Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
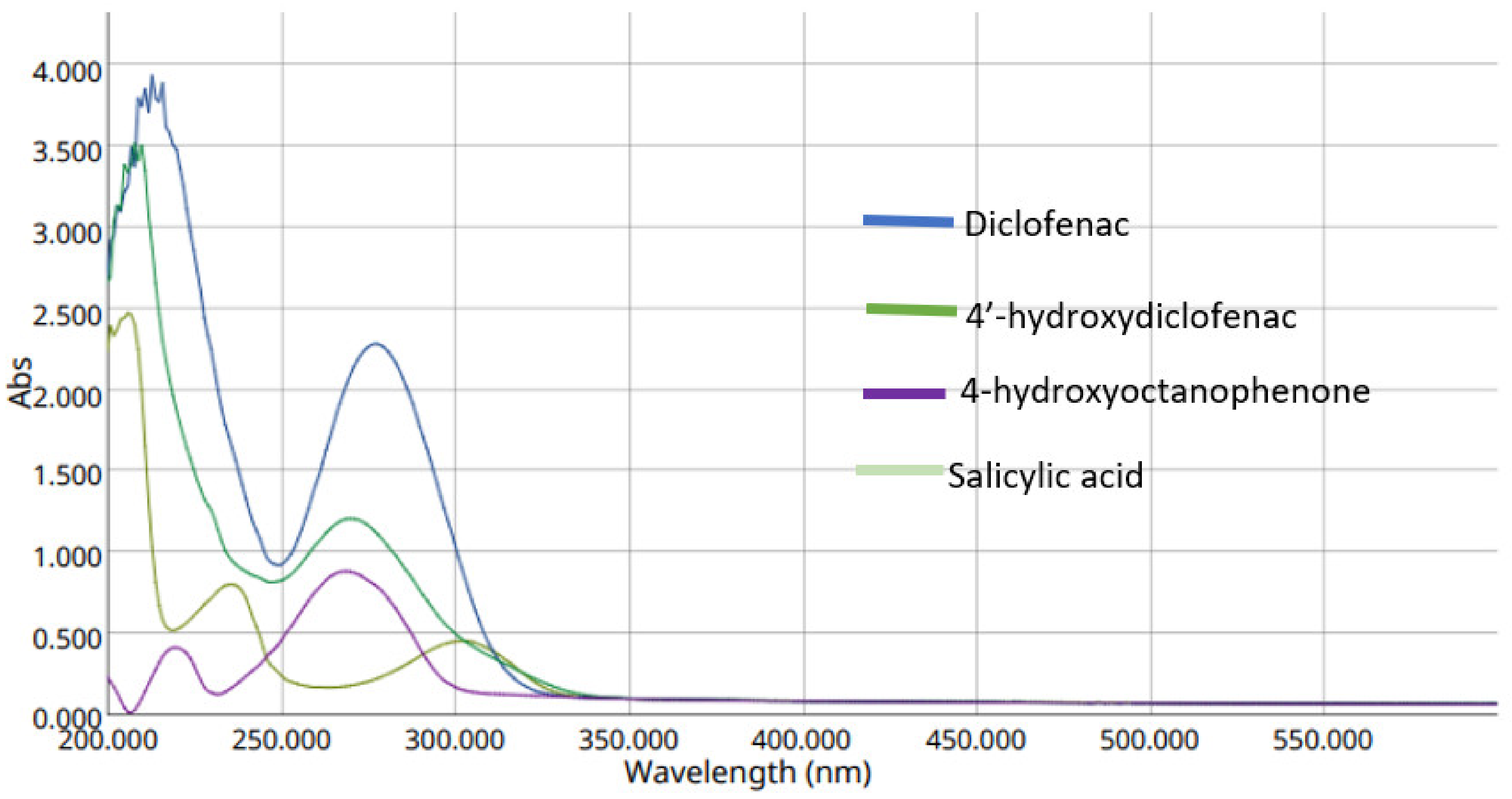
2.1.1. UV–VIS (Ultraviolet-Visible) Spectroscopy
2.1.2. Robustness Test
Variation in Column Temperature
Variation in Flow Rate
2.2. Method Validation
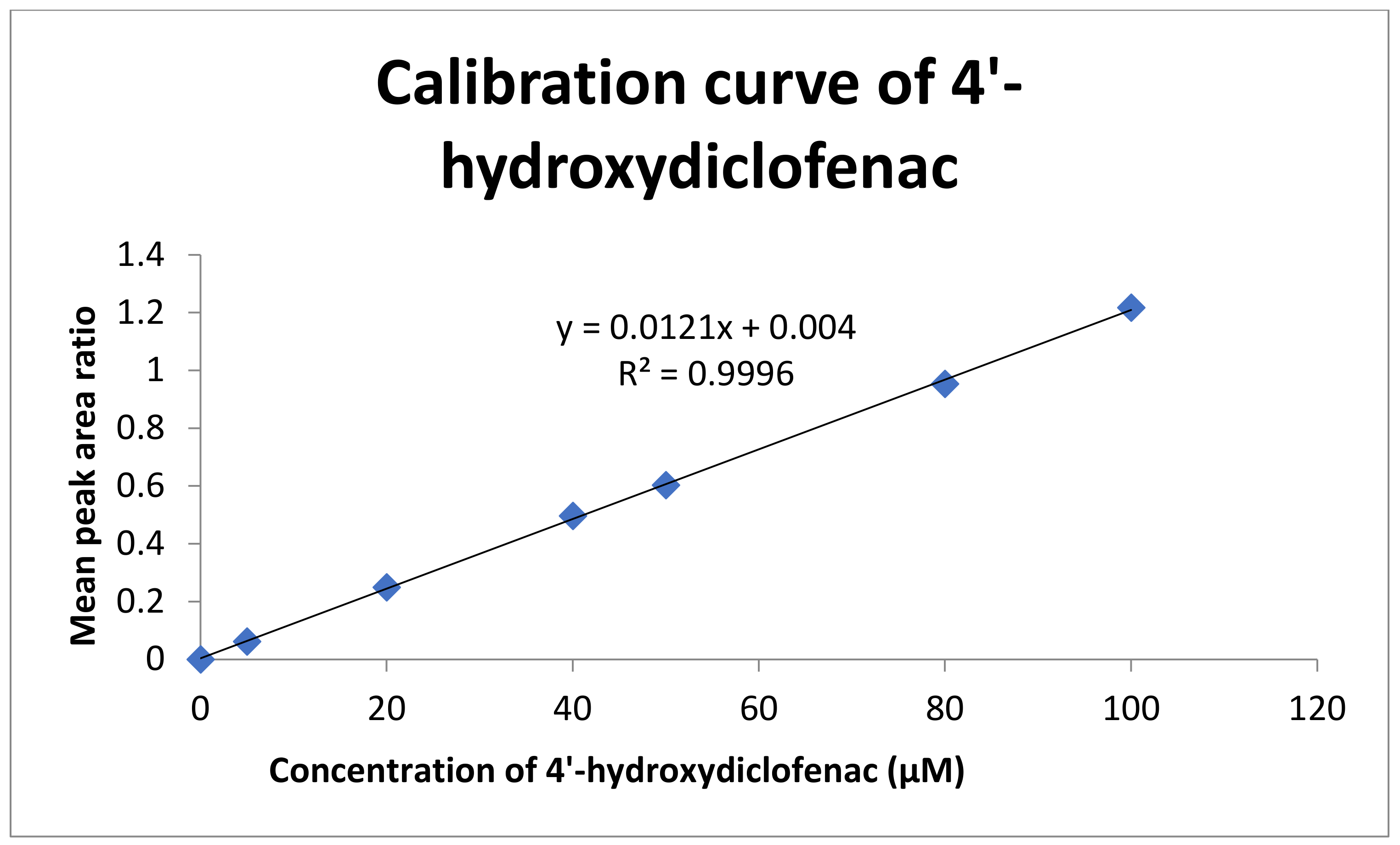
2.2.1. Linearity and Range
2.2.2. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
2.2.3. Precision
Intra-Assay Variation of 4′-Hydroxydiclofenac in Rat Liver Microsomes
Inter-Assay Variation of 4′-Hydroxydiclofenac in Rat Liver Microsomes
2.2.4. Specificity and Selectivity
2.2.5. Chromatography Fundamentals Calculations for CYP2C9 Components
2.2.6. Stability Test
Stability Test of 4′-hydroxydiclofenac in Rat Liver Microsomes
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Analytical Wavelength Selection
3.4. High Performance Liquid Chromatography (HPLC) Analysis
3.4.1. Rat Liver Microsomes Sample Preparation
3.4.2. Preparation of Salicylic Acid, Diclofenac, 4-Hydroxyoctanophenone Stock and Standard Solutions in Rat Liver Microsomes
3.4.3. Preparation of 4′-Hydroxydiclofenac Stock and Standard Solutions in Rat Liver Microsomes
3.4.4. Liquid Chromatography (HPLC) Conditions
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Dalvie, D.K. Cytochrome P450 and Non-Cytochrome P450 Oxidative Metabolism: Contributions to the Pharmacokinetics, Safety, and Efficacy of Xenobiotics. Drug Metab. Dispos. 2016, 44, 1229–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flockhart, D.A.; Tanus-Santos, J.E. Implications of cytochrome P450 interactions when prescribing medication for hypertension. Arch. Intern. Med. 2002, 162, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogu, C.C.; Maxa, J.L. Drug Interactions Due to Cytochrome P450. Bayl. Univ. Med. Cent. Proc. 2000, 13, 421–423. [Google Scholar] [CrossRef]
