Abstract
The availability of natural substances able to fulfill the role of antioxidants in a physiologic environment is important for the development of therapies against diseases associated with excessive production of reactive oxygen species and ensuing oxidative stress. Antioxidant properties have been reported episodically for sericin, a proteinaceous constituent of the silk thread in the cocoons generated by the larvae of the Lepidoptera order. We investigated the sericin fractions isolated from the cocoons spun by the domesticated (Bombyx mori) silkworm. Three fractions were isolated and evaluated, including two peptidoid fractions, the crude sericin and the purified (dialyzed) sericin, and the non-peptidoid methanolic extract of the crude fraction. When subjected to Trolox equivalent antioxidant capacity (TEAC) assay, the extract showed much higher antioxidant capacity as compared to the crude or purified sericin fractions. The three fractions were also evaluated in cultures of murine retinal photoreceptor cells (661 W), a cell line that is highly susceptible to oxidants and is crucially involved in the retinopathies primarily caused by oxidative stress. The extract displayed a significant dose-dependent protective effect on the cultured cells exposed to hydrogen peroxide. In identical conditions, the crude sericin showed a certain level of antioxidative activity at a higher concentration, while the purified sericin did not show any activity. We concluded that the non-peptidoid components accompanying sericin were chiefly responsible for the previously reported antioxidant capacity associated with sericin fractions, a conclusion supported by the qualitative detection of flavonoids in the extract but not in the purified sericin fraction.
1. Introduction
Sericin is the second major (∼25%) protein in the composition of the silk thread produced by the domesticated silkworm Bombyx mori and related species, accompanying fibroin (∼75%) as an adhesive coating layer. Both proteins can be readily regenerated from raw silk (either cocoons or silk yarn) in laboratory conditions. The silk cocoons of the genus Bombyx contain the highest amounts of sericin of all cocoons produced by the Lepidopteran larvae [1]. While silk fibroin has found many applications as a biomaterial, B. mori silk sericin (henceforth, BMSS) has been largely neglected on account of causing immunogenic tissue responses. However, other investigators [2,3,4] have suggested the lack of such responses. More recent studies [5,6,7,8], based on a critical examination of the extant literature or on further experiments, have proved that BMSS did not induce cytopathologic effects and also have suggested that the earlier opinions were due to insufficient documentation or poor experimental design, occasionally aggravated by instances of misinterpretation or misquotation of the reported results. Investigations of the beneficial effect of BMSS as a supplement in cell culture media [9,10,11,12,13,14,15,16,17,18,19] were carried on consistently over the past two decades leading to the current commercial availability of sericin-based supplements. In the meantime, the biocompatibility of BMSS became generally accepted, triggering increased interest in the potential applications of BMSS in tissue engineering and biomedicine [20,21,22,23,24,25,26,27,28,29,30]. Apart from sericin’s reported enzyme inhibiting and cytoprotective effects, its activity as an antioxidant (also termed antioxidative capacity) is another of its remarkable biological properties, which was first noticed about two decades ago [31].
Oxidative stress is commonly defined as a state of imbalance between the amount of the oxidant reactive oxygen species (ROS) generated by cells and the endogenous antioxidants within the cell’s physiological environment, resulting in excess production of ROS. Although frequently used, the generality of this concept has been debated [32,33,34], and the validity of the oxidative stress theory of disease has been lately challenged [35,36,37].
The antioxidants (either endogenous or exogenous) are substances able to delay or prevent the formation of ROS (free radicals, ions, radical-ions, and other reactive molecular species) from oxidizable substrates (proteins, lipids, carbohydrates, and nucleic acids) by any of such actions as inhibiting the formation of free radicals or scavenging them, binding to substances that may promote the formation of ROS, or involving more complex mechanisms [38,39,40,41,42]. Certain processes involving ROS are necessary for life [34,43,44]. However, most ROS are in a chemically unstable state, and they trade electrons with the surrounding matter, including the intracellular organelles, to reach a stable state. Such processes lead to oxidative stress and injury to cells, tissues or organs, especially manifested when ROS levels have escaped from under homeostatic control and become in excess.
As recounted in some seminal reviews [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], there is a vast body of evidence showing that for organisms living in an atmosphere containing oxygen, oxidative stress is an unavoidable consequence that leads to aging and diseases. However, if antioxidants are present, they can partially control the production of ROS when the cells’ own defense mechanisms no longer can act efficaciously. Out of all our organs (besides skin), the eye is associated with the most auspicious circumstances for oxidative damage. It is relentlessly exposed to the oxygen permeating from the atmosphere and to the electromagnetic radiation provided by the sun, notwithstanding that light is essential for our vision. In the ocular tissues, the “oxidative photodegradation triangle” [57]―an analogy of the “fire triangle”―is fulfilled perhaps like nowhere else in the body, as it comprises all necessary elements, namely radiation (the “ignition source”), tissular radiation-absorbing chromophores (the “fuel”), and oxygen (as itself), an ideal synergy for generating excessive amounts of ROS, all enclosed in a relatively small organ of our body.
BMSS has been reported as an antioxidant in preparations isolated directly from cocoons, generally followed by additional purification, and then assessing its antioxidant capacity either by a variety of chemical assays [31,58,59,60,61,62,63,64,65] or in cell cultures [61,63,65,66,67,68,69,70]. Antioxidant activity has also been demonstrated in the non-peptidoid compounds associated with sericin that were isolated by various separation techniques [60,65,71,72,73,74]. It was reported that the antioxidative capacity of isolated sericin was not affected by its chemical modification [75] nor by additional enzymatic degradation [76,77]. However, when retinal cells were cultured on solid fibroin-sericin substrates (as hydrogel films), no antioxidative protection induced by the presence of sericin was noticed [78]. BMSS has also been applied as a potential therapeutic agent (administered either by diet, topically, or by perfusion/injection) in animal pathologic models of skin damage, tumors, hyperglycemia, or myocardial infarction [60,79,80,81,82,83,84], where the observed therapeutic effects were attributed to a decline in the level of oxidative stress.
We can conclude that, amongst the two major constitutive proteins of silk, the antioxidant activity is specific to sericin, at least in B. mori species. However, it is still unclear whether this activity can be attributed (a) solely to sericin, with or without the accompanying peptidoid entities resulting from the hydrolytic degradation during isolation procedures; (b) solely to the non-protein substances associated with sericin (if so, this may imply that routine purification procedures for sericin may not be sufficient to remove such compounds); (c) to both previous components; or (d) it is a combined effect of a gland-secreted complex that consists of the main constituent native sericinoid polypeptides, non-sericinoid polypeptides, and non-protein organic substances. To investigate the alternative (d) above is both difficult and impractical, as it would involve the isolation of native sericin directly from the silkworm’s middle gland through an aspirating device, precisely at a stage prior to coating the fibroin filaments, and assuring that no alteration takes place during its experimental manipulation.
Here, we report results that may contribute to further understanding of some aspects of the topic. In the present study, hydrogen peroxide was used as an oxidant agent. The antioxidant effects of sericin fractions and of its methanolic extract were evaluated employing cultures of a murine retinal photoreceptor cell line (661 W) in parallel with a routine chemical assay. This cell line had been cloned [85] from retinal tumors generated in a transgenic mouse expressing the simian virus SV 40 T-antigen under the control of human interphotoreceptor retinoid-binding protein (IRBP) promoter. The 661 W cells express cone markers, with no rod markers detectable, are sensitive to light [86], and appear to be a valuable model for the investigation of various retinopathies where oxidative stress is believed to be a major causal factor [87,88,89].
2. Materials and Methods
2.1. Materials
Silk cocoons (B. mori) were supplied by Tajima Shoji Co. Ltd. (Yokohama, Japan), with the pupae removed. According to the supplier, these silkworms were fed at their early stages of life with an artificial diet consisting of mulberry leaf powder, starch, and defatted soybean powder. At later stages, they were fed exclusively on fresh, natural mulberry leaves.
The chemical reagents were all supplied by MilliporeSigma (St Louis, MO, USA), which also supplied the dialysis tubes with an MMCO of 3.5 kDa. High-purity water (Milli-Q or equivalent) was used in all procedures. The Minisart®-GF pre-filters (0.7 μm) and sterile Minisart® High-Flow filters (0.22 μm) were supplied by Sartorius Stedim Biotech (Göttingen, Germany). All cell culture reagents and supplements were purchased from Thermo Fisher Scientific (Rockford, IL, USA), except for the fetal bovine serum (FBS) that was supplied by Cytiva (Sydney, Australia).
The 661 W murine retinal photoreceptor cell line originated in Professor Muayyad Al-Ubaidi’s laboratory at the University of Oklahoma Health Science Center (Oklahoma City, OK, USA). Dr. Krisztina Valter-Kocsi (Australian National University Medical School, Canberra, Australia) provided this line for our experiments.
2.2. Isolation of BMSS from Silk Cocoons
Autoclave extraction was applied according to a published protocol [90], with some modifications. In the current study, the cocoon material (unwashed, 10 g), placed in 200 mL water, was autoclaved at 121 °C for 4 h. Two different sample variants were further processed. A purified sericin powder (henceforth, PS) was prepared by dialysis of the solution using tubes with MMCO of 3.5 kDa in water for 2 days at constant 30 °C, with continuous stirring. Prior to dialysis, the solution was passed through a paper filter (Whatman #4), and after dialysis through successive sterile Minisart® filters (0.7 and 0.22 μm). Following the last filtration step, the solution was frozen at –80 °C and concentrated to a powder in a freeze-dryer/vacuum concentrator (Alpha 1-2 LDplus, Martin Chris GmbH, Osterode, Germany). To obtain the crude sericin powder (henceforth, CS), the same procedure was followed but omitting the dialysis stage and post-dialysis filtration. The sericin powder samples were all stored at room temperature until further use.
