Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
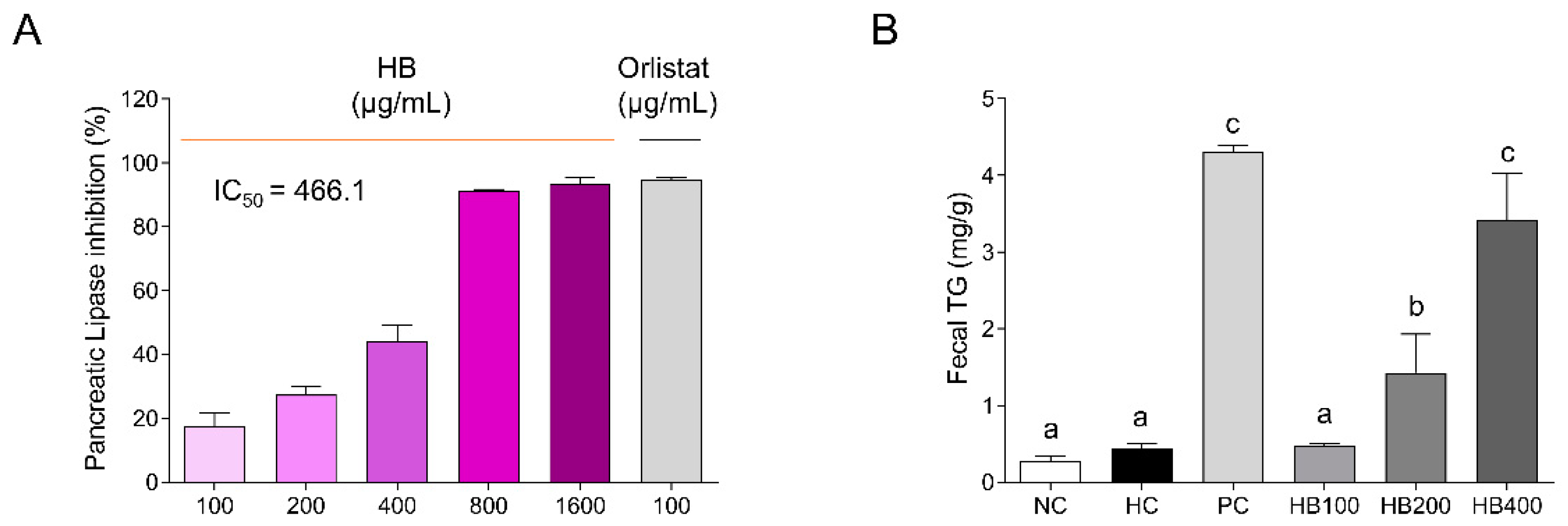
2.1. Effects of HB on Pancreatic Lipase Inhibition and TG Contents in the Feces
2.2. Effects of HB on Fat Accumulation in the Mice Model of HFD
2.3. Effects of HB on Lipid Metabolism in Adipose Tissue
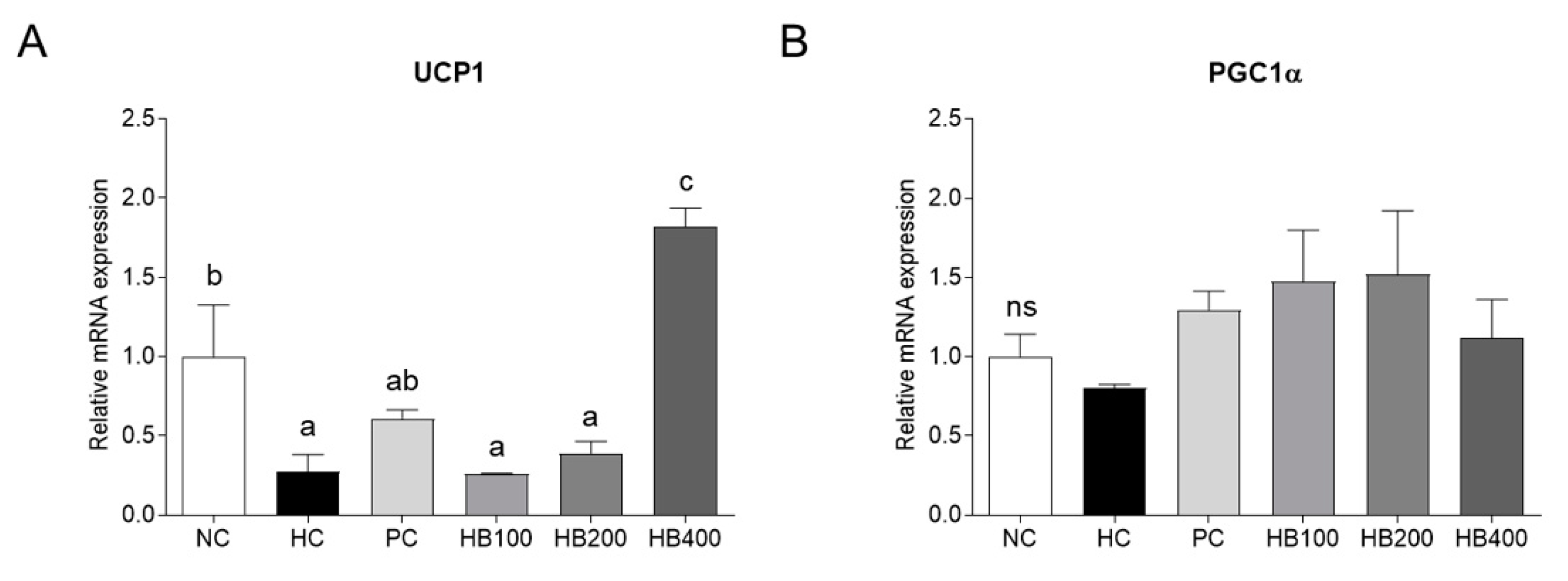
2.4. Effects of HB on Thermogenesis-Related Genes Expression in Adipose Tissue
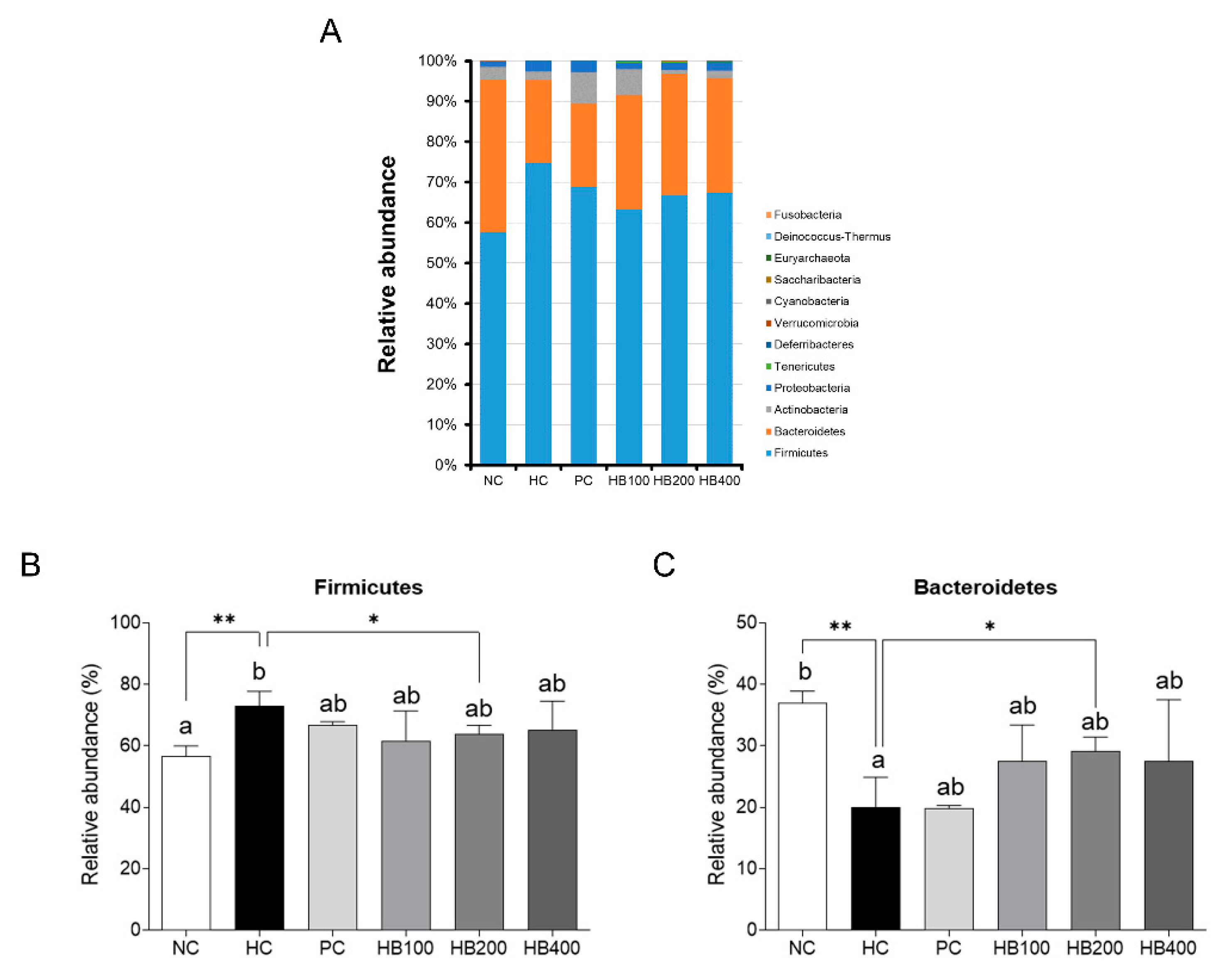
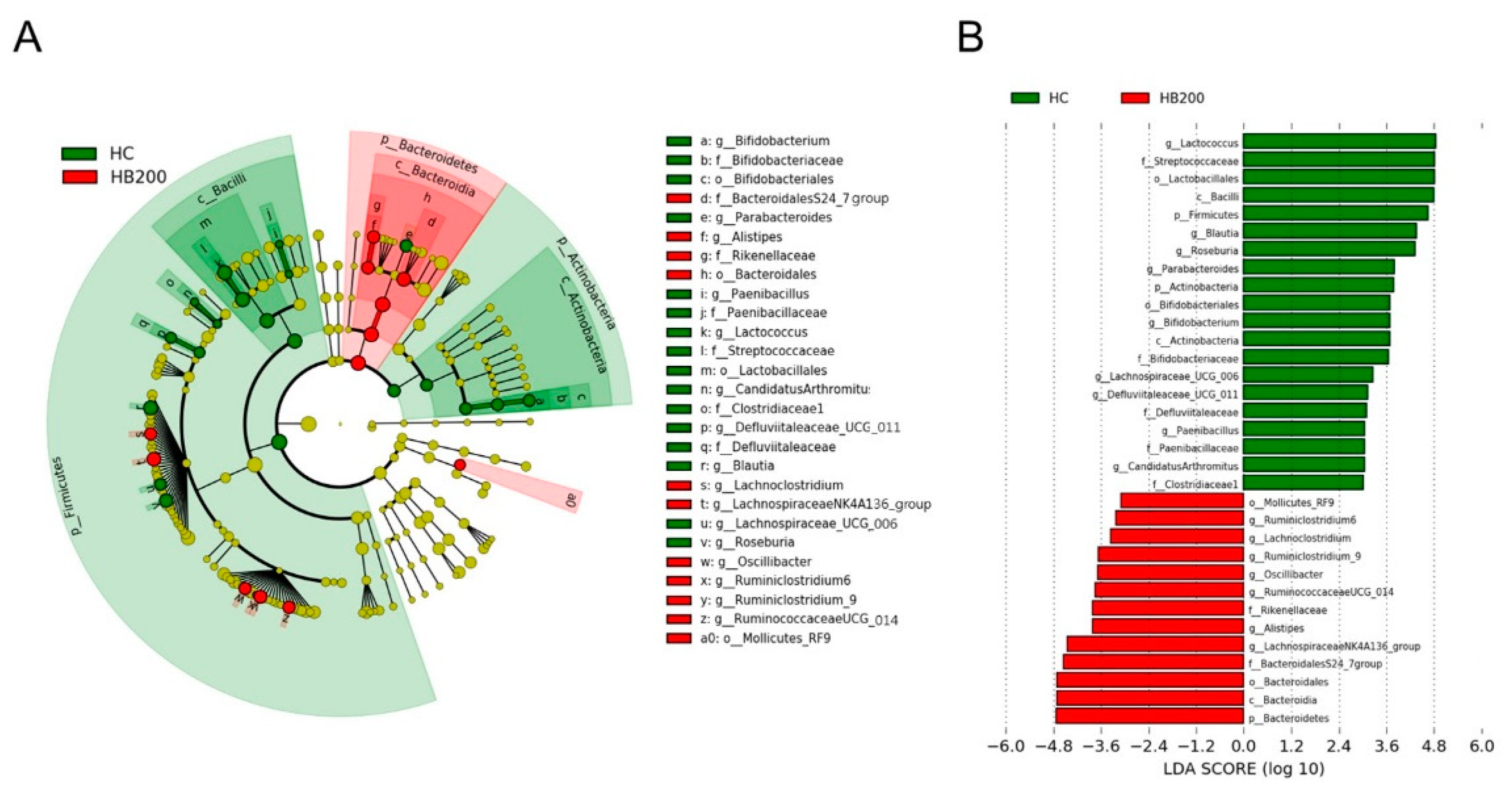
2.5. Effects of HB on the Composition of Fecal Microbiota
3. Discussion
4. Materials and Methods
4.1. Preparation of HB Extract
4.2. Pancreatic Lipase Inhibition Assay
4.3. Animals and Treatment
4.4. Epididymal Fat Tissue and Feces Collection
4.5. Fecal Triglyceride Analysis
4.6. Histological Analysis
4.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Microbiota Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatric Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaun, H.; Thariat, J.; Vignot, M.; Merrouche, Y.; Vignot, S. Obesity and cancer. Bull. Cancer 2017, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-Obesity Effect of DKB-117 through the Inhibition of Pancreatic Lipase and alpha-Amylase Activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.R.; Calis, K.A.; Booth, S.L.; Uwaifo, G.I.; Yanovski, J.A. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy 2002, 22, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, W.; Wang, H.L.; Dai, L.L.; Zong, W.H.; Wang, Y.Z.; Bi, J.; Han, W.; Dong, G.J. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef]
