Trityl Cation-Catalyzed Hosomi-Sakurai Reaction of Allylsilane with β,γ-Unsaturated α-Ketoester to Form γ,γ-Disubstituted α-Ketoesters
Abstract
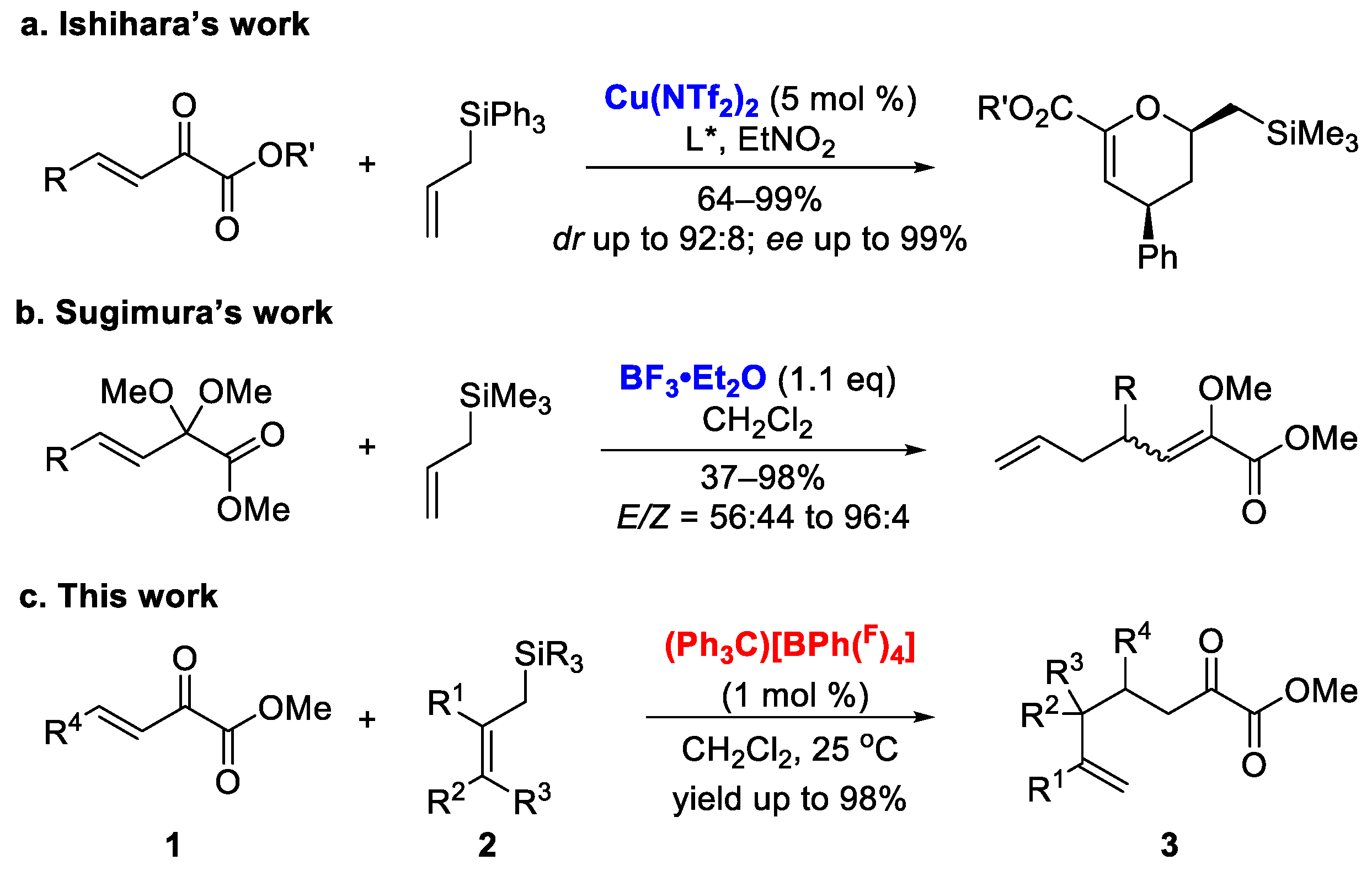
:1. Introduction
2. Results and Discussion
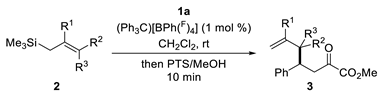
2.1. Synthesis
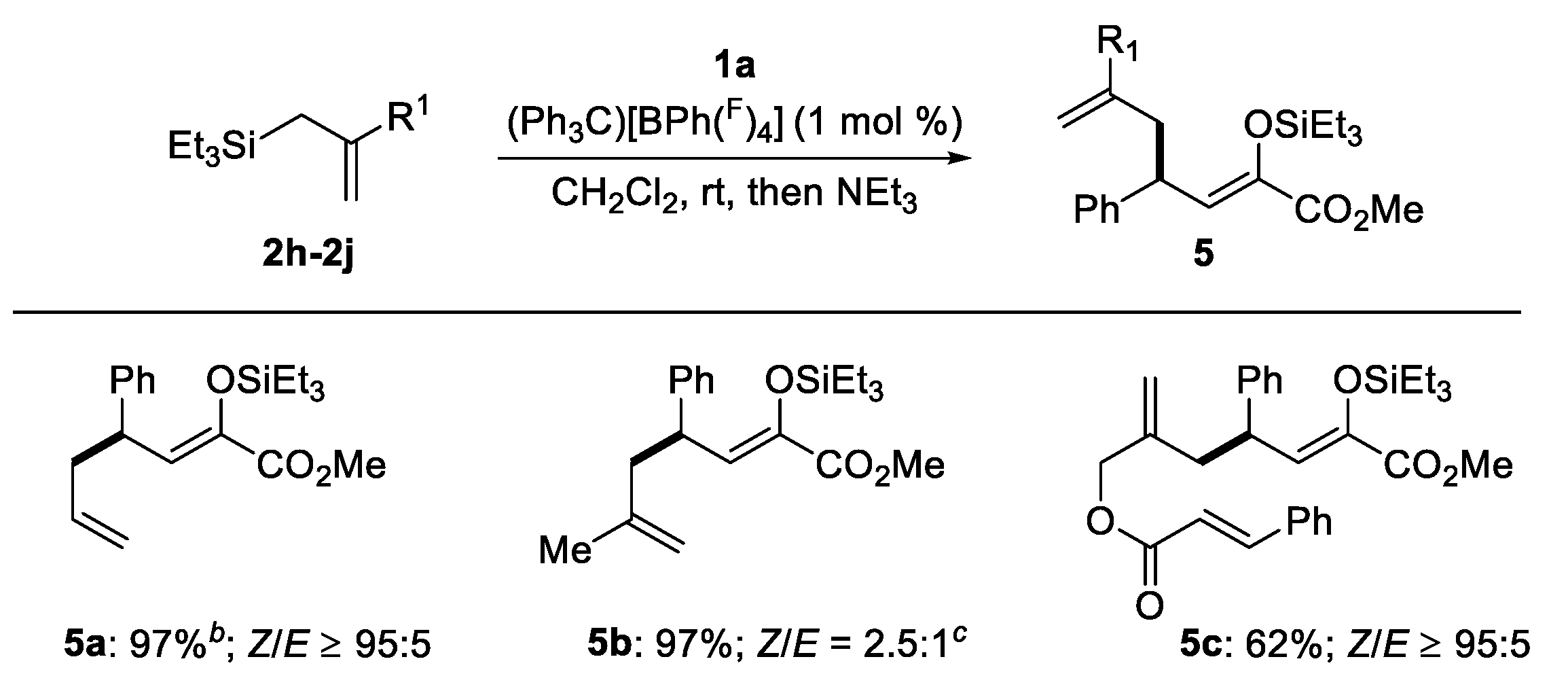
2.2. Mechanistic Investigations
3. Materials and Methods
3.1. General Procedures for the Synthesis of γ,γ-Disubstituted α-Ketoesters 3a–3v
3.2. General Procedures for the Synthesis of Silyl Enol Ethers 5a–5e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References and Notes
- Deng, R.; Han, T.-J.; Gao, X.; Yang, Y.-F.; Mei, G.-J. Further Developments of β,γ-Unsaturated α-Ketoesters as Versatile Synthons in Asymmetric Catalysis. iScience 2022, 25, 103913. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, K.; Morimoto, H.; Mashima, K.; Ohshima, T. Direct Enantioselective Alkynylation of α-Ketoesters and α-Ketiminoesters Catalyzed by [bis(Oxazoline)phenyl]rhodium(III) Complexes. Heterocycles 2017, 95, 637–661. [Google Scholar]
- Blaser, H.-U.; Jalett, H.-P.; Muller, M.; Studer, M. Enantioselective Hydrogenation of α-ketoesters Using Cinchona Modified Platinum Catalysts and Related Systems: A Review. Catal. Today 1997, 37, 441–463. [Google Scholar] [CrossRef]
- Kambale, D.A.; Thorat, S.S.; Pratapure, M.S.; Gonnade, R.G. Lewis Acid Catalyzed Cascade Annulation of Alkynols with α-Ketoesters: A Facile Access to γ-Spiroketal-γ-lactones. Chem. Commun. 2017, 53, 6641–6644. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Gloer, J.B.; Shearer, C.A. Massarinolins A−C: New Bioactive Sesquiterpenoids from the Aquatic Fungus Massarina tunicate. J. Nat. Prod. 1999, 62, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, K.; Zhang, B.; Guo, W.; Liu, Y.; Li, H. Water Enables an Asymmetric Cross Reaction of α-Keto Acids with α-Keto Esters for the Synthesis of Quaternary Isotetronic Acids. Chem. Commun. 2019, 55, 12813–12816. [Google Scholar] [CrossRef] [PubMed]
- Haritakun, R.; Rachtawee, P.; Chanthaket, R.; Boonyuen, N.; Isaka, M. Butyrolactones from the Fungus Aspergillus terreus BCC 4651. Chem. Pharm. Bull. 2010, 58, 1545–1548. [Google Scholar] [CrossRef] [Green Version]