- Miners, J.O.; Birkett, D.J. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 1998, 45, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Kirchheiner, J.; Meineke, I.; Steinbach, N.; Meisel, C.; Roots, I.; Brockmöller, J. Pharmacokinetics of diclofenac and inhibition of cyclooxygenases 1 and 2: No relationship to the CYP2C9 genetic polymorphism in humans. Br. J. Clin. Pharmacol. 2003, 55, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Gunes, A.; Bilir, E.; Zengil, H.; Babaoğlu, M.O.; Bozkurt, A.; Yasar, U.; Bozkurt, A. Inhibitory Effect of Valproic Acid on Cytochrome P450 2C9 Activity in Epilepsy Patients. Basic Clin. Pharmacol. Toxicol. 2007, 100, 383–386. [Google Scholar] [CrossRef]
- Ismail, S.; Mansor, S.; Hanapi, N. Inhibitory effect of mitragynine on human cytochrome P450 enzyme activities. Pharmacogn. Res. 2013, 5, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Lansdorp, D.; Janssen, T.; Guelen, P.; Vree, T. High-performance liquid chromatographic method for the determination of diclofenac and its hydroxy metabolites in human plasma and urine. J. Chromatogr. B Biomed. Sci. Appl. 1990, 528, 487–494. [Google Scholar] [CrossRef]
- Usman, M.; Zhen-Han, Z.; Ze-Na, C.; Jun-Ping, H.; Wen, Q.; Chang-Qing, Y.; Miyu, N.; Toshiyuki, S. Effect of iguratimod on diclofenac metabolism by CYP2C9 in rats and human recombinant CYP2C9 yeast cells. Braz. J. Pharm. Sci. 2019, 55, e17240. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhang, J.-X.; Zhang, X.-Q.; Qi, M.-J.; Shi, M.-Z.; Yang, J.; Zhang, K.-Z.; Guo, C.; Han, Y.-L. Effects of diosmetin on nine cytochrome P450 isoforms, UGTs and three drug transporters in vitro. Toxicol. Appl. Pharmacol. 2017, 334, 1–7. [Google Scholar] [CrossRef]
- Shi, X.; Yang, S.; Zhang, G.; Song, Y.; Su, D.; Liu, Y.; Guo, F.; Shan, L.; Cai, J. The different metabolism of morusin in various species and its potent inhibition against UDP-glucuronosyltransferase (UGT) and cytochrome p450 (CYP450) enzymes. Xenobiotica 2015, 46, 467–476. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Wu, H.; Xu, Y.; Zhang, L.; Su, K.; Cui, Y.; Wang, H. In vitro inhibitory effect of lysionotin on the activity of cytochrome P450 enzymes. Pharm. Biol. 2020, 58, 695–700. [Google Scholar] [CrossRef]
