rank=6 (accessed on 6 May 2020).
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Study to Evaluate the Safety of Intravitreal APL-2 in Patients Diagnosed with Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT03777332?term=APL-2&cond=AMD&draw=2&rank=5 (accessed on 6 May 2020).
- Study to Compare the Efficacy and Safety of Intravitreal APL-2 Therapy with Sham Injections in Patients with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03525600?term=APL-2&cond=AMD&draw=2&rank=3 (accessed on 6 May 2020).
- A Study to Compare the Efficacy and Safety of Intravitreal APL-2 Therapy with Sham Injections in Patients with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03525613?term=APL-2&cond=Age-Related+Macular+Degeneration&draw=2&rank=5 (accessed on 7 April 2022).
- An Extension Study to Evaluate the Long-Term Safety and Efficacy of Pegcetacoplan (APL-2) in Subjects with Geographic Atrophy Secondary to AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT04770545 (accessed on 8 April 2022).
- Apellis Announces Top-Line Results from Phase 3 DERBY and OAKS Studies in Geographic Atrophy (GA) and Plans to Submit NDA to FDA in the First Half of 2022|Apellis Pharmaceuticals, Inc. Available online: https://investors.apellis.com/news-releases/news-release-details/apellis-announces-top-line-results-phase-3-derby-and-oaks (accessed on 8 April 2022).
- U.S. Food and Drug Administration. Soliris; Alexion Pharmaceuticals, Inc.: Cheshire, CT, USA, 2007. [Google Scholar]
- Complement Inhibition with Eculizumab for the Treatment of Non-Exudative Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00935883?term=Eculizumab&cond=AMD&draw=2&rank=1 (accessed on 28 April 2020).
- Stetson, P.F.; Yehoshua, Z.; Garcia Filho, C.A.A.; Nunes, R.P.; Gregori, G.; Rosenfeld, P.J. OCT Minimum Intensity as a Predictor of Geographic Atrophy Enlargement. Investig. Ophthalmol. Vis. Sci. 2014, 55, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Yehoshua, Z.; Alexandre De Amorim Garcia Filho, C.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic Complement Inhibition with Eculizumab for Geographic Atrophy in Age-Related Macular Degeneration: The COMPLETE Study. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef]
- Intravitreal LFG316 in Patients with Advanced Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01255462?term=LFG316&cond=AMD&draw=2&rank=4 (accessed on 4 May 2020).
- Intravitreal LFG316 in Patients with Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01527500?term=NCT01527500&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- CLG561 Proof-of-Concept Study as a Monotherapy and in Combination with LFG316 in Subjects with Geographic Atrophy (GA). Available online: https://clinicaltrials.gov/ct2/show/NCT02515942?term=NCT02515942&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Chen, J.Y.; Cortes, C.; Ferreira, V.P. Properdin: A Multifaceted Molecule Involved in Inflammation and Diseases. Mol. Immunol. 2018, 102, 58–72. [Google Scholar] [CrossRef]
- Pharmacokinetics of CLG561 in Patients with Advanced Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01835015?term=CLG561&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 11 April 2022).
- Glazer, L.C.; Williams, J.G.; Gordon, C.M.; Dugel, P.U.; Milton, M.; Valencia, T.; Klein, U.; Kretz, S.; Gedif, K.; Grosskreutz, C.L.; et al. A First in Human Study of Intravitreal (IVT) CLG561 in Subjects with Advanced Age-Related Macular Degeneration (AMD). IOVS 2016, 57, 2672. [Google Scholar]
- Park, E.J.; Choi, J.; Lee, K.C.; Na, D.H. Emerging PEGylated Non-Biologic Drugs. Expert Opin. Emerg. Drugs 2019, 24, 107–119. [Google Scholar] [CrossRef]
- A Study of ARC1905 (Anti-C5 Aptamer) in Subjects with Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00950638?term=NCT00950638&cond=AMD&draw=2&rank=1 (accessed on 17 July 2020).
- Zimura in Subjects with Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02686658?term=ARC-1905&cond=Geographic+AMD&draw=2&rank=1 (accessed on 29 April 2020).
- A Phase 3 Safety and Efficacy Study of Intravitreal Administration of Zimura (Complement C5 Inhibitor). Available online: https://clinicaltrials.gov/ct2/show/NCT04435366?term=Avacincaptad&cond=AMD&draw=2&rank=2 (accessed on 5 July 2021).
- Schnabolk, G.; Parsons, N.; Obert, E.; Annamalai, B.; Nasarre, C.; Tomlinson, S.; Lewin, A.S.; Rohrer, B. Delivery of CR2-FH Using AAV Vector Therapy as Treatment Strategy in the Mouse Model of Choroidal Neovascularization. Mol. Ther. Methods Clin. Dev. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Advanced Dry Age Related Macular Degeneration with AAVCAGsCD59. Available online: https://clinicaltrials.gov/ct2/show/NCT03144999?term=NCT03144999&draw=2&rank=1 (accessed on 27 April 2020).
- Pravin, U. Dugel CLINICAL TRIAL DOWNLOAD: Data on a Gene Therapy for Dry and Wet AMDrapy for Dry and Wet AMD. Available online: https://www.retinalphysician.com/issues/2020/april-2020/clinical-trial-download-data-on-a-gene-therapy-for (accessed on 25 April 2020).
- Jaffe, G.J.; Sahni, J.; Fauser, S.; Geary, R.S.; Schneider, E.; McCaleb, M. Development of IONIS-FB-LRx to Treat Geographic Atrophy Associated with AMD. IOVS 2020, 61, 4305. [Google Scholar]
- Safety and Efficacy of IONIS-FB-Lrx in up to 120 Patients 55 and Older with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT03446144?term=Ionis&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 11 April 2022).
- GOLDEN STUDY: A Study to Assess Safety and Efficacy of Multiple Doses of IONIS-FB-LRx in Participants with Geographic Atrophy Secondary to Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT03815825?term=Ionis&cond=Age-Related+Macular+Degeneration&draw=2&rank=2 (accessed on 11 April 2022).
- FocuS: First in Human Study to Evaluate the Safety and Efficacy of GT005 Administered in Subjects with Dry AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03846193 (accessed on 7 July 2021).
- EXPLORE: A Phase II Study to Evaluate the Safety and Efficacy of Two Doses of GT005. Available online: https://clinicaltrials.gov/ct2/show/NCT04437368 (accessed on 7 July 2021).
- HORIZON: A Phase II Study to Evaluate the Safety and Efficacy of Two Doses of GT005. Available online: https://clinicaltrials.gov/ct2/show/NCT04566445 (accessed on 7 July 2021).
- First in Human Study to Evaluate the Safety and Tolerability of GEM103 in Geographic Atrophy Secondary to Dry Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04246866?term=GEM103&cond=Age-Related+Macular+Degeneration&draw=2&rank=3 (accessed on 12 April 2022).
- A Multiple Dose Study of Repeat Intravitreal Injections of GEM103 in Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04643886?term=GEM103&cond=Age-Related+Macular+Degeneration&draw=2&rank=2 (accessed on 12 April 2022).
- A Multiple Dose Study of Repeat Intravitreal Injections of GEM103 in Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04684394?term=GEM103&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 12 April 2022).
- Napoli, K.L.; Taylor, P.J. From Beach to Bedside: History of the Development of Sirolimus. Ther. Drug Monit. 2001, 23, 559–586. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Rapamune; Pfizer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Intravitreal Injections of Sirolimus in the Treatment of Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT01675947?term=Sirolimus&cond=AMD&draw=2&rank=9 (accessed on 18 June 2020).
- Phase 1/2 Study of an Ocular Sirolimus (Rapamycin) Formulation in Patients with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00712491?term=NCT00712491&draw=2&rank=1 (accessed on 18 June 2020).
- Sirolimus for Advanced Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01445548?term=Sirolimus&cond=AMD&draw=2&rank=5 (accessed on 18 June 2020).
- Petrou, P.A.; Cunningham, D.; Shimel, K.; Harrington, M.; Hammel, K.; Cukras, C.A.; Ferris, F.L.; Chew, E.Y.; Wong, W.T. Intravitreal Sirolimus for the Treatment of Geographic Atrophy: Results of a Phase I/II Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2014, 56, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Raj, Y.; Mehra, N.; Khan, F.; Alam, O.; Iqubal, A.; Kumar, G.; Kohli, K. Sirolimus Loaded Chitosan Functionalized Poly (Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles for Potential Treatment of Age-Related Macular Degeneration. Int. J. Biol. Macromol. 2021, 191, 548–559. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Copaxone; Teva Neuroscience, Inc.: North Wales, PA, USA, 2018. [Google Scholar]
- Arnon, R.; Sela, M. The Chemistry of the Copaxone Drug. Chem. Isr. 1999, 1, 12–17. [Google Scholar]
- Yong, V.W. Differential Mechanisms of Action of Interferon-b and Glatiramer Acetate in MS. Neurology 2002, 59, 802–808. [Google Scholar] [CrossRef]
- Copaxone in Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00466076?term=Glatiramer+acetate&cond=AMD&draw=2&rank=1 (accessed on 29 April 2020).
- Weekly Vaccination with Copaxone as a Potential Therapy for Dry Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00541333?term=Glatiramer+acetate&cond=AMD&draw=2&rank=2 (accessed on 29 April 2020).
- Landa, G.; Butovsky, O.; Shoshani, J.; Schwartz, M.; Pollack, A. Weekly Vaccination with Copaxone (Glatiramer Acetate) as a Potential Therapy for Dry Age-Related Macular Degeneration. Curr. Eye Res. 2008, 22, 1011–1013. [Google Scholar] [CrossRef]
- Foye, W.O.; Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; ISBN 978-0-7817-6879-5. [Google Scholar]
- U.S. Food and Drug Administration. Iluvien; Alimera Sciences, Inc.: Alpharetta, GA, USA, 2016. [Google Scholar]
- Fluocinolone Acetonide Intravitreal Inserts in Geographic Atrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT00695318?term=Fluocinolone&cond=Geographic+AMD&draw=2&rank=1 (accessed on 28 April 2020).
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem Cell Based Therapies for Age-Related Macular Degeneration: The Promises and the Challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Sahibzada, N.; Golestaneh, N. Human Induced Pluripotent Stem-Derived Retinal Pigment Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. Stem Cells 2011, 29, 825–835. [Google Scholar] [CrossRef]
- Bracha, P.; Moore, N.A.; Ciulla, T.A. Induced Pluripotent Stem Cell-Based Therapy for Age-Related Macular Degeneration. Expert Opin. Biol. Ther. 2017, 17, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Kokkinaki, M.; Sahibzada, N.; Golestaneh, N. Human IPS-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized VEGF Secretion and Gene Expression Pattern Similar to Native RPE. Stem Cells 2011, 29, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Surendran, H.; Rathod, R.J.; Pal, R. Generation of Transplantable Retinal Pigmented Epithelial (RPE) Cells for Treatment of Age-Related Macular Degeneration (AMD). Stem Cells Aging 2018, 2045, 283–298. [Google Scholar]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed SIRT1/PGC-1α Pathway and Mitochondrial Disintegration in IPSC-Derived RPE Disease Model of Age-Related Macular Degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef]
- Production of IPSC Derived RPE Cells for Transplantation in AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02464956?term=iPSC&cond=dry+AMD&draw=2&rank=1 (accessed on 23 April 2020).
- Generation of Induced Pluripotent Stem (IPS) Cell Lines from Skin Fibroblast Cells of Participants with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03372746?term=iPSC&cond=AMD&draw=2&rank=2 (accessed on 10 April 2020).
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Safety and Tolerability of Sub-Retinal Transplantation of HESC Derived RPE (MA09-HRPE) Cells in Patients with Advanced Dry Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01344993?term=MA09-hRPE&draw=2&rank=6 (accessed on 24 April 2020).
- Long Term Follow Up of Sub-Retinal Transplantation of HESC Derived RPE Cells in Patients with AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02463344?term=NCT02463344&draw=2&rank=1 (accessed on 17 June 2020).
- Koss, M.J.; Falabella, P.; Stefanini, F.R.; Pfister, M.; Thomas, B.B.; Kashani, A.H.; Brant, R.; Zhu, D.; Clegg, D.O.; Hinton, D.R.; et al. Subretinal Implantation of a Monolayer of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium: A Feasibility and Safety Study in Yucatán Minipigs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1553–1565. [Google Scholar] [CrossRef]
- Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02590692?term=NCT02590692&draw=2&rank=1 (accessed on 24 April 2020).
