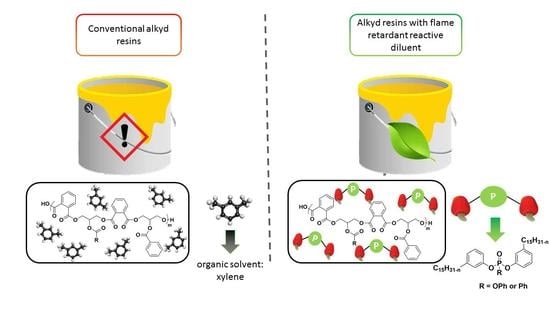
Phosphorus Modified Cardanol: A Greener Route to Reduce VolaTile Organic Compounds and Impart Flame Retardant Properties to Alkyd Resin Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nuclear Magnetic Resonance
2.2.2. Acid Value (AV)
2.2.3. Rheological Analysis
2.2.4. Size-Exclusion Chromatography (SEC)
2.2.5. Gel Content (GC)
2.2.6. Differential Scanning Calorimetry
2.2.7. Thermogravimetric Analysis (TGA)
2.2.8. Pyrolysis Combustion Flow Calorimeter (PCFC)
2.2.9. Cone Calorimeter
2.2.10. Film Properties
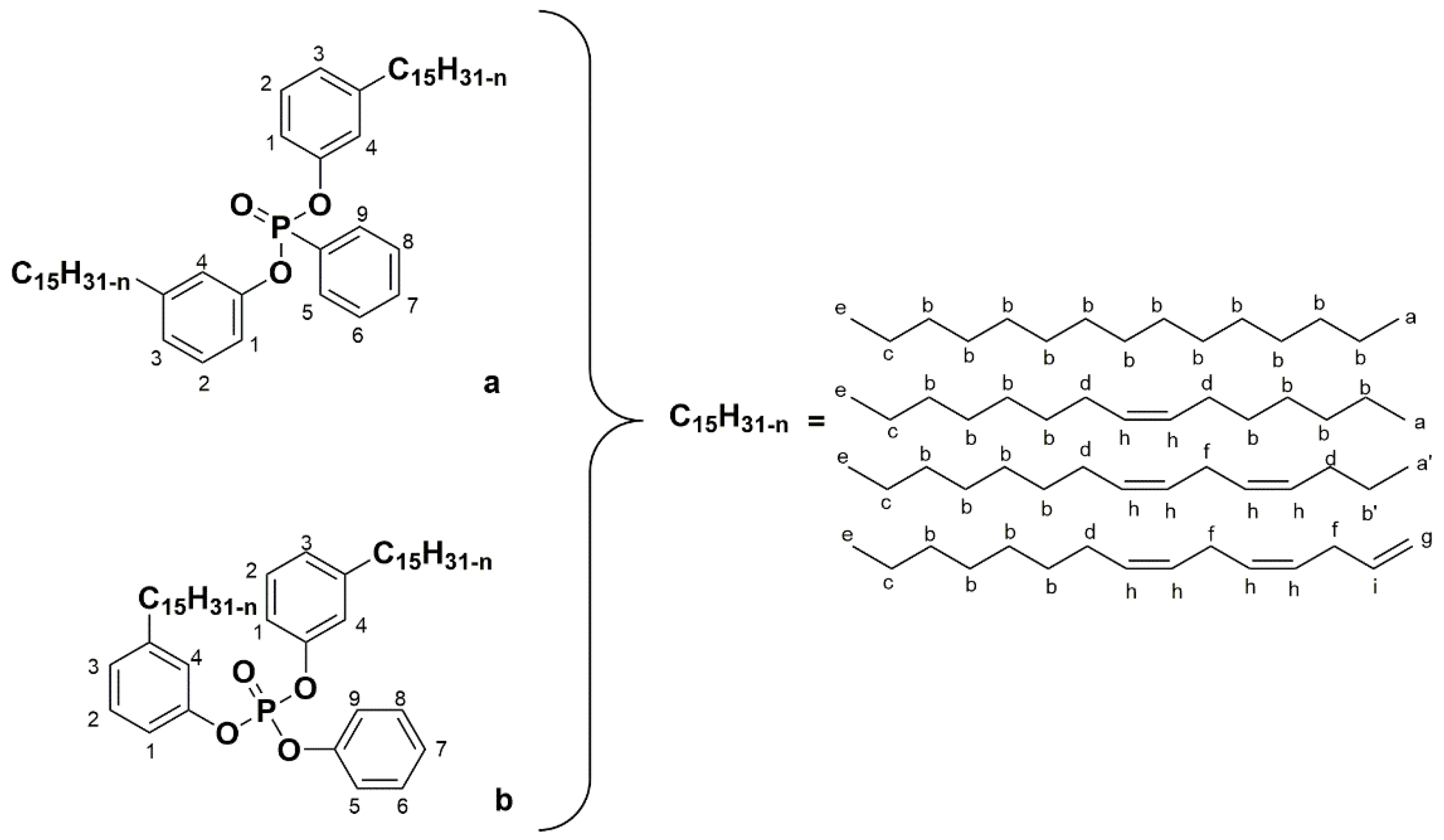
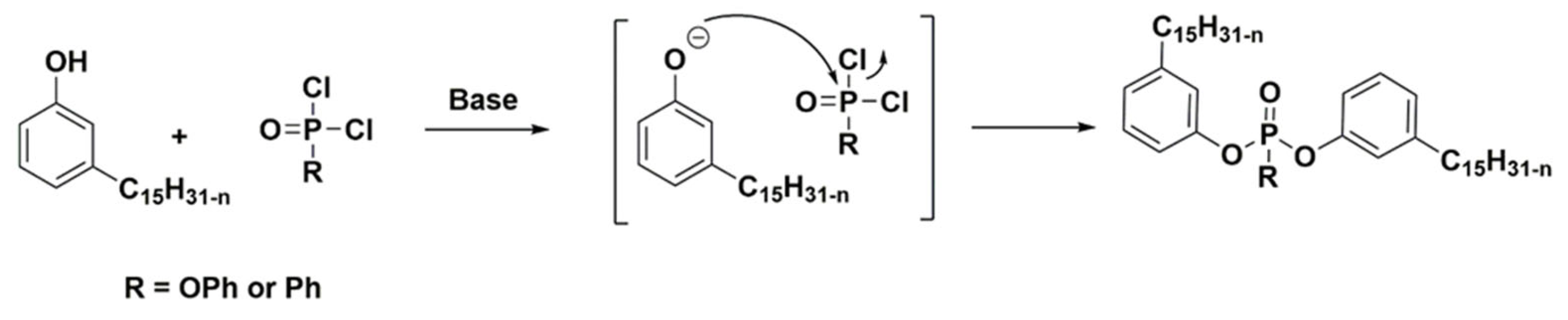
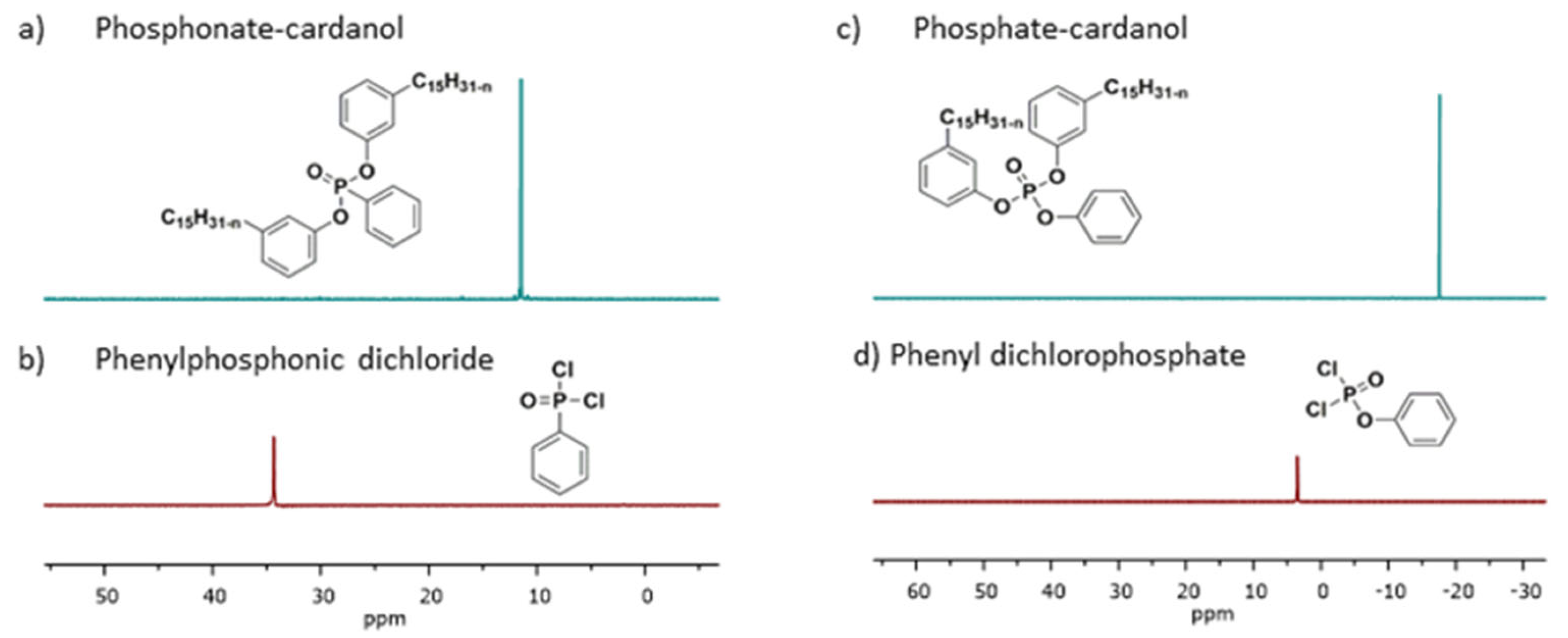
2.2.11. Synthesis of Phosphonate Cardanol Diluent (PO3RC)
31P NMR (161.6 MHz, CDCl3, ppm): δ: 11.5
2.2.12. Synthesis of Phosphate Cardanol Diluent (PO4RC)
31P NMR (161.6 MHz, CDCl3, ppm): δ: −17.7
2.2.13. Synthesis of Tall Oil Fatty Acids (TOFA) Alkyd Resin
2.2.14. Preparation of the Mixture Alkyd Resin—Reactive Diluent
3. Results and Discussion
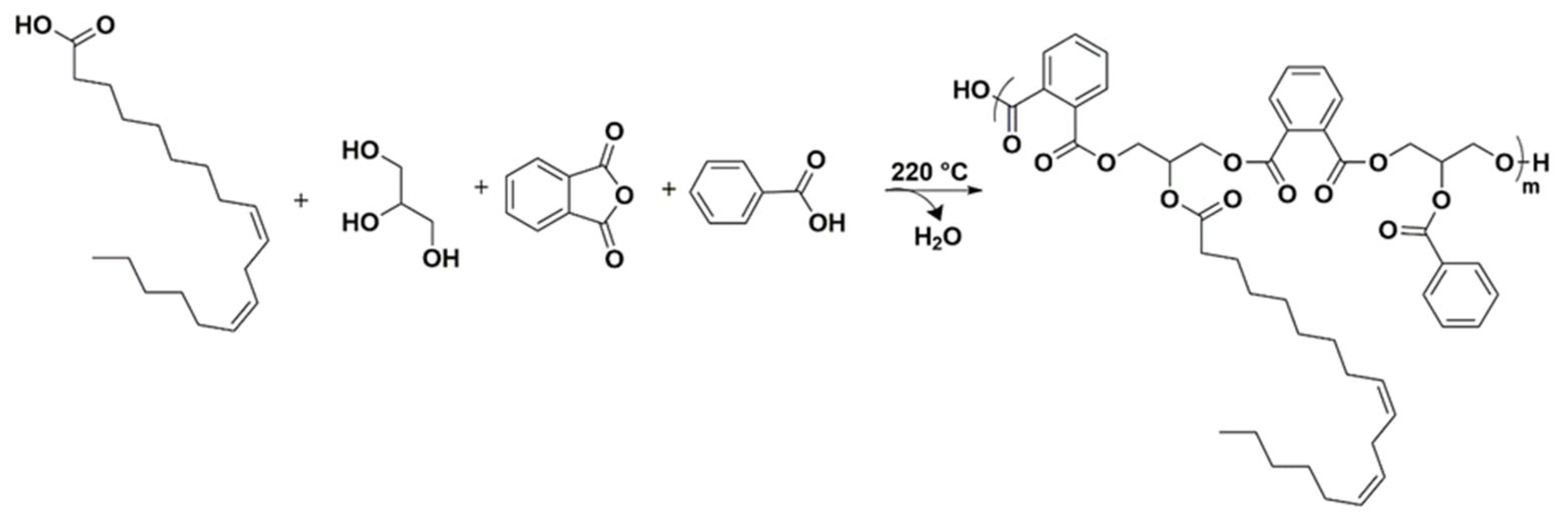
3.1. Synthesis of Phosphorus Cardanol Reactive (POxCR) Diluent Containing TOFA Alkyd Resin
3.2. Thermal and Flame Retardant Properties
3.3. Film Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hofland, A. Alkyd Resins: From down and out to Alive and Kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- La Nasa, J.; Degano, I.; Modugno, F.; Colombini, M.P. Alkyd Paints in Art: Characterization Using Integrated Mass Spectrometry. Anal. Chim. Acta 2013, 797, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Wicks, Z.W. Alkyd Resins. Kirk-Othmer Encycl. Chem. Technol. 2000, 2, 147–169. [Google Scholar]
