Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate
Abstract
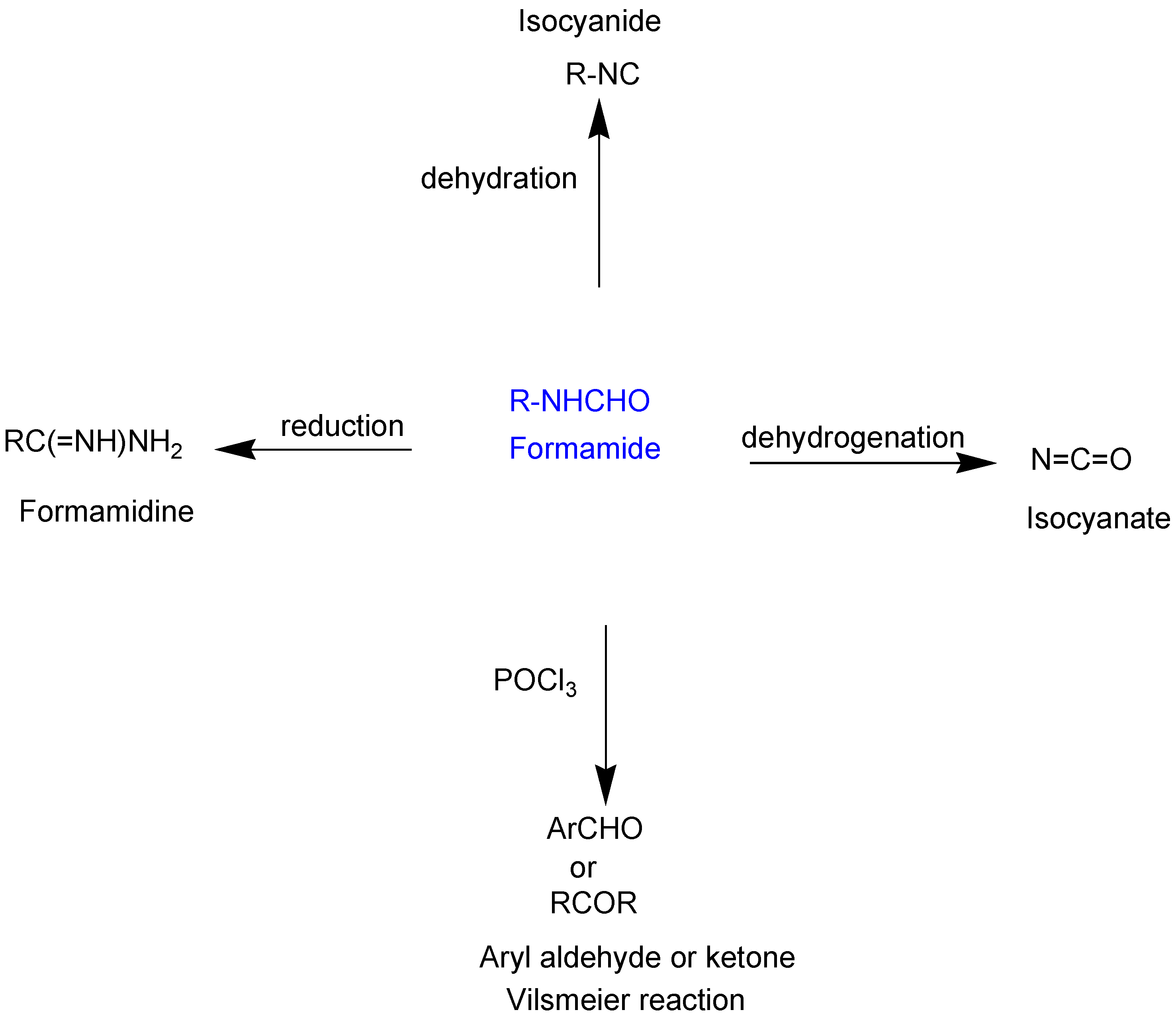
1. Introduction
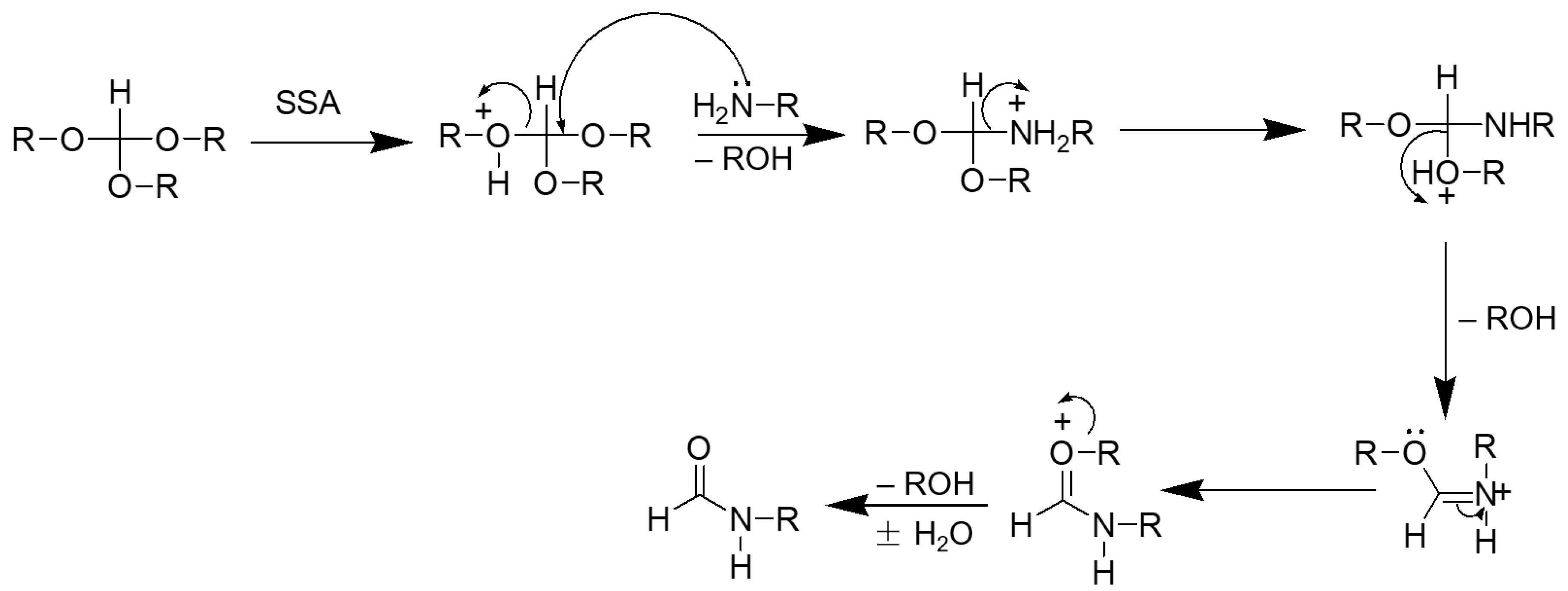
2. Results and Discussion
3. Reusability of Catalyst
4. Materials and Methods
4.1. Preparation of Sulfuric Acid Adsorbed on Silica Gel (H2SO4–SiO2)
4.2. A General Procedure for N-Formylation of Amines with Triethyl Orthoformate Promoted by Immobilized H2SO4 on Silica Gel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Habibi, D.; Sahebekhtiari, H.; Nasrollahzadeh, M.; Taghipour, A. A Very Simple, Highly Efficient and Catalyst-free Procedure for the N-Formylation of Amines Using Triethyl orthoformate in Water Under Ultrasound-irradiation. Lett. Org. Chem. 2013, 10, 209–212. [Google Scholar] [CrossRef]
- Kaboudin, B.; Khodamorady, M. Organic reactions in water: A practical and convenient method for the N-formylation of amines in water. Synlett 2010, 19, 2905–2907. [Google Scholar] [CrossRef]
- Khatri, C.K.; Chaturbhuj, G.U. Sulfated polyborate-catalyzed N-formylation of amines: A rapid, green and efficient protocol. J. Iran. Chem. Soc. 2017, 14, 2513–2519. [Google Scholar] [CrossRef]
- Han, Y.; Cai, L. An efficient and convenient synthesis of formamidines. Tetrahedron Lett. 1997, 38, 5423–5426. [Google Scholar] [CrossRef]
- Gould-Fogerite, S.; Mannino, R.J. Protein or peptide-cochleate vaccines and methods of immunizing using the same. U.S. Patent 5,643,574, 1 July 1997. [Google Scholar]
- Pravin, P.; Maryam, A.M.; Alexander, D. Isocyanide 2.0. Green Chem. 2020, 22, 6902–6911. [Google Scholar] [CrossRef]
- Roberts, R.M.; Vogt, P.J. Ortho esters, imidic esters and amidines: N-alkylformanilides from alkyl orthoformates and primary aromatic amines; Rearrangement of alkyl N-arylformimidates. J. Am. Chem. Soc. 1956, 78, 4778–4782. [Google Scholar] [CrossRef]
- de la Mare, P.B.D. Kinetics of thermal addition of halogens to olefinic compounds. Q. Rev. Chem. Soc. 1949, 3, 126–145. [Google Scholar] [CrossRef]
- Swaringen, R.A.; Eaddy, J.F.; Henderson, T.R. Reaction of Ortho Esters with Secondary Amines. J. Org. Chem. 1980, 45, 3986–3989. [Google Scholar] [CrossRef]