- Strauss, O.; Stumpffa, S.; Mergler, S.; Wienrich, M.; Wiederholt, M. The Royal College of Surgeons Rat: An Animal Model for Inherited Retinal Degeneration with a Still Unknown Genetic Defect. Acta Anat. 1998, 162, 101–111. [Google Scholar] [CrossRef]
- McGill, T.J.; Cottam, B.; Lu, B.; Wang, S.; Girman, S.; Tian, C.; Huhn, S.L.; Lund, R.D.; Capela, A. Transplantation of Human Central Nervous System Stem Cells—Neuroprotection in Retinal Degeneration. Eur. J. Neurosci. 2012, 35, 468–477. [Google Scholar] [CrossRef]
- McGill, T.J.; Osborne, L.; Lu, B.; Stoddard, J.; Huhn, S.; Tsukamoto, A.; Capela, A. Subretinal Transplantation of Human Central Nervous System Stem Cells Stimulates Controlled Proliferation of Endogenous Retinal Pigment Epithelium. Transl. Vis. Sci. Technol. 2019, 8, 43. [Google Scholar] [CrossRef]
- Study of Human Central Nervous System Stem Cells (HuCNS-SC) in Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01632527?term=HuCNS-SC&cond=AMD&draw=2&rank=1 (accessed on 10 April 2020).
- Study of HUCNS-SC Subretinal Transplantation in Subjects with GA of AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02467634?term=HuCNS-SC&cond=AMD&draw=2&rank=2 (accessed on 10 April 2020).
- StemCells, Inc. Reports Top Line Results for Its Phase I/II Study in Dry Age Related Macular Degeneration Nasdaq:STEM. Available online: https://www.globenewswire.com/news-release/2015/06/26/747839/10139889/en/StemCells-Inc-Reports-Top-Line-Results-for-Its-Phase-I-II-Study-in-Dry-Age-Related-Macular-Degeneration.html (accessed on 18 September 2020).
- Long-Term Follow-up Safety Study of Human Central Nervous System Stem Cells in Subjects with Geographic Atrophy of Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02137915?term=HuCNS-SC&cond=AMD&draw=2&rank=3 (accessed on 10 April 2020).
- L’Esperance, F.A. Clinical Applications of the Organic Dye Laser. Ophthalmology 1985, 92, 1592–1600. [Google Scholar] [CrossRef]
- Kliffen, M.; Sharma, H.S.; Mooy, C.M.; Kerkvliet, S.; De Jong, P.T.V.M. Increased Expression of Angiogenic Growth Factors in Age-Related Maculopathy. Br. J. Ophthalmol. 1997, 81, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Rhu, H.W.; Kang, S.; Roh, Y.J. New Approach of Anti-VEGF Agents for Age-Related Macular Degeneration. J. Ophthalmol. 2012, 2012, 637316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macular Photocoagulation Study (MPS). Available online: https://clinicaltrials.gov/ct2/show/NCT00000158?term=MPS&cond=AMD&draw=2&rank=2 (accessed on 17 March 2020).
- Argon Laser Photocoagulation for Neovascular Maculopathy. Arch. Ophthalmol. 1991, 109, 1109. [CrossRef]
- Saini, R.; Lee, N.V.; Liu, K.Y.P.; Poh, C.F. Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions. Cancers 2016, 8, 83. [Google Scholar] [CrossRef]
- Houle, J.M.; Strong, A. Clinical Pharmacokinetics of Verteporfin. J. Clin. Pharmacol. 2002, 42, 547–557. [Google Scholar] [CrossRef]
- Aveline, B.; Hasan, T.; Redmond, R.W. Photophysical and Photosensitizing Properties of Benzoporphyrin Derivative Monoacid Ring a (Bpd-Ma). Photochem. Photobiol. 1994, 59, 328–335. [Google Scholar] [CrossRef]
- Richter, A.M.; Yip, S.; Meadows, H.; Jain, A.K.; Neyndorff, H.; Moreno, G.; Salet, C.; Levy, J.G. Photosensitizing Potencies of the Structural Analogues of Benzoporphyrin Derivative in Different Biological Test Systems. J. Clin. Laser Med. Surg. 1996, 14, 335–341. [Google Scholar] [CrossRef]
- Lim, J.I. Photodynamic Therapy for Choroidal Neovascular Disease: Photosensitizers and Clinical Trials. Ophthalmol. Clin. N. Am. 2002, 15, 473–478. [Google Scholar] [CrossRef]
- Blinder, K.J.; Bradley, S.; Bressler, N.M.; Bressler, S.B.; Donati, G.; Hao, Y.; Ma, C.; Menchini, U.; Miller, J.; Potter, M.J.; et al. Effect of Lesion Size, Visual Acuity, and Lesion Composition on Visual Acuity Change with and without Verteporfin Therapy for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration: TAP and VIP Report No. 1. Am. J. Ophthalmol. 2003, 136, 407–418. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Visudyne; MEDWATCH, HF-2 FDA: Rockville, MD, USA, 2002. [Google Scholar]
- Haute Autorité de Santé. VISUDYNE; Haute Autorité de Santé: Saint-Denis, France, 2006. [Google Scholar]
- Haute Autorité de Santé. Place Dans La Stratégie Thérapeutique de LUCENTIS, EYLEA et de Leurs Comparateurs Cliniquement Pertinents Dans La Forme Néovasculaire (Humide) de La Dégénérescence Maculaire Liée à l’âge (DMLA); Haute Autorité de Santé: Saint-Denis, France, 2017. [Google Scholar]
- Ribatti, D. Napoleone Ferrara and the Saga of Vascular Endothelial. Endothelium 2008, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eng, V.A.; Id, N.R.; Id, H.V.N.; Id, T.L. Complete RPE and Outer Retinal Atrophy in Patients Receiving Anti-VEGF Treatment for Neovascular Age-Related Macular Degeneration. PLoS ONE 2020, 15, e0232353. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Amano, S.; Ogura, Y.; Hida, T.; Oguchi, Y.; Ambati, J.; Miller, J.W.; et al. VEGF164-Mediated Inflammation Is Required for Pathological, but Not Physiological, Ischemia-Induced Retinal Neovascularization. J. Exp. Med. 2003, 198, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical Pharmacology of Intravitreal Anti-VEGF Drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R.; Cunningham, E.T. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Macugen; Gilead Sciences, Inc.: San Dimas, CA, USA, 2011. [Google Scholar]
- A Study to Evaluate RhuFab V2 in Subjects with Minimally Classic or Occult Subfoveal Neovascular Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00056836?term=NCT00056836&cond=AMD&draw=2&rank=1 (accessed on 3 April 2020).
- An Extension Study to Evaluate Safety and Tolerability of Ranibizumab in Macular Edema Secondary to Retinal Vein Occlusion (Cohort 2). Available online: https://clinicaltrials.gov/ct2/show/NCT01442064?term=NCT01442064&cond=AMD&draw=2&rank=1 (accessed on 3 April 2020).
- An Extension Study to Evaluate the Safety and Tolerability of Ranibizumab in Subjects with Choroidal Neovascularization Secondary to AMD or Macular Edema Secondary to RVO. Available online: https://clinicaltrials.gov/ct2/show/NCT00379795?term=NCT01442064&cond=AMD&draw=2&rank=2 (accessed on 3 April 2020).
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Ph, D.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- A Study to Compare RhuFab V2 with Verteporfin Photodynamic in Treating Subfoveal Neovascular Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00061594?term=NCT00061594&cond=AMD&draw=2&rank=1 (accessed on 3 April 2020).
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A Multicenter Cohort Study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Ho, A.C.; Busbee, B.G.; Regillo, C.D.; Wieland, M.R.; Van Everen, S.A.; Li, Z.; Rubio, R.G.; Lai, P. Twenty-Four-Month Efficacy and Safety of 0.5 Mg or 2.0 Mg Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration. Ophthalmology 2014, 121, 2181–2192. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Croft, D.E.; Brown, D.M.; Wang, R.; Payne, J.F.; Clark, L.; Abdelfattah, N.S.; Sadda, S.R. Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: TREX-AMD 1-Year Results. Ophthalmology 2015, 122, 2514–2522. [Google Scholar] [CrossRef]
- Yan, J.; Peng, X.; Cai, Y.; Cong, W. Development of Facile Drug Delivery Platform of Ranibizumab Fabricated PLGA-PEGylated Magnetic Nanoparticles for Age-Related Macular Degeneration Therapy. J. Photochem. Photobiol. B Biol. 2018, 183, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.R.; Kaiser, P.K. Therapeutic Potential of the Ranibizumab Port Delivery System in the Treatment of AMD: Evidence to Date. Clin. Ophthalmol. 2020, 14, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé. Ranibizumab; Haute Autorité de Santé: Saint-Denis, France, 2018. [Google Scholar]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-Related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Vascular Endothelial Growth Factor VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration(AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00509795?term=NCT00509795&draw=2&rank=1 (accessed on 9 April 2020).
- Vascular Endothelial Growth Factor (VEGF) Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00637377?term=NCT00637377&draw=2&rank=1 (accessed on 9 April 2020).
- U.S. Food and Drug Administration. EYLEA® (Aflibercept); Regeneron Pharmaceuticals, Inc.: Tarrytown, NY, USA, 2018. [Google Scholar]
- Zuo, W.; Mei, D.; Sun, W.; Tang, X.; Niu, Z.; Gao, D.; Zhang, B. The Interpretation of China National Essential Medicines List 2018. Expert Rev. Clin. Pharmacol. 2020, 13, 191–200. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. AVASTIN (Bevacizumab); Genentech, Inc.: South San Francisco, CA, USA, 2014. [Google Scholar]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-Related Macular Degeneration: Two-Year Results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L.; Fine, S.L. Five-Year Outcomes with Anti–Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef]
- French Evaluation Group Avastin Versus Lucentis. Available online: https://clinicaltrials.gov/ct2/show/NCT01170767?term=NCT01170767&draw=2&rank=1 (accessed on 9 April 2020).
- Kodjikian, L.; Souied, E.H.; Mimoun, G.; Mauget-Faÿsse, M.; Behar-Cohen, F.; Decullier, E.; Huot, L.; Aulagner, G. Ranibizumab versus Bevacizumab for Neovascular Age-Related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 2013, 120, 2300–2309. [Google Scholar] [CrossRef]
- Heier, J.S. Ask the Doctor: For Macular Degeneration, Which Is Better, Avastin or Lucentis? Harvard Health. Available online: https://www.health.harvard.edu/diseases-and-conditions/for-macular-degeneration-which-is-better-avastin-or-lucentis (accessed on 9 September 2020).
- Yannuzzi, N.A.; Freund, K.B. Brolucizumab: Evidence to Date in the Treatment of Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2019, 13, 1323–1329. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report Beovu; Novartis Europharm Limited: Dublin, Ireland, 2019. [Google Scholar]
- Safety and Pharmacokinetics of RTH258 in Subjects with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02507388?term=Brolucizumab&cond=AMD&draw=2&rank=8 (accessed on 1 July 2020).
- Study of the Safety of Brolucizumab 6 Mg in Prefilled Syringe in Patients with Neovascular Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03930641?term=Brolucizumab&cond=AMD&draw=2&rank=4 (accessed on 1 July 2020).
- Study to Collect Safety and ECG Data on Brolucizumab 6 Mg Intravitreal Treatment in Patients with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03954626?term=Brolucizumab&cond=AMD&draw=2&rank=6 (accessed on 1 July 2020).
- Study of Safety and Efficacy of Brolucizumab 6 Mg Drug Product Intended for Commercialization in Patients with NAMD. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03386474?term=Brolucizumab&cond=AMD&draw=2&rank=7 (accessed on 1 July 2020).
- Efficacy and Safety of RTH258 Versus Aflibercept—Study 1. Available online: https://clinicaltrials.gov/ct2/show/NCT02307682?term=HAWK&cond=AMD&draw=2&rank=1 (accessed on 1 July 2020).
- Efficacy and Safety of RTH258 Versus Aflibercept—Study 2. Available online: https://clinicaltrials.gov/ct2/show/NCT02434328?term=HARRIER&cond=AMD&draw=2&rank=1 (accessed on 1 July 2020).