- Yu, D.; Meng, X.; de Vos, W.M.; Wu, H.; Fang, X.; Maiti, A.K. Implications of Gut Microbiota in Complex Human Diseases. Int. J. Mol. Sci. 2021, 22, 12661. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- An, M.Y.; Eo, H.J.; Son, H.J.; Geum, N.G.; Park, G.H.; Jeong, J.B. Antiinflammatory effects of leaf and branch extracts of honeyberry (Lonicera caerulea) on lipopolysaccharidestimulated RAW264.7 cells through ATF3 and Nrf2/HO1 activation. Mol. Med. Rep. 2020, 22, 5219–5230. [Google Scholar] [CrossRef] [PubMed]
- Golba, M.; Sokol-Letowska, A.; Kucharska, A.Z. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An Update on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Lee, H.J. Lonicera caerulea: An updated account of its phytoconstituents and health-promoting activities. Trends Food Sci. Technol. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, J.W.; Ku, S.K.; Choi, J.S.; Lee, H.J. Anti-diabetic effects of blue honeyberry on high-fed-diet-induced type II diabetic mouse. Nutr. Res. Pract. 2019, 13, 367–376. [Google Scholar] [CrossRef]
- Park, M.; Yoo, J.H.; Lee, Y.S.; Lee, H.J. Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice. Nutrients 2019, 11, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Park, E.J.; Kim, S.M.; Kim, J.Y.; Lee, H.J. Anti-Sarcopenic Obesity Effects of Lonicera caerulea Extract in High-Fat Diet-Fed Mice. Antioxidants 2021, 10, 1633. [Google Scholar] [CrossRef]