- Dachineni, R.; Kumar, D.R.; Callegari, E.; Kesharwani, S.S.; Sankaranarayanan, R.; Seefeldt, T.; Tummala, H.; Bhat, G.J. Salicylic acid metabolites and derivatives inhibit CDK activity: Novel insights into aspirin’s chemopreventive effects against colorectal cancer. Int. J. Oncol. 2017, 51, 1661–1673. [Google Scholar] [CrossRef] [Green Version]
- Voruganti, D.; Bassareo, P.P.; Calcaterra, G.; Mehta, J.L. Does aspirin save lives in patients with COVID-19? Heart 2021, 108, 88–89. [Google Scholar] [CrossRef]
- Salhab, H.; Naughton, D.P.; Barker, J. Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme. Molecules 2019, 24, 4294. [Google Scholar] [CrossRef] [Green Version]
- Salhab, H.; Naughton, D.P.; Barker, J. Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2E1 Enzyme Activity: Inhibitory Effect of Cytochrome P450 Enzyme CYP2E1 by Salicylic Acid in Rat Liver Microsomes. Molecules 2020, 25, 932. [Google Scholar] [CrossRef] [Green Version]
- Salhab, H.; Naughton, D.P.; Barker, J. Potential assessment of UGT2B17 inhibition by salicylic acid in human supersomes in vitro. Molecules 2021, 26, 4410. [Google Scholar] [CrossRef]
- Kaphalia, L.; Kaphalia, B.S.; Kumar, S.; Kanz, M.F.; Treinen-Moslen, M. Efficient high performance liquid chromatograph/ultraviolet method for determination of diclofenac and 4′-hydroxydiclofenac in rat serum. J. Chromatogr. B 2006, 830, 231–237. [Google Scholar] [CrossRef]
- Payán, M.R.; López, M.B.; Fernández-Torres, R.; Bernal, J.L.P.; Mochón, M.C. HPLC determination of ibuprofen, diclofenac and salicylic acid using hollow fiber-based liquid phase microextraction (HF-LPME). Anal. Chim. Acta 2009, 653, 184–190. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kwon, J.-H.; Yoon, S.-H. An Improved Synthesis of 4′-Hydroxydiclofenac. Bull. Korean Chem. Soc. 2010, 31, 3007–3009. [Google Scholar] [CrossRef] [Green Version]