- Agostini, H.; Mulyukov, Z.; Tsilimbaris, M.; Calvo, P.; Bucher, F.; Gaucher, D.; Pigeolet, E.; Colafrancesco, V.; Clemens, A. Comparison of the Efficacy of Brolucizumab with Natural Disease Progression in Wet AMD Using Clinical Data from the Phase III HAWK and HARRIER Trials and Modelled Placebo Data. Curr. Eye Res. 2020, 45, 1298–1301. [Google Scholar] [CrossRef]
- Novartis Novartis Receives FDA Approval for Beovu®, Offering Wet AMD Patients Vision Gains and Greater Fluid Reductions vs Aflibercept. Available online: https://novartis.gcs-web.com/Novartis-receives-FDA-approval-for-Beovu-offering-wet-AMD-patients-vision-gains-and-greater-fluid-reductions-vs-aflibercept (accessed on 23 March 2020).
- Yorston, D. Intravitreal Injection Technique. Community Eye Health J. 2014, 27, 47. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, J.; Yan, M.; Luo, D.; Zhu, W.; Kaiser, P.K.; Yu, D.C. A Phase 1 Study of KH902, a Vascular Endothelial Growth Factor Receptor Decoy, for Exudative Age-Related Macular Degeneration. Ophthalmology 2011, 118, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, X. Profile of Conbercept in the Treatment of Neovascular Age-Related Macular Degeneration. Drug Des. Devel. Ther. 2015, 9, 2311. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Guymer, R. Conbercept (KH-902) for the Treatment of Neovascular Age-Related Macular Degeneration. Expert Rev. Clin. Pharmacol. 2015, 8, 541–548. [Google Scholar] [CrossRef] [PubMed]
- A Study Assessing the Safety and Efficacy of Multiple Intravitreal KH902 in Patients with CNV Due to AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01242254?term=KH902&recrs=eh&cond=AMD&draw=2&rank=2 (accessed on 2 July 2020).
- A Randomized, Double-Masked, Multicenter, Controlled Study of Intravitreal KH902 in Patients with Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01157715?term=NCT+01157715&recrs=eh&cond=AMD&draw=2&rank=1 (accessed on 2 July 2020).
- Li, X.; Xu, G.; Wang, Y.; Xu, X.; Liu, X.; Tang, S.; Zhang, F.; Zhang, J.; Tang, L.; Wu, Q.; et al. Safety and Efficacy of Conbercept in Neovascular Age-Related Macular Degeneration: Results from a 12-Month Randomized Phase 2 Study: AURORA Study. Ophthalmology 2014, 121, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- A Randomized, Double-Masked, Multicenter, Sham-Controlled, Safety and Efficacy Study of KH902 in Patients with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01436864?term=Conbercept&recrs=eh&cond=AMD&draw=2&rank=2 (accessed on 19 March 2020).
- Head to Head Study of Anti-VEGF Treatment. Available online: https://clinicaltrials.gov/ct2/show/NCT02577107?term=Conbercept&recrs=eh&cond=AMD&draw=2&rank=1 (accessed on 19 March 2020).
- Efficacy and Safety Trial of Conbercept Intravitreal Injection for Neovascular AMD (PANDA-1). Available online: https://clinicaltrials.gov/ct2/show/NCT03577899?term=Conbercept&cond=AMD&draw=2&rank=1 (accessed on 5 July 2021).
- Efficacy and Safety Trial of Conbercept Intravitreal Injection for Neovascular AMD (PANDA-2). Available online: https://clinicaltrials.gov/ct2/show/NCT03630952?term=Conbercept&cond=AMD&draw=2&rank=2 (accessed on 5 July 2021).
- Zhang, J.; Liang, Y.; Xie, J.; Li, D.; Hu, Q.; Li, X.; Zheng, W.; He, R. Conbercept for Patients with Age-Related Macular Degeneration: A Systematic Review. BMC Ophthalmol. 2018, 18, 142. [Google Scholar] [CrossRef]
- Kunimoto, D.; Ohji, M.; Maturi, R.K.; Sekiryu, T.; Wang, Y.; Pan, G.; Li, X.Y.; Schneider, S. Evaluation of Abicipar Pegol (an Anti-VEGF DARPIN Therapeutic) in Patients with Neovascular Age-Related Macular Degeneration: Studies in Japan and the United States. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, E10–E22. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Mason, M.; Christie, L.A.; Hansen, C.; Hernandez, L.M.; Burke, J.; Luhrs, K.A.; Hohman, T.C. Functional Characterization of Abicipar-Pegol, an Anti-VEGF DARPin Therapeutic That Potently Inhibits Angiogenesis and Vascular Permeability. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5836–5846. [Google Scholar] [CrossRef]
- Safety and Pharmacokinetics of Abicipar Pegol Intravitreal Injections in Patients with Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02859766?term=Abicipar+pegol&cond=AMD&draw=2&rank=1 (accessed on 3 July 2020).
- Safety and Pharmacokinetics of Abicipar Pegol Intravitreal Injections in Japanese Patients with Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03335852?term=Abicipar+pegol&cond=AMD&draw=2&rank=2 (accessed on 3 July 2020).
- A Study of Abicipar Pegol in Patients with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02181517?term=CYPRESS&cond=AMD&draw=2&rank=1 (accessed on 3 July 2020).
- A Study of Abicipar Pegol in Japanese Patients with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02181504?term=BAMBOO&cond=AMD&draw=2&rank=1 (accessed on 3 July 2020).
- Evaluation of AGN-150998 in Exudative Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01397409?term=NCT01397409&cond=AMD&draw=2&rank=1 (accessed on 3 July 2020).
- Callanan, D.; Kunimoto, D.; Maturi, R.K.; Patel, S.S.; Staurenghi, G.; Wolf, S.; Cheetham, J.K.; Hohman, T.C.; Kim, K.; López, F.J.; et al. Double-Masked, Randomized, Phase 2 Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Neovascular Age-Related Macular Degeneration. J. Ocul. Pharmacol. Ther. 2018, 34, 700–709. [Google Scholar] [CrossRef]
- Allergen A Safety and Efficacy Study of Abicipar Pegol in Patients with Neovascular Age-Related Macular Degeneration—CDER. Available online: https://clinicaltrials.gov/ct2/show/NCT02462928?term=NCT02462928&draw=2&rank=1 (accessed on 1 April 2020).
- Safety and Efficacy of Abicipar Pegol in Patients with Neovascular Age-Related Macular Degeneration—SEQUOIA. Available online: https://clinicaltrials.gov/ct2/show/NCT02462486?term=NCT02462486&draw=2&rank=1 (accessed on 1 April 2020).
- Kunimoto, D.; Yoon, Y.H.; Wykoff, C.C.; Chang, A.; Khurana, R.N.; Maturi, R.K.; Agostini, H.; Souied, E.; Chow, D.R.; Lotery, A.J.; et al. Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration: 52-Week Results of Phase 3 Randomized Controlled Study. Ophthalmology 2020, 127, 1331–1344. [Google Scholar] [CrossRef]
- Evaluating Abicipar for Safety and Treatment Effect in Patients with Neovascular Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT03539549?term=Abicipar+pegol&cond=AMD&draw=2&rank=5 (accessed on 3 July 2020).
- Study Evaluating the Safety, Pharmacokinetics and Pharmacodynamics of OPT-302 with or without LucentisTM in Patients with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02543229?term=OPT-302&cond=AMD&draw=2&rank=2 (accessed on 9 April 2020).
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 Study of OPT-302 Inhibition of Vascular Endothelial Growth Factors C and D for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 250–263. [Google Scholar] [CrossRef] [PubMed]
- A Dose Ranging Study of OPT-302 with Ranibizumab in Neovascular (Wet) AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03345082?term=OPT-302&cond=AMD&draw=2&rank=1 (accessed on 9 April 2020).
- Stein, M.N.; Flaherty, K.T. Sorafenib and Sunitinib in Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 3765–3770. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. NEXAVAR (Sorafenib); Bayer HealthCare Pharmaceuticals Inc.: Leverkusen, Germany, 2011. [Google Scholar]
- Haute Autorité de Santé. Nexavar; Haute Autorité de Santé: Saint-Denis, France, 2008. [Google Scholar]
- Kernt, M.; Neubauer, A.S.; Liegl, R.G.; Hirneiss, C.; Alge, C.S.; Wolf, A.; Ulbig, M.W.; Kampik, A. Sorafenib Prevents Human Retinal Pigment Epithelium Cells from Light-Induced Overexpression of VEGF, PDGF and PlGF. Br. J. Ophthalmol. 2010, 94, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Kernt, M.; Staehler, M.; Stief, C.; Kampik, A.; Neubauer, A.S. Resolution of Macular Oedema in Occult Choroidal Neovascularization under Oral Sorafenib® Treatment. Acta Ophthalmol. 2008, 86, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Diago, T.; Pulido, J.S.; Molina, J.R.; Collet, L.C.; Link, T.P.; Ryan, E.H. Ranibizumab Combined with Low-Dose Sorafenib for Exudative Age-Related Macular Degeneration. Mayo Clin. Proc. 2008, 83, 231–234. [Google Scholar] [CrossRef]
- Zhao, H.L.; Yang, F.; Huang, X.; Zhou, Q.H. Overview of Fundamental Study of Pazopanib in Cancer. Thorac. Cancer 2014, 5, 487–493. [Google Scholar] [CrossRef][Green Version]
- U.S. Food and Drug Administration. Votrient; GlaxoSmithKline: Research Triangle Park, NC, USA, 2012. [Google Scholar]
- A Study to Evaluate the Safety, Tolerability and Pk of Pazopanib Eye Drops in Healthy Adult and Elderly Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT00463320?term=pazopanib&cond=AMD&draw=2&rank=6 (accessed on 30 June 2020).
- Study of the Repeat Dosing of Ketoconazole on the Pharmacokinetics of a Single Dose of Pazopanib (GW786034) Eye Drops. Available online: https://clinicaltrials.gov/ct2/show/NCT00659555?term=pazopanib&cond=AMD&draw=2&rank=7 (accessed on 30 June 2020).
- A Study to Evaluate Pazopanib Tablets in Patients Who Have Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01154062?term=pazopanib&cond=AMD&draw=2&rank=4 (accessed on 30 June 2020).
- A Safety Study in Healthy Volunteers to Evaluate Safety, How Fast the Drug Is Absorbed, and the Side Effects of the Drug in Humans. Available online: https://clinicaltrials.gov/ct2/show/NCT01051700?term=pazopanib&cond=AMD&draw=2&rank=9 (accessed on 30 June 2020).
- A Safety Study to Evaluate Pazopanib Eye Drops in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT01072214?term=pazopanib&cond=AMD&draw=2&rank=8 (accessed on 30 June 2020).
- To Evaluate the Pharmacodynamics, Safety, and Pharmacokinetics of Pazopanib Drops in Adult Subjects with Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT00612456?term=NCT00612456&draw=2&rank=1 (accessed on 30 June 2020).
- An Extension to Study MD7108240. Available online: https://clinicaltrials.gov/ct2/show/NCT00733304?term=pazopanib&cond=AMD&draw=2&rank=5 (accessed on 30 June 2020).
- Dose Ranging Study of Pazopanib to Treat Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01134055?term=pazopanib&cond=wet+AMD&draw=2&rank=1 (accessed on 30 June 2020).
- 12 Week Patient Study in Neovascular Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01362348?term=pazopanib&cond=AMD&draw=2&rank=2 (accessed on 30 June 2020).