2.3. Electrophoretic Analysis of Sericin Fractions
The molecular mass distributions in CS (i.e., non-dialyzed) and PS (i.e., dialyzed) samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) employing a Novex® XCell Sure Lock™ Mini-Cell system (Life Technologies, Carlsbad, CA, USA), according to a previously published protocol [90] that has been slightly modified. Thus, the aqueous solution of sericin (10 mg/mL) was mixed with both NuPAGE® LDS sample buffer and NuPAGE® sample reducing agent and heated at 70 °C for 10 min. A volume of 10 μL BMSS solution containing about 20 μg protein was then loaded into 1 mm thick 4–12% NuPAGE® Bis-Tris gel in NuPAGE® MES SDS running buffer. The gels were run at a voltage of 200 V for 35 min together with the SeeBlue® Pre-stained Protein Standard. The remaining procedure was carried out as detailed previously [90].
2.4. Extraction of Non-Sericin Fraction from Crude BMSS
Freeze-dried CS powder (1.5–2 g) was placed in a screw-capped bottle with 150 mL methanol and shaken at 240 rpm for 2 days at room temperature on a shaker (Model 130 Basic, IKA, Staufen, Germany). The resulting liquid was filtered through a filter paper (Whatman #4) and then through successive Minisart® filters. The solution (henceforth, CS-E) was concentrated to about 15 mL in a rotary evaporator (Rotavapor R-215, BÜCHI Labortechnik AG, Flawil, Switzerland), subsequently dried in an oven at 60 °C overnight, and then kept in a vacuum oven at 40 °C for 2–3 days under moderate vacuum. The dried CS-E was stored at 4 °C until further use.
2.5. Analysis of Flavonoids in Sericin Fractions and Extract
To check the presence of flavonoids and their distribution in the sericin fractions or the extract, two chromogenic methods were employed.
(a) The first method was based on the staining induced by the reaction of polyphenolic compounds, such as flavonoids, with 4-(dimethylamino)cinnamaldehyde (DAC) (Sigma Cat. D4506; IUPAC name: (E)-3-[4-(dimethylamino)phenyl]prop-2-enal). This reagent is to be stored in a freezer, and its solutions shall be kept in cool dark conditions. According to our protocol, 0.1 g DAC was dissolved in a pre-cooled mixture of 25 mL concentrated hydrochloric acid and 70 mL methanol. The same amounts (10 mg) of CS, PS, or CS-E were dissolved in 0.1 mL water in test tubes, and the resulting solutions were mixed each with 2 mL DAC solution and vortexed briefly. Color change was observed after keeping the test tubes on the bench for 1 h at room temperature.
(b) The visualization of flavonoids was based on the fluorescence generated by their complexation reaction with 2-aminoethyl diphenylborinate (2-APB) (Sigma Cat. D9754; IUPAC name: 2-[(diphenylboranyl)oxy]ethan-1-amine). The reagent was dissolved in water containing 0.3% dimethyl sulfoxide to achieve a concentration of 0.2% (w/v). The samples (CS, PS, and CS-E) were dissolved in water to a concentration of 5 mg/mL. Equal volumes of 2-APB and sample solutions were mixed in Eppendorf tubes and vortexed thoroughly. Volumes of 100 μL of each mixture were placed in the wells of a black 96-well plate and read on a fluorescent microplate reader (FLUOstar OPTIMA, BMG Labtech Pty Ltd., Mornington, Australia) at an excitation wavelength of 360 nm and an emission wavelength of 415–425 nm. A solution of 2-APB as such was used as a blank, and its intensity was subtracted from the sample intensities to provide the background level.
2.6. Protein Quantitation in Extract
The dried CS-E was dissolved in water to make up a 1 mg/mL solution and then diluted 10-fold to obtain a concentration suitable for analysis. The quantitation of proteinaceous matter was carried out using the Micro BCA protein assay (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific, Rockford, IL, USA) and following the manufacturer’s guidelines. Briefly, sample solutions (150 µL) were mixed with the working reagent (150 µL) in a 96-well plate and incubated for 2 h at 37 °C. Absorbance was then measured at 562 nm using the microplate spectrometer AC200D (Paradigm Absorbance Detection, Beckman Coulter, Brea, CA, USA). Bovine serum albumin standard solution (included in the kit) was sequentially diluted and used to obtain a standard curve (0–200 µg/mL). Each sample was assessed in triplicate.
2.7. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
The sericin powders, both CS and PS, and the dried CS-E were each dissolved in water to a concentration of 10 mg/mL. These solutions were assessed for their intrinsic antioxidant capacity with the TEAC assay employing a dedicated kit (Cayman Chemical, Ann Arbor, MI, USA) and following the manufacturer’s instructions. Essentially, this assay indicates the capacity of a substance to prevent the formation of the radical cation of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+·) compared to the standard antioxidant 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (a substance related to tocopherols and commonly known as Trolox). Briefly, 10 µL sample solution was mixed with metmyoglobin (10 µL) and chromogen solutions (150 µL), and a reaction was initiated by adding 40 µL hydrogen peroxide (H2O2). After incubating on a shaker for 5 min at room temperature, the absorbance was recorded at 405 nm using the microplate spectrometer AC200D. Trolox solutions of varying concentrations were used as standards for a calibration plot (from 0 to 0.33 mM), and the Trolox equivalents of antioxidant capacity were calculated per 1 mg of sample. Each sample was assessed in triplicate.
2.8. Cell Culture and the Effect of Oxidant on Cell Viability
Using a previously reported protocol [87], the initial culture of 661 W cells was established using T75 tissue culture flasks in Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% FBS, 2 mM L-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin. The harvested cells were seeded onto 24-well plates at a density of 25,000 cells/well and kept in an incubator for 24 h. A CO2 incubator Model MCO-170AICL (PHCbi, Tokyo, Japan) was used for all cell culture experiments.
The powder samples CS, PS, and CS-E were each dissolved in DMEM supplemented with 1% FBS and then filter-sterilized by passing through a sterile 0.22 μm filter. The medium in each well was replaced with 0.5 mL of the test sample solutions and incubated for 24 h, using 6 wells for each sample and DMEM/1% FBS as a control. Within each group, three wells served as controls, while the other three wells were supplemented with 1.2 mM H2O2 as a solution of 60 mM in DMEM/1% FBS (made by diluting the as-supplied H2O2 of 30% in water) and further incubated for 24 h. The medium was then removed, and all cultures were washed briefly with Hank’s balanced salt solution (HBSS).
The MTT assay was used to evaluate quantitatively the cell viability following the H2O2-induced oxidative treatment. Briefly, stock solutions of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were prepared in Dulbecco’s phosphate buffered saline, filter-sterilized, and stored at –20 °C until required. Prior to running the assay, the stock solution was diluted 10-fold with DMEM/1% FBS medium to achieve a working solution of 0.5 mg/mL. HBSS was aspirated from wells, 1 mL of MTT solution was added to each well, and the samples were all incubated for 3 h at 37 °C. After this, the MTT supernatant was aspirated and discarded, and 1 mL of a 0.04 N solution of hydrochloric acid in isopropanol was added to each well and shaken gently for 5 min. From each well, 200 μL of isopropanol solution was transferred to wells of a new 96-well plate, and the absorbance was measured at the 570-nm wavelength in an AC200D microplate spectrophotometer. The percent cell viability was then estimated against the readings for the controls taken as 100% viability.
2.9. Microscopic Procedure
The morphology of proliferating cells was examined and photographed by using a bright-field Nikon Eclipse® TS100 microscope (Nikon, Tokyo, Japan) equipped with a Nikon Digital Sight camera and using the NIS Elements® F4.00.00 software.