- Elliott, W.T. Alkyd Resins. In Surface Coatings; Springer: Dordrecht, The Netherlands, 1993; Volume 5, pp. 76–109. [Google Scholar]
- Assanvo, E.F.; Gogoi, P.; Dolui, S.K.; Baruah, S.D. Synthesis, Characterization, and Performance Characteristics of Alkyd Resins Based on Ricinodendron Heudelotii Oil and Their Blending with Epoxy Resins. Ind. Crops Prod. 2015, 65, 293–302. [Google Scholar] [CrossRef]
- Chiplunkar, P.P.; Pratap, A.P. Utilization of Sunflower Acid Oil for Synthesis of Alkyd Resin. Prog. Org. Coat. 2016, 93, 61–67. [Google Scholar] [CrossRef]
- Chardon, F.; Denis, M.; Negrell, C.; Caillol, S. Hybrid Alkyds, the Glowing Route to Reach Cutting-Edge Properties? Prog. Org. Coat. 2021, 151, 106025. [Google Scholar] [CrossRef]
- Nabuurs, T. Alkyd-Acrylic Composite Emulsions Polymerization and Morphology; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 1997. [Google Scholar]
- Ling, J.S.; Ahmed Mohammed, I.; Ghazali, A.; Khairuddean, M. Novel Poly(Alkyd-Urethane)s from Vegetable Oils: Synthesis and Properties. Ind. Crops Prod. 2014, 52, 74–84. [Google Scholar] [CrossRef]
- Nalawade, P.P.; Soucek, M.D. Modified Soybean Oil as a Reactive Diluent: Coating Performance. J. Coat. Technol. Res. 2015, 12, 1005–1021. [Google Scholar] [CrossRef]