- Blicke, F.F.; Lu, C.-J. Formylation of Amines with Chloral and Reduction of the N-Formyl Derivatives with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1952, 74, 3933–3934. [Google Scholar] [CrossRef]
- Kiho, T.; Yoshida, I.; Katsuragawa, M.; Sakushima, M.; Usui, S.; Ukai, S. Polysaccharides in Fungi. XXXIV. A Polysaccharide from the Fruiting Bodies of Amanita muscaria and the Antitumor Activity of Its Carboxymethylated Product. Biol. Pharm. Bull. 1994, 17, 1460–1462. [Google Scholar] [CrossRef][Green Version]
- Jung, S.H.; Ahn, J.H.; Park, S.K.; Choi, J.K. A practical and convenient procedure for the N-formylation of amines using formic acid. Bull. Korean Chem. Soc. 2002, 23, 149–150. [Google Scholar] [CrossRef]
- Ganapati Reddy, P.; Kishore Kumar, G.D.; Baskaran, S. A convenient method for the N-formylation of secondary amines and anilines using ammonium formate. Tetrahedron Lett. 2000, 41, 9149–9151. [Google Scholar] [CrossRef]
- Rupesh Patre, E.; Sanjib Mal, A.; Pankaj, R.; Nilkanth, R.; Sujit Ghorai, K.; Sudhindra Deshpande, H.; Myriem Qacemi, E.I.; Smejkal, T.; Pal, S.; Manjunath, B.N. First report on bio-catalytic N-formylation of amines using ethyl formate. Chem. Commun. 2017, 53, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.; Danks, T.N.; Wagner, G. Thermal and microwave-assisted N-formylation using solid-supported reagents. Tetrahedron Lett. 2005, 46, 955–957. [Google Scholar] [CrossRef]
- Dhake, K.P.; Tambade, P.J.; Singhal, R.S.; Bhanage, B.M. An efficient, catalyst- and solvent-free N-formylation of aromatic and aliphatic amines. Green Chem. Lett. Rev. 2011, 4, 151–157. [Google Scholar] [CrossRef]
- Das, B.; Krishnaiah, M.; Balasubramanyam, P.; Veeranjaneyulu, B.; Nandan Kumar, D. A remarkably simple N-formylation of anilines using polyethylene glycol. Tetrahedron Lett. 2008, 49, 2225–2227. [Google Scholar] [CrossRef]