- Van Midwoud, P.M.; Janssen, J.; Merema, M.T.; de Graaf, I.A.M.; Groothuis, G.M.M.; Verpoorte, E. On-line HPLC Analysis System for Metabolism and Inhibition Studies in Precision-Cut Liver Slices. Anal. Chem. 2010, 83, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Barker, J.; Naughton, D.P.; Barton, S.J.; Ashraf, S.S. Determination of diclofenac concentrations in human plasma using a sensitive gas chromatography mass spectrometry method. Chem. Cent. J. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higton, D.; Palmer, M.E.; Vissers, J.P.C.; Mullin, L.G.; Plumb, R.S.; Wilson, I.D. Use of Cyclic Ion Mobility Spectrometry (cIM)-Mass Spectrometry to Study the Intramolecular Transacylation of Diclofenac Acyl Glucuronide. Anal. Chem. 2021, 93, 7413–7421. [Google Scholar] [CrossRef]
- Yilmaz, B.; Asci, A.; Palabiyik, S.S. HPLC method for determination of diclofenac in human plasma and its application to a pharmacokinetic study in Turkey. J. Chromatogr. Sci. 2011, 49, 422–427. [Google Scholar] [CrossRef] [Green Version]
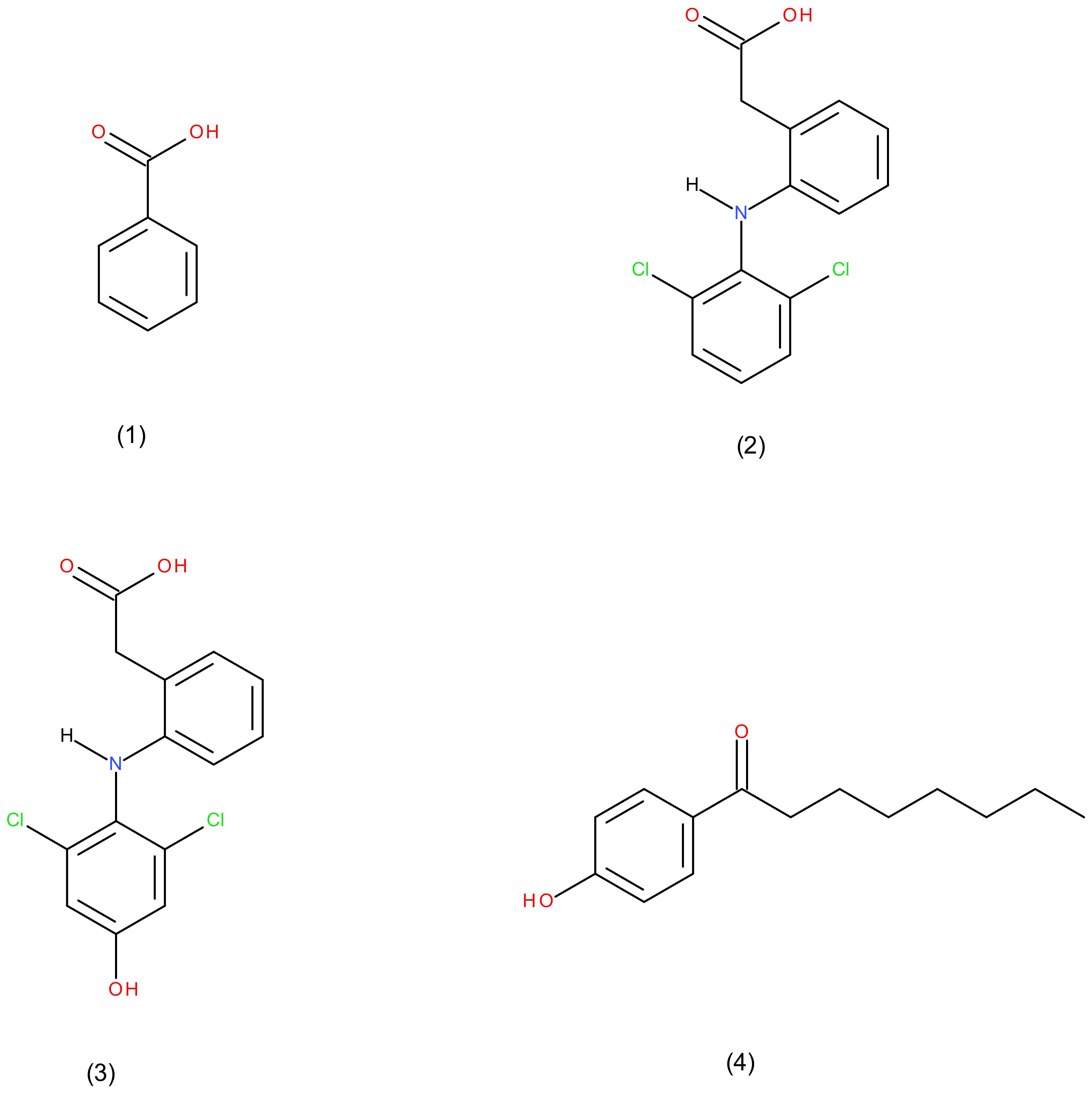


| Analytical Parameters | Analytes in Sample | Average Retention Times (min) | Average Area Peak (Mean ± RSD) | Comment on Resolution |
|---|---|---|---|---|
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), T = 30 °C | Salicylic acid (100 µM) | 3.982 | 44,815.67 ± 0.3081 | The resolution between 4′-hydroxydiclofenac and Diclofenac is 2.701 min (better resolution). |
| 4-hydroxyoctanophenone (50 µM) | 10.094 | 203,645.70 ± 0.1387 | ||
| Diclofenac (200 µM) | 9.374 | 627,869.70 ± 0.0813 | ||
| 4′-hydroxydiclofenac (100 µM) | 6.673 | 235,636.30 ± 0.1327 | ||
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), T = 25 °C | Salicylic acid (100 µM) | 4.093 | 45,668.67 ± 0.0599 | The resolution between 4′-hydroxydiclofenac and Diclofenac is 2.446 min (good resolution). |
| 4-hydroxyoctanophenone (50 µM) | 10.200 | 205,369.30 ± 0.0884 | ||
| Diclofenac (200 µM) | 9.478 | 633,525.00 ± 0.0322 | ||
| 4′-hydroxydiclofenac (100 µM) | 7.032 | 233,145.30 ± 0.0952 |
| Analytical Parameters | Analytes in Sample | Average Retention Times (min) | Average Area Peak (Mean ± RSD) | Comment on Resolution |