- Overbeek, A. Polymer Heterogeneity in Waterborne Coatings. J. Coat. Technol. Research 2010, 7, 1. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent Advances in Vegetable Oils Based Environment Friendly Coatings: A Review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zhou, Y.; Zhou, Q. Improvement of Corrosion Resistance and Solid Content of Zinc Phosphate Pigmented Alkyd Coating by Methacrylated Cardanol. Mater. Today Commun. 2020, 24, 101139. [Google Scholar] [CrossRef]
- Athawale, V.D.; Nimbalkar, R.V. Waterborne Coatings Based on Renewable Oil Resources: An Overview. JAOCS. J. Am. Oil Chem. Soc. 2011, 88, 159–185. [Google Scholar] [CrossRef]
- Wang, H.; Hu, G.; Liu, X.; Guo, L.; Li, X.; Guo, R.; Li, Y. Concurrent Alkylation and Crosslinking of Polyaniline for Enhanced Anticorrosive Performance of Waterborne Alkyd Coating. Prog. Org. Coat. 2022, 168, 106865. [Google Scholar] [CrossRef]
- Njuku, F.W.; Mwangi, P.M.; Thiong’o, G. Evaluation of Cardanol Acetate as a Reactive Diluent for Alkyd Coatings. Int. J. Adv. Res. 2014, 2, 928–941. [Google Scholar]
- Jagtap, A.R.; More, A. Developments in Reactive Diluents: A Review. Polym. Bull. 2021, 79, 5667–5708. [Google Scholar] [CrossRef]
- Jones, F.N. Alkyd Resins. Ullman’s Ecyclopedia Ind. Chem. 2012, 2, 429–446. [Google Scholar]
- Bora, M.M.; Deka, R.; Ahmed, N.; Kakati, D.K. Karanja (Millettia pinnata (L.) Panigrahi) Seed Oil as a Renewable Raw Material for the Synthesis of Alkyd Resin. Ind. Crops Prod. 2014, 61, 106–114. [Google Scholar] [CrossRef]
- Lee, R.; Gryn’ova, G.; Ingold, K.U.; Coote, M.L. Why Are Sec-Alkylperoxyl Bimolecular Self-Reactions Orders of Magnitude Faster than the Analogous Reactions of Tert-Alkylperoxyls? The Unanticipated Role of CH Hydrogen Bond Donation. R. Soc. Chem. 2016, 18, 23673–23679. [Google Scholar] [CrossRef]