3. Results
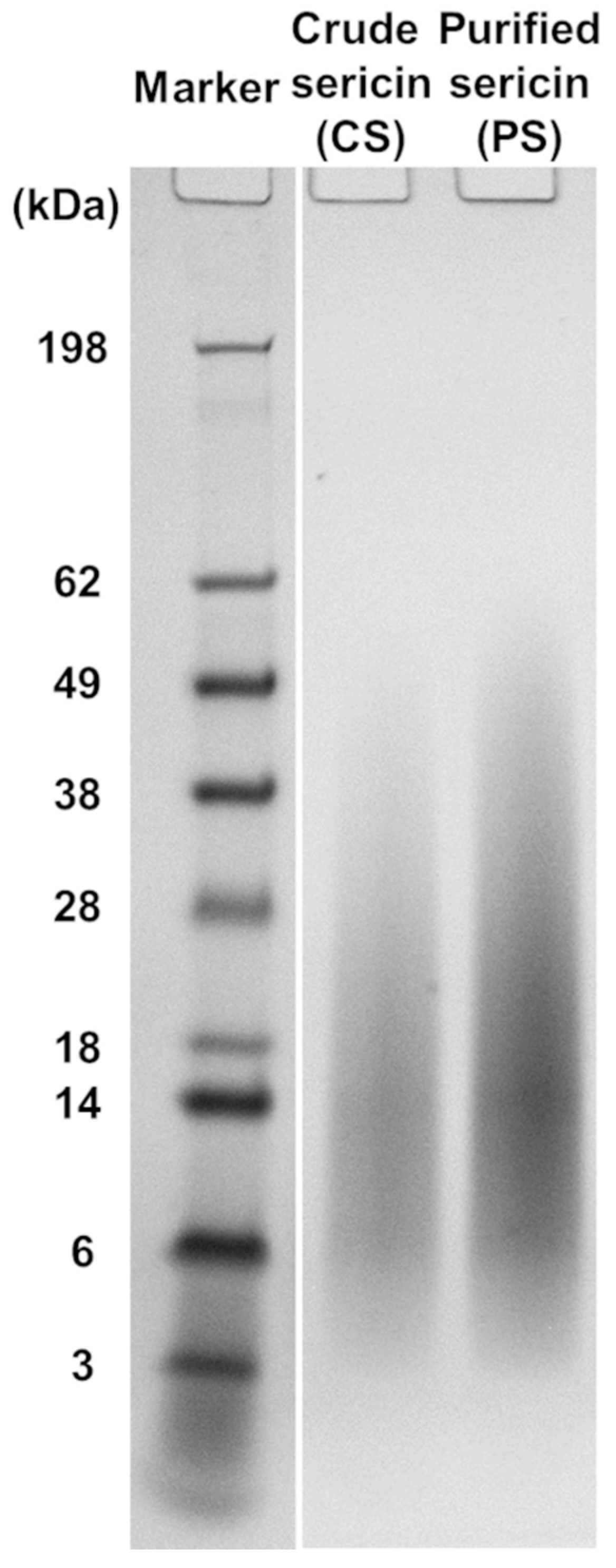
Two BMSS samples were regenerated from silk cocoons for this study: a crude product (CS) resulting directly from autoclaving as an aqueous solution and a purified fraction (PS) that was obtained after extensive dialysis of CS, in a process that had removed most of non-peptidoid and some of peptidoid substances of low molecular mass. The yields of sericin fractions relative to the initial weight of cocoon material were determined gravimetrically and found to be 15.49% for CS and 10.28% for PS. The electrophoretic analysis of CS and PS samples resulted in similar smear patterns (Figure 1), each consisting of a contiguous array of peptidoid components with molecular mass values ranging from ∼5 kDa to ∼60 kDa. Such a diffuse distribution pattern indicates a significant hydrothermal degradation of the native polypeptides during autoclaving. When compared to the electrophoretogram of PS, the CS displayed a slightly more limited distribution, but this might be due to a quantitative concentration of the components in PS due to removal through dialysis of the low molecular mass components. The other material evaluated in this study was an extract in methanol of the CS powder (CS-E), which was concentrated to dryness and then re-dissolved in an aqueous medium. The yield of extract was 5.34% relative to the weight of fraction CS and 0.83% relative to the total weight of cocoon material. Analysis carried out by using the BCA assay indicated the presence of 29% proteinaceous matter in this extract.
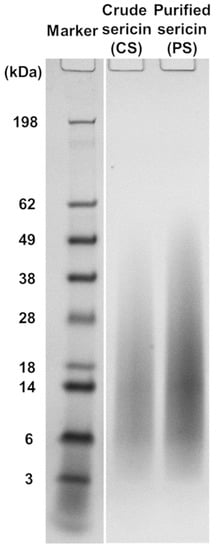
Figure 1.
Molecular mass distribution in the sericin regenerated from B. mori cocoons, as determined by SDS-PAGE. The electrophoretic patterns of non-dialyzed (crude) and dialyzed (purified) products are shown.
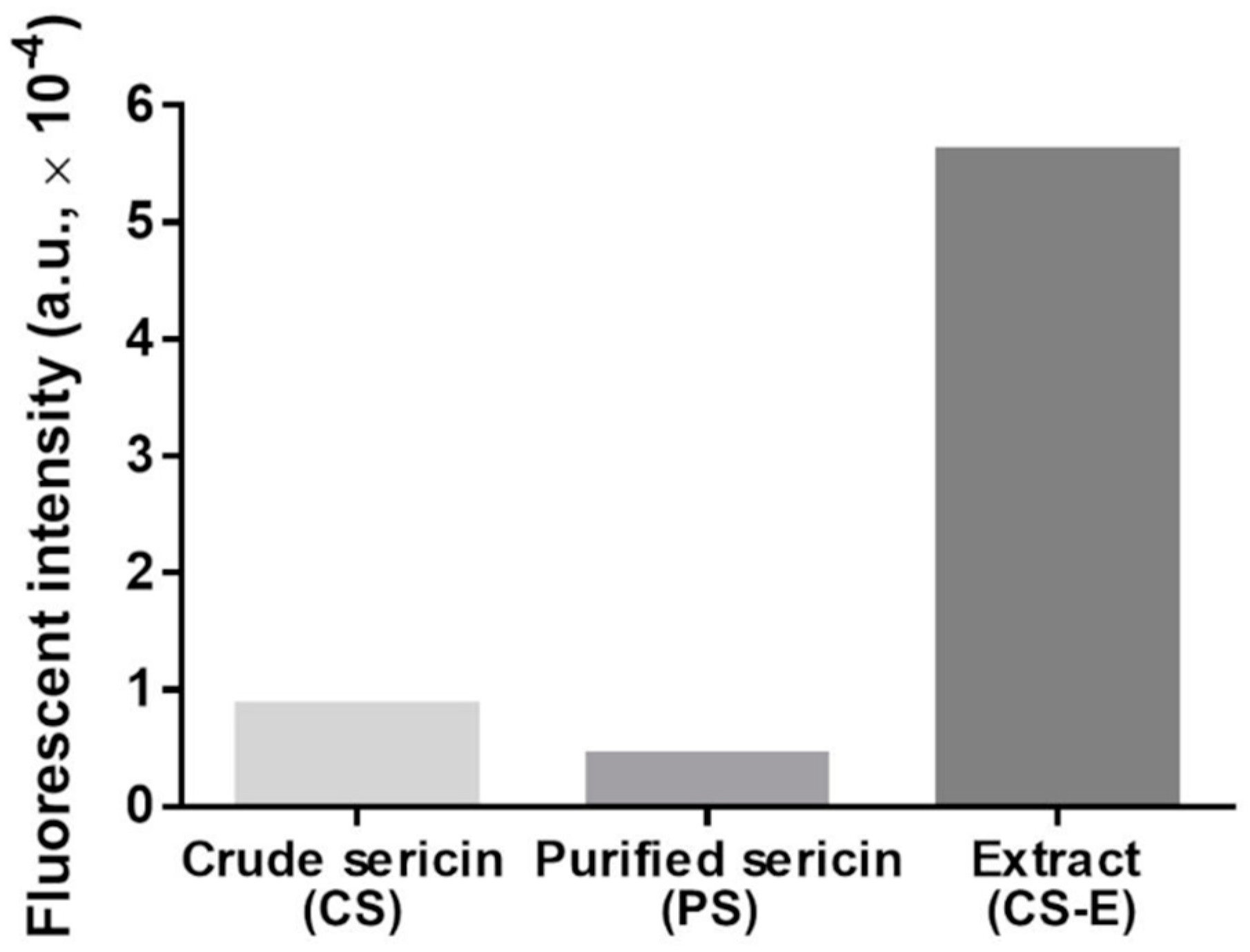
To assess qualitatively and semi-quantitatively the presence of flavonoids, we employed two chromogenic methods. By reacting flavonoids with DAC, a deep red color shall develop [91]. The results of this test are shown in Figure 2, where such coloration is seen only in CS-E. In the other sericin fractions, a yellow-green coloration appeared along with precipitated matter that likely consists of sericinoid peptides. To bring additional proof of the presence of flavonoids in CS-E, a fluorescence method was applied based on the complexation reaction between flavonoids and 2-APB [92,93,94,95,96,97]. The resulting fluorescein measurements clearly indicated the presence of a substantial amount of flavonoids in CS-E as compared to CS or PS (Figure 3).

Figure 2.
Staining of aqueous solutions of crude sericin (CS), purified sericin (PS), and extract of crude sericin (CS-E) following treatment with the DAC reagent in methanolic acidic medium. Photographs were taken after 1 h at room temperature. The red-brown coloration denotes the presence of flavonoids in the sample.
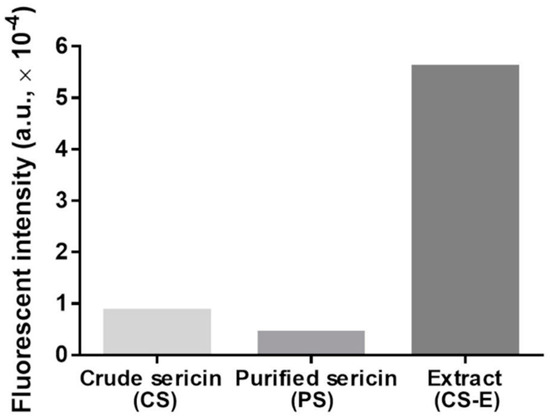
Figure 3.
The fluorescence intensities of aqueous solutions of crude sericin (CS), purified sericin (PS), and extract of crude sericin (CS-E) following treatment with the 2-APB reagent, irradiation with 360-nm wavelength (excitation), and recording the emission spectra at 415–425 nm. The higher fluorescence intensity indicates the presence of flavonoids. Each bar is the result of one measurement.
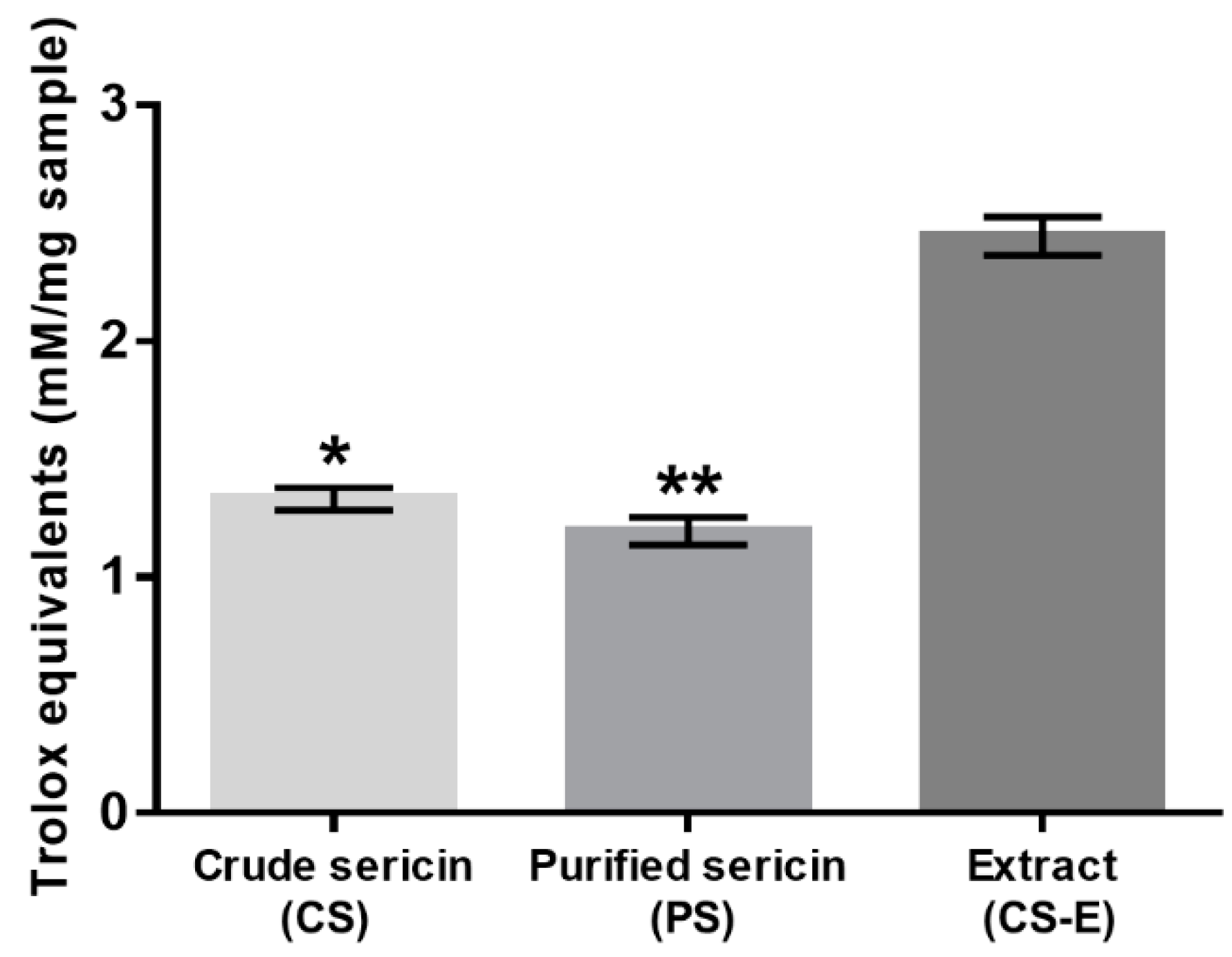
All three samples were evaluated with the TEAC assay to compare their intrinsic antioxidant capacity, and the results are shown in Figure 4. The sample CS-E displayed significantly higher antioxidant activity when compared to CS and PS, the latter two showing virtually the same levels of activity.
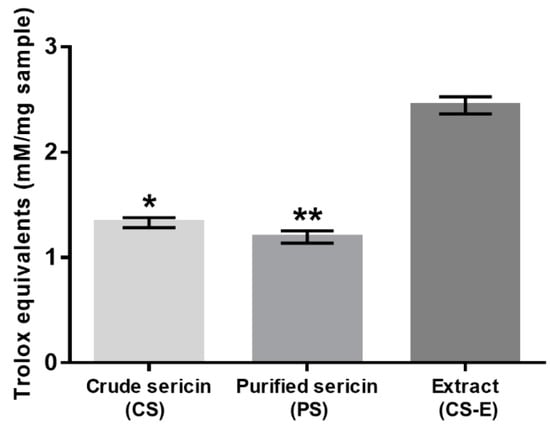
Figure 4.
Antioxidant capacity of non-dialyzed (crude, CS) and dialyzed (purified, PS) sericin and of methanolic extract from crude sericin (CS-E) as determined by TEAC (ABTS+·) assay and expressed in Trolox equivalents. Bars represent mean values ± s. d. for n = 10. Statistical analysis was performed using the non-parametric Friedman test with Dunn’s multiple comparisons test. * p < 0.05; ** p < 0.0001. The difference between crude and purified sericins is not statistically significant (p > 0.2).
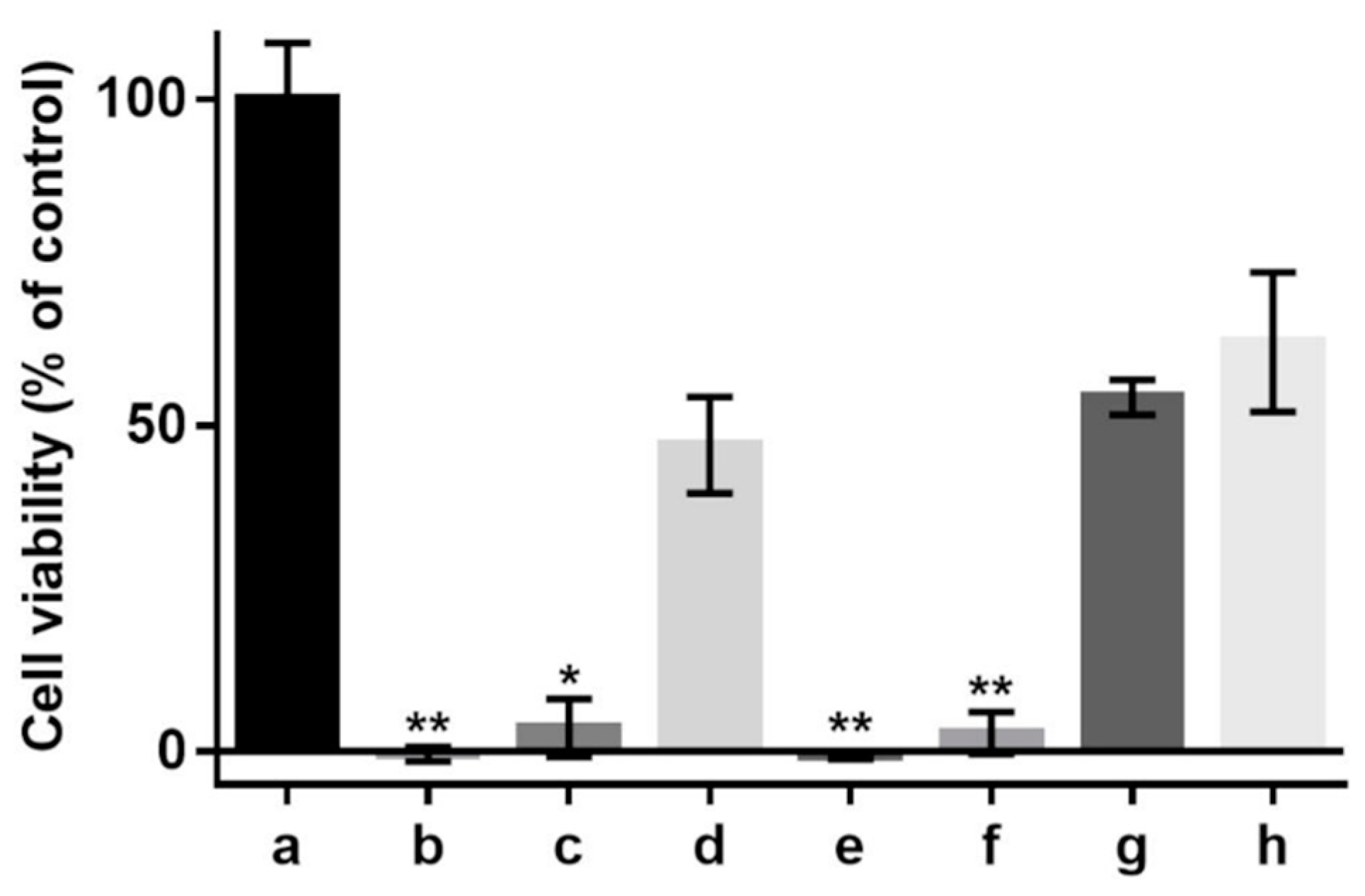
In the cell cultures investigated in this study, the antioxidant effects of various sericin fractions were found to be dose-dependent (Figure 5). While CS-E displayed statistically significant antioxidant activity at any concentration and CS showed such activity only at the higher concentration, PS did provide negligible protection, if any, to the cells against oxidative death.