- Nishina, A.; Itagaki, M.; Suzuki, Y.; Koketsu, M.; Ninomiya, M.; Sato, D.; Suzuki, T.; Hayakawa, S.; Kuroda, M.; Kimura, H. Effects of Flavonoids and Triterpene Analogues from Leaves of Eleutherococcus sieboldianus (Makino) Koidz. ‘Himeukogi‘ in 3T3-L1 Preadipocytes. Molecules 2017, 22, 671. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, E.J.; Kim, S.M.; Lee, H.J. Optimization of adipogenic differentiation conditions for canine adipose-derived stem cells. J. Vet. Sci. 2021, 22, e53. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, M.G.; Choung, S.Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Palacios-Gonzalez, B.; Vargas-Castillo, A.; Velazquez-Villegas, L.A.; Vasquez-Reyes, S.; Lopez, P.; Noriega, L.G.; Aleman, G.; Tovar-Palacio, C.; Torre-Villalvazo, I.; Yang, L.J.; et al. Genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice. J. Nutr. Biochem. 2019, 68, 59–68. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.S.; Lee, H.J. Anti-obesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef] [Green Version]
- D‘Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, S.; Mepani, R.J.; Laznik, D.; Ye, L.; Jurczak, M.J.; Jornayvaz, F.R.; Estall, J.L.; Chatterjee Bhowmick, D.; Shulman, G.I.; Spiegelman, B.M. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 9635–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.M.; Kim, Y.S.; Lee, Y.; Kim, J.; Sun, H.; Kim, J.H.; Kim, J.S. Inhibitory activities of pancreatic lipase and phosphodiesterase from Korean medicinal plant extracts. Phytother. Res. 2012, 26, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.J.; Lee, H.Y.; Kim, T.H.; Kim, S.G. Antioxidant and pancreatic lipase inhibitory activities of Anemarrhena asphodeloides. Korean J. Food Preserv. 2014, 21, 421–426. [Google Scholar] [CrossRef]