|---|---|---|---|---|
| Gradient elution (50% H2O + 15% MeOH+ 35% ACN), T = 30 °C | Salicylic acid (100 µM) | 3.982 | 44,815.67 ± 0.3081 | The resolution between 4′-hydroxydiclofenac and salicylic acid is 2.691 min (better resolution). |
| 4-hydroxyoctanophenone (50 µM) | 10.094 | 203,645.70 ± 0.1387 | ||
| Diclofenac (200 µM) | 9.374 | 627,869.70 ± 0.0813 | ||
| 4′-hydroxydiclofenac (100 µM) | 6.673 | 235,636.30 ± 0.1327 | ||
| Gradient elution (50% H2O + 15% MeOH + 35% ACN), T = 35 °C | Salicylic acid (100 µM) | 3.918 | 44,525.00 ± 0.5865 | The resolution between 4′-hydroxydiclofenac and salicylic acid is 2.447 min (good resolution). |
| 4-hydroxyoctanophenone (50 µM) | 9.975 | 205,037.00 ± 0.2982 | ||
| Diclofenac (200 µM) | 9.245 | 626,595.30 ± 0.0729 | ||
| 4′-hydroxydiclofenac (100 µM) | 6.365 | 232,149.00 ± 0.9973 |
| Analytes in Sample | Average Retention Times (min) | Average Area Peak (Mean ± RSD) | Comment on Resolution | |
|---|---|---|---|---|
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), flow rate = 1.00 mL/min | Salicylic acid (100 µM) | 3.982 | 44,815.67 ± 0.3081 | The resolution between 4-hydroxyoctanophenone and Diclofenac is 0.720 min (better resolution). |
| 4-hydroxyoctanophenone (50 µM) | 10.094 | 203,645.70 ± 0.1387 | ||
| Diclofenac (200 µM) | 9.374 | 627,869.70 ± 0.0813 | ||
| 4′-hydroxydiclofenac (100 µM) | 6.673 | 235,636.30 ± 0.1327 | ||
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), flow rate = 0.8 mL/min | Salicylic acid (100 µM) | 5.017 | 53,803.50 ± 0.9971 | The resolution between 4-hydroxyoctanophenone and Diclofenac is 0.709 min (good resolution). |
| 4-hydroxyoctanophenone (50 µM) | 11.213 | 250,260.50 ± 0.9400 | ||
| Diclofenac (200 µM) | 10.504 | 779,628.00 ± 0.8101 | ||
| 4′-hydroxydiclofenac (100 µM) | 8.193 | 289,811 ± 1.0234 |
| Analytes in Sample | Average Retention Times (min) | Average Area Peak (Mean ± RSD) | Comment on Resolution | |
|---|---|---|---|---|
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), flow rate = 1.00 mL/min | Salicylic acid (100 µM) | 3.982 | 44,815.67 ± 0.3081 | The resolution between 4′-hydroxydiclofenac and salicylic acid is 2.691 min (better resolution). |
| 4-hydroxyoctanophenone (50 µM) | 10.094 | 203,645.70 ± 0.1387 | ||
| Diclofenac (200 µM) | 9.374 | 627,869.70 ± 0.0813 | ||
| 4′-hydroxydiclofenac (100 µM) | 6.673 | 235,636.30 ± 0.1327 | ||
| Gradient elution (50% H2O+ 15% MeOH+ 35% ACN), flow rate = 1.2 mL/min | Salicylic acid (100 µM) | 3.338 | 36,188.67 ± 0.2316 | The resolution between 4′-hydroxydiclofenac and salicylic acid is 2.232 min (good resolution). |
| 4-hydroxyoctanophenone (50 µM) | 9.256 | 171,764.30 ± 0.1622 | ||
| Diclofenac (200 µM) | 8.517 | 521,222.00 ± 0.0992 | ||
| 4′-hydroxydiclofenac (100 µM) | 5.570 | 191,386.00 ± 1.0291 |
| CYP2C9 Metabolite | 4′-Hydroxydiclofenac |
|---|---|
| Limit of Detection (LOD) | 1.54 |
| Limit of Quantitation (LOQ) | 4.67 |
| 4′-Hydroxydiclofenac Standard (μΜ) | Calculated Concentration (μM) | Standard Deviation | % Relative Standard Deviation (RSD) | Matrix Effect (%) |
|---|---|---|---|---|
| Low-activity standard (C = 10 μM) | 10.0949 | 0.0447 | 0.4430 | 0.94 |
| Medium-activity standard (C = 30 μM) | 30.8203 | 0.1147 | 0.1145 | 2.73 |
| High-activity standard (C = 60 μΜ) | 57.8351 | 0.0587 | 0.1016 | 3.61 |
| 4′-Hydroxydiclofenac Standard (μΜ) | Mean Area Peak (n = 3 Each Level) | Calculated Concentration (μM) | Standard Deviation | % Relative Standard Deviation (RSD | Matrix Effect (%) | |
|---|---|---|---|---|---|---|
| Low-activity standard (C = 10μM) | Day 1 | 0.1358 | 10.2351 | 0.5348 | 5.2254 | 2.35 |
| Day 2 | 0.1369 | |||||
| Day 3 | 0.1185 | |||||
| Medium-activity standard (C = 30 μM) | Day 1 | 0.4172 | 30.3758 | 1.0015 | 3.2970 | 1.25 |
| Day 2 | 0.3857 | |||||
| Day 3 | 0.3446 | |||||
| High-activity standard (C = 60 μΜ) | Day 1 | 0.7854 | 58.7187 | 0.9750 | 1.6605 | 2.14 |
| Day 2 | 0.7246 | |||||
| Day 3 | 0.7000 | |||||