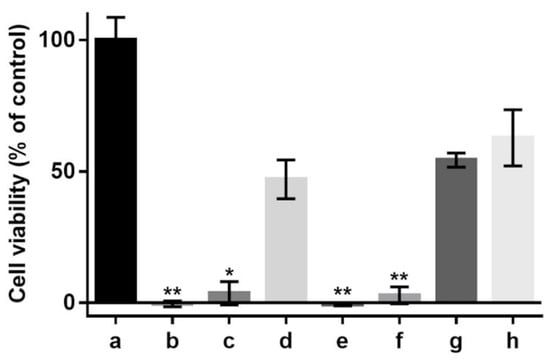
Figure 5.
Effect of supplementation with sericin fractions on viability of 661 W murine retinal photoreceptor cells cultured in DMEM/10% FBS, without or in the presence of hydrogen peroxide (H202, 1.2 mM). (a) Control (no sericin, no oxidant); (b) control (no sericin, +oxidant); (c) CS, 1 mg/mL (+oxidant); (d) CS, 5 mg/mL (+oxidant); (e) PS, 1 mg/mL (+oxidant); (f) PS, 5 mg/mL (+oxidant); (g) CS-E, 1 mg/mL (+oxidant); (h) CS-E, 5 mg/mL (+oxidant). Bars represent mean values ± s. d. for n = 6. Statistical analysis was performed using the non-parametric Friedman test with Dunn’s multiple comparisons test. * p < 0.02; ** p < 0.002. The differences between cell viabilities in the control culture (a) and test culture samples (d,g,h) are not statistically significant (p > 0.2).
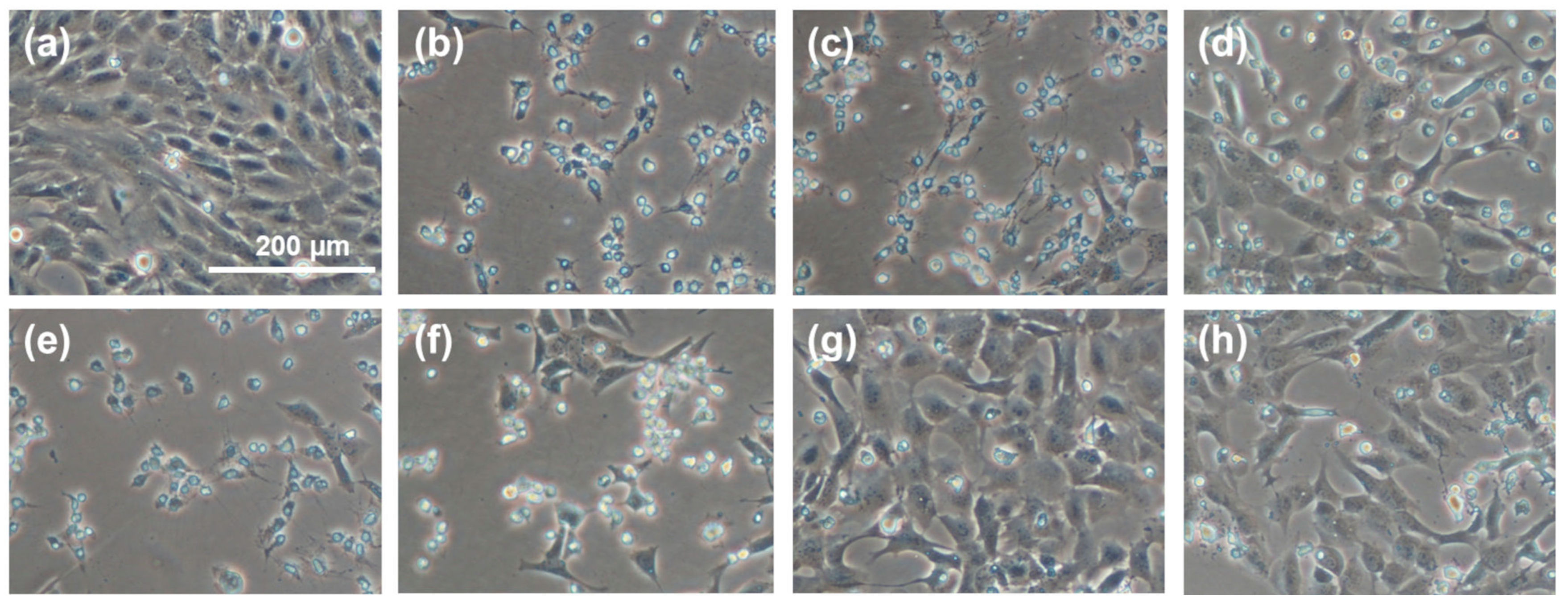
The representative images in Figure 6 show a regional comparison between normally proliferating cells (Figure 6a), cells grown in the presence of an oxidant (H2O2) without any sericin fraction as a protective antioxidant (Figure 6b), cells grown in the presence of H2O2 in a medium supplemented with CS (Figure 6c,d) or PS (Figure 6e,f), and cells grown in the presence of H2O2 in a medium supplemented with CS-E (Figure 6g,h). The superior protective effect of fraction CS-E is convincingly reflected in the cellular morphology and growth patterns.
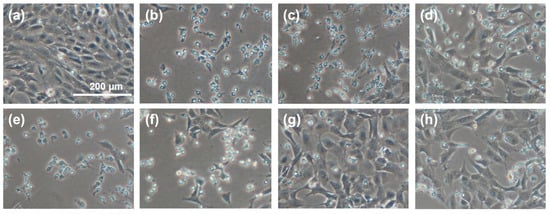
Figure 6.
Micrographs of randomly selected cell growth areas illustrating the effect of supplemental sericin fractions on the proliferation and morphology of 661 W murine retinal photoreceptor cells under oxidative stress induced by hydrogen peroxide (H202, 1.2 mM by total media volume). (a) Control (no sericin, no oxidant); (b) control (no sericin, +oxidant); (c) CS, 1 mg/mL (+oxidant); (d) CS, 5 mg/mL (+oxidant); (e) PS, 1 mg/mL (+oxidant); (f) PS, 5 mg/mL (+oxidant); (g) CS-E, 1 mg/mL (+oxidant); (h) CS-E, 5 mg/mL (+oxidant). The scale bar in (a) is the same for all images.
4. Discussion
The antioxidant capacity of BMSS may be regarded as a vestigial evolutionary feature contributing to the protective activities that the silk cocoon must perform. First, the cocoons, which are immobile and non-metabolizing entities, must be protected from infection and ensuing decay. This explains the presence in their composition of defense antimicrobial proteins [98,99,100,101]. Second, the cocoons are themselves responsible for the survival of the species by protecting the pupae from pathogens, predators, sunlight, humidity, and heat. A variety of proteins and non-protein substances in the silk thread composition fulfill such tasks. For instance, sericin in the B. mori cocoon material was found to be responsible for absorbing UV-A radiation, such as protecting the cocoon structure from oxidative damage during the pupal stage of development [102]. It is, therefore, to be expected that certain substances contained in the silk cocoon material will display identical or similar protective properties, including antioxidative capacity.
The composition of non-protein matter in B. mori silk cocoon is traditionally reported [103,104] as 1.2–1.6% carbohydrates, ∼0.7% inorganic matter, 0.4–0.8% wax matter, and ∼0.2% pigments. The rest consists of the two major proteins in the silk thread, fibroin, and sericin, which are accompanied by much lower amounts of other proteins having either identifiable defense functions (enzymes, seroins, protease inhibitors) or other roles yet to be determined [99,100,101,104]. Regarding BMSS itself, the number and the distribution of constituting peptidoid units are still disputed. Between 2 and 15 polypeptides have been reported in the literature over the past century [6]. It has been also suggested [105] that the presence of many peptidoid fractions in BMSS is, in fact, an artifact due to hydrolytic degradative processes during isolation procedures. Closer to our times, however, genomic analysis has shown that BMSS must contain at least six major native polypeptides that are all distinctly biosynthesized in the middle gland of silkworms [106,107,108,109,110]. The molecular mass distribution of polypeptides in regenerated BMSS has been widely reported between 20 and 400 kDa [6].
The secondary metabolites in the plants that are eaten by silkworms are the main source of the non-protein substances in the cocoons they have spun. These metabolites are produced by plants without being directly involved in their own normal development, but they have essential protective and defensive roles that contribute to enhanced survival and reproduction for the host plant. All species that feed on plants, from insects to humans, use indirectly the secondary metabolites contained in the vegetable matter [111]. The range of secondary metabolites produced by plants is vast and includes terpenoids, carotenoids, phenolics (e.g., flavonoids, tannins), plant steroids (e.g., sterols), alkaloids (e.g., caffeine, nicotine), carbohydrates (e.g., saponins), and hydrocarbons. When the silkworms consume leaves, some of these phytochemicals can be selectively sequestered to the silk cocoon material, where they remain either as such [104,112,113] or as derivatives resulting from biosynthetic modifications occurring within the larvae’s gut [114,115,116]. Phytochemical sequestration is a highly selective process, as illustrated by the significant differences in the nature of non-protein components throughout the silkworm species. For instance, saponins and steroids have been identified in the silk cocoons produced by Antheraea mylitta silkworms but not in those produced by B. mori silkworms. In addition, the latter do not contain tannins or terpenoids.
There is a considerable diversity of metabolites detected in the B. mori silk cocoons. No less than 45 metabolites have been identified in the wild B. mandarina cocoons, 28 of them being also present in the cocoons of its domesticated version, B. mori [104]. The following non-protein metabolites were found so far in the B. mori silk cocoons [104,112,113]: fatty acids and other carboxylic acids; carotenoids; flavonoids and other phenolics; carbohydrates and derivatives; amines; amino acids; urea and derivatives; hydrocarbons; and other organic compounds (e.g., hexanal, isopropanol, and glycerol). It is believed that many metabolites have defined roles in fulfilling the tasks required for protecting the larvae. For instance, flavonoids can shield the cocoons from UV-induced damage [114,117] and can enhance their resistance against oxidative damage [73,118].
In the present study, three types of samples were prepared from B. mori silk cocoons and were investigated in relation to their antioxidant capacity: crude BMSS (CS, non-dialyzed), purified BMSS (PS, dialyzed), and a methanolic extract of the crude sericin fraction (CS-E). To assure the solubility of BMSS fractions in aqueous media, the autoclaving stage was run for at least 4 h leading to the molecular mass distribution shown in Figure 1. Although shorter durations in the autoclave or employing procedures carried out at lower temperatures can lead to advanced preservation of the higher molecular mass components [90], such resulting sericin fractions are insoluble or only partially soluble in water, therefore unsuitable for evaluation in cell culture media. Regarding the extract (CS-E), it must be devoid of methanol and be soluble in an aqueous medium to enable its evaluation as an antioxidant. Therefore, methanol was removed by gentle evaporation to dryness, and the resulting residue was re-dissolved in water (for TEAC assay) or in DMEM (for cell culture). The bicinchonic acid (BCA) assay showed that the extract contained 29% proteinaceous matter that likely would include oligopeptides and amino acids that were dissolved and retained in the methanolic phase. This confirms the results of a previous study [60], where the ethanolic extracts of the crude sericin in five different strains of B. mori cocoons contained between 20 and 32% non-precipitable amino acids and low molecular mass peptides, the rest being non-sericin components such as flavonoids and other unidentified compounds.
TEAC assay indicated (Figure 4) that the antioxidative capacity of the extracted non-sericin components in CS-E was significantly higher than that of the water-soluble BMSS powder samples (CS and PS), the latter two showing almost the same levels of activity. While the identification of the individual chemical compounds in the CS-E was beyond the purpose of this study, it can be assumed that the non-sericin fractions include mostly flavonoids, as reported previously [60,65,71,72,73], substances known for their remarkable antioxidative properties. Flavonoids are indeed a major category of secondary metabolites in the mulberry leaves; for instance, 17 flavonoids have been identified in Korean mulberry leaves [119]. To investigate the status of flavonoids in CS, PS, and CS-E, we used two chromogenic analytical methods, which proved the presence of flavonoids, and that they exist predominantly in CS-E (see Figure 2 and Figure 3). As the contribution of flavonoids to the antioxidant properties of sericin formulations is a tenet of this study, we employed two different methods for the qualitative and semi-quantitative analysis of the samples in order to raise the level of confidence for proving the presence of flavonoids and their prevalence in CS-E. CS contains chaperone non-sericin substances that, after extraction, are retrieved within CS-E, while PS is supposed to contain only traces of them, if any.
We cannot assert whether, or to what extent, the peptidoid components co-extracted in methanol have contributed to the total antioxidant activity of the CS-E fraction. However, by examining the antioxidant activities in cell culture (Figure 5), it becomes evident that the peptidoid substances in CS-E may not have any contribution and that the activity displayed by CS at higher concentration (Figure 5d) is likely due to the presence of non-sericin substances that would be eventually transferred to CS-E by solvent extraction. The purified sericin, PS, where such substances were removed by the process of dialysis, did not display antioxidant properties in cell culture (Figure 5e,f). Our previous results [78], where insoluble gels containing sericin were used as solid substrates for cell growth, have shown no antioxidative protection for the cells suggesting that pure sericin is not an antioxidant per se. On another note, the fact that PS fraction has shown, by the TEAC assay (Figure 4), a level of antioxidant activity similar to that of CS might be attributed to the difference between a chemical test and an evaluation in cell culture. The TEAC assay reflects straightforward the ability of a substance to participate in a specific chemical reaction, while the assessment in cell culture of the same substance is an intricate process influenced by factors such as number of cells, amounts of antioxidant or oxidant, and differences in the reaction mechanisms involving the antioxidant. Therefore, a direct correspondence between the two assays is rather incongruous. As a related recommendation [32], a substance shall not be called an antioxidant at cellular level or in vivo only because any of the available chemical assays has revealed antioxidant properties.
The choice of a cell line in our study was purposeful: the photoreceptor cells (cones and rods) are crucially involved in the pathophysiology of age-related macular degeneration (AMD), currently the major cause of visual impairment and blindness in the Western world [120]. AMD is a complicated neurodegenerative process affecting the aging retina and leading to progressive visual loss and irreversible blindness. The currently available therapies are limited and effective only in certain categories of patients. Although the pathomechanisms leading to AMD are not fully elucidated, it is believed that oxidative stress, associated with an excessive generation of ROS, peroxidative processes, and chronic inflammation, is the significant factor triggering damage to cells that results in dysfunction of the retina in AMD and in other retinal degenerations [120,121,122,123,124]. The retinal photoreceptor cells, primarily the cones, are specifically amenable to oxidative damage as they are nonproliferative cells that lack detection systems for ROS-induced damage to nucleic acids at the checkpoints in the cell cycle. They have a high demand for oxygen and are under continuous exposure to light.
Discovering antioxidants that can be placed in the subretinal space, with an aim to reduce oxidative stress and protect the sensitive photoreceptor cells, is pivotal to potential treatments for AMD and other blinding diseases. However, more extensive knowledge of their in vivo mechanism of action, absorption, modification, distribution, and real benefits is imperative. While evaluation in single-cell culture systems is essential to this aim, a lengthy and laborious process is still needed in order to achieve clinically useful antioxidants.
5. Conclusions
Certain sericin fractions isolated from B. mori silk cocoons possess antioxidative capacity, as demonstrated in the present study by a photochemical assay and in cultures of murine retinal photoreceptor cells. While the crude sericin shows some activity at higher concentration, the highest level of antioxidant activity is displayed at cellular level by the sericin-associated non-peptidoid components extracted in a solvent from crude sericin, where the presence of flavonoids can be demonstrated chromogenically. The purified sericin fraction shows negligible activity, if any. Antioxidants able to protect the photoreceptor cells are relevant for the development of therapeutic strategies against age-related macular degeneration (AMD) and other eye pathologic conditions associated with oxidative stress. The extracts of sericin are promising natural antioxidants that may be effective in an in vivo pathologic situation.
Author Contributions
T.V.C. initiated and coordinated the study, carried out the literature search, and wrote the manuscript. S.S. contributed to the experimental design, carried out the isolation and processing of silk materials, assisted with the cell culture experiments, and executed the graphic matter. O.S. conducted cell culture experiments. All authors contributed to the interpretation of results. All data were generated in-house. All authors have read and agreed to the published version of the manuscript.
Funding
The submitted work did not receive any specific grant from funding agencies in the public or commercial sector.
Institutional Review Board Statement
Not applicable: no animal or human experimentation or trials were associated with this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors can confirm that all relevant data are included in this published article. The authors can provide upon request samples of the materials described in the article.
Acknowledgments
The authors acknowledge the ongoing support from the Queensland Eye Institute Foundation (Brisbane, Australia) through the Viertel’s Vision Program. This organization had no input in the design and conclusions of the study, interpretation of data, or the process of writing the article. The authors also wish to express their gratitude to Cassie Rayner and Nigel Barnett (Bond University, Gold Coast, Australia) for their input on the initial stages of the project and to Krisztina Valter-Kocsi (Australian National University Medical School, Canberra, Australia) for a gift of the 661 W cell line.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial relationships, and they have no conflict of interest or financial interests to declare that are relevant to the content of this article.
Sample Availability
Samples of the compounds are not available from the authors.