- Chien, J.C.W.; Tsai, W.M.; Raush, M.D. Isospecific Polymerization of Propylene Catalyzed by rac-Ethylenebis(indenyl)methylzirconium Cation. J. Am. Chem. Soc. 1991, 113, 8570–8571. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kobayashi, S.; Murakami, M. Trityl Perchlorate as an Efficient Catalyst in the Aldol-Type Reaction. Chem. Lett. 1984, 13, 1759–1762. [Google Scholar] [CrossRef] [Green Version]
- Mukaiyama, T.; Kobayashi, S.; Murakami, M. An Efficient Method for the Preparation of Threo Cross-Aldols from Silyl Enol Ethers and Aldehydes Using Trityl Perchlorate as a Catalyst. Chem. Lett. 1985, 14, 447–550. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, M.; Mukaiyama, T. Trityl Salts as Efficient Catalysts in the Aldol Reaction. Chem. Lett. 1985, 14, 1535–1538. [Google Scholar] [CrossRef]
- Kobayashi, S.; Matsui, S.; Mukaiyama, T. Trityl Salt Catalyzed Aldol Reaction between α,β-Acetylenic Ketones and Silyl Enol Ethers. Chem. Lett. 1988, 17, 1491–1494. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Akamatsu, H.; Han, J.S. A Convenient Method for Stereoselective Synthesis of β-Aminoesters. Iron(II) Iodide or Trityl Hexachloroantimonate as an Effective Catalyst in the Reaction of Ketene Silyl Acetals with Imines. Chem. Lett. 1990, 19, 889–892. [Google Scholar] [CrossRef]
- Denmark, S.E.; Chen, C. Triarylcarbenium Ions as Catalysts in the Mukaiyama Aldol Addition: A Mechanistic Investigation. Tetrahedron Lett. 1994, 35, 4327–4330. [Google Scholar] [CrossRef]
- Chen, C.; Chao, S.; Yen, K. Functionalized Triarylcarbenium Ions as Catalysts in Mukaiyama Aldol Addition: Effects of Counter Ions and Silyl Groups on the Intervention of Silyl Catalysis. Synlett 1998, 1998, 924–926. [Google Scholar] [CrossRef]
- Chen, C.; Chao, S.; Yen, K.; Chen, C.; Chou, I.; Hon, S. Chiral Triarylcarbenium Ions in Asymmetric Mukaiyama Aldol Additions. J. Am. Chem. Soc. 1997, 119, 11341–11342. [Google Scholar] [CrossRef]
- Kosugi, M.; Sumiya, T.; Ohhashi, K.; Sano, H.; Migita, T. Novel Hydroxymethylation of Aryl Bromides by Means of Oraganotin Reagents. Chem. Lett. 1985, 14, 997–998. [Google Scholar] [CrossRef]
- Hollis, T.K.; Bosnich, B. Homogeneous Catalysis. Mechanisms of the Catalytic Mukaiyama Aldol and Sakurai Allylation Reactions. J. Am. Chem. Soc. 1995, 117, 4570–4581. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, M.; Mukaiyama, T. The Trityl Perchlorate Catalyzed Michael Reaction. Chem. Lett. 1985, 14, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Riant, O.; Samuel, O.; Kagan, H.B. A General Asymmetric Synthesis of Ferrocenes with Planar Chirality. J. Am. Chem. Soc. 1993, 115, 5835–5836. [Google Scholar] [CrossRef]
- Brunner, A.; Taudien, S.; Riant, O.; Kagan, H.B. Stereoselective Synthesis of Some Chiral α-Ferrocenyl Carbenium Ions. Chirality 1997, 9, 478–486. [Google Scholar] [CrossRef]
- Taudien, S.; Riant, O.; Kagan, H.B. Synthesis of Chiral Carbocations Linked to a Ferrocene Unit. Tetrahedron Lett. 1995, 36, 3513–3516. [Google Scholar] [CrossRef]
- Sammakia, T.; Latham, H.A. On the Use of Ferrocenyl Cations as Chiral Lewis Acids: Evidence for Protic Acid Catalysis. Tetrahedron Lett. 1995, 36, 6867–6870. [Google Scholar] [CrossRef]
- Klare, H.F.T.; Bergander, K.; Oestreich, M. Taming the Silylium Ion for Low-Temperature Diels–Alder Reactions. Angew. Chem. Int. Ed. 2009, 48, 9077–9079. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.K.; Müther, K.; Mück-Lichtenfeld, C.; Grimme, S.; Oestreich, M. Silylium Ion-Catalyzed Challenging Diels–Alder Reactions: The Danger of Hidden Proton Catalysis with Strong Lewis Acids. J. Am. Chem. Soc. 2012, 134, 4421–4428. [Google Scholar] [CrossRef] [PubMed]
- Bah, J.; Franzén, J. Carbocations as Lewis Acid Catalysts in Diels–Alder and Michael Addition Reactions. Chem. A Eur. J. 2014, 20, 1066–1072. [Google Scholar] [CrossRef]
- Bah, J.; Naidu, V.R.; Teske, J.; Franzén, J. Carbocations as Lewis Acid Catalysts: Reactivity and Scope. Adv. Synth. Catal. 2015, 357, 148–158. [Google Scholar] [CrossRef]
- EI Remaily, M.A.E.A.; Naidu, V.R.; Ni, S.; Franzen, J. Carbocation Catalysis: Oxa-Diels–Alder Reactions of Unactivated Aldehydes and Simple Dienes. Eur. J. Org. Chem. 2015, 2015, 6610–6614. [Google Scholar] [CrossRef]
- Ni, S.; Naidu, V.R.; Franzén, J. Chiral Anion Directed Asymmetric Carbocation-Catalyzed Diels–Alder Reactions. Eur. J. Org. Chem. 2016, 2016, 1708–1713. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Li, Z.; Huang, Y.; Wang, H.; Gao, Y.; Guo, T.; Ouyang, P.; Guo, K. Carbocation Organocatalysis in Interrupted Povarov Reactions to cis-Fused Pyrano- and Furanobenzodihydropyrans. Eur. J. Org. Chem. 2017, 2017, 3996–4003. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, J.; Li, S.; Luo, S. Carbocation Lewis Acid Catalyzed Diels–Alder Reactions of Anthracene Derivatives. Org. Lett. 2018, 20, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; EI Remaily, M.A.E.A.; Franzén, J. Carbocation Catalyzed Bromination of Alkyl Arenes, a Chemoselective sp3 vs. sp2 C−H functionalization. Adv. Synth. Catal. 2018, 360, 4197–4204. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Q.; Zhong, X.; Luo, S. Asymmetric Latent Carbocation Catalysis with Chiral Trityl Phosphate. J. Am. Chem. Soc. 2015, 137, 15576–15583. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, Y.; Horinouchi, R.; Nishiyama, N.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Trityl Cation Catalyzed Intramolecular Carbonyl-Ene Cyclization and [2+2] Cycloaddition. Synlett 2017, 28, 265–269. [Google Scholar]
- Ni, S.; Franzén, J. Carbocation Catalysed Ring Closing Aldehyde–Olefin Metathesis. Chem. Commun. 2018, 54, 12982–12985. [Google Scholar] [CrossRef] [Green Version]
- Rulev, Y.A.; Gugkaeva, Z.T.; Lokutova, A.V.; Maleev, V.I.; Peregudov, A.S.; Wu, X.; North, M.; Belokon, Y.N. Carbocation/Polyol Systems as Efficient Organic Catalysts for the Preparation of Cyclic Carbonates. ChemSusChem 2017, 10, 1152–1159. [Google Scholar] [CrossRef]
- Mosaferi, E.; Ripsman, D.; Stephan, D.W. The Air-stable Carbocation Salt [(MeOC6H4)CPh2][BF4] in Lewis Acid Catalyzed Hydrothiolation of Alkenes. Chem. Commun. 2016, 52, 8291–8293. [Google Scholar] [CrossRef]
- Veluru, R.N.; Bah, J.; Franzén, J. Direct Organocatalytic Oxo-Metathesis, a trans-Selective Carbocation-Catalyzed Olefination of Aldehydes. Eur. J. Org. Chem. 2015, 2015, 1834–1839. [Google Scholar] [CrossRef]
- Xiao, P.H.; Cao, Y.J.; Gui, Y.Y.; Gao, L.; Song, Z.L. Me3Si−SiMe2[oCON(iPr)2−C6H4]: An Unsymmetrical Disilane Reagent for Regio- and Stereoselective Bis-Silylation of Alkynes. Angew. Chem. Int. Ed. 2018, 57, 4769–4773. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Guo, Q.Y.; Sun, X.W.; Lu, J.; Cao, Y.J.; Pu, Q.; Chu, Z.W.; Gao, L.; Song, Z.L. Total Synthesis of Bryostatin 8 Using an Organosilane-Based Strategy. Angew. Chem. Int. Ed. 2018, 57, 942–946. [Google Scholar] [CrossRef]
- Yang, W.Y.; Gao, L.; Lu, J.; Song, Z.L. Chemoselective Deoxygenation of Ether-substituted Alcohols and Carbonyl Compounds by B(C6F5)3-catalyzed Reduction with (HMe2SiCH2)2. Chem. Commun. 2018, 54, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Tang, X.X.; Liu, S.F.; Wang, R.P.; Hu, T.B.; Gao, L.; Song, Z.L. Rhodium-Catalyzed Reaction of Silacyclobutanes with Unactivated Alkynes to Afford Silacyclohexenes. Angew. Chem. Int. Ed. 2019, 58, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.W.; Wang, K.; Gao, L.; Song, Z.L. Chiral Crotyl Geminal Bis(silane): A Useful Reagent for Asymmetric Sakurai Allylation by Selective Desilylation-enabled Chirality Transfer. Chem. Commun. 2017, 53, 3078–3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosomi, A. Characteristics in the Reactions of Allylsilanes and Their Applications to Versatile Synthetic Equivalents. Acc. Chem. Res. 1988, 21, 200–206. [Google Scholar] [CrossRef]
- Langkopf, E.; Schinzer, D. Uses of Silicon-Containing Compounds in the Synthesis of Natural Products. Chem. Rev. 1995, 95, 1375–1408. [Google Scholar] [CrossRef]
- Masse, C.E.; Panek, J.S. Diastereoselective Reactions of Chiral Allyl and Allenyl Silanes with Activated C: X. pi.-Bonds. Chem. Rev. 1995, 95, 1293–1316. [Google Scholar] [CrossRef]
- Monti, H.; Audran, G.; Le´andri, G.; Monti, J. ZnI2 Catalyzed [2+2] versus [3+2] Cycloaddition of an Allyltrimethylsilane with 3-butyn-2-one: Confirmation of a Cyclobutene By-product Formation. Tetrahedron Lett. 1994, 35, 3073–3076. [Google Scholar] [CrossRef]
- Brengel, G.P.; Rithner, C.; Meyers, A.I. [2+2] and [3+2] Cycloadditions of Triisopropylallylsilane to alpha.,. beta.-Unsaturated Bicyclic Lactams. J. Org. Chem. 1994, 59, 5144–5146. [Google Scholar] [CrossRef]
- Akiyama, T.; Yamanaka, M. Stereoselective Synthesis of Cyclopentanols by Lewis Acid-mediated [3+2] Annulation of Allyldiisopropylphenylsilane with α,β-Unsaturated Diesters. Tetrahedron Lett. 1998, 39, 7885–7888. [Google Scholar] [CrossRef]
- Organ, M.G.; Dragan, V.; Miller, M.; Froese, R.D.J.; Goddard, J.D. Sakurai Addition and Ring Annulation of Allylsilanes with α,β-Unsaturated Esters. Experimental Results and ab Initio Theoretical Predictions Examining Allylsilane Reactivity. J. Org. Chem. 2000, 65, 3666–3678. [Google Scholar] [CrossRef]
- Takasu, K.; Hosokawa, N.; Inanaga, K.; Ihara, M. Cyclobutane Ring Formation by Triflic Imide Catalyzed [2+2]-Cycloaddition of Allylsilanes. Tetrahedron Lett. 2006, 47, 6053–6056. [Google Scholar] [CrossRef]
- Matsumura, Y.; Suzuki, T.; Sakakura, A.; Ishihara, K. Catalytic Enantioselective Inverse Electron Demand Hetero-Diels–Alder Reaction with Allylsilanes. Angew. Chem. Int. Ed. 2014, 53, 6131–6134. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, H.; Miyazaki, H.; Makita, Y. Lewis Acid-promoted Reaction of β,γ-Unsaturated α,α-Dimethoxy Esters with Silyl Nucleophiles. Tetrahedron Lett. 2012, 53, 4584–4587. [Google Scholar] [CrossRef]
- The stereochemistry of determined according to the similar structures reported in the reference: Yamamoto, Y.; Nishii, S. The Anti-selective Michael Addition of Allylic Organometals to Ethylidenemalonates and Related Compounds. J. Org. Chem. 1988, 53, 3597–3603. [Google Scholar] [CrossRef]
- The 1H NMR titration experiment of [(Ph3C)[BPh(F)4]] with β,γ-unsaturated α-ketoester 1a and allylsilane 2a have been per-formed, respectively. However, both of them did not show strong interaction between [(Ph3C)[BPh(F)4]] and 1a or 2a. These results suggest that if the mechanism shown Scheme 3d works, weak activation of 1a by [(Ph3C)[BPh(F)4]] effectively promotes the Hosomi-Sakurai reaction with allylsilane, despite such activation appears being too weak to be probed by 1H NMR.




| Entry | Cat. | Time | 3a[(±)-4a] [%] b,c | 3a/(±)-4a d |
|---|---|---|---|---|
| 1 | TiCl4 (10 mol %) | 10 min | 0 (97) | ≤5:95 |
| 2 | SnCl4 (10 mol %) | 10 min | 22 (74) | 23:77 |
| 3 | AlCl3 (10 mol %) | 10 min | 32 (63) | 33:67 |
| 4 | FeCl3 (10 mol %) | 10 min | 43 (51) | 45:55 |
| 5 | BF3•Et2O (10 mol %) | 4 days | 0 (10) | ≤5:95 |
| 6 | TMSOTf (10 mol %) | 4 days | 20 (<5) | 17:83 |
| 7 | Sc(OTf)3 (10 mol %) | 8 h | 0 (95) | ≤5:95 |
| 8 | Yb(OTf)3 (10 mol %) | 24 h | N.R. | N.D. |
| 9 | (Ph3C)[BPh(F)4] (10 mol %) | 10 min | 97 (0) | ≥95:5 |
| 10 | (Ph3C)[BPh(F)4] (1 mol %) | 10 min | 97 (0) | ≥95:5 |
| Entry | Allysilanes | Products | Yield |
|---|---|---|---|
| 1 | 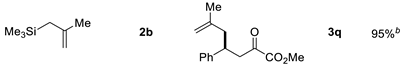 | ||
| 2 | 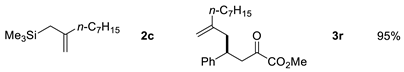 | ||
| 3 | 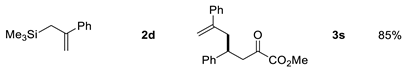 | ||
| 4 | 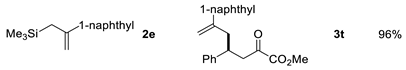 | ||
| 5 | 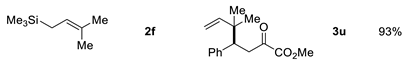 | ||
| 6 | 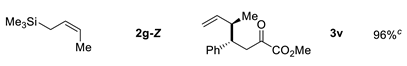 | ||
| 7 | 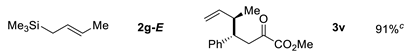 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Z.; Cui, D.; Zhang, H.; Feng, Y.; Huang, L.; Gui, Y.; Gao, L.; Song, Z. Trityl Cation-Catalyzed Hosomi-Sakurai Reaction of Allylsilane with β,γ-Unsaturated α-Ketoester to Form γ,γ-Disubstituted α-Ketoesters. Molecules 2022, 27, 4730. https://doi.org/10.3390/molecules27154730
Gan Z, Cui D, Zhang H, Feng Y, Huang L, Gui Y, Gao L, Song Z. Trityl Cation-Catalyzed Hosomi-Sakurai Reaction of Allylsilane with β,γ-Unsaturated α-Ketoester to Form γ,γ-Disubstituted α-Ketoesters. Molecules. 2022; 27(15):4730. https://doi.org/10.3390/molecules27154730
Chicago/Turabian StyleGan, Zubao, Deyun Cui, Hongyun Zhang, Ying Feng, Liying Huang, Yingying Gui, Lu Gao, and Zhenlei Song. 2022. "Trityl Cation-Catalyzed Hosomi-Sakurai Reaction of Allylsilane with β,γ-Unsaturated α-Ketoester to Form γ,γ-Disubstituted α-Ketoesters" Molecules 27, no. 15: 4730. https://doi.org/10.3390/molecules27154730
APA StyleGan, Z., Cui, D., Zhang, H., Feng, Y., Huang, L., Gui, Y., Gao, L., & Song, Z. (2022). Trityl Cation-Catalyzed Hosomi-Sakurai Reaction of Allylsilane with β,γ-Unsaturated α-Ketoester to Form γ,γ-Disubstituted α-Ketoesters. Molecules, 27(15), 4730. https://doi.org/10.3390/molecules27154730





