Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Optimization of Chromatographic Condition with QbD Concept
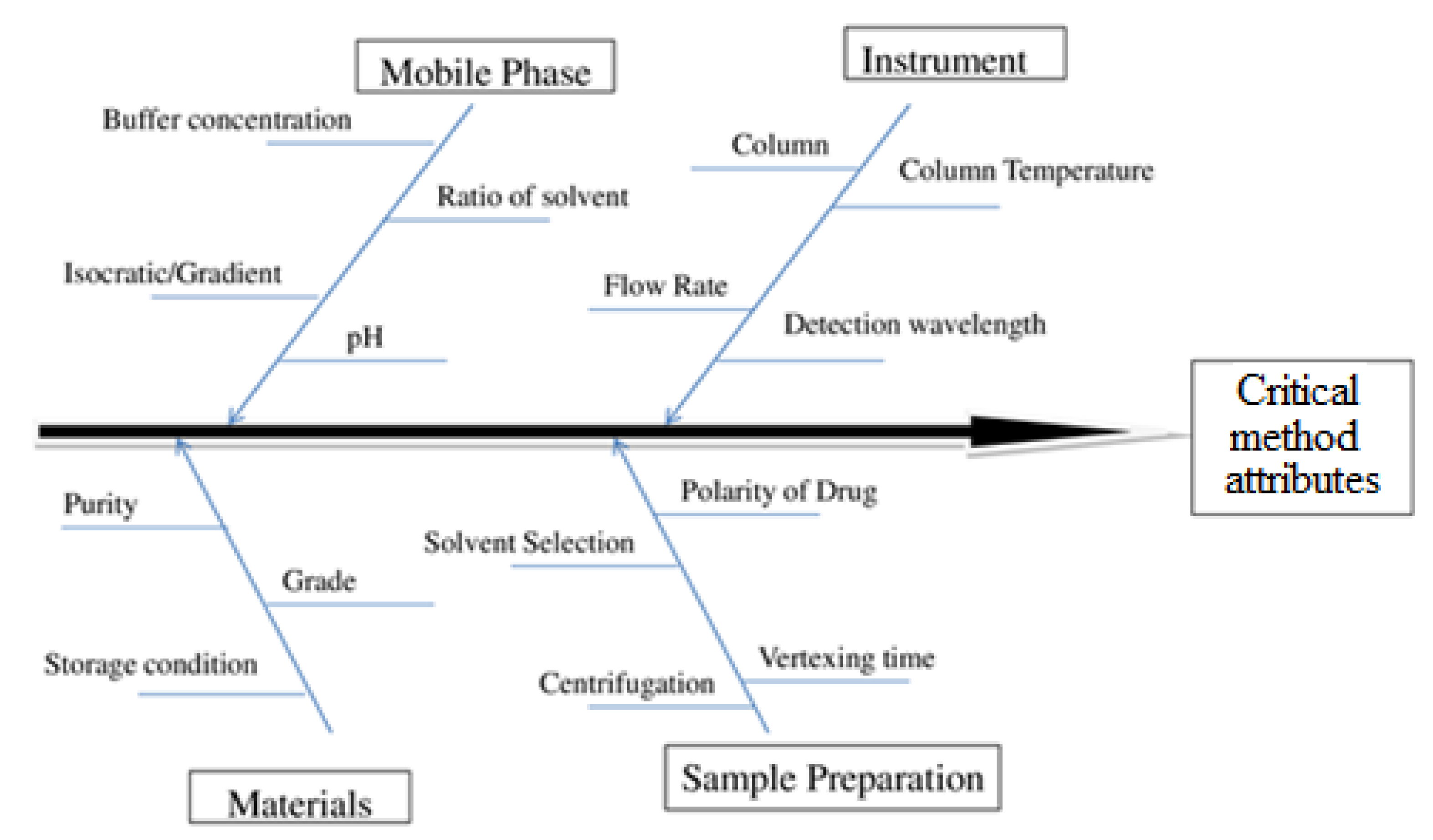
2.4. Risk Assessment
2.5. Optimization of Chromatographic Conditions Using BBD
2.6. Method Development
2.7. Preparation of Standard Solutions
2.8. Determination of λmax
2.9. Preparation of Calibration Curve
2.10. Method Validation
2.11. Assay of the Tablet
2.12. Forced Degradation Study
2.12.1. Acid Hydrolysis
2.12.2. Alkali Hydrolysis
2.12.3. Oxidative Degradation
2.12.4. Thermal Degradation
3. Results and Discussion
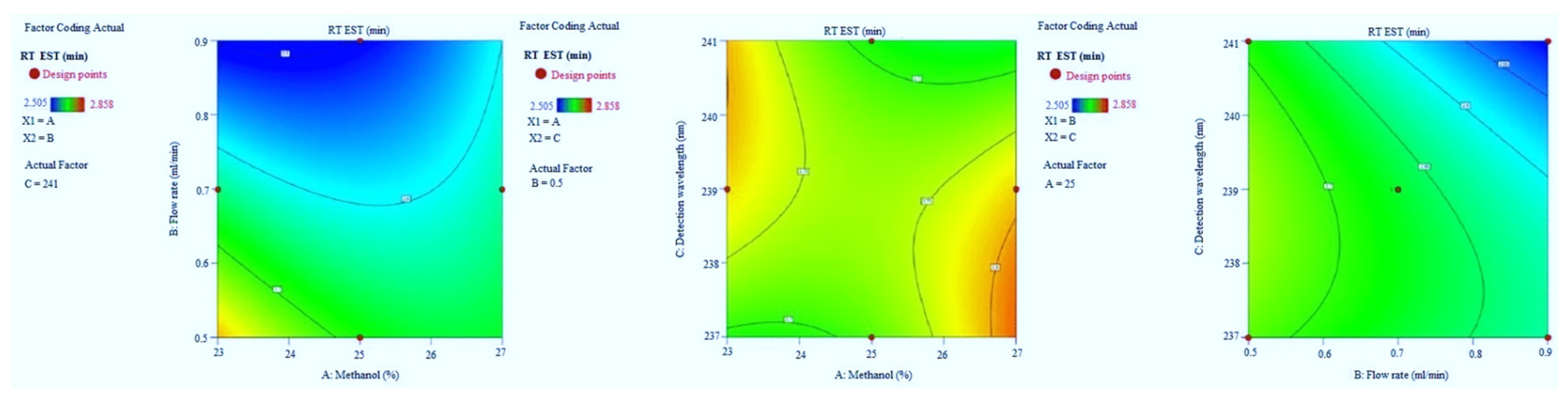
3.1. Optimization
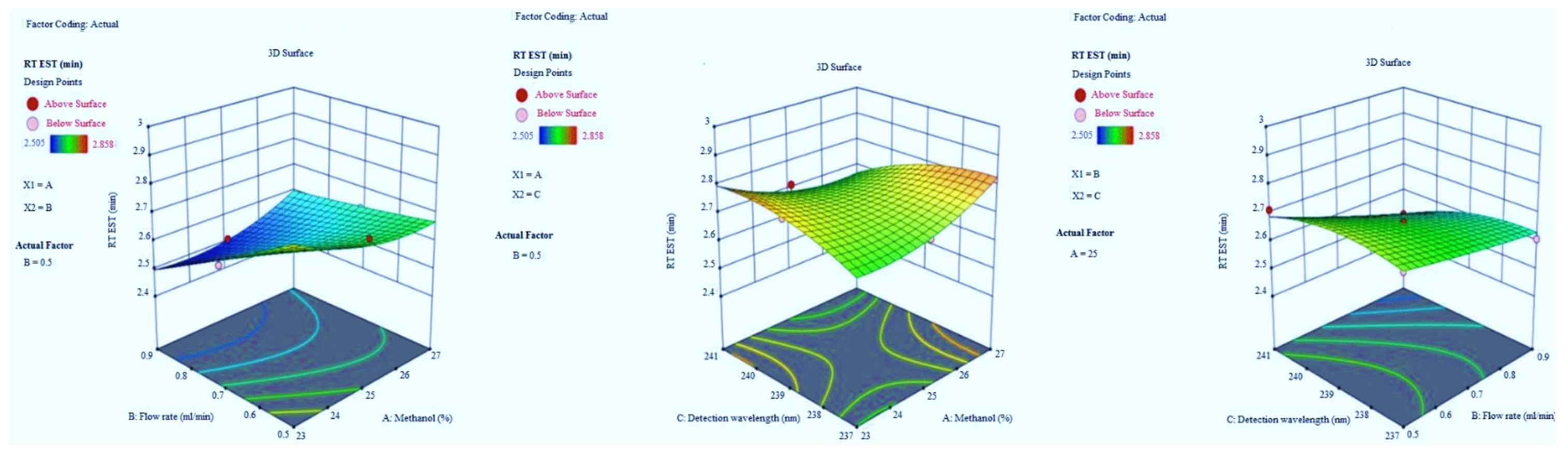
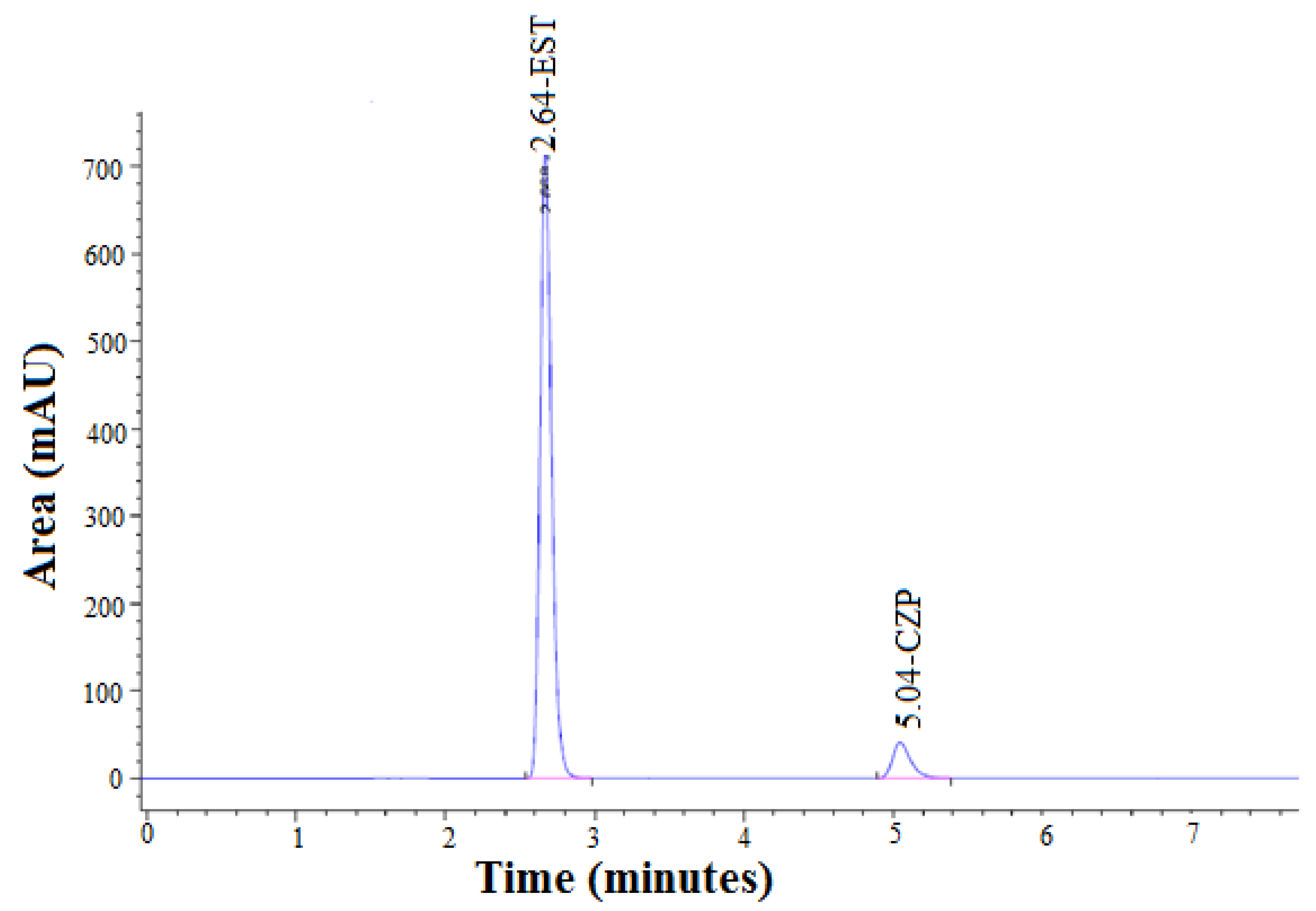
3.1.1. The Retention Time of EST
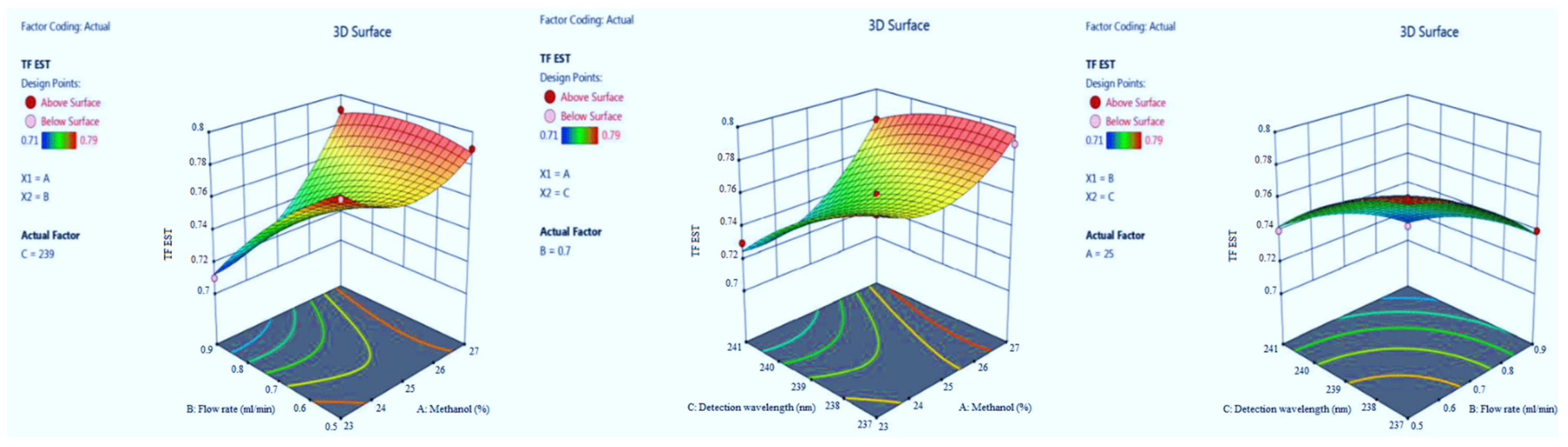
3.1.2. Tailing Factor of EST
3.1.3. The Retention Time of CZP
3.1.4. Tailing Factor of CZP
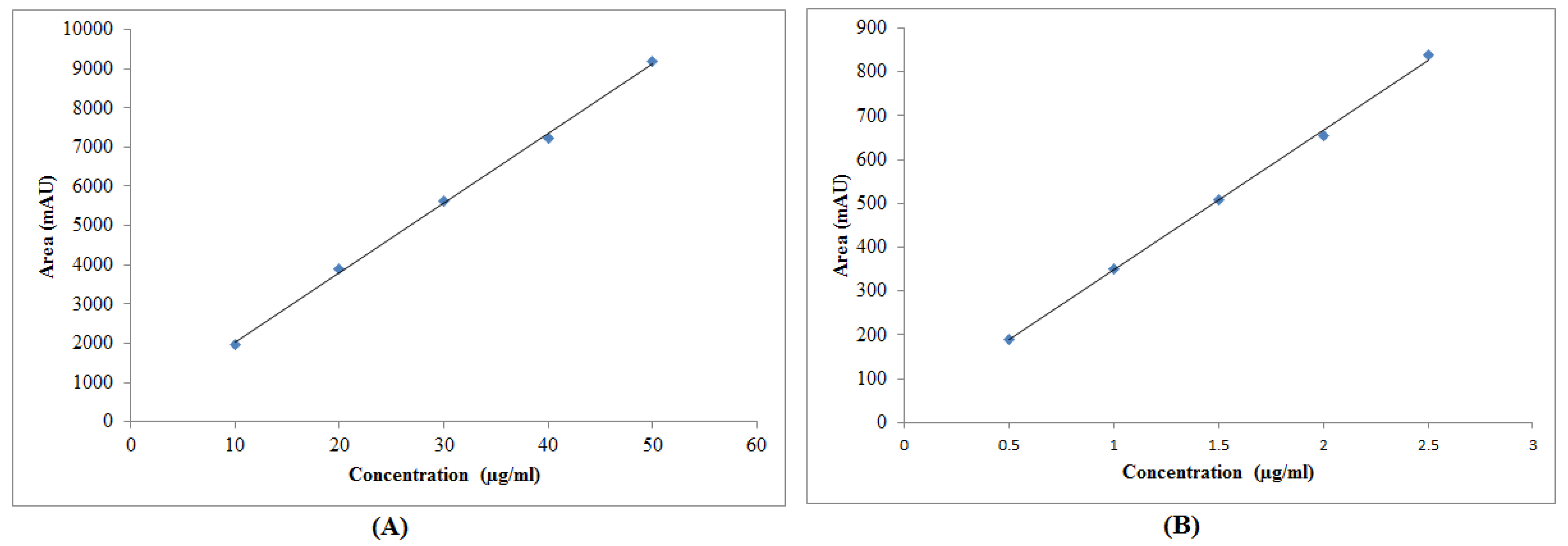
3.2. Calibration Curve
3.3. Method Validation
3.3.1. System Suitability Test
3.3.2. Linearity
3.3.3. Precision and Accuracy
3.3.4. Solution Stability
3.3.5. Robustness Study
3.4. Assay of Tablet
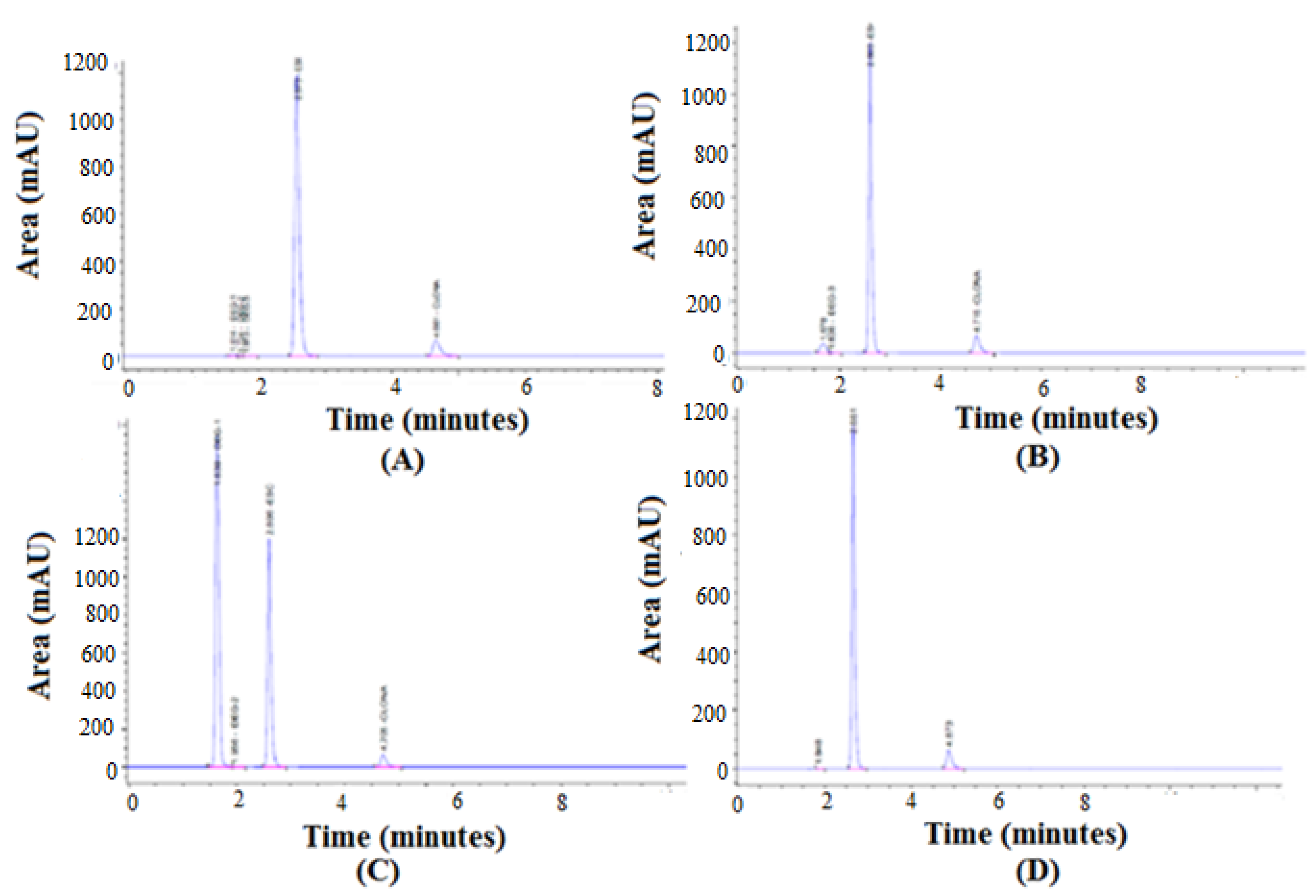
3.5. Forced Degradation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dhillon, S.; Scott, L.J.; Plosker, G.L. Escitalopram: A review of its use in the management of anxiety disorders. CNS Drugs 2006, 20, 763–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Kim, J.-B.; Sakong, J.K.; Suh, H.