Abbreviations
| 2-APB | 2-Aminoethyl diphenylborinate |
| AMD | Age-related macular degeneration |
| BMSS | Bombyx mori silk sericin |
| CS | Crude sericin |
| CS-E | Methanolic extract from crude sericin |
| DAC | 4-(Dimethylamino)cinnamaldehyde |
| DMEM | Dulbecco modified Eagle’s medium |
| FBS | Fetal bovine serum |
| HBSS | Hank’s balanced salt solution |
| MMCO | Molecular mass cut-off |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PS | Purified sericin |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| TEAC | Trolox equivalent antioxidant capacity |
References
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556561. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Gheysens, T.; Vollrath, F.; Grahn, M.F.; Knight, D.P.; Vadgama, P. Modulation of cell growth on exposure to silkworm and spider silk fibers. J. Biomed. Mater. Res. 2010, 92A, 1366–1372. [Google Scholar] [CrossRef]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory potential of silk sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Chirila, T.V.; Suzuki, S.; Bray, L.; Barnett, N.L.; Harkin, D.G. Evaluation of sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P. Bio-Response to Silk Sericin. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 299–329. [Google Scholar]
- Jiao, Z.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.; Chang, P.; Kundu, S.C.; Wang, G.; Wang, Z.; et al. In vivo characterizations of the immune properties of sericin: An ancient material with emerging value in biomedical applications. Macromol. Biosci. 2017, 17, 1700229. [Google Scholar] [CrossRef]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar] [CrossRef]
- Terada, S.; Sasaki, M.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef]
- Takahashi, M.; Tsujimoto, K.; Yamada, H.; Takagi, H.; Nakamori, S. The silk protein, sericin, protects against cell death caused by acute serum deprivation in insect cell culture. Biotechnol. Lett. 2003, 25, 1805–1809. [Google Scholar] [CrossRef]
- Ogawa, A.; Terada, S.; Kanayama, T.; Miki, M.; Morikawa, M.; Kimura, T.; Yamaguchi, A.; Sasaki, M.; Yamada, H. Improvement of islet culture with sericin. J. Biosci. Bioeng. 2004, 98, 217–219. [Google Scholar] [CrossRef]
- Sato, W.; Fukumoto, K.; Yanagihara, K.; Sasaki, M.; Kunitomi, Y.; Terada, S. Mitogenic effect of sericin on mammalian cells. BMC Proc. 2011, 5 (Suppl. 8), 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, J.; Duan, S.; Chen, L.; Xiang, H.; Dong, Y.; Wang, W. Systematic evaluation of sericin protein as a substitute for fetal bovine serum in cell culture. Sci. Rep. 2016, 6, 31516. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Pal, S.; Sapru, S.; Kundu, J.; Talukdar, S.; Singh, N.I.; Yao, J.; Kundu, S.C. Non-mulberry and mulberry silk protein sericins as potential media supplement for animal cell culture. BioMed Res. Int. 2016, 2016, 7461041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, T.T.; Zhang, Y.Q. Viability and proliferation of L929, tumour and hybridoma cells in the culture media containing sericin protein as a supplement or serum substitute. Appl. Microbiol. Biotechnol. 2015, 99, 7219–7228. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. The potential of silk sericin protein as a serum substitute or an additive in cell culture and cryopreservation. Amino Acids 2017, 49, 1029–1039. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, T.T.; Wei, Z.G.; Zhang, Y.Q. Silk sericin hydrolysate is a potential candidate as a serum-substitute in the culture of Chinese hamster ovary and Henrietta Lacks cells. J. Insect Sci. 2019, 19, 10. [Google Scholar] [CrossRef]
- Chelladurai, K.S.; Christyraj, J.D.S.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Vasantha, N.C.; Christyraj, J.R.S.S. Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Tsukada, M. Electrospun Silk Sericin Nanofibers for Biomedical Applications. In Silk Biomaterials for Tissue Eengineering and Regenerative Medicine; Kundu, S.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–156. [Google Scholar]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Costa Brancalhão, R.M.; Chasko Ribeiro, L.D.F.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M., Jr.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. I. Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, R.S.; Nambiar, K.S.; Haragannavar, V.C.; Augustine, D.; Sowmya, S.V. Sericin, a dietary additive: Minireview. J. Med. Radiol. Pathol. Surg. 2019, 6, 4–8. [Google Scholar] [CrossRef]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1115–1129. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kweon, H.Y.; Oh, J.H. Sericin for tissue engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Suryawanshi, R.; Kanoujia, J.; Parashar, P.; Saraf, S. Sericin: A versatile protein biopolymer with therapeutic significance. Curr. Pharmac. Des. 2020, 26, 5414–5429. [Google Scholar] [CrossRef]
- Elahi, M.; Ali, S.; Tahir, H.M.; Mushtaq, R.; Bhatti, M.F. Sericin and fibroin nanoparticles—Natural product for cancer therapy: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 256–269. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A.; Davies, K.J.A.; Kelly, F. Free radical biology—Terminology and critical thinking. FEBS Lett. 2004, 558, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A. Oxidative stress: A dead end or a laboratory hypothesis? Biochem. Biophys. Res. Commun. 2007, 362, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Pinchuk, I. Oxidative stress, the term and the concept. Biochem. Biophys. Res. Commun. 2015, 461, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. Myths that will not die. Nature 2015, 528, 322–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghezzi, P.; Ghiara, V.; Davies, K. Epistemological Challenges of the Oxidative Stress Theory of Disease and the Problem of Biomarkers. In Oxidative Stress: Eustress and Distress; Sies, H., Ed.; Academic Press: London, UK, 2020; pp. 13–27. [Google Scholar]
- Ghezzi, P.; Mooradian, A.D. Demystifying Oxidative Stress. In Reactive Oxygen Species; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 3–26. [Google Scholar]
- Halliwell, B. How to characterize a biological antioxidant. Free Radic. Res. Commun. 1990, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Drug antioxidant effects. A basis for drug selection? Drugs 1991, 42, 569–605. [Google Scholar] [CrossRef]
- Diplock, A.T. Antioxidants and disease prevention. Mol. Aspects Med. 1994, 15, 293–376. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signalling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [Green Version]
- Omran, B.; Baek, K.H. Nanoantioxidants: Pioneer types, advantages, limitations, and future insights. Molecules 2021, 26, 7031. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Eustress and Oxidative Distress: Introductory Remarks. In Oxidative Stress: Eustress and Distress; Sies, H., Ed.; Academic Press: London, UK, 2020; pp. 3–12. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Davies, K.J.A. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar]
- Davies, K.J.A. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O. Oxidative stress: A theoretical model or a biological reality? C. R. Biol. 2004, 327, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochim. Polonica 2013, 60, 1–16. [Google Scholar] [CrossRef]
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols. J. Mex. Chem. Soc. 2015, 59, 231–262. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Johnson, B.A.; George, E.R. Oxidative photodegradation of ocular tissues: Beneficial effects of filtering and exogenous antioxidants. Exp. Eye Res. 2014, 129, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.B.; Wu, L.P.; Chen, L.S.; Mao, X.Y.; Ren, F.Z. Antioxidant activities of silk sericin from silkworm Bombyx mori. J. Food Biochem. 2009, 33, 74–88. [Google Scholar] [CrossRef]
- Manosroi, A.; Boonpisuttinant, K.; Winitchai, S.; Manosroi, W.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori). Pharm. Biol. 2010, 48, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Y.; Wang, Y.J.; Zhou, L.X.; Zhu, L.; Zhang, Y.Q. Isolation and bioactivities of a non-sericin component from cocoon shell silk sericin of the silkworm Bombyx mori. Food Funct. 2012, 3, 150–158. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Faragò, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef]
- Takechi, T.; Wada, R.; Fukuda, T.; Harada, K.; Takamura, H. Antioxidant activities of two sericin proteins extracted from cocoon of silkworm (Bombyx mori) measured by DPPH, chemiluminescence, ORAC and ESR methods. Biomed. Rep. 2014, 2, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Micheal, A.S.; Subramanyam, M. Influence of sericin in alleviating the hydrogen peroxide induced oxidative stress in silkworm Bombyx mori: Role of the amino acids. Invertebr. Surviv. J. 2014, 11, 257–272. [Google Scholar]
- Sanwong, G.; Sumida, M.; Sutthikhum, V. Antioxidant activity of chemically and enzymatically modified sericin extracted from cocoons of Bombyx mori. Biocatal. Agric. Biotechnol. 2016, 5, 155–161. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in medicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef]
- Dash, R.; Acharya, C.; Bindu, P.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, T.; Ikebata, Y.; Onitsuka, T.; Wittayarat, M.; Sato, Y.; Taniguchi, M.; Otoi, T. Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology 2012, 78, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kitisin, T.; Maneekan, P.; Luplertlop, N. In-vitro characterization of silk sericin as an anti-aging agent. J. Agric. Sci. 2013, 5, 54–62. [Google Scholar] [CrossRef]
- Eidet, J.R.; Reppe, S.; Pasovic, L.; Olstad, O.; Lyberg, T.; Khan, A.Z.; Fostad, I.G.; Chen, D.F.; Utheim, T.P. The silk-protein sericin induces rapid melanisation of cultured primary human retinal pigment epithelial cells by activating the NF-ĸB pathway. Sci. Rep. 2016, 6, 22671. [Google Scholar] [CrossRef] [Green Version]