- McLaughlin, M.M.; Paglione, M.G.; Slakter, J.; Tolentino, M.; Ye, L.; Xu, C.F.; Suttle, A.B.; Kim, R.Y. Initial Exploration of Oral Pazopanib in Healthy Participants and Patients with Age-Related Macular Degeneration. JAMA Ophthalmol. 2013, 131, 1595–1601. [Google Scholar] [CrossRef]
- Singh, R.; Wurzelmann, J.I.; Ye, L.; Henderson, L.; Hossain, M.; Trivedi, T.; Kelly, D.S. Clinical Evaluation of Pazopanib Eye Drops in Healthy Subjects and in Subjects with Neovascular Age-Related Macular Degeneration. Retina 2014, 34, 1787–1795. [Google Scholar] [CrossRef]
- Csaky, K.G.; Dugel, P.U.; Pierce, A.J.; Fries, M.A.; Kelly, D.S.; Danis, R.P.; Wurzelmann, J.I.; Xu, C.F.; Hossain, M.; Trivedi, T. Clinical Evaluation of Pazopanib Eye Drops versus Ranibizumab Intravitreal Injections in Subjects with Neovascular Age-Related Macular Degeneration. Ophthalmology 2015, 122, 579–588. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; François, L.; Quivy, A.; Ravaud, A. Axitinib: A Review of Its Safety and Efficacy in the Treatment of Adults with Advanced Renal Cell Carcinoma. Clin. Med. Insights Oncol. 2013, 7, 269–277. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Inlyta; Pfizer: New York, NY, USA, 2020. [Google Scholar]
- Kang, S.; Roh, C.R.; Cho, W.K.; Park, K.C.; Yang, K.J.; Choi, H.S.; Kim, S.H.; Roh, Y.J. Antiangiogenic Effects of Axitinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinase, on Laser-Induced Choroidal Neovascularization in Mice. Curr. Eye Res. 2013, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Giddabasappa, A.; Lalwani, K.; Norberg, R.; Gukasyan, H.J.; Paterson, D.; Schachar, R.A.; Rittenhouse, K.; Klamerus, K.; Mosyak, L.; Eswaraka, J. Axitinib Inhibits Retinal and Choroidal Neovascularization in in Vitro and in Vivo Models. Exp. Eye Res. 2016, 145, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Safety and Tolerability Study of Suprachoroidal Injection of CLS-AX Following Anti-VEGF Therapy in Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT04626128?term=Axitinib&cond=AMD&draw=2&rank=1 (accessed on 5 July 2021).
- Extension Study to Evaluate the Long-Term Outcomes of Subjects in the CLS-AX CLS1002-101 Study. Available online: https://clinicaltrials.gov/ct2/show/NCT05131646?term=axitinib&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 25 April 2022).
- Study Evaluating the Treatment of OTX-TKI for Subjects with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04989699?term=axitinib&cond=Age-Related+Macular+Degeneration&draw=2&rank=3 (accessed on 25 April 2022).
- Adams, C.M.; Anderson, K.; Artman, G.; Bizec, J.; Cepeda, R.; Elliott, J.; Fassbender, E.; Ghosh, M.; Hanks, S.; Hardegger, L.A.; et al. The Discovery of N-(1-Methyl-5-(Trifluoromethyl)-1H-pyrazol-3-Yl)-5- ((6- ((Methylamino)Methyl)Pyrimidin-4-Yl)Oxy)-1H-indole-1- Carboxamide (Acrizanib), a VEGFR-2 Inhibitor Specifically Designed for Topical Ocular Delivery, as a Therapy for Neovascular Ag. J. Med. Chem. 2018, 61, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- First-in-Human Study of LHA510 in Elderly Subjects and Patients with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02076919?term=Acrizanib&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 19 April 2022).
- LHA510 Proof-of-Concept Study as a Maintenance Therapy for Patients with Wet Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02355028?term=Acrizanib&cond=Age-Related+Macular+Degeneration&draw=2&rank=2 (accessed on 19 April 2022).
- Poor, S.H.; Adams, C.M.; Ferriere, M.; Weichselberger, A.; Grosskreutz, C.L.; Weissgerber, G. Topical VEGF Receptor Inhibitor, LHA510, Did Not Demonstrate Efficacy in a Proof-of-Concept Study in Patients with Neovascular Age-Related Macular Degeneration (Nv AMD). Investig. Ophthalmol. Vis. Sci. 2018, 59, 2394. [Google Scholar]
- Carr, B.I.; D’Alessandro, R.; Refolo, M.G.; Iacovazzi, P.A.; Lippolis, C.; Messa, C.; Cavallini, A.; Correale, M.; Di Carlo, A. Effects of Low Concentrations of Regorafenib and Sorafenib on Human HCC Cell AFP, Migration, Invasion, and Growth in Vitro. J. Cell. Physiol. 2013, 228, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Stivarga; Bayer HealthCare Pharmaceuticals Inc.: Whippany, NJ, USA, 2017. [Google Scholar]
- Regorafenib Eye Drops: Investigation of Efficacy and Safety in Neovascular Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02222207?term=NCT02222207&draw=2&rank=1 (accessed on 9 April 2020).
- Joussen, A.M.; Wolf, S.; Kaiser, P.K.; Boyer, D.; Schmelter, T.; Sandbrink, R.; Zeitz, O.; Deeg, G.; Richter, A.; Zimmermann, T.; et al. The Developing Regorafenib Eye Drops for Neovascular Age-Related Macular Degeneration (DREAM) Study: An Open-Label Phase II Trial. Br. J. Clin. Pharmacol. 2019, 85, 347–355. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Merrigan, S.; Quigley, J.; Qi, X.; Lee, B.; Boulton, M.E.; Kennedy, B.; Seo, S.; Corson, T.W. A Novel Small Molecule Ameliorates Ocular Neovascularisation and Synergises with Anti-VEGF Therapy. Sci. Rep. 2016, 6, 25509. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Park, B.; Pasha, S.; Pran, S.; Si, Y.; Kharwadkar, R.; Mitter, S.K.; Lee, B.; Sun, W.; Qi, X.; et al. Chemical Proteomics Reveals Soluble Epoxide Hydrolase as a Therapeutic Target for Ocular Neovascularization. ACS Chem. Biol. 2018, 13, 45–52. [Google Scholar] [CrossRef]
- Rudolf, M.; Roizman, K. Treatment of Age-Related Macular Degeneration and Other Eye Diseases with Apolipoprotein Mimetics. US Patent 2018/0207233 A1, 1 October 2019. [Google Scholar]
- Tolentino, M.J.; Dennrick, A.; John, E.; Tolentino, M.S. Drugs in Phase II Clinical Trials for the Treatment of Age-Related Macular Degeneration. Expert Opin. Investig. Drugs 2015, 24, 183–199. [Google Scholar] [CrossRef]
- Phase 1 Study of Topical Ocular PAN-90806 for Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02022540?term=NCT02022540&draw=2&rank=1 (accessed on 9 April 2020).
- Study of PAN-90806 Eye Drops, Suspension for Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03479372?term=NCT03479372&draw=2&rank=1 (accessed on 9 April 2020).
- Jackson, T.L.; Boyer, D.; Brown, D.M.; Chaudhry, N.; Elman, M.; Liang, C.; O’Shaughnessy, D.; Parsons, E.C.; Patel, S.; Slakter, J.S.; et al. Oral Tyrosine Kinase Inhibitor for Neovascular Age-Related Macular Degeneration: A Phase 1 Dose-Escalation Study. JAMA Ophthalmol. 2017, 135, 761–767. [Google Scholar] [CrossRef]
- EyePoint Pharmaceuticals, Inc. Securities and Exchange Commission; EyePoint Pharmaceuticals, Inc.: Washington, DC, USA, 2020; Volume 1933. [Google Scholar]
- Pilot Study of X-82 in Patients with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT01674569?term=NCT01674569&draw=1&rank=1 (accessed on 9 April 2020).
- First in Human Study to Evaluate the Safety and Tolerability of EYP-1901 in Patients with Wet Age Related Macular Degeneration (WAMD). Available online: https://clinicaltrials.gov/ct2/show/NCT04747197?term=EYP-1901&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 25 April 2022).
- A Depot Formulation of Sunitinib Malate (GB-102) in Subjects with Neovascular (Wet) Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03249740?term=GB102&cond=Age-Related+Macular+Degeneration&draw=2&rank=1 (accessed on 25 April 2022).
- A Depot Formulation of Sunitinib Malate (GB-102) Compared to Aflibercept in Subjects with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03953079?term=GB102&cond=Age-Related+Macular+Degeneration&draw=2&rank=2 (accessed on 25 April 2022).
- Graybug Vision Releases Preliminary Topline Results of Phase 2b ALTISSIMO Trial. Available online: https://www.ophthalmologytimes.com/view/graybug-vision-releases-preliminary-topline-results-of-phase-2b-altissimo-trial (accessed on 25 April 2022).
- U.S. Food and Drug Administration. LENVIMA; Eisai Inc.: Woodcliff Lake, NJ, USA, 2018. [Google Scholar]
- Costa, R.; Carneiro, B.A.; Chandra, S.; Pai, S.G.; Chae, Y.K.; Kaplan, J.B.; Garrett, H.B.; Agulnik, M.; Kopp, P.A.; Giles, F.J. Spotlight on Lenvatinib in the Treatment of Thyroid Cancer: Patient Selection and Perspectives. Drug Des. Devel. Ther. 2016, 10, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Korb, D.; Thalheimer, A.; Kämmerer, U.; Allmanritter, J.; Matthes, N.; Linnebacher, M.; Schlegel, N.; Klein, I.; Ergün, S.; et al. E7080 (Lenvatinib), a Multi-Targeted Tyrosine Kinase Inhibitor, Demonstrates Antitumor Activities Against Colorectal Cancer Xenografts. Neoplasia 2014, 16, 972–981. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, T.; Yao, Y.; Zeng, S.; Li, M.; Xiang, H.; Zhao, C.; Cao, G.; Li, M.; Wan, R.; et al. Efficacy of Lenvatinib, a Multitargeted Tyrosine Kinase Inhibitor, on Laser-Induced CNV Mouse Model of Neovascular AMD. Exp. Eye Res. 2018, 168, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a Dual FGF/VEGF Inhibitor, Is Active Both 1st and 2nd Line against Mouse Pancreatic Neuroendocrine Tumors (PNET) Developing Adaptive/Evasive Resistance to VEGF Inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef] [PubMed]
- Bhide, R.S.; Lombardo, L.J.; Hunt, J.T.; Cai, Z.W.; Barrish, J.C.; Galbraith, S.; Jeyaseelan, R.; Mortillo, S.; Wautlet, B.S.; Krishnan, B.; et al. The Antiangiogenic Activity in Xenograft Models of Brivanib, a Dual Inhibitor of Vascular Endothelial Growth Factor Receptor-2 and Fibroblast Growth Factor Receptor-1 Kinases. Mol. Cancer Ther. 2010, 9, 369–378. [Google Scholar] [CrossRef]
- Li, L.; Zhu, M.; Wu, W.; Qin, B.; Gu, J.; Tu, Y.; Chen, J.; Liu, D.; Shi, Y.; Liu, X.; et al. Brivanib, a Multitargeted Small-Molecule Tyrosine Kinase Inhibitor, Suppresses Laser-Induced CNV in a Mouse Model of Neovascular AMD. J. Cell. Physiol. 2020, 235, 1259–1273. [Google Scholar] [CrossRef]
- Brycki, B.; Koenig, H.; Pospieszny, T. Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies. Molecules 2015, 20, 20887–20900. [Google Scholar] [CrossRef]
- Pietras, R.J.; Weinberg, O.K. Antiangiogenic Steroids in Human Cancer Therapy. Evid.-Based Complement. Altern. Med. 2005, 2, 49–57. [Google Scholar] [CrossRef]
- A Safety and Efficacy Study of MSI-1256F (Squalamine Lactate) to Treat “Wet” Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00089830?term=Squalamine&cond=AMD&draw=2&rank=7 (accessed on 30 June 2020).
- A Study of MSI-1256F (Squalamine Lactate) to Treat “Wet” Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00333476?term=Squalamine&cond=AMD&draw=2&rank=6 (accessed on 30 June 2020).
- MSI-1256F (Squalamine Lactate) in Combination with Verteporfin in Patients with “Wet” Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00094120?term=Squalamine&cond=AMD&draw=2&rank=3 (accessed on 30 June 2020).
- A Safety and Efficacy Study of Squalamine Lactate for Injection (MSI-1256F) for “Wet” Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00139282?term=NCT00139282&draw=2&rank=1 (accessed on 10 April 2020).
- Efficacy and Safety of Squalamine Lactate Eye Drops in Subjects with Neovascular (Wet) Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01678963?term=NCT01678963&draw=2&rank=1 (accessed on 10 April 2020).
- Cunningham, E.T., Jr. Impact of the OHR-102 IMPACT Data. Available online: https://www.retina-specialist.com/article/impact-of-the-ohr102-impact-data (accessed on 30 March 2020).