- Honzíček, J. Curing of Air-Drying Paints: A Critical Review. Ind. Eng. Chem. Res. 2019, 58, 12485–12505. [Google Scholar] [CrossRef]
- Li, W.S.J.; Cuminet, F.; Ladmiral, V.; Lacroix-Desmazes, P.; Caillol, S.; Negrell, C. Phosphonated and Methacrylated Biobased Cardanol Monomer: Synthesis, Characterization and Application. Prog. Org. Coat. 2021, 153, 106093. [Google Scholar] [CrossRef]
- Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S. Polyols and Rigid Polyurethane Foams from Cashew Nut Shell Liquid. J. Polym. Environ. 2012, 20, 647–658. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Prasad, V.S.; Sudha, J.D.; Bera, S.C.; Menon, A.R. Polymeric Resins from Renewable Resources. II Synthesis and Characterization of Flame- Retardant Prepolymers from Cardanol. J. Appl. Polym. Sci. 1990, 41, 2487–2501. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zeng, W.; Zhou, Q. Making Alkyd Greener: Modified Cardanol as Bio-Based Reactive Diluents for Alkyd Coating. Prog. Org. Coat. 2019, 135, 281–290. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Ecochard, Y.; Decostanzi, M.; Negrell, C.; Sonnier, R.; Caillol, S. Cardanol and Eugenol Based Flame Retardant Epoxy Monomers for Thermostable Networks. Molecules 2019, 24, 9. [Google Scholar] [CrossRef]
- Phalak, G.; Patil, D.; Patil, A.; Mhaske, S. Synthesis of acrylated cardanol diphenyl phosphate for UV curable flame-retardant coating application. Eur. Polym. J. 2019, 121, 109320. [Google Scholar] [CrossRef]
- Mestry, S.; Kakatkar, R.; Mhaske, S.T. Cardanol derived P and Si based precursors to develop flame retardant PU coating. Prog. Org. Coat. 2019, 129, 59–68. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New Prospects in Flame Retardant Polymer Materials: From Fundamentals to Nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of Rate of Heat Release by Means of Oxygen Consumption Measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Williamson, W. On Etherification. Q. J. Chem. Soc. Lond. 1852, 4, 229. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell-Guirao, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of Biobased Phosphate Flame Retardants. Pure Appl. Chem. 2014, 86, 1637–1650. [Google Scholar] [CrossRef]
- Illy, N.; Fache, M.; Ménard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of Bio-Based Compounds: The State of the Art. Polym. Chem. 2015, 6, 6257–6291. [Google Scholar] [CrossRef]
- Mustafa, S.F.M.; Gan, S.N.; Yahya, R. Synthesis and Characterization of Novel Alkyds Derived from Palm Oil Based Polyester Resin. Asian J. Chem. 2013, 25, 8737–8740. [Google Scholar] [CrossRef]
- Waitara, F.N. Evaluation of Cashew Nut Shell Liquid Based Products as Reactive Diluents for Alkyd Coatings. Ph.D. Thesis, Jomo Kenyatta University of Agriculture, Nairobi, Kenya, 2014. Scientific Conference Prosiding. [Google Scholar]
- Green, J. A Review of Phosphorus-Containing Flame Retardants. J. Fire Sci. 1996, 14, 353–366. [Google Scholar] [CrossRef]