- Noh, H.W.; An, Y.; Lee, S.; Jung, J.; Son, S.U.; Jang, H.Y. Metal-free Carbon Monoxide (CO) Capture and Utilization: Formylation of Amines. Adv. Synth. Catal. 2019, 361, 3068–3073. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Zhao, X.; Wang, Z.; Ding, K. Highly efficient ruthenium-catalyzed N-formylation of amines with H2 and CO2. Angew. Chem. Int. Ed. 2015, 54, 186–6189. [Google Scholar] [CrossRef]
- Rasheed, S.; Rao, D.N.; Reddy, A.S.; Shankar, R.; Das, P. Sulphuric acid immobilized on silica gel (H2SO4-SiO2) as an eco-friendly catalyst for transamidation. RSC Adv. 2015, 5, 10567–10574. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Abdollahi, M. The immobilized NaHSO4·H2O on activated charcoal: A highly efficient promoter system for N-formylation of amines with ethyl formate. Curr. Chem. Lett. 2016, 5, 51–58. [Google Scholar] [CrossRef]
- Das, V.K.; Devi, R.R.; Raul, P.K.; Thakur, A.J. Nano rod-shaped and reusable basic Al2O3 catalyst for N-formylation of amines under solvent-free conditions: A novel, practical and convenient NOSE’ approach. Green Chem. 2012, 14, 847–854. [Google Scholar] [CrossRef]
- Madthukur Bhojegowd, M.R.; Nizam, A.; Pasha, M.A. Amberlite IR-120: A reusable catalyst for N-formylation of amines with formic acid using microwaves. Cuihua Xuebao/Chin. J. Catal. 2010, 31, 518–520. [Google Scholar] [CrossRef]
- Hosseini-sarvari, M.; Sharghi, H. ZnO as a New Catalyst for N-Formylation of Amines under Solvent-Free Conditions. Tetrahedron 2006, 8, 6652–6654. [Google Scholar]
- Zeynizadeh, B. Catalytic Performance. J. Chem. Soc. Pak. 2017, 39, 1–11. [Google Scholar]
- Nasrollahzadeh, M.; Motahharifar, N.; Sajjadi, M.; Aghbolagh, A.M.; Shokouhimehr, M.; Varma, R.S. Recent advances in N -formylation of amines and nitroarenes using efficient (nano)catalysts in eco-friendly media. Green Chem. 2019, 21, 5144–5167. [Google Scholar] [CrossRef]
- Bahari, S.; Sajadi, S.M. Natrolite zeolite: A natural and reusable catalyst for one-pot synthesis of α-aminophosphonates under solvent-free conditions. Arab. J. Chem. 2012, 10, 700–704. [Google Scholar] [CrossRef]
- Kim, J.G.; Jang, D.O. Indium-catalyzed N-formylation of amines under solvent-free conditions. Synlett 2010, 8, 1231–1234. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Swaminathan, M. A convenient method for the N-formylation of amines at room temperature using TiO2-P25 or sulfated titania. J. Mol. Catal. A Chem. 2011, 334, 98–102. [Google Scholar] [CrossRef]