-S.; Oh, K.S.; Woo, J.-M.; Yoo, S.-W.; Lee, S.M.; Lee, S.-Y.; Lim, S.-W.; et al. The efficacy and safety of clonazepam in patients with anxiety disorder taking newer antidepressants: A multicenter naturalistic study. Clin. Psychopharmacol. Neurosci. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakde, R.B.; Satone, D.D. Spectrophotometric method for simultaneous estimation of escitalopram oxalate and clonazepam in tablet dosage form. Indian J. Pharm. Sci. 2009, 71, 702–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadge, S.S.; Game, M.D.; Salode, V.L. Simultaneous spectrophotometric estimation of paroxetine hydrochlorides and clonazepam in bulk and tablet dosage form. Res. J. Pharm. Technol. 2021, 14, 2497–2501. [Google Scholar] [CrossRef]
- Sharma, S.; Rajpurohit, H.; Sonwal, C.; Bhandari, A.; Choudhary, V.; Jain, T. Zero order spectrophotometric method for estimation of escitalopram oxalate in tablet formulations. J. Young Pharm. 2010, 2, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.B.; Dave, J.B.; Patel, F.M.; Patel, C.N. UV spectrophotometric method for identification and estimation of clonazepam in tablet dosage form. Int. J. Pharm. Res. Biosci. 2012, 1, 62–70. [Google Scholar]
- Soliman, S.M. Enantiomeric assay of escitalopram S (+)-enantiomer and its “in-process impurities” using two different techniques. Chirality 2019, 31, 185–201. [Google Scholar] [CrossRef]
- Kalia, B.; Baghel, U.S. Method development and validation of stability indicating RP-HPLC method for simultaneous estimation of escitalopram oxalate and clonazepam in bulk and its pharmaceutical formulations. J. Drug Deliv. Ther. 2019, 9, 265–274. [Google Scholar] [CrossRef]
- Patil, P.M.; Wankhede, S.B.; Chaudhari, P.D. A validated stability–indicating HPLC method estimation of clonazepam in the bulk drug and pharmaceutical dosage form. Pharm. Anal. Acta 2015, 6, E332. [Google Scholar]
- Spell, J.C.; Stewart, J.T. Analysis of clonazepam in a tablet dosage form using small bore HPLC. J. Pharm. Biomed. Anal. 1998, 18, 453–460. [Google Scholar] [CrossRef]
- Panchale, W.A.; Nimbokar, S.W.; Gudalwar, B.R.; Bakal, R.L.; Manwar, J.V. RP-HPLC method for simultaneous determination of escitalopram oxalate and flupentixol HCl in tablet dosage form. GSC Biol. Pharm. Sci. 2021, 14, 169–174. [Google Scholar] [CrossRef]
- Singh, S.; Shah, H.; Gupta, S.; Jain, M.; Sharma, K.; Thakkar, P.; Shah, R. Liquid chromatography–electrospray ionisation mass spectrometry method for the determination of escitalopram in human plasma and its application in bio-equivalence study. J. Chromatogr. B 2004, 811, 209–215. [Google Scholar] [CrossRef]
- Thakur, D.; Kaur, A.; Sharma, S. Application of QbD based approach in method development of RP-HPLC for simultaneous estimation of antidiabetic drugs in pharmaceutical dosage form. J. Pharm. Investig. 2017, 47, 229–239. [Google Scholar] [CrossRef]
- Beg, S.; Sharma, G.; Katare, O.P.; Lohan, S.; Singh, B. Development and validation of a stability-indicating liquid chromatographic method for estimating olmesartan medoxomil using quality by design. J. Chromatogr. Sci. 2015, 53, 1048–1059. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO nanoparticles of Rubia cordifolia extract formulation developed and optimized with QbD application, considering ex vivo skin permeation, antimicrobial and antioxidant properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef]
- Stamenković, O.S.; Kostić, M.D.; Radosavljević, D.B.; Veljković, V.B. Comparison of Box-Behnken, face central composite and full factorial designs in optimization of hempseed oil extraction by n-hexane: A case study. Period. Polytech. Chem. Eng. 2018, 62, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Gundogdu, T.K.; Deniz, I.; Caliskan, G.; Sahin, E.S.; Azbar, N. Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 2016, 36, 368–388. [Google Scholar] [CrossRef]
- Final Concept Paper, ICH Q14: Analytical Procedure Development and Revision of Q2 (R1) Analytical Validation, (November 2018); ICH: Geneva, Switzerland, 2018.
- Parab Gaonkar, V.; Mannur, V.K.; Hullatti, K. Quality assessment and analytical quality by design-based RP-HPLC method development for quantification of piperine in Piper nigrum L. Future J. Pharm. Sci. 2022, 8, 16. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Shirkhedkar, A.A. Design of experiments in liquid chromatography (HPLC) analysis of pharmaceuticals: Analytics, applications, implications and future prospects. Rev. Anal. Chem. 2017, 36, 20160025. [Google Scholar] [CrossRef]
- Palakurthi, A.K.; Dongala, T.; Katakam, N.L.R. QbD based development of HPLC method for simultaneous quantification of telmisartan and hydrochlorothiazide impurities in tablets dosage form. Pract. Lab. Med. 2020, 21, e00169. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M. Optimized Box-Behnken experimental design based response surface methodology and Youden’s robustness test to develop and validate methods to determine nateglinide using kinetic spectrophotometry. Spectrochim. Acta A 2022, 268, 120712. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Patel, J.; Patel, K.; Gandhi, T. Development and validation of an HPTLC method for the simultaneous estimation of clonazepam and paroxetine hydrochloride using a DOE approach. J. Taibah Uni. Sci. 2017, 11, 121–132. [Google Scholar] [CrossRef]
- Bairagi, S.H.; Ghosh, R.S. Development and evaluation of novel estimation techniques for in vitro dissolution study and validation protocol for escitalopram as antidepressant drug and their formulation. Int. J. Pharm. Pharm. Sci. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- ICH Q2(R2) Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures (accessed on 16 May 2022).
- FDA; CDER; Beers; Donald. Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry; FDA: Silver Spring, MD, USA, 2015.
- Alquadeib, B.T. Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm. J. 2019, 27, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Thangabalan, B.; Kahsay, G.; Eticha, T. Development and validation of a high-performance liquid chromatographic method for the determination of cinitapride in human plasma. J. Anal. Methods Chem. 2018, 2018, 8280762. [Google Scholar] [CrossRef]
- Ali, S.; Taleuzzaman, M.; Gilani, S.J.; Ahmed, M.L.; Hafeez, A. Quantitative estimation of Donepenzil hydrochloride tablet by HPLC. Int. J. Pharm. Chem. 2016, 6, 62–65. [Google Scholar]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. development and optimization of liquid chromatography analytical methods by using a QbD principles: Overview and recent advances. Org. Proc. Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef] [Green Version]
- Al-Rimawi, F. Development and validation of a simple reversed-phase HPLC-UV method for determination of oleuropein in olive leaves. J. Food Drug Anal. 2014, 22, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, A.; Pandey, A.; Gupta, V.; Malasoni, R.; Srivastava, A.; Pandey, R.R.; Satyanarayana, M.; Pratap, R.; Dwivedi, A.K. Assay method for quality control and stability studies of a new anti-diabetic and anti-dyslipidemic flavone (S002-853). Pharmacogn. Mag. 2015, 11, S53–S59. [Google Scholar] [PubMed] [Green Version]
- Bhimanadhuni, C.N.; Garikapati, D.R.; Usha, P. Development and validation of an RP-HPLC method for the simultaneous determination of escitalopram oxalate and clonazepam in bulk and its pharmaceutical formulations. Int. Curr. Phram. J. 2012, 1, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Jahan, M.S.; Islam, M.J.; Begum, R.; Kayesh, R.; Rahman, A. A Study of method development, validation, and forced degradation for simultaneous quantification of paracetamol and ibuprofen in pharmaceutical dosage form by RP-HPLC method. Anal. Chem. Insights 2014, 9, 75–81. [Google Scholar] [PubMed]
- Eldin, A.B.; Shalaby, A.; Abdallah, M.S.; Shaldam, M.A.; Abdallah, M.A. Applying green analytical chemistry (GAC) for development of stability indicating HPLC method for determining clonazepam and its related substances in pharmaceutical formulations and calculating uncertainty. Arabian J. Chem. 2019, 12, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Awni, K.J.; Aboktifa, M.A.; Salman, M.A.; Jasim, A.M. Characterization and synthesis of new model of derivative colonazepam and clinical trial to inspection of adverse effect in male mice. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 553, p. 012020. [Google Scholar]

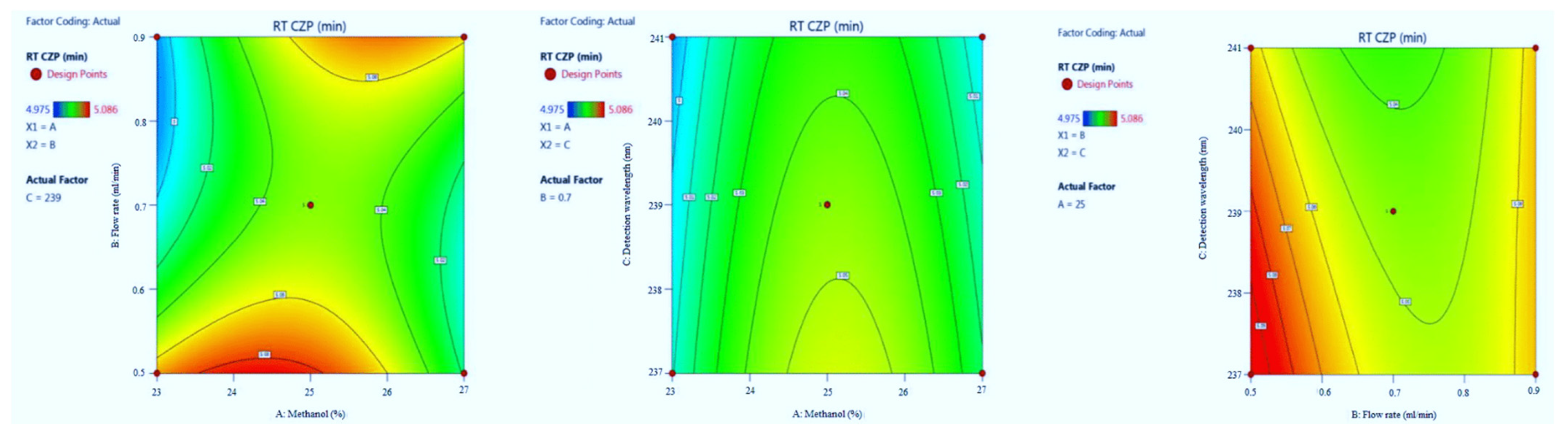
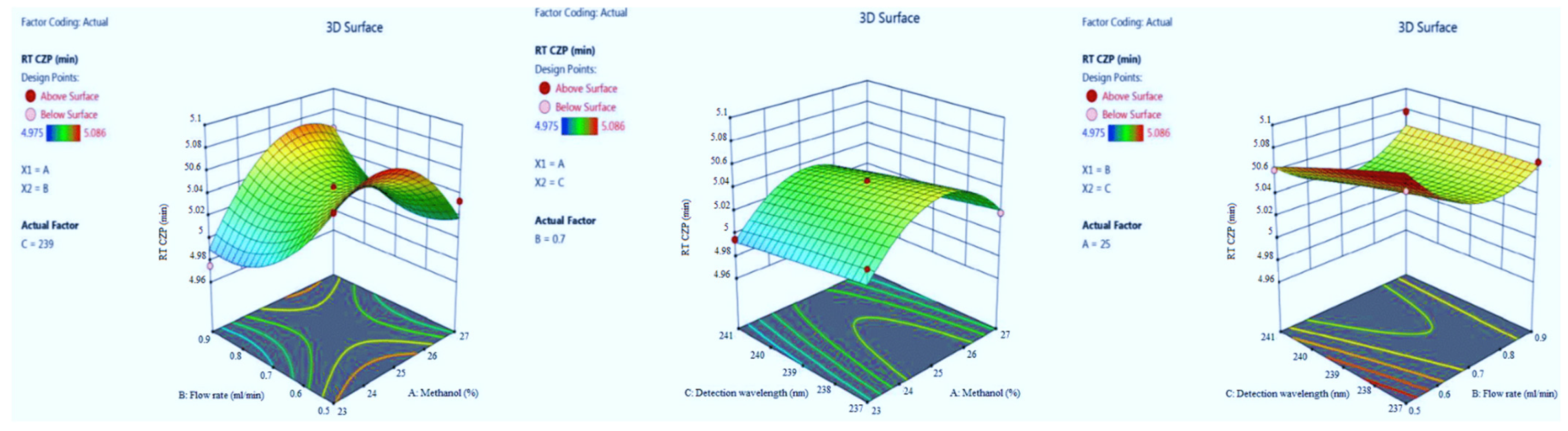
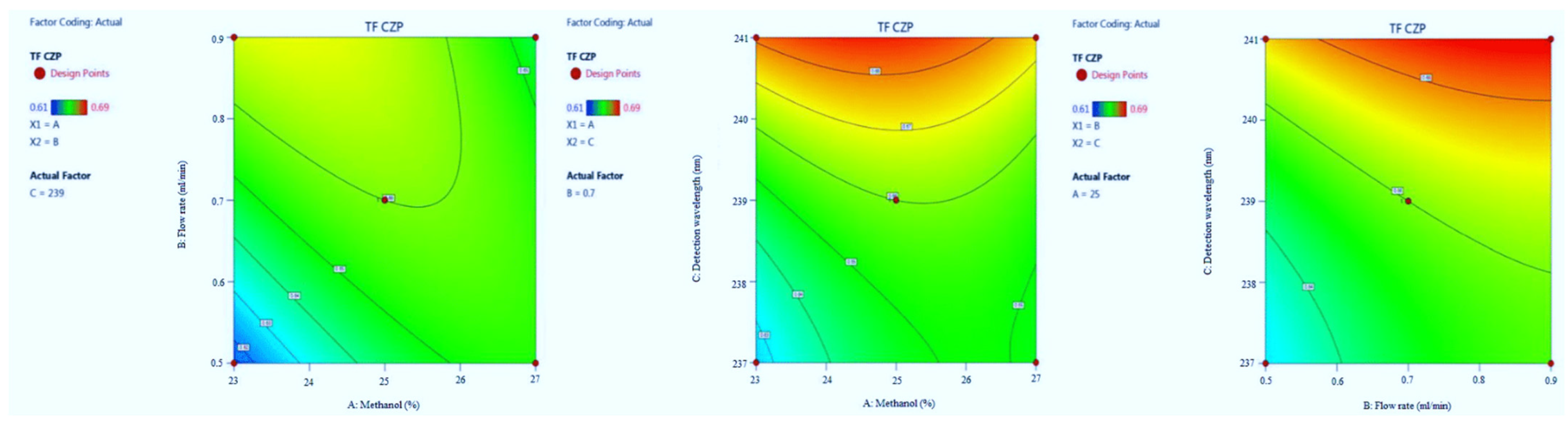
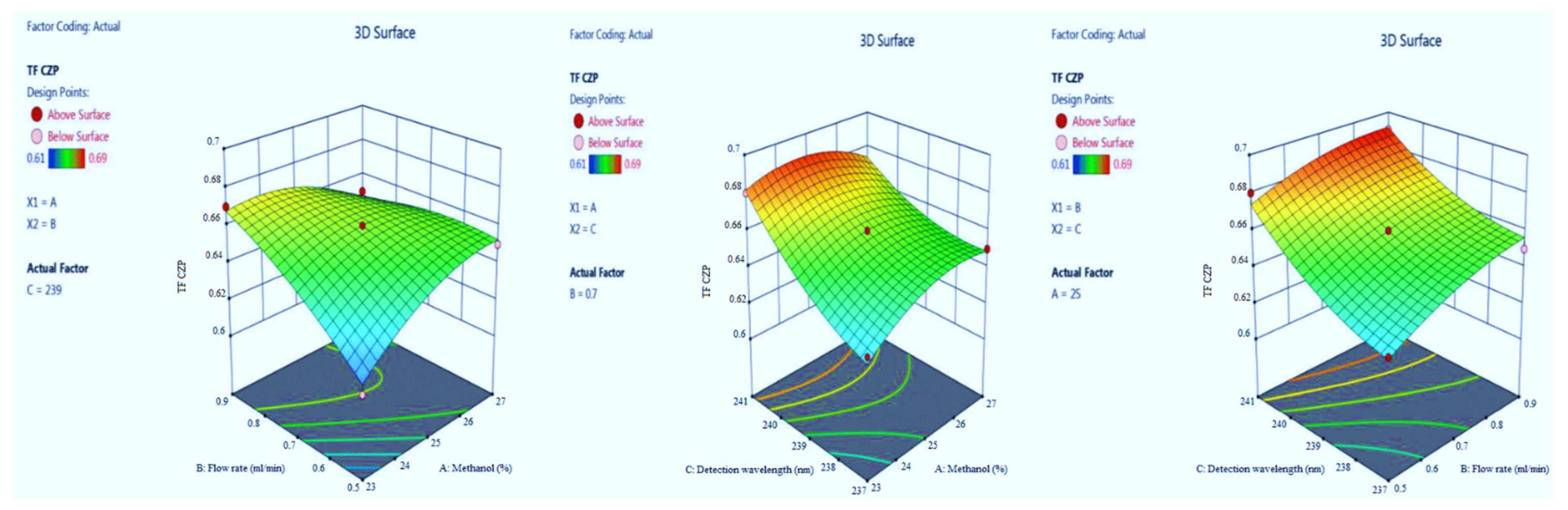
| Run | Factor A (%Metnaol in Mobile Phase) | Factor B (Flow Rate) | Factor C (λmax) | Response 1 (Retention Time of EST) | Response 2 (Tailing Factor of EST) | Response 3 (Retention Time of CZP) | Response 4 (Tailing Factor of CZP) |
|---|---|---|---|---|---|---|---|
| 1. | 23 | 0.5 | 239 | 2.779 | 0.79 | 5.069 | 0.61 |
| 2. | 27 | 0.5 | 239 | 2.769 | 0.79 | 5.034 | 0.65 |
| 3. | 23 | 0.9 | 239 | 2.552 | 0.71 | 4.975 | 0.67 |
| 4. | 27 | 0.9 | 239 | 2.772 | 0.79 | 5.062 | 0.65 |
| 5. | 23 | 0.7 | 237 | 2.596 | 0.78 | 5.022 | 0.63 |
| 6. | 27 | 0.7 | 237 | 2.858 | 0.79 | 5.019 | 0.65 |
| 7. | 23 | 0.7 | 241 | 2.622 | 0.73 | 4.995 | 0.68 |
| 8. | 27 | 0.7 | 241 | 2.624 | 0.78 | 4.989 | 0.67 |
| 9. | 25 | 0.5 | 237 | 2.711 | 0.79 | 5.086 | 0.63 |
| 10. | 25 | 0.9 | 237 | 2.608 | 0.74 | 5.068 | 0.65 |
| 11. | 25 | 0.5 | 241 | 2.712 | 0.74 | 5.061 | 0.68 |
| 12. | 25 | 0.9 | 241 | 2.505 | 0.71 | 5.078 | 0.69 |
| 13 *. | 25 | 0.7 | 239 | 2.668 | 0.76 | 5.046 | 0.66 |
| 14 *. | 25 | 0.7 | 239 | 2.668 | 0.76 | 5.046 | 0.66 |
| 15 *. | 25 | 0.7 | 239 | 2.668 | 0.76 | 5.046 | 0.66 |
| 16 *. | 25 | 0.7 | 239 | 2.668 | 0.76 | 5.046 | 0.66 |
| 17 *. | 25 | 0.7 | 239 | 2.668 | 0.76 | 5.046 | 0.66 |
| EST | CZP | |||
|---|---|---|---|---|
| Retention Time | Tailing Factor | Retention Time | Tailing Factor | |
| Source | Quadratic | Quadratic | Quadratic | Quadratic |
| Std.dev. | 0.0196 | 0.0038 | 0.0132 | 0.0050 |
| R2 | 0.9786 | 0.9809 | 0.9238 | 0.9726 |
| Adjusted R2 | 0.9510 | 0.9917 | 0.8259 | 0.9374 |
| Predicted R2 | 0.6568 | 08664 | −0.2185 | 0.5617 |
| Sequential p-value | 0.0034 | <0.0001 | 0.0012 | 0.0066 |
| Adequate precision | 21.766 | 14.112 | 10.756 | 8.2412 |
| Precision | ||||||||
|---|---|---|---|---|---|---|---|---|
| EST | CZP | |||||||
| QC Sample (μg/mL) | Intraday | Inter-Day | Intraday | Inter-day | ||||
| SD | CV (%) | SD | CV (%) | SD | CV (%) | SD | CV (%) | |
| LOQ | 0.96 | 0.23 | 5.65 | 0.64 | 1.12 | 0.32 | 1.43 | 0.41 |
| MOQ | 4.73 | 0.08 | 2.76 | 0.02 | 1.02 | 0.23 | 2.04 | 0.40 |
| HQC | 3.40 | 0.05 | 1.47 | 0.17 | 1.95 | 0.29 | 7.84 | 1.19 |
| Accuracy | ||||||||
| Excess drug added (%) | EST | CZP | ||||||
| Avg. recovered (%) | CV (%) | SD | Avg. recovered (%) | SD | CV (%) | |||
| 80 | 101.06 | 1.44 | 1.43 | 100.02 | 0.01 | 0.01 | ||
| 100 | 100.39 | 0.21 | 0.21 | 102.24 | 0.45 | 0.44 | ||
| 120 | 102.36 | 0.26 | 0.26 | 101.68 | 0.22 | 0.22 | ||
| EST | CZP | |||||
|---|---|---|---|---|---|---|
| Mean Area (µAU) | SD | CV (%) | Mean Area (µAU) | SD | CV (%) | |
| Flow rate at 0.6 mL/min | 8563.44 | 2.96 | 0.03 | 771.22 | 1.69 | 0.22 |
| Flow rate at 0.8 mL/min | 6409.23 | 22.3 | 0.35 | 580.10 | 1.52 | 0.26 |
| Methanol (24%) + OPA (76%) | 7295.60 | 30.26 | 0.41 | 662.60 | 1.87 | 0.28 |
| Methanol (26%) + OPA (74%) | 7303.25 | 31.11 | 0.43 | 661.12 | 2.78 | 0.42 |
| Wavelength 238 nm | 7176.40 | 2.57 | 0.04 | 701.40 | 1.81 | 0.26 |
| Wavelength 240 nm | 7527 | 9.03 | 0.12 | 622.61 | 3.82 | 0.61 |
| S. No. | Degradation | Conc. of Standard (µg/mL) | Conc. of Drug Remaining (µg/mL) | Actual Degradation (%) |
|---|---|---|---|---|
| EST | ||||
| 1. | Acid degradation | 30.00 | 29.81 | 0.63 |
| 2. | Basic degradation | 30.00 | 29.97 | 0.10 |
| 3. | H2O2 degradation | 30.00 | 29.75 | 0.83 |
| 4. | Thermal degradation | 30.00 | 29.81 | 0.63 |
| CZP | ||||
| 1. | Acid degradation | 30.00 | 25.84 | 13.86 |
| 2. | Basic degradation | 30.00 | 24.94 | 16.86 |
| 3. | H2O2 degradation | 30.00 | 28.01 | 6.63 |
| 4. | Thermal degradation | 30.00 | 29.77 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foudah, A.I.; Alshehri, S.; Shakeel, F.; Alqarni, M.H.; Aljarba, T.M.; Alam, P. Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design. Molecules 2022, 27, 4209. https://doi.org/10.3390/molecules27134209
Foudah AI, Alshehri S, Shakeel F, Alqarni MH, Aljarba TM, Alam P. Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design. Molecules. 2022; 27(13):4209. https://doi.org/10.3390/molecules27134209
Chicago/Turabian StyleFoudah, Ahmed I., Sultan Alshehri, Faiyaz Shakeel, Mohammed H. Alqarni, Tariq M. Aljarba, and Prawez Alam. 2022. "Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design" Molecules 27, no. 13: 4209. https://doi.org/10.3390/molecules27134209
APA StyleFoudah, A. I., Alshehri, S., Shakeel, F., Alqarni, M. H., Aljarba, T. M., & Alam, P. (2022). Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design. Molecules, 27(13), 4209. https://doi.org/10.3390/molecules27134209