- Fang, F.; Huo, S.; Shen, H.; Ran, S.; Wang, H.; Song, P.; Fang, Z. A bio-based ionic complex with different oxidation states of phosphorus for reducing flammability and smoke release of epoxy resins. Compos. Commun. 2020, 17, 104–108. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R. Fire Retardancy of Polymeric Materials; Taylor & Francis Groups: Santa Barbara, CA, USA, 2009; Chapter 2; pp. 15–42. [Google Scholar]
- Qi, J.; Wen, Q.; Zhu, W. Research Progress on Flame-Retarded Silicone Rubber. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 032007. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame Retardancy of Silicone-Based Materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- McNally, T.; Pötschke, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene Multiwalled Carbon Nanotube Composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Alkyd Based Resin from Non-Drying Oil. Procedia Eng. 2014, 90, 78–88. [Google Scholar] [CrossRef]
- Ikhuoria, E.U.; Aigbodion, A.I.; Okieimen, F.E. Enhancing the Quality of Alkyd Resins Using Methyl Esters of Rubber Seed Oil. Trop. J. Pharm. Res. 2003, 3, 311. [Google Scholar] [CrossRef][Green Version]

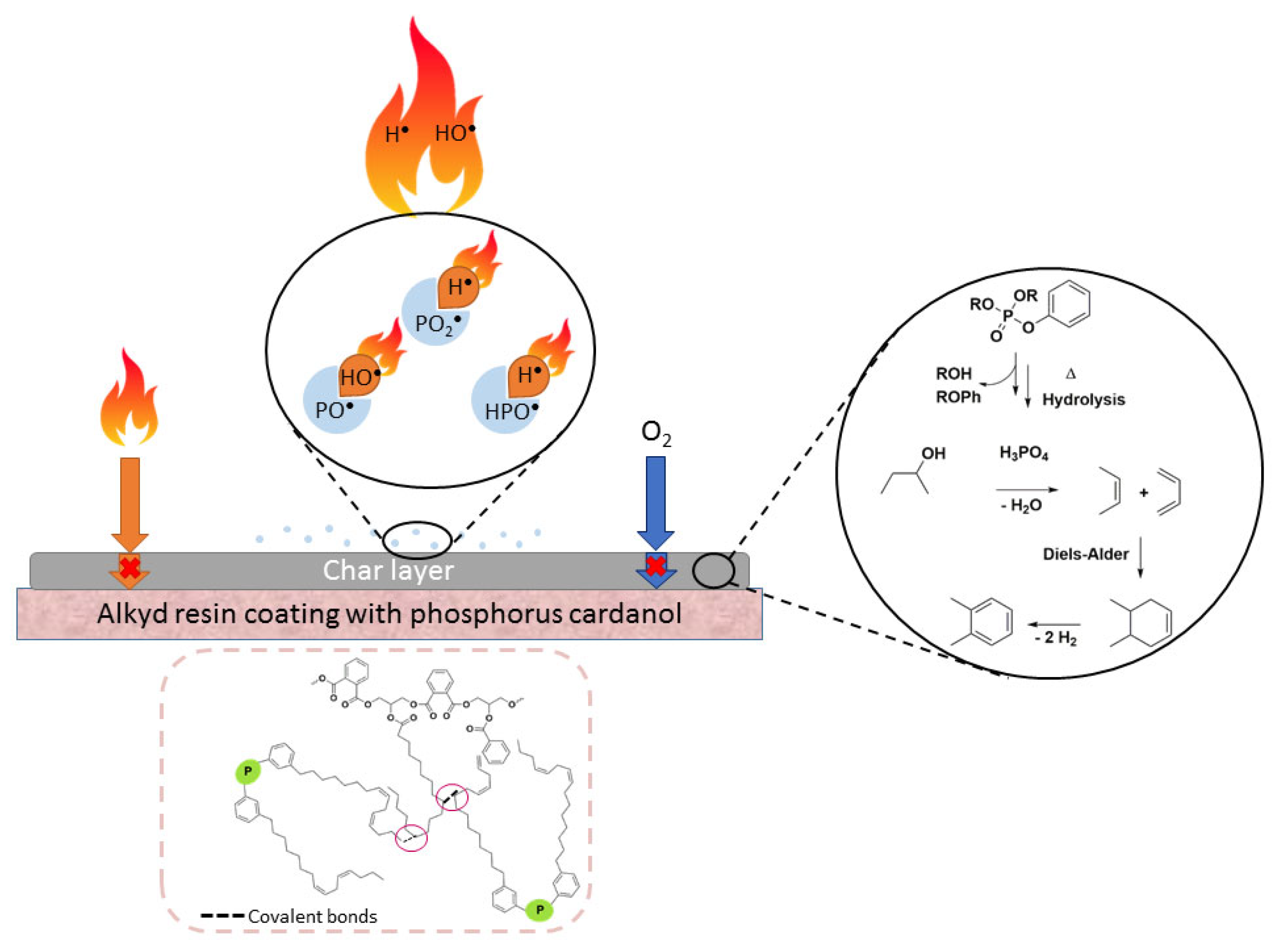
| Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| Xylene content (wt%) | 40 | 31 | 20 | 9 | 29 | 20 | 9 |
| Reactive diluent content (wt%) | 0 | 16 | 37 | 63 | 17 | 38 | 64 |
| Alkyd resin content (wt%) | 60 | 54 | 43 | 27 | 53 | 42 | 26 |
| N.V.C. (wt%) | 60 | 70 | 80 | 90 | 70 | 80 | 90 |
| Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| η (Pa.s) (xylene: 40 wt%) | 5.3 | 1.67 | 0.17 | 0.03 | 1.65 | 0.16 | 0.03 |
| Tg(°C) | 40 | 27 | −3 | −11 | 26 | −3 | −12 |
| Gel content (%) | 95 | 94 | 93 | 91 | 94 | 92 | 91 |
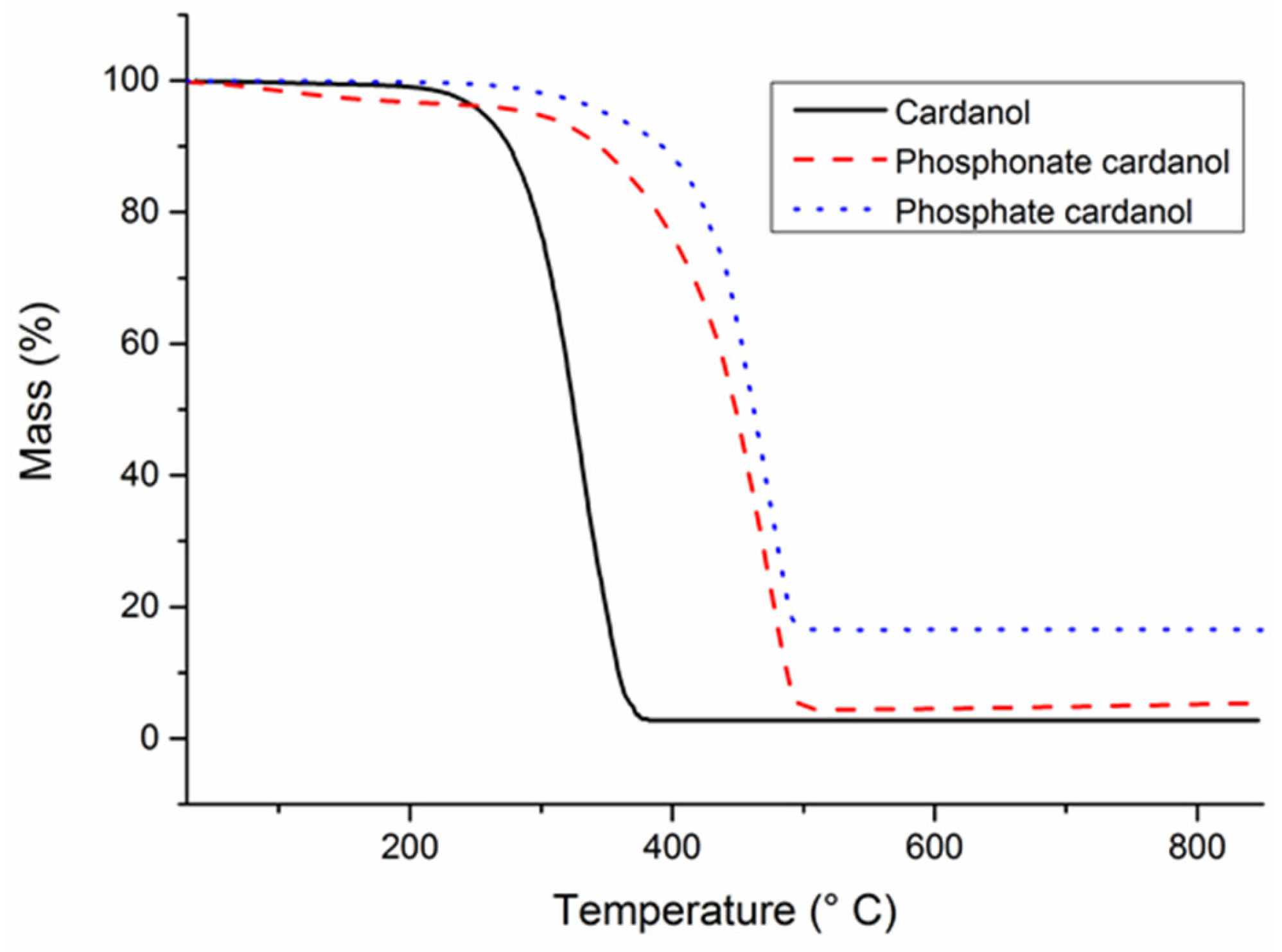
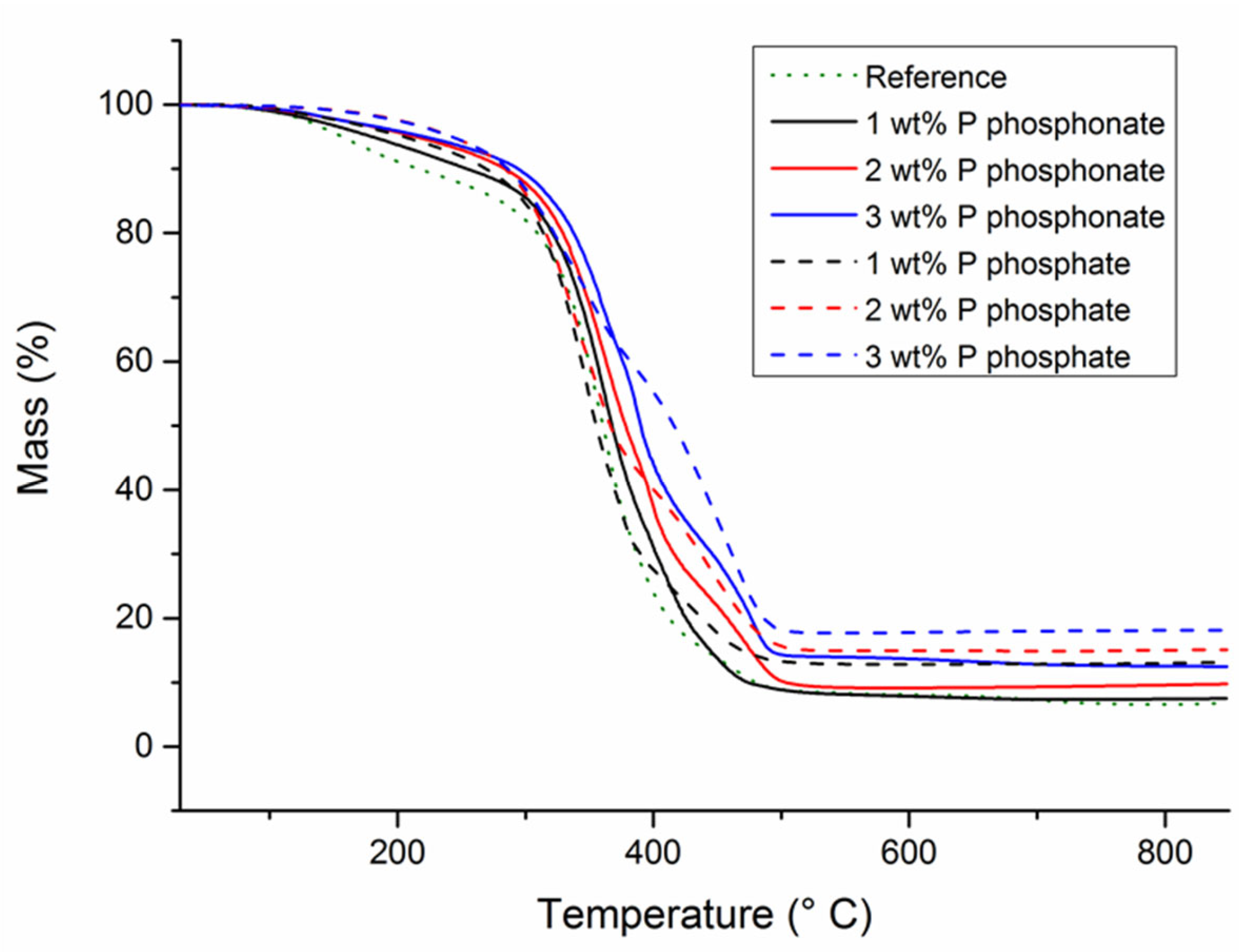
| Wt% P | Td,5wt% (°C) | Td,50wt% (°C) | Residue at 850 °C (%) | |
|---|---|---|---|---|
| Reference | 0 | 140 | 350 | 5.0 |
| Phosphonate-cardanol | Monomer | 294 | 448 | 6.4 |
| 1 | 180 | 368 | 7.5 | |
| 2 | 214 | 378 | 9.8 | |
| 3 | 221 | 390 | 12.5 | |
| Phosphate-cardanol | Monomer | 350 | 462 | 16.5 |
| 1 | 204 | 357 | 14.1 | |
| 2 | 242 | 367 | 15.2 | |
| 3 | 242 | 416 | 18.7 |
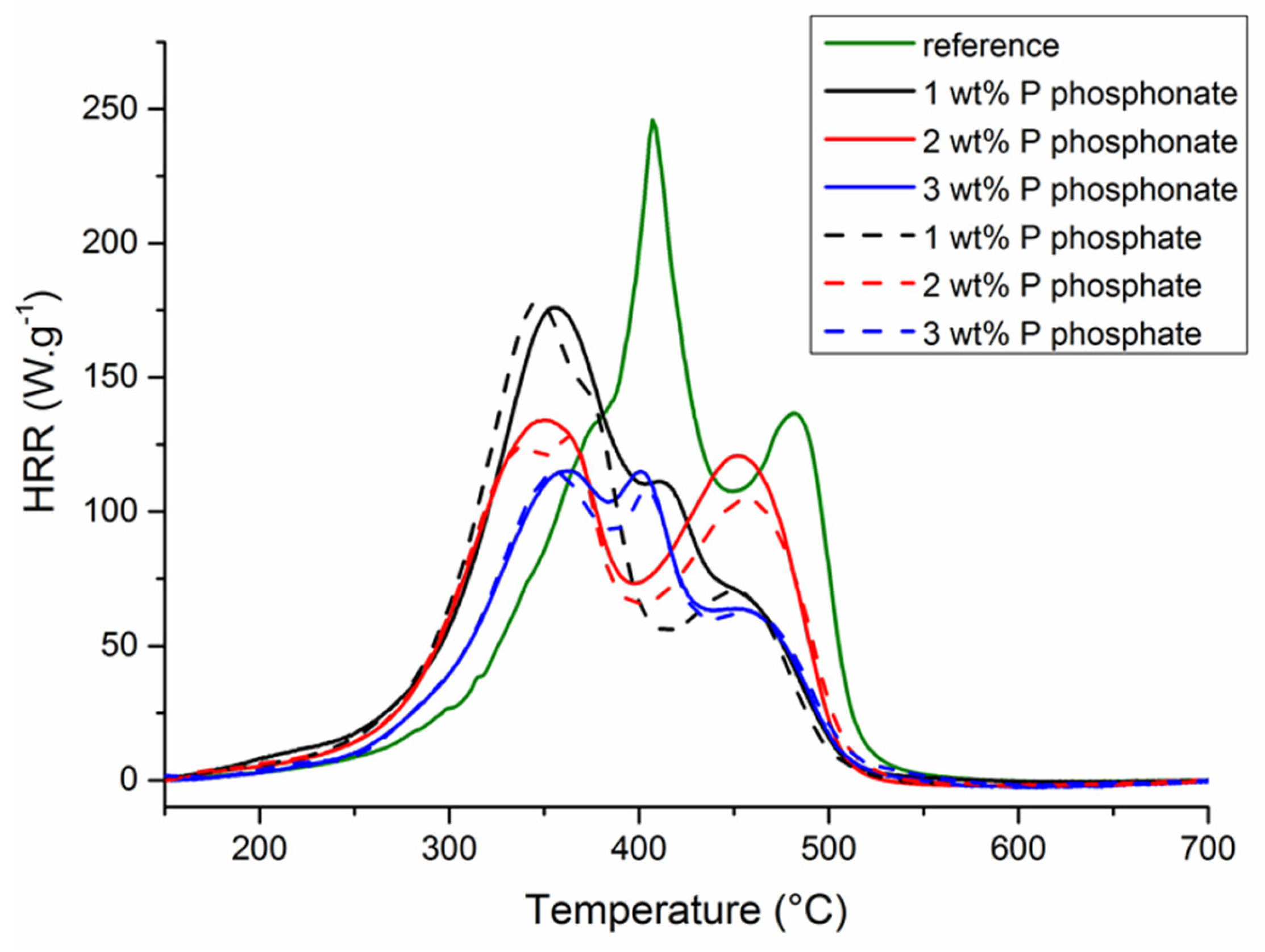
| Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| pHRR (W.g−1) | 246 137 | 176 110 | 134 120 | 115 114 | 178 71 | 125 105 | 115 108 |
| T at pHRR (°C) | 406 482 | 353 417 | 350 453 | 356 399 | 347 450 | 341 454 | 358 402 |
| THR (KJ.g−1) | 25.7 | 23.2 | 21.7 | 17.3 | 21.1 | 20.9 | 17.1 |
| Residue content (%) | 0.5 | 5.3 | 8.1 | 12.0 | 10.4 | 12.3 | 15.5 |
| Δh (KJ.g−1) | 25.8 | 24.5 | 23.6 | 19.7 | 23.5 | 23.8 | 20.2 |
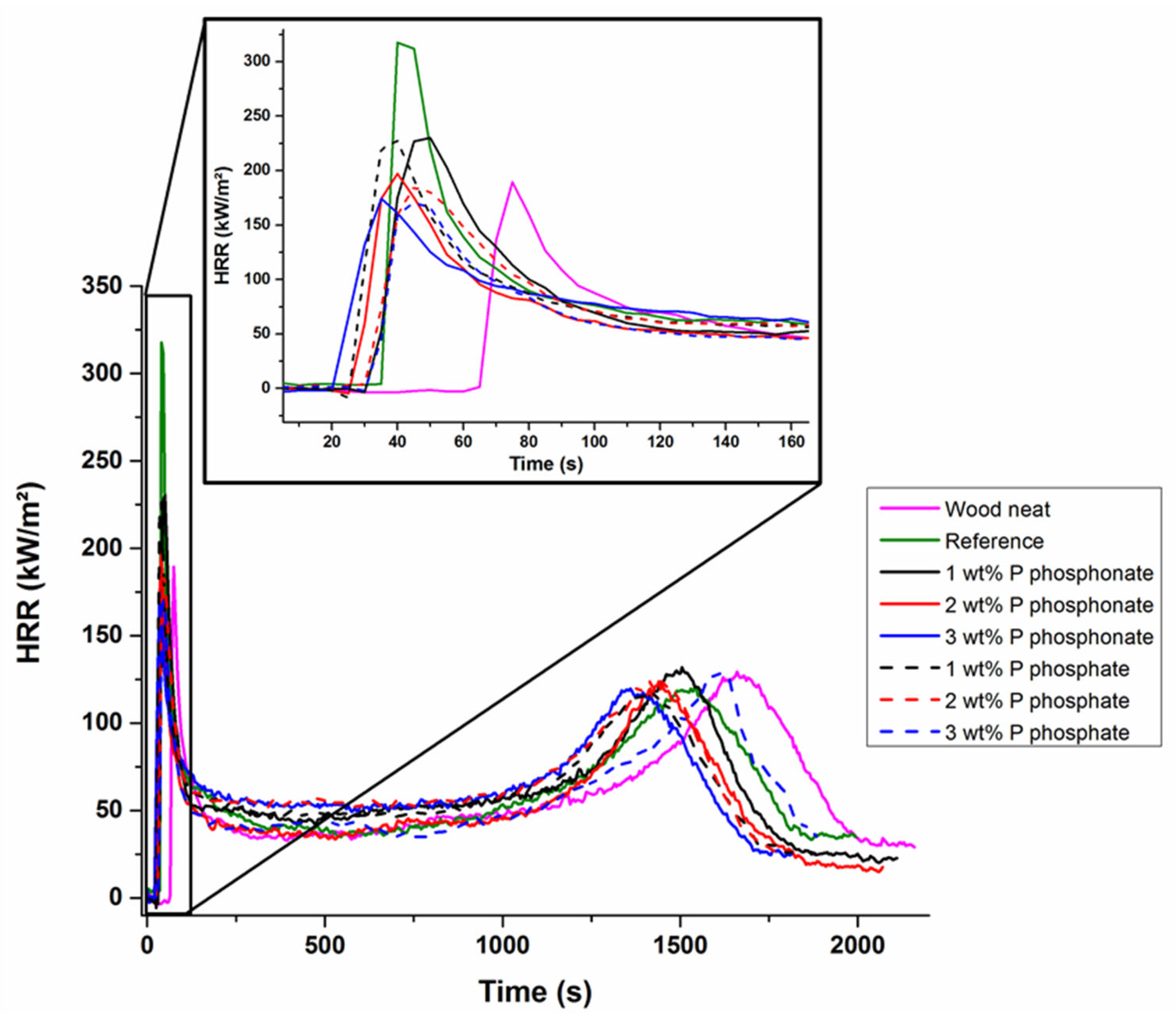
| Wood Neat | Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| TTI (s) | 65 | 35 | 30 | 26 | 22 | 26 | 32 | 31 |
| pHRR1 (KW.m−2) | 190 ± 10 | 316 ± 11 | 229 ± 9 | 197 ± 10 | 174 ± 13 | 227 ± 11 | 186 ± 8 | 170 ± 7 |
| THR (KJ.g−1) | 9.4 | 10.0 | 10.0 | 8.7 | 8.9 | 8.6 | 8.8 | 8.4 |
| Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hardness (s) | 67 | 58 | 48 | 48 | 67 | 58 | 48 |
| Gloss 20°/60° | 81/96 | 82/98 | 79/97 | 83/98 | 84/98 | 80/97 | 80/95 |
| Drying time (min) | 90 | 180 | 340 | 420 | 180 | 340 | 420 |
| Reference | Phosphonate | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| Wt% P | 0 | 1 | 2 | 3 | 1 | 2 | 3 |