- Vijayaraj, P.; Nakagawa, H.; Yamaki, K. Cyanidin and cyanidin-3-glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. J. Food Biochem. 2019, 43, e12774. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef]
- Lin, C.S.; Chang, C.J.; Lu, C.C.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D.; Lai, H.C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed. J. 2014, 37, 259–268. [Google Scholar]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Kapoor, P.; Kumari, A.; Sheoran, B.; Sharma, S.; Kaur, S.; Bhunia, R.K.; Rajarammohan, S.; Bishnoi, M.; Kondepudi, K.K.; Garg, M. Anthocyanin biofortified colored wheat modifies gut microbiota in mice. J. Cereal Sci. 2022, 104, 103433. [Google Scholar] [CrossRef]
- Wu, S.; Hu, R.; Nakano, H.; Chen, K.; Liu, M.; He, X.; Zhang, H.; He, J.; Hou, D.X. Modulation of Gut Microbiota by Lonicera caerulea L. Berry Polyphenols in a Mouse Model of Fatty Liver Induced by High Fat Diet. Molecules 2018, 23, 3213. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.F.; Murphy, E.F.; O‘Sullivan, O.; Ross, R.P.; O‘Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Cheng, D.; Peng, C.; Li, Y.; Zhu, Y.; Lu, N. High-Fat Diet Induces Dysbiosis of Gastric Microbiota Prior to Gut Microbiota in Association With Metabolic Disorders in Mice. Front. Microbiol. 2018, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, Y.S.; Kim, S.M.; Park, G.S.; Lee, Y.H.; Jeong, D.Y.; Kang, J.; Lee, H.J. Beneficial Effects of Lactobacillus plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients 2020, 12, 542. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Inui, R.; Akamatsu, Y.; Goto, M.; Doi, H.; Matsuoka, S. Illumina iSeq 100 and MiSeq exhibit similar performance in freshwater fish environmental DNA metabarcoding. Sci. Rep. 2021, 11, 15763. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]



| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession Number |
|---|---|---|---|
| Pparγ | TTTTCAAGGGTGCCAGTTT | AATCCTTGGCCCTCTGAGAT | NM_001127330.2 |
| C/ebpα | TTACAACAGGCCAGGTTTCC | GGCTGGCGACATACAGTACA | NM_001287523 |
| Fabp4 | TCACCTGGAAGACAGCTCCT | AATCCCCATTTACGCTGATG | NM_024406.3 |
| Srebp1c | ATCGCAAACAAGCTGACCTG | AGATCCAGGTTTGAGGTGGG | NM_011480 |
| Fas | TTGCTGGCACTACAGAATGC | AACAGCCTCAGAGCGACAAT | NM_007988.3 |
| Ucp1 | CTTTGCCTCACTCAGGATTGG | ACTGCCACACCTCCAGTCATT | NM_009463.3 |
| Ppargc1α | ATGTGTCGCCTTCTTGCTCT | ATCTACTGCCTGGGGACCTT | NM_008904.2 |
| Actb | CCACAGCTGAGAGGAAATC | AAGGAAGGCTGGAAAAGAGC | NM_007393.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Lee, Y.-S.; Park, E.-J.; Lee, H.-J. Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Molecules 2022, 27, 4731. https://doi.org/10.3390/molecules27154731
Kim J-Y, Lee Y-S, Park E-J, Lee H-J. Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Molecules. 2022; 27(15):4731. https://doi.org/10.3390/molecules27154731
Chicago/Turabian StyleKim, Jong-Yeon, You-Suk Lee, Eun-Jung Park, and Hae-Jeung Lee. 2022. "Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice" Molecules 27, no. 15: 4731. https://doi.org/10.3390/molecules27154731
APA StyleKim, J.-Y., Lee, Y.-S., Park, E.-J., & Lee, H.-J. (2022). Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Molecules, 27(15), 4731. https://doi.org/10.3390/molecules27154731