- Gustina, S.; Katja, N.W.K.; Hasbi, H.; Setiadi, M.A.; Supriatna, I. Hydrogen peroxide concentration and DNA fragmentation of buffalo oocytes matured in sericin-supplemented maturation medium. S. Afr. J. Anim. Sci. 2019, 49, 227–234. [Google Scholar] [CrossRef]
- Prommuak, C.; De-Eknamkul, W.; Shotipruk, A. Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Separ. Purif. Technol. 2008, 62, 444–448. [Google Scholar] [CrossRef]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Butimal, J. Phenolic composition and antioxidant activity of Thai and Eri silk sericins. Food Sci. Biotechnol. 2012, 21, 389–398. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Lutz, O.; Fischnaller, M.; Jakschitz, T.; Bonn, G.; Aramwit, P. Identification and quantification and antioxidant activity of flavonoids in different strains of silk cocoon, Bombyx mori. Arch. Biochem. Biophys. 2017, 631, 58–65. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhao, J.G.; Zhang, Y.Q. The flavonoid-rich ethanolic extract from the green cocoon shell of silkworm has excellent antioxidation, glucosidase inhibition, and cell protective effects in vitro. Food Nutr. Res. 2020, 64, 1637. [Google Scholar] [CrossRef]
- Lim, K.S.; Kundu, J.; Reeves, A.; Poole-Warren, L.A.; Kundu, S.C.; Martens, P.J. The influence of silkworm species on cellular interactions with novel PVA/silk sericin hydrogels. Macromol. Biosci. 2012, 12, 322–332. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Enzymatic production of bioactive peptides from sericin recovered from silk industry wastewater. Process Biochem. 2008, 43, 480–487. [Google Scholar] [CrossRef]
- Fan, J.B.; Zheng, L.H.; Wang, F.; Guo, H.Y.; Jiang, L.; Ren, F.Z. Enzymatic hydrolysis of silk sericin by proteases and antioxidant activities of the hydrolisates. J. Food Biochem. 2010, 34, 382–398. [Google Scholar] [CrossRef]
- Suzuki, S.; Rayner, C.L.; Chirila, T.V. Silk fibroin/sericin native blends as potential biomaterial templates. Adv. Tissue Eng. Regen. Med. 2019, 5, 11–19. [Google Scholar] [CrossRef]
- Zhaorigetu, S.; Sasaki, M.; Watanabe, H.; Kato, N. Supplemental silk protein, sericin, suppresses colon tumorigenesis in 1,2-dimethylhydrazine-treated mice by reducing oxidative stress and cell proliferation. Biosci. Biotechnol. Biochem. 2001, 65, 2181–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhaorigetu, S.; Yanaka, N.; Sasaki, M.; Watanabe, H.; Kato, N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J. Photochem. Photobiol. B Biol. 2003, 71, 11–17. [Google Scholar] [CrossRef]
- Zhaorigetu, S.; Sasaki, M.; Kato, N. Consumption of sericin suppresses colon oxidative stress and aberrant crypt foci in 1,2-dimethylhydrazine-treated rats by colon undigested sericin. J. Nutr. Sci. Vitaminol. 2007, 53, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; He, Y.; Fu, W.; Xue, J. Effects of sericin on heme oxygenase-1 expression in the hippocampus and cerebral cortex of type 2 diabetes mellitus rats. Neural Regen. Res. 2011, 6, 423–427. [Google Scholar]
- Chen, Z.; He, Y.; Song, C.; Dong, Z.; Su, Z.; Xue, J. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen. Res. 2012, 7, 197–201. [Google Scholar]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016, 41, 210–223. [Google Scholar] [CrossRef]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.; Al-Ubaidi, M.R. Expression of cone-photoreceptor–specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Kuse, Y.; Tsuruma, K.; Kanno, Y.; Shimazawa, M.; Hara, H. CCR3 is associated with the death of a photoreceptor cell-line induced by light exposure. Front. Pharmacol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, C.L.; Bottle, S.E.; Gole, G.A.; Ward, M.S.; Barnett, N.L. Real-time quantification of oxidative stress and the protective effect of nitroxide oxidants. Neurochem. Int. 2016, 92, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheway, G.; Nazlamova, L.; Turner, D.; Cross, S. 661W photoreceptor cell line as a cell model for studying retinal ciliopathies. Front. Genet. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Chirila, T.V.; Suzuki, S.; McKirdy, N.C. Further development of silk sericin as a biomaterial: Comparative investigation of the procedures for its isolation from Bombyx mori silk cocoons. Prog. Biomater. 2016, 5, 135–145. [Google Scholar] [CrossRef] [Green Version]
- McMurrough, I.; McDowell, J. Chromatographic separation and automated analysis of flavanols. Anal. Biochem. 1978, 91, 92–100. [Google Scholar] [CrossRef]
- Sheahan, J.J.; Rechnitz, G.A. Differential visualization of transparent testa mutants in Arabidopsis thaliana. Anal. Chem. 1993, 65, 961–963. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of arabidopsis roots to gravity and light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef] [Green Version]
- Matteini, P.; Agati, G.; Pinelli, P.; Goti, A. Modes of complexation of rutin with the flavonoid reagent diphenylborinic acid 2-aminoethyl ester. Monatsh. Chem. 2011, 142, 885–893. [Google Scholar] [CrossRef]
- Grootaert, C.; Gonzales, G.B.; Vissenaekens, H.; Van de Wiele, T.; Raes, K.; Smagghe, G.; Van Camp, J. Flow cytometric method for the detection of flavonoids in cell lines. J. Biomol. Screen. 2016, 21, 858–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandiarajan, J.; Cathrin, B.; Pratheep, T.; Krishnan, M. Defense role of the cocoon in the silk worm Bombyx mori L. Rapid Commun. Mass Spectrom. 2011, 25, 3202–3206. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Song, Q.; Zhang, Y.; Chen, S.; Zhang, X.; Zhao, P.; Xia, Q. Structure, evolution, and expression of antimicrobial silk proteins, seroins in Lepidoptera. Insect Biochem. Mol. Biol. 2016, 75, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhao, P.; Zhang, Y.; Song, Q.; Zhang, X.; Guo, P.; Wang, D.; Xia, Q. Analysis of proteome dynamics inside the silk gland lumen of Bombyx mori. Sci. Rep. 2016, 6, 21158. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhang, X.; Lu, M.; Chen, H.; Chen, S.; Han, J.; Zhang, Y.; Zhao, P.; Dong, Z. Antibacterial mechanism of silkworm seroins. Polymers 2020, 12, 2985. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Tsuzuki, T.; Millington, K.; Zhang, J.; Wang, X. Photoprotection by silk cocoons. Biomacromolecules 2013, 14, 3660–3667. [Google Scholar] [CrossRef]
- Rangi, A.; Jaipura, L. The biopolymer sericin: Extraction and applications. J. Text. Sci. Eng. 2015, 5, 1000188. [Google Scholar]
- Zhang, Y.; Zhao, D.; Meng, Z.; Dong, Z.; Lin, Y.; Chen, S.; Xia, Q.; Zhao, P. Wild silkworm cocoon contains more metabolites than domestic silkworm cocoon to improve its protection. J. Insect Sci. 2017, 17, 105. [Google Scholar] [CrossRef]
- Rutherford, H.A.; Harris, M. Concerning the existence of fractions of the sericin in raw silk. Text. Res. 1940, 10, 221–228. [Google Scholar] [CrossRef]
- Gamo, T. Genetic variants of the Bombyx mori silkworm encoding sericin proteins of different length. Biochem. Genet. 1982, 20, 165–177. [Google Scholar] [CrossRef]
- Michaille, J.J.; Couble, P.; Prudhomme, J.C.; Garel, A. A single gene produces multiple sericin messenger RNA in the silk gland of Bombyx mori. Biochimie 1986, 68, 1165–1173. [Google Scholar] [CrossRef]
- Michaille, J.J.; Garel, A.; Prudhomme, J.C. The expression of five middle silk gland specific genes is territorially regulated during the larval development of Bombyx mori. Insect Biochem. 1989, 19, 19–27. [Google Scholar] [CrossRef]
- Grzelak, K. Control of expression of silk protein genes. Comp. Biochem. Physiol. 1995, 110B, 671–681. [Google Scholar] [CrossRef]
- Julien, E.; Coulon-Bublex, M.; Garel, A.; Royer, C.; Chavancy, G.; Prudhomme, J.C.; Couble, P. Silk Gland Development and Regulation of Silk Protein Genes. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 369–384. [Google Scholar]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Ma, M.; Hussain, M.; Dong, S.; Zhou, W. Characterization of the pigment in naturally yellow-coloured domestic silk. Dyes Pigments 2016, 124, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Ahamad, S.I.; Neetha, K.; Vootla, S.K. Non-Protein Chemical Compounds from Lepidopteran Insect Cocoons. In Natural Materials and Products from Insects: Chemistry and Applications; Kumar, D., Shahid, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 137–156. [Google Scholar]
- Kurioka, A.; Yamazaki, M. Purification and identification of flavonoids from the yellow green cocoon shell (Sasamayu) of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 1396–1399. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Nakajima, K.; Nagayasu, K.; Takabayashi, C. Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochemistry 2002, 59, 275–278. [Google Scholar] [CrossRef]
- Hirayama, C.; Ono, H.; Tamura, Y.; Nakamura, M. C-Prolinylquercetins from the yellow cocoon shell of the silkworm, Bombyx mori. Phytochemistry 2006, 67, 579–583. [Google Scholar] [CrossRef]
- Daimon, T.; Hirayama, C.; Kanai, M.; Ruike, Y.; Meng, Y.; Kosegawa, E.; Nakamura, M.; Tsujimoto, G.; Katsuma, S.; Shimada, T. The silkworm Green b locus encodes a quercetin 5-O-glucosyltransferase that produces green cocoons with UV-shielding properties. Proc. Natl. Acad. Sci. USA 2010, 107, 11471–11476. [Google Scholar] [CrossRef] [Green Version]
- Bungthong, C.; Siriamornpun, S. Changes in amino acid profiles and bioactive compounds of Thai silk cocoons as affected by water extraction. Molecules 2021, 26, 2033. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.T.; Kwom, O.C.; Kim, H.B.; Sung, G.B.; Kim, H.W.; Kim, Y.S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadziahmetovic, M.; Malek, G. Age-related macular degeneration revisited: From pathology and cellular stress to potential therapies. Front. Cell Dev. Biol. 2021, 8, 612812. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [Green Version]
- Toma, C.; De Cillà, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and nitrosative stress in age-related macular degeneration: A review of their role in different stages of disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).