| Water | 86 ± 2 | 96 ± 2 | 95± 3 | 95 ± 1 | 99 ± 1 | 98 ± 1 | 96 ± 2 |
| HCl (0.1 M) | 80 ± 4 | 91 ± 2 | 91 ± 1 | 92 ± 3 | 92 ± 1 | 91 ± 1 | 91 ± 1 |
| NaOH (0.1 M) | 69 ± 1 | 74 ± 2 | 75 ± 2 | 73 ± 3 | 74 ± 3 | 74 ± 2 | 73 ± 2 |
| NaCl (5%) | 95 ± 3 | 97 ± 1 | 96 ± 1 | 96 ± 1 | 95 ± 2 | 95 ± 4 | 97 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denis, M.; Le Borgne, D.; Sonnier, R.; Caillol, S.; Totee, C.; Negrell, C. Phosphorus Modified Cardanol: A Greener Route to Reduce VolaTile Organic Compounds and Impart Flame Retardant Properties to Alkyd Resin Coatings. Molecules 2022, 27, 4880. https://doi.org/10.3390/molecules27154880
Denis M, Le Borgne D, Sonnier R, Caillol S, Totee C, Negrell C. Phosphorus Modified Cardanol: A Greener Route to Reduce VolaTile Organic Compounds and Impart Flame Retardant Properties to Alkyd Resin Coatings. Molecules. 2022; 27(15):4880. https://doi.org/10.3390/molecules27154880
Chicago/Turabian StyleDenis, Maxinne, Damien Le Borgne, Rodolphe Sonnier, Sylvain Caillol, Cédric Totee, and Claire Negrell. 2022. "Phosphorus Modified Cardanol: A Greener Route to Reduce VolaTile Organic Compounds and Impart Flame Retardant Properties to Alkyd Resin Coatings" Molecules 27, no. 15: 4880. https://doi.org/10.3390/molecules27154880
APA StyleDenis, M., Le Borgne, D., Sonnier, R., Caillol, S., Totee, C., & Negrell, C. (2022). Phosphorus Modified Cardanol: A Greener Route to Reduce VolaTile Organic Compounds and Impart Flame Retardant Properties to Alkyd Resin Coatings. Molecules, 27(15), 4880. https://doi.org/10.3390/molecules27154880