| Analytical Components | Retention Time (Rt) | Width at Half Peak Height (W0.5) | Column Efficiency (N) | Asymmetry Factor (AsF) | Plate Height (H) (cm) | Resolution (Rs) |
|---|---|---|---|---|---|---|
| Salicylic acid | 4.00 | 0.18 | 127 | 1.47 | 0.196 | 6.63 7.48 3.30 |
| 4-hydroxydiclofenac | 6.67 | 0.29 | 129 | 1.10 | 0.194 | |
| Diclofenac | 9.34 | 0.12 | 431 | 1.40 | 0.058 | |
| 4′-hydroxyoctanophenone | 10.07 | 0.12 | 457 | 1.36 | 0.055 |
| Actual Concentration of 4′-Hydroxydiclofenac (µM) | 10 | 30 | 60 | |
|---|---|---|---|---|
| Calculated concentration (µM) | 0 h | 9.3721 | 30.4148 | 59.5072 |
| 24 h | 10.4285 | 33.2619 | 64.2936 | |
| 48 h | 11.2703 | 33.5320 | 63.3625 | |
| % Recovery a | 24 h | 111.2717 | 109.3609 | 108.0433 |
| 48 h | 120.0000 | 110.2489 | 106.4787 | |
| % Accuracy b | 0 h | 106.2790 | 101.3826 | 100.8213 |
| 24 h | 95.7150 | 89.1270 | 92.8440 | |
| 48 h | 87.2970 | 88.2266 | 94.3958 |
| Time (min) | % H2O (0.1% Formic Acid) | % Methanol | % Acetonitrile |
|---|---|---|---|
| 0–3 | 50 | 15 | 35 |
| 3–4 | 45 | 27.5 | 27.5 |
| 4–5 | 35 | 32.5 | 32.5 |
| 5–7 | 10 | 45 | 45 |
| 7–9 | 5 | 47.5 | 47.5 |
| 9–10 | 20 | 40 | 40 |
| 10–10.5 | 50 | 15 | 35 |
| 10.5–13 | 50 | 15 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhab, H.; Barker, J. Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes. Molecules 2022, 27, 3587. https://doi.org/10.3390/molecules27113587
Salhab H, Barker J. Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes. Molecules. 2022; 27(11):3587. https://doi.org/10.3390/molecules27113587
Chicago/Turabian StyleSalhab, Hassan, and James Barker. 2022. "Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes" Molecules 27, no. 11: 3587. https://doi.org/10.3390/molecules27113587
APA StyleSalhab, H., & Barker, J. (2022). Development and Validation of an HPLC-UV Method for the Quantification of 4′-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug–Drug Interaction in Rat Liver Microsomes. Molecules, 27(11), 3587. https://doi.org/10.3390/molecules27113587