- Efficacy and Safety Study of Squalamine Ophthalmic Solution in Subjects with Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02727881?term=NCT02727881&cond=AMD&draw=2&rank=1 (accessed on 14 September 2020).
- Tura, A.; Ranjbar, M.; Grisanti, S.; Grisanti, S. Squalamine and Age-Related Macular Degeneration. Did the Shark Lose Its Teeth? New Front. Ophthalmol. 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Garba, A.O.; Mousa, S.A. Bevasiranib for the Treatment of Wet, Age-Related Macular Degeneration. Ophthalmol. Eye Dis. 2010, 2, OED.S4878. [Google Scholar] [CrossRef]
- Safety & Efficacy Study Evaluating the Combination of Bevasiranib & Lucentis Therapy in Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00499590?term=Bevasiranib&cond=AMD&draw=2&rank=1 (accessed on 20 March 2020).
- Shen, J.; Samul, R.; Silva, R.L.; Akiyama, H.; Liu, H.; Saishin, Y.; Hackett, S.F.; Zinnen, S.; Kossen, K.; Fosnaugh, K.; et al. Suppression of Ocular Neovascularization with SiRNA Targeting VEGF Receptor 1. Gene Ther. 2006, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- A Dose Escalation Trial of an Intravitreal Injection of Sirna-027 in Patients with Subfoveal Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00363714?term=siRNA-027&cond=AMD&draw=2&rank=1 (accessed on 20 March 2020).
- A Study Using Intravitreal Injections of a Small Interfering RNA in Patients with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00395057?term=siRNA-027&cond=AMD&draw=2&rank=2 (accessed on 20 March 2020).
- AGN-745 (Sirna-027)—Wet AMD Development Was Halted|Www.Amdbook.Org. Available online: https://amdbook.org/content/agn-745-sirna-027-wet-amd-development-was-halted (accessed on 22 September 2020).
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Hum. Gene Ther. 2017, 28, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Phase I Dose Escalation Safety Study of RetinoStat in Advanced Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01301443?term=RetinoStat&cond=AMD&draw=2&rank=1 (accessed on 3 July 2020).
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J. Highly Efficient RNA-Guided Genome Editing in Human Cells via Delivery of Purified Cas9 Ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.Y.; Liu, D.R. Cationic Lipid-Mediated Delivery of Proteins Enables Efficient Protein-Based Genome Editing in Vitro and in Vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.W.; Kim, J.H.; Lee, S.H.; Kim, D.; Koo, T.; Kim, K.E.; Kim, J.H.; Kim, J.S. Genome Surgery Using Cas9 Ribonucleoproteins for the Treatment of Age-Related Macular Degeneration. Genome Res. 2017, 27, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy Study of RAAV.SFlt-1 in Patients with Exudative Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01494805?term=NCT01494805&draw=2&rank=1 (accessed on 30 June 2020).
- Constable, I.J.; Lai, C.M.; Magno, A.L.; French, M.A.; Barone, S.B.; Schwartz, S.D.; Blumenkranz, M.S.; Degli-Esposti, M.A.; Rakoczy, E.P. Gene Therapy in Neovascular Age-Related Macular Degeneration: Three-Year Follow-up of a Phase 1 Randomized Dose Escalation Trial. Am. J. Ophthalmol. 2017, 177, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Safety and Tolerability Study of AAV2-SFLT01 in Patients with Neovascular Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01024998?term=NCT01024998&draw=2&rank=1 (accessed on 30 June 2020).
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous Injection of AAV2-SFLT01 in Patients with Advanced Neovascular Age-Related Macular Degeneration: A Phase 1, Open-Label Trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef]
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef]
- ADVM-022 Intravitreal Gene Therapy for Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03748784 (accessed on 7 July 2021).
- Armstrong, M. Adverum Halt Raises More Gene Therapy Questions; Evaluate Vantage: London, UK, 2021. [Google Scholar]
- RGX-314 Gene Therapy Administered in the Suprachoroidal Space for Participants with Neovascular Age-Related Macular Degeneration (NAMD). Available online: https://clinicaltrials.gov/ct2/show/NCT04514653 (accessed on 7 July 2021).
- Pivotal 1 Study of RGX-314 Gene Therapy in Participants with NAMD. Available online: https://clinicaltrials.gov/ct2/show/NCT04704921 (accessed on 7 July 2021).
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: A Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Cunha, S.I.; Ma, H.; Åhgren, A.; Cedervall, J.; Olsson, A.K.; Heldin, C.H.; Lennartsson, J. Specific Targeting of PDGFRβ in the Stroma Inhibits Growth and Angiogenesis in Tumors with High PDGF-BB Expression. Theranostics 2020, 10, 1122–1135. [Google Scholar] [CrossRef]
- Sadiq, M.A.; Hanout, M.; Sarwar, S.; Hassan, M.; Do, D.V.; Nguyen, Q.D.; Sepah, Y.J. Platelet Derived Growth Factor Inhibitors: A Potential Therapeutic Approach for Ocular Neovascularization. Saudi J. Ophthalmol. 2015, 29, 287–291. [Google Scholar] [CrossRef] [PubMed]
- A Phase 1, Safety, Tolerability and Pharmacokinetic Profile of Intravitreous Injections of E10030 (Anti-PDGF Pegylated Aptamer) in Subjects with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00569140 (accessed on 9 April 2020).
- A Safety and Efficacy Study of E10030 (Anti-PDGF Pegylated Aptamer) Plus Lucentis for Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01089517 (accessed on 9 April 2020).
- Study of Intravitreal REGN2176-3 in Patients with Neovascular (“Wet”) Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT02418754?term=REGN2176-3&cond=AMD&draw=2&rank=2 (accessed on 9 April 2020).
- Scholz, A.; Plate, K.H.; Reiss, Y. Angiopoietin-2: A Multifaceted Cytokine That Functions in Both Angiogenesis and Inflammation. Ann. N. Y. Acad. Sci. 2015, 1347, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.M. The Angiopoietin-Tie2 Signaling Axis in Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 1973–1982. [Google Scholar] [CrossRef]
- Phase 1 Safety Study with Intravitreal (IVT) REGN2176-3 in Patients Aged 50 Years and Older with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02061865?term=REGN2176-3&cond=AMD&draw=2&rank=1 (accessed on 9 April 2020).
- Wolf, A.; Langmann, T. Anti-VEGF-A/ ANG 2 Combotherapy Limits Pathological Angiogenesis in the Eye: A Replication Study. EMBO Mol. Med. 2019, 11, 10–11. [Google Scholar] [CrossRef] [PubMed]
- A Study of RO6867461 Administered in Single- and Multiple-Ascending Doses in Patients with Wet Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01941082?term=NCT01941082&draw=2&rank=1 (accessed on 10 April 2020).
- A Proof-of-Concept Study of Faricimab (RO6867461) in Participants with Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT02484690?term=NCT02484690&draw=2&rank=1 (accessed on 10 April 2020).
- A Study to Evaluate the Efficacy and Safety of Faricimab in Participants with Neovascular Age-Related Macular Degeneration (LUCERNE). Available online: https://clinicaltrials.gov/ct2/show/NCT03823300?term=NCT03823300&draw=2&rank=1 (accessed on 10 April 2020).
- A Study to Evaluate the Efficacy and Safety of Faricimab in Participants with Neovascular Age-Related Macular Degeneration (TENAYA). Available online: https://clinicaltrials.gov/ct2/show/NCT03823287?term=RG7716&cond=wet+AMD&draw=2&rank=1 (accessed on 1 April 2020).
- A Study to Evaluate the Long-Term Safety and Tolerability of Faricimab in Participants with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04777201?term=Faricimab&cond=AMD&draw=3&rank=3 (accessed on 5 July 2021).
- Khanani, A.M.; Russell, M.W.; Aziz, A.A.; Danzig, C.J.; Weng, C.Y.; Eichenbaum, D.A.; Singh, R.P. Angiopoietins as Potential Targets in Management of Retinal Disease. Clin. Ophthalmol. 2021, 15, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Study of Intravitreal (IVT) REGN910-3 and IVT REGN910 in Patients with Either Neovascular (“Wet”) Age Related Macular Degeneration (AMD) or Diabetic Macular Edema (DME). Available online: https://clinicaltrials.gov/ct2/show/NCT01997164?term=NCT01997164&draw=2&rank=1 (accessed on 10 April 2020).
- Anti-AngiOpoeitin 2 Plus Anti-Vascular ENdothelial Growth Factor as a TherapY for Neovascular Age Related Macular Degeneration: Evaluation of a FiXed Combination Intravitreal Injection. Available online: https://clinicaltrials.gov/ct2/show/NCT02713204?term=NCT02713204&draw=2&rank=1 (accessed on 10 April 2020).
- Ishikawa, M.; Jin, D.; Sawada, Y.; Abe, S.; Yoshitomi, T. Future Therapies of Wet Age-Related Macular Degeneration. J. Ophthalmol. 2015, 2015, 138070. [Google Scholar] [CrossRef] [PubMed]
- Anecortave Acetate in Patients with Exudative Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00299507?term=Anecortave+acetate&cond=AMD&draw=2&rank=2 (accessed on 24 June 2020).
- Triple Combination Therapy of Choroidal Neovascularization in AMD, a Cost Effect and Efficient Therapeutic Treatment. Available online: https://clinicaltrials.gov/ct2/show/NCT02287298?term=Triple+combination+therapy&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Khatol, P.; Saraf, S.; Jain, A. Peroxisome Proliferated Activated Receptors (PPARs): Opportunities and Challenges for Ocular Therapy. Crit. Rev. Ther. Drug Carrier Syst. 2018, 35, 65–97. [Google Scholar] [CrossRef]
- Choudhary, M.; Ding, J.D.; Qi, X.; Boulton, M.E.; Yao, P.L.; Peters, J.M.; Malek, G. PPARβ/δ Selectively Regulates Phenotypic Features of Age-Related Macular Degeneration. Aging 2016, 8, 1952–1978. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. PPARγ Agonists: Potential Treatments for Exudative Age-Related Macular Degeneration. Life Sci. 2017, 188, 123–130. [Google Scholar] [CrossRef]
- A Safety and Efficacy Study of ALG-1001 in Human Subjects with Wet Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01749891?term=ALG-1001&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- A Phase 1 Safety Study of Single and Repeated Doses of JSM6427 (Intravitreal Injection) to Treat AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT00536016?term=JSM6427&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- A Phase 1 Ascending and Parallel Group Trial to Establish the Safety, Tolerability and Pharmacokinetics Profile of Volociximab (Alpha 5 Beta 1 Integrin Antagonist) in Subjects with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00782093?term=Volociximab&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Safety and Efficacy of ATG003 in Patients with AMD Receiving Anti-VEGF. Available online: https://clinicaltrials.gov/ct2/show/NCT00607750?term=ATG003&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Safety and Efficacy of ATG003 in Patients with Wet Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00414206?term=ATG003&cond=AMD&draw=2&rank=2 (accessed on 24 June 2020).
- Lim, J.I. Neovascular Age-Related Macular Degeneration, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN1 10: 1841849499. ISBN2 13: 9781841849492. [Google Scholar]
- Kurup, S.K.; Gee, C.; Greven, C.M. Intravitreal Methotrexate in Therapeutically Resistant Exudative Age-Related Macular Degeneration. Acta Ophthalmol. 2009, 88, e145–e146. [Google Scholar] [CrossRef]
- Soheilian, M.; Movaseghi, M.; Ramezani, A.; Peyman, G.A. Pilot Study of Safety and Effect of Combined Intravitreal Bevacizumab and Methotrexate for Neovascular Age-Related Macular Degeneration. Eur. J. Ophthalmol. 2011, 21, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Evaluate the Effects and Safety of ALK4290 in Patients with Newly Diagnosed Wet Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03558061?term=ALK4290&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Evaluate the Therapeutic Effects and Safety of ALK4290 in Patients with Refractory Wet Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT03558074?term=ALK4290&cond=AMD&draw=2&rank=2 (accessed on 24 June 2020).
- Infliximab, Sirolimus and Daclizumab to Treat Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00304954?term=Sirolimus&cond=AMD&draw=2&rank=7 (accessed on 18 June 2020).
- Phase 1/2 Study of an Ocular Sirolimus (Rapamycin) Formulation in Patients with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00712491?term=Sirolimus&cond=AMD&draw=2&rank=4 (accessed on 18 June 2020).
- Sirolimus Versus Anti-Vascular Endothelial Growth Factor (AntiVEGF) for Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02357342?term=Sirolimus&cond=AMD&draw=2&rank=2 (accessed on 18 June 2020).
- Sirolimus in Conjunction with Eylea vs. Eylea Alone for Exudative AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02732899?term=Sirolimus&cond=AMD&draw=2&rank=1 (accessed on 18 June 2020).
- Anecortave Acetate Versus Placebo in AMD Patients Following PDT. Available online: https://clinicaltrials.gov/ct2/show/NCT00346866?term=Anecortave+acetate&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- TAC-PF, Avastin® in Combination with Photodynamic Therapy to Treat Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00464347?term=Triamcinolone+acetonide&cond=wet+AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Study of the Combination of Anecortave Acetate and Triamcinolone Acetonide for the Treatment of Exudative Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00211419?term=Triamcinolone+acetonide&cond=wet+AMD&draw=2&rank=4 (accessed on 24 June 2020).
- Safety and Efficacy of Intravitreal LFG316 in Wet Age Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01535950?term=NCT01535950&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Safety and Tolerability of Intravenous LFG316 in Wet Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT01624636?term=NCT01624636&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- ARC1905 (ANTI-C5 APTAMER) Given Either In Combination Therapy with Lucentis® 0.5 Mg/Eye In Subjects with Neovascular Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00709527?term=NCT00709527&cond=AMD&draw=2&rank=1 (accessed on 17 July 2020).
- ZIMURA in Combination with LUCENTIS in Patients with Neovascular Age Related Macular Degeneration (NVAMD). Available online: https://clinicaltrials.gov/ct2/show/NCT03362190?term=Zimura&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Safety of Intravitreal POT-4 Therapy for Patients with Neovascular Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00473928?term=POT-4&cond=AMD&draw=2&rank=1 (accessed on 4 May 2020).
- Evaluation of AL-78898A in Exudative Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01157065?term=AL-78898A&cond=AMD&draw=2&rank=1 (accessed on 8 July 2020).
- Assessment of Safety, Tolerability and Pharmacokinetics of Intravitreal APL-2 for Patients with Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT02461771?term=APL-2&cond=AMD&draw=2&rank=1 (accessed on 6 May 2020).
- APL-2 in Neovascular AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03465709?term=APL-2&cond=AMD&draw=2&rank=2 (accessed on 6 May 2020).
- AAVCAGsCD59 for the Treatment of Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03585556?term=AAVCAGsCD59&cond=AMD&draw=2&rank=1 (accessed on 24 June 2020).
- Kanemura, H.; Go, M.J.; Shikamura, M.; Nishishita, N.; Sakai, N.; Kamao, H.; Mandai, M.; Morinaga, C.; Takahashi, M.; Kawamata, S. Tumorigenicity Studies of Induced Pluripotent Stem Cell (IPSC)-Derived Retinal Pigment Epithelium (RPE) for the Treatment of Age-Related Macular Degeneration. PLoS ONE 2014, 9, e85336. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, S. An Eye to the Future: Researchers Debate Best Path for Stem Cell-Derived Therapies. Nat. Med. 2016, 22, 116–119. [Google Scholar] [CrossRef] [PubMed]
- A Study of Implantation of Retinal Pigment Epithelium in Subjects with Acute Wet Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01691261?term=Stem+cells&cond=wet+AMD&draw=2&rank=1 (accessed on 10 April 2020).
- Retinal Pigment Epithelium Safety Study for Patients in B4711001. Available online: https://clinicaltrials.gov/ct2/show/NCT03102138?term=Stem+cells&cond=wet+AMD&draw=2&rank=2 (accessed on 10 June 2020).
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-Related Macular Degeneration and Blindness Due to Neovascular Maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- X-82 to Treat Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02348359?term=NCT02348359&draw=2&rank=1 (accessed on 9 April 2020).
- A Phase 3 Safety and Efficacy Study of Fovista® (E10030) Intravitreous Administration in Combination with Lucentis® Compared to Lucentis® Monotherapy. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01940900 (accessed on 9 April 2020).
- A Phase 3 Safety and Efficacy Study of Fovista® (E10030) Intravitreous Administration in Combination with Lucentis® Compared to Lucentis® Monotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT01944839?term=E10030&cond=AMD&draw=2&rank=3 (accessed on 9 April 2020).
- A Phase 3 Safety and Efficacy Study of Fovista® (E10030) Intravitreous Administration in Combination with Either Avastin® or Eylea® Compared to Avastin® or Eylea® Monotherapy. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01940887?term=E10030&cond=AMD&draw=2&rank=5 (accessed on 9 April 2020).
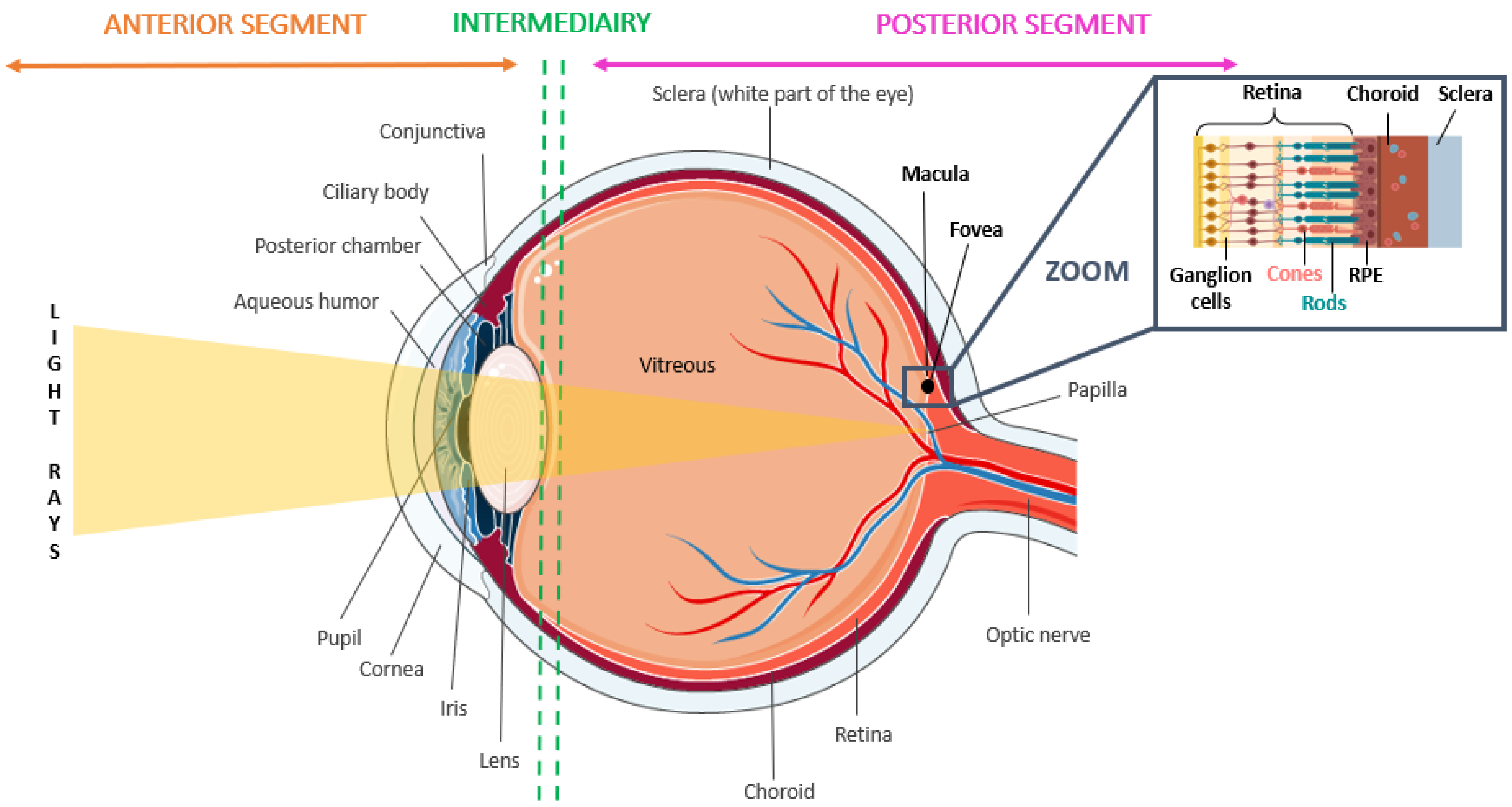



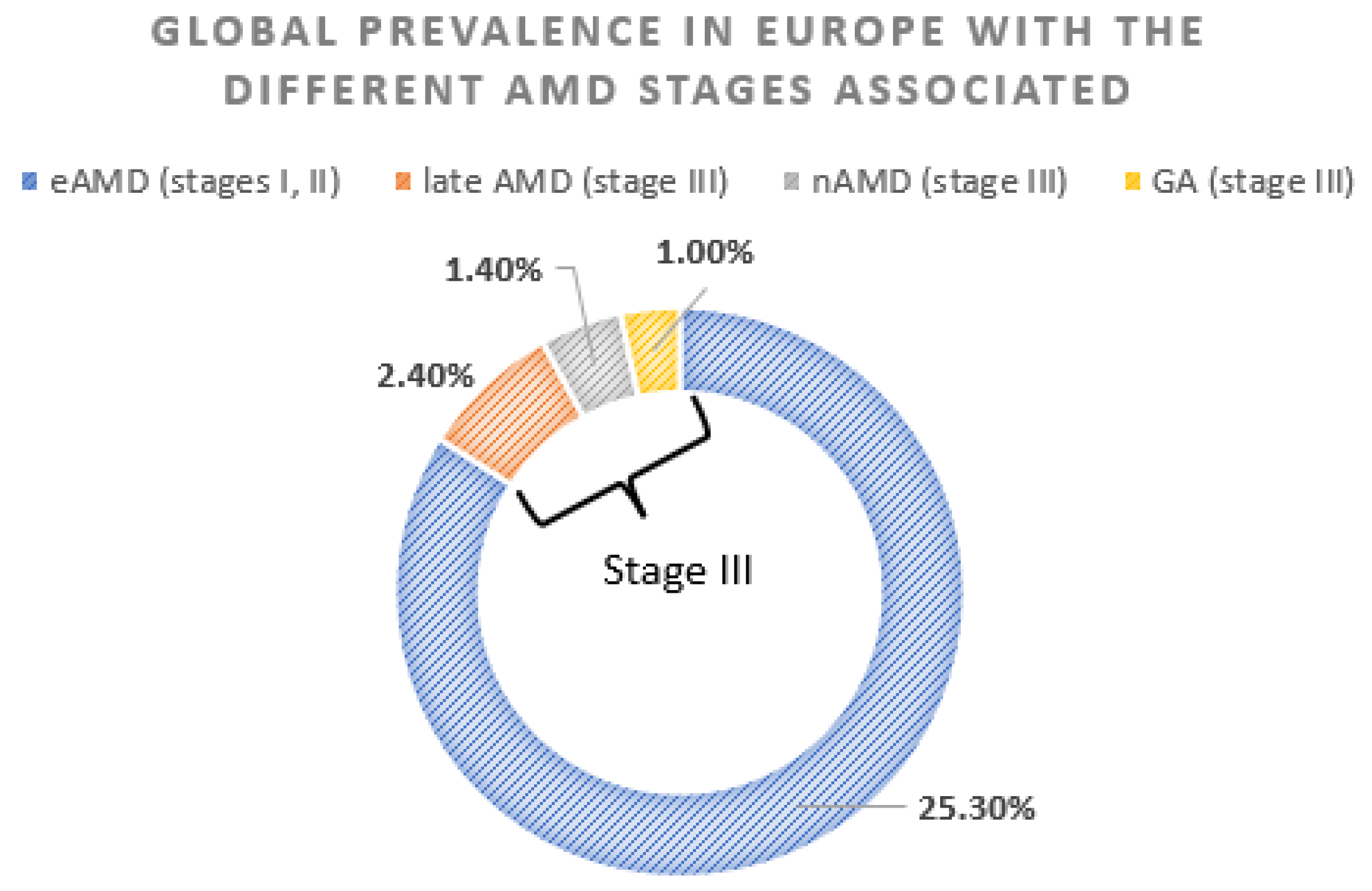


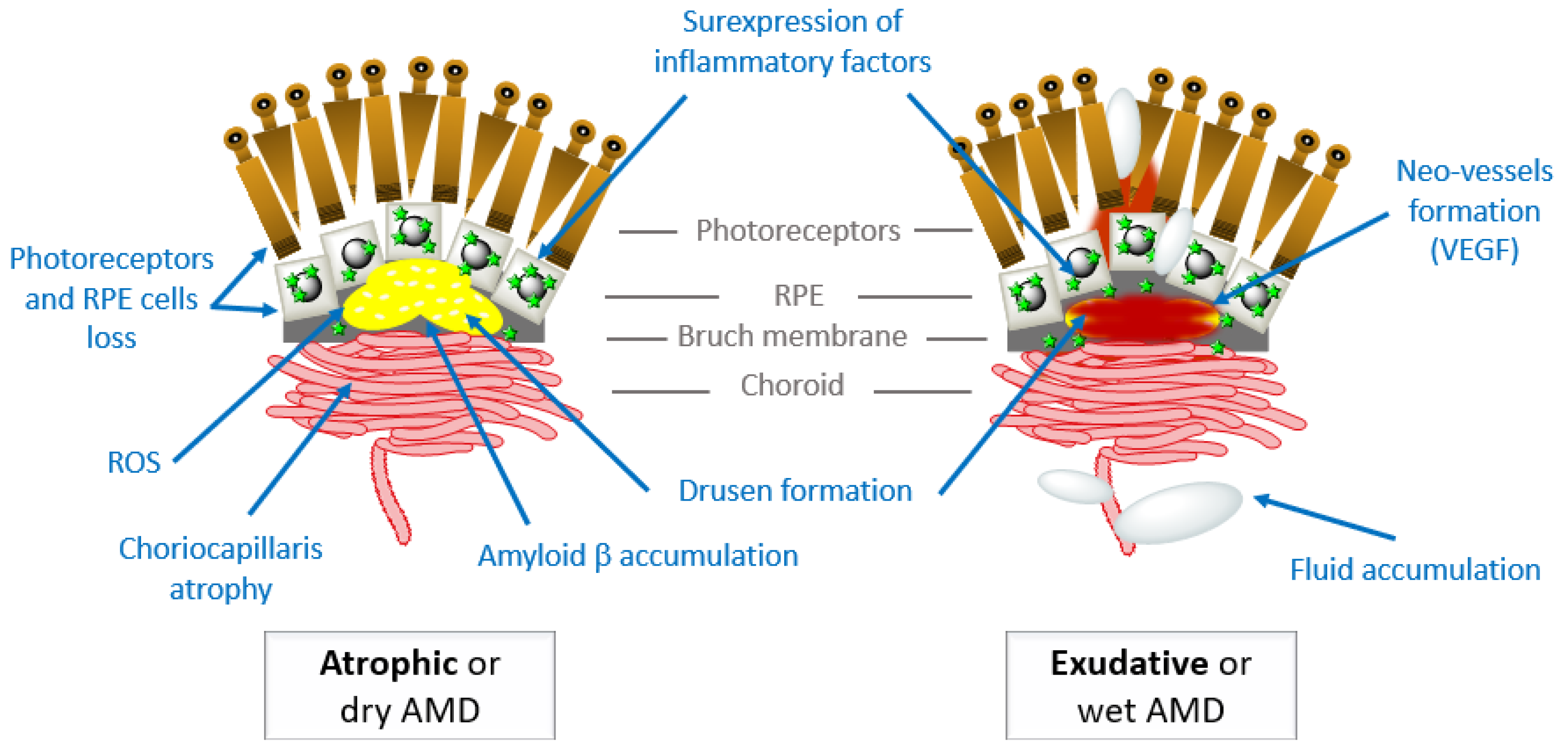




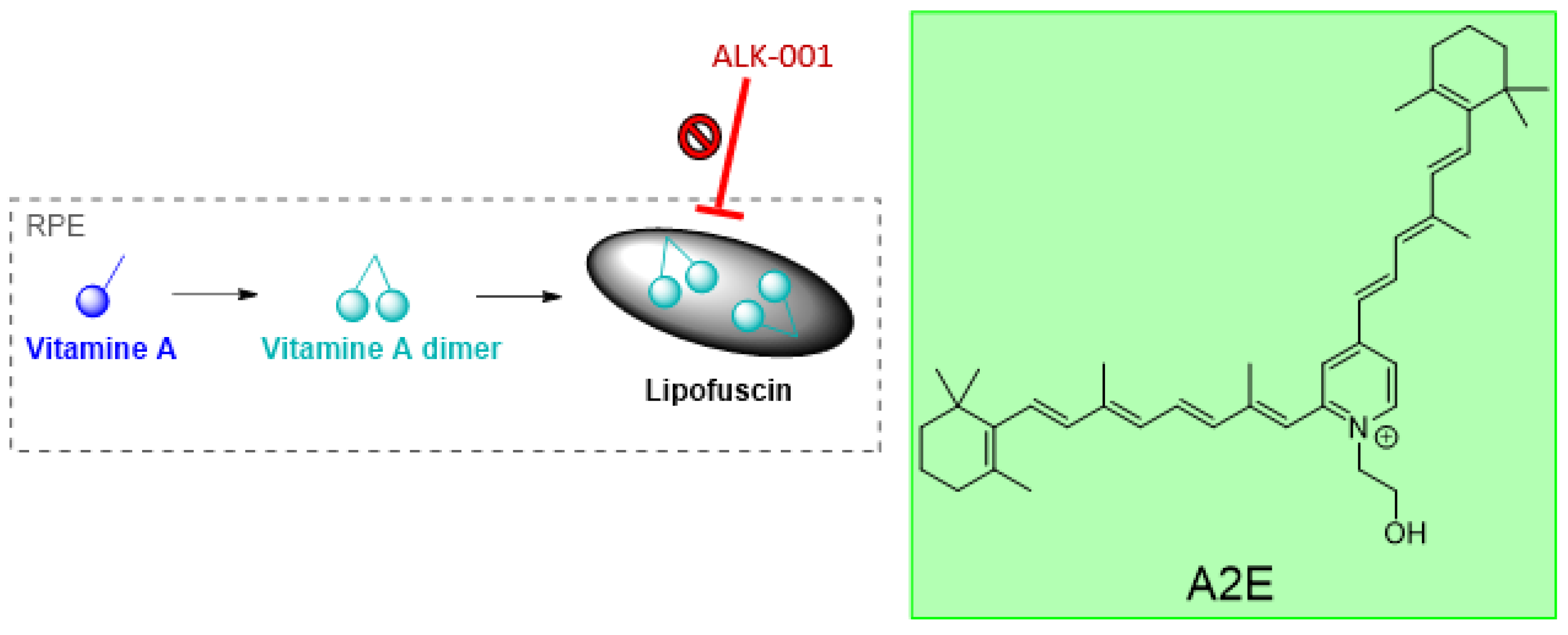




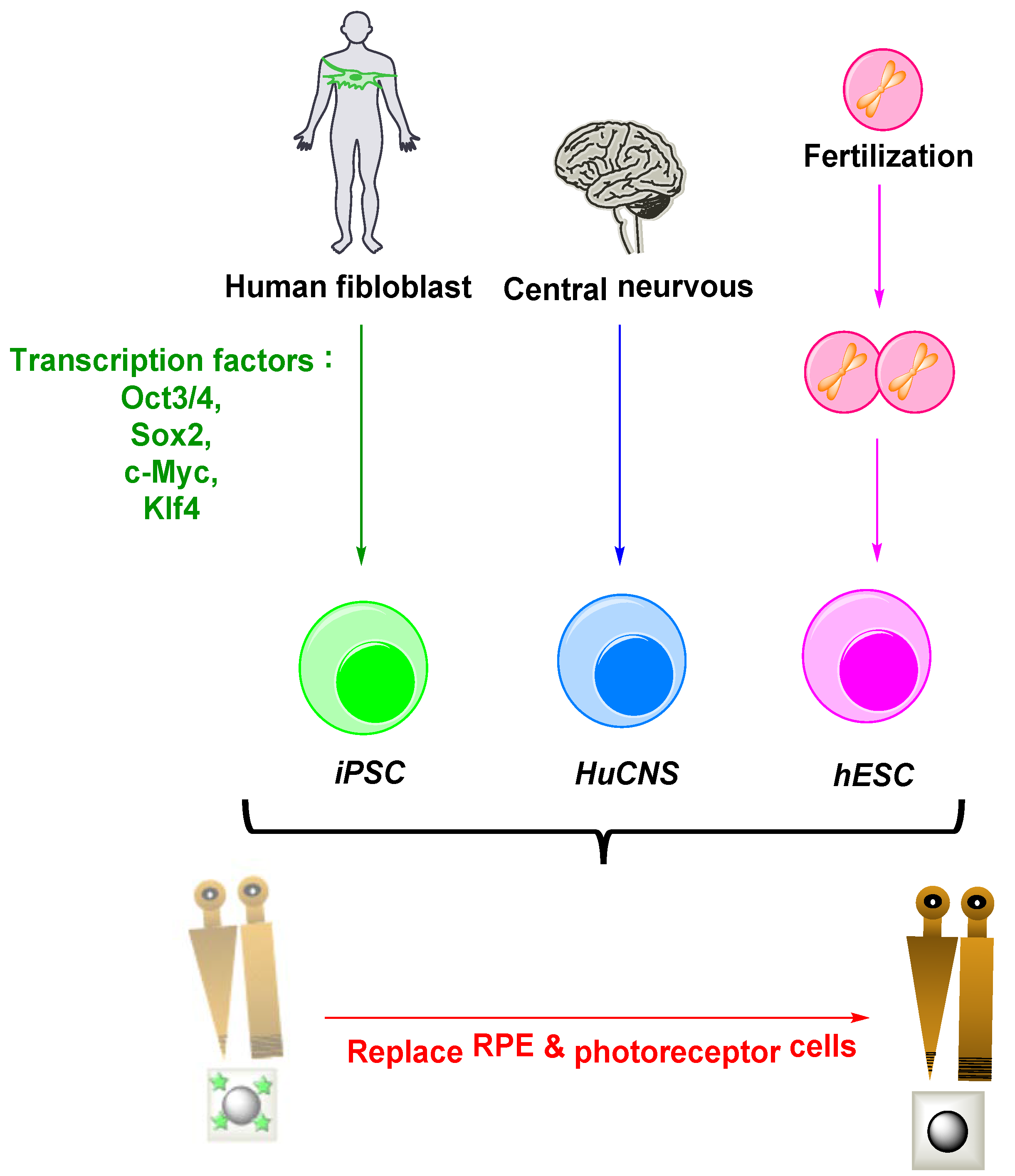




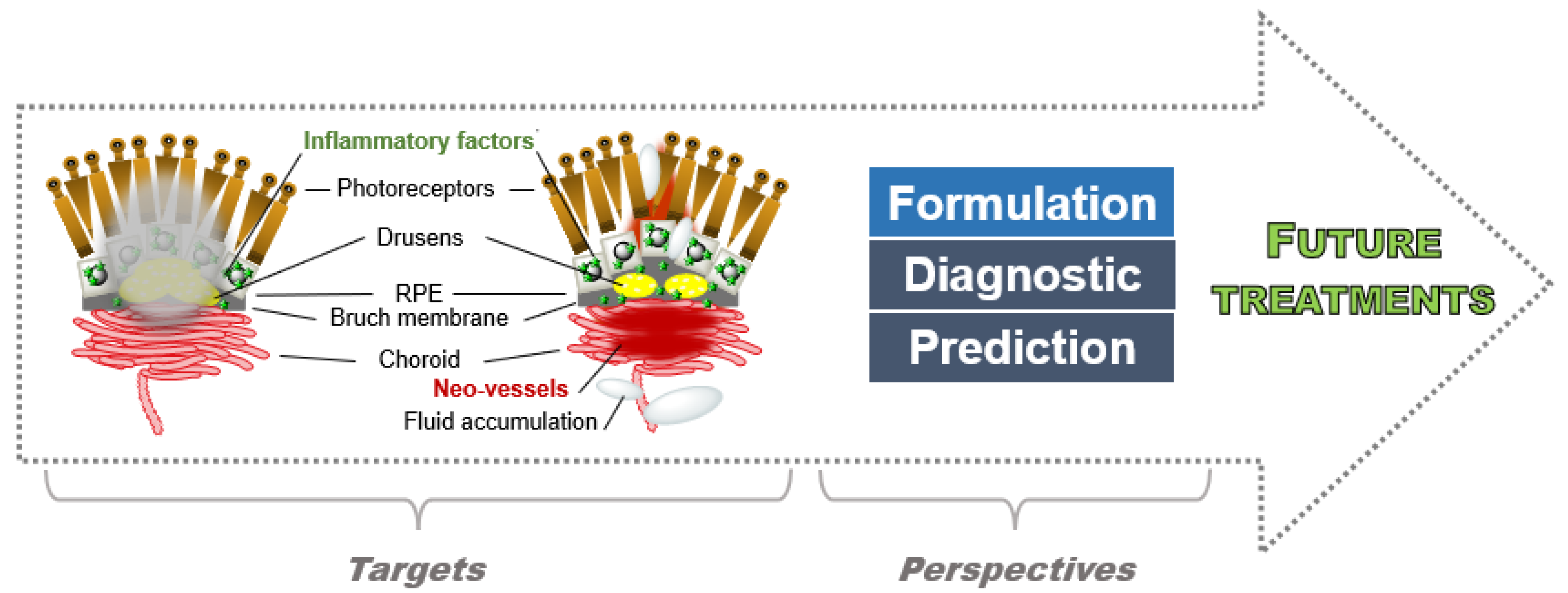
| Drugs | Structure |
|---|---|
| Lutein |  |
| Zeaxanthin |  |
| meso-zeaxanthin |  |
| Curcumin |  |
| Resveratrol |  |
| Drugs | Structure | Target | Visual Acuity Efficiency in Phase 3 | Formulation | Prices (US; France) | Ref. |
|---|---|---|---|---|---|---|
| Pegaptanib | Aptamer (50 kDa) | VEGF-A 165 | - | Intravitreal injections (0.3 mg/6 weeks) | $5300 per 5 doses | VISION [255] |
| Ranibizumab | Monoclonal Antibody, Fab Fragment (48 kDa) | All isoforms of VEGF-A | + | Intravitreal injections (0.5 mg/month) | $2023 per dose; 738 euros/monthly dose | NCT00056836 [257] NCT01442064 [258] NCT00379795 [259] NCT00061594 [261] |
| Aflibercept | Fusion Protein (115 kDa) | All isoforms of VEGF-A + PIGF | + | Intravitreal injections (2 mg/month) | $1940.90 per dose; 730 euros/monthly dose | NCT00509795 [270] NCT00637377 [271] |
| Bevacizumab | Monoclonal Antibody (149 kDa) | All isoforms of VEGF-A | + | Intravitreal injections (1.25 mg) | $55 per dose; 100 euros per dose | NCT01170767 [277] |
| Brolucizumab | Monoclonal Antibody (26 kDa) | All isoforms of VEGF-A | + | Intravitreal injections (6 mg/month for the first three doses, followed by 6 mg/8–12 weeks) | $1940.90 per dose | NCT02507388 [282] NCT03930641 [283] NCT03954626 [284] NCT03386474 [285] NCT02307682 [286] NCT02434328 [287] |
| Drugs | Structure | Target | Clinic | Formulation | Ref. |
|---|---|---|---|---|---|
| Triple combination therapy | Verteporfin-Ranibizumab-Dexamethasone | Multiple components | 2 | PDT, Intravitreal injections | NCT02287298 [423] |
| PPAR inhibitors | Nuclear transcription factors | PPAR receptors | R | - | [424,425,426] |
| ALG-1001 |  | Integrin receptors | 1/2 | Intravitreal injections | NCT01749891 [427] |
| JSM6427 | 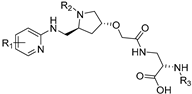 | Integrin α5β1 | 1 | Intravitreal injections | NCT00536016 [428] |
| Volociximab | Monoclonal Antibody | Integrin α5β1 | 1 | Intravitreal injections | NCT00782093 [429] |
| ATG003 | Mecamylamine | Nicotinic acetylcholine receptor (nAchR) | 2 | Eye drops | NCT00607750 [430] NCT00414206 [431] |
| AdPEDF.11 | Adenoviral PEDF | PEDF | 1 | Intravitreal injections | [432] |
| Methotrexate |  | Antifolate drug | R | Intravitreal injections | [433,434] |
| ALK4290 |  | CCL11 | 2 | Oral | NCT03558061 [435] NCT03558074 [436] |
| Sirolimus |  | mTOR | 2 | Intravitreal injections | NCT00304954 [437] NCT00712491 [438] NCT02357342 [439] NCT02732899 [440] |
| Anecortave acetate |  | Inflammatory | 3 | Intravitreal injections | NCT00299507 [422] NCT00346866 [441] |
| Triamcinolone |  | Inflammatory | 2 | Intravitreal Injections | NCT00464347 [442] NCT00211419 [443] |
| Infliximab | Monoclonal Antibody | TNF-α | 1 | Intravitreal injections | NCT00304954 [437] |
| Tesidolumab | Monoclonal Antibody | C5 | 2 | Intravitreal injections | NCT01255462 [178] NCT01535950 [444] NCT01624636 [445] |
| Avacincaptad pegol | Aptamer | C5 | 1 2 | Intravitreal injections | NCT00709527 [446] NCT03362190 [447] |
| POT-4/APL-2 | Monomer/Dimer cyclic peptide | C3 | 2/1/2/3 | Intravitreal injections | NCT00473928 [448] NCT01157065 [449] NCT02461771 [450] NCT03465709 [451] |
| AAVCAGsCD59 | Ocular gene therapy product | MAC | 1 | Intravitreal injections | NCT03585556 [452] |
| GEM103 | Recombinant complement factor H protein | Factor H | 2 | Intravitreal injections | NCT04684394 [199] |
| Drugs | Structure | Target | Clinic | Formulation | Ref. |
|---|---|---|---|---|---|
| Conbercept | Recombinant fusion protein | all isoforms of VEGF A, + VEGF B + PIGF | 1 | Intravitreal injections | NCT01242254 [295] |
| 2 | NCT01157715 [296] | ||||
| 3 | NCT01436864 [298] | ||||
| NCT03577899 [300] | |||||
| NCT03630952 [301] | |||||
| 4 | NCT02577107 [299] | ||||
| Abicipar pegol | DARpin | VEGF | 1 | Intravitreal injections | NCT02859766 [305] |
| NCT03335852 [306] | |||||
| 2 | NCT02181517 [307] | ||||
| NCT02181504 [308] | |||||
| NCT01397409 [309] | |||||
| 3 | NCT02462928 [311] | ||||
| NCT02462486 [312] | |||||
| 2 | NCT03539549 [314] | ||||
| OPT-302 | soluble form of VEGFR-3 | VEGF C and D | 2b | Intravitreal injections | NCT02543229 [315] NCT03345082 [317] |
| Sorafenib | 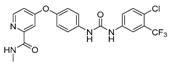 | Raf, PDGF, VEGF receptor kinases and c Kit | R | Eye drops | [321,322,323] |
| Pazopanib |  | VEGFR, PDGFR | Eye drops | NCT00463320 [326] | |
| 1 | NCT00659555 [327] | ||||
| NCT01154062 [328] | |||||
| NCT01051700 [329] | |||||
| NCT01072214 [330] | |||||
| NCT00612456 [331] | |||||
| 2 | NCT00733304 [332] | ||||
| NCT01134055 [333] | |||||
| Axitinib |  | VEGFR, PDGFR, FGFR and colony-stimulating factor receptor | 1/2 | Eye drops | NCT04626128 [342] NCT05131646 [343] NCT04989699 [344] |
| Acrizanib |  | VEGFR-2 | 1 | Topical formulation | NCT02076919 [346] |
| 2 | NCT02355028 [347] | ||||
| Regorafenib |  | VEGFR, PDGFR | 2 | Eye drops | NCT02222207 [351] |
| SH-11037 |  | VEGF sEH | R | Eye drops | [353,354] |
| PAN-90806 |  | VEGF-R2 | 1 1/2 | Eye drops | NCT02022540 [357] NCT03479372 [358] |
| Vorolanib |  | VEGFR, PDGFR | 2 | Oral tablet Intravitreal injection | NCT01674569 [361] NCT02348359 [458] NCT04747197 [362] |
| Lenvatinib |  | VEGFR, PDGFR and FGFR | R | [369] | |
| Brivanib |  | VEGFR2 and FGFR1 | R | [372] | |
| Squalamine |  | VEGF + PDGF | 3 | Eye drops | NCT00089830 [375] NCT00333476 [376] NCT00094120 [377] NCT00139282 [378] NCT01678963 [379] |
| Bevasiranib | Small interfering RNA | siRNA | 3 | Intravitreal injections | NCT00499590 [384] |
| siRNA-027 | siRNA | Intravitreal injections | NCT00363714 [386] NCT00395057 [387] | ||
| RetinoStat | Lentivirus expressing endostatin/angiostatin | Gene therapy (VEGF) | 1 | Subretinal Injections | GEM STUDY [389] |
| Cas9 | Ribonucleoproteins | Gene therapy (VEGF) | R | Subretinal injections | [393] |
| rAAV.sFLT-1 | AAV expressing sFLT | Gene therapy (VEGF) | 1/2 | Subretinal injections | NCT01494805 [394,395] |
| AAV2.sFLT01 | AAV expressing sFLT | Gene therapy (VEGF) | 1 | Intravitreous injections | NCT01024998 [396,397] |
| ADVM-022 | AAV expressing aflibercept | Gene therapy (VEGF) | 1 | Intravitreal injections | NCT03748784 [399] |
| RGX-314 | AAV8 expressing monoclonal antibody fragment | Gene therapy (VEGF) | 1 2/3 | Suprachoroidal Subretinal injections | NCT04514653 [401] NCT04704921 [402] |
| Pegleranib | PEGylated aptamer | PDGF | 1 2 3 | Intravitreal injections | NCT00569140 [406] NCT01089517 [407] NCT01940900 [459] NCT01944839 [460] NCT01940887 [461] |
| Rinucumab | Monoclonal Antibody | PDGF | 1 2 | Intravitreal injections | NCT02061865 [411] NCT02418754 [408] |
| Faricimab | Monoclonal Antibody | Ang-2 | 1 2 3 | Intravitreal injections | NCT01941082 [413] NCT02484690 [414] NCT03823300 [415] NCT03823287 [416] NCT04777201 [417] |
| Nesvacumab | Fully human antibody | Ang-2 | 1 2 | Intravitreal injections | NCT01997164 [419] NCT02713204 [420] |
| iPSC | Human fibroblast | RPE cells | R | Implant | [453,454] |
| PF-05206388 | Stem cell | RPE cells | 1 | Implant | NCT01691261 [455] NCT03102138 [456] |
| Atrophic AMD | Exudative AMD | |
|---|---|---|
| Characteristics and specificities |
|
|
| Approved treatments | No current treatment |
|
| Future trends |
|
|
|
| |
|
| |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. https://doi.org/10.3390/molecules27165089
Fabre M, Mateo L, Lamaa D, Baillif S, Pagès G, Demange L, Ronco C, Benhida R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules. 2022; 27(16):5089. https://doi.org/10.3390/molecules27165089
Chicago/Turabian StyleFabre, Marie, Lou Mateo, Diana Lamaa, Stéphanie Baillif, Gilles Pagès, Luc Demange, Cyril Ronco, and Rachid Benhida. 2022. "Recent Advances in Age-Related Macular Degeneration Therapies" Molecules 27, no. 16: 5089. https://doi.org/10.3390/molecules27165089
APA StyleFabre, M., Mateo, L., Lamaa, D., Baillif, S., Pagès, G., Demange, L., Ronco, C., & Benhida, R. (2022). Recent Advances in Age-Related Macular Degeneration Therapies. Molecules, 27(16), 5089. https://doi.org/10.3390/molecules27165089