- Veer, S.D.; Pathare, S.P.; Akamanchi, K.G. Sulfated tungstate catalyzed hydration of alkynes. Ark. 2016, 2016, 59–66. [Google Scholar] [CrossRef]
- Thirunarayanan, G.; Muthuvel, I.; Sathiyendiran, V. Spectral LFER studies in some N-(substituted phenyl) formamides. Ann. Chem. 2015, 70, 31. [Google Scholar] [CrossRef]
- Kim, J.G.; Jang, D.O. Facile and highly efficient N-formylation of amines using a catalytic amount of iodine under solvent-free conditions. Synlett 2010, 14, 2093–2096. [Google Scholar] [CrossRef]
- Lei, M.; Ma, L.; Hu, L. A convenient one-pot synthesis of formamide derivatives using thiamine hydrochloride as a novel catalyst. Tetrahedron Lett. 2010, 51, 4186–4188. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Soleimani, E.; Norouzi, P.; Adnan, R.; Sepahvand, H. Preparation of trifluoroacetic acid-immobilized Fe3O4@SiO2-APTES nanocatalyst for synthesis of quinolines. J. Fluor. Chem. 2015, 178, 219–224. [Google Scholar] [CrossRef]
- Pathare, S.P.; Sawant, R.V.; Akamanchi, K.G. Sulfated tungstate catalyzed highly accelerated N-formylation. Tetrahedron Lett. 2012, 53, 3259–3263. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A.; Salaris, M. A new, simple procedure for the synthesis of formyl amides. Synlett 2004, 14, 2570–2572. [Google Scholar] [CrossRef]
- Deutsch, J.; Eckelt, R.; Köckritz, A.; Martin, A. Catalytic reaction of methyl formate with amines to formamides. Tetrahedron 2009, 65, 10365–10369. [Google Scholar] [CrossRef]
- Baghbanian, S.M.; Farhang, M. Protic [TBD][TFA] ionic liquid as a reusable and highly efficient catalyst for N-formylation of amines using formic acid under solvent-free condition. J. Mol. Liq. 2013, 183, 45–49. [Google Scholar] [CrossRef]
- Chandra Shekhar, A.; Ravi Kumar, A.; Sathaiah, G.; Luke Paul, V.; Sridhar, M.; Shanthan Rao, P. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Hong, M.; Xiao, G. Hafnium(IV) bis(perfluorooctanesulfonyl)imide complex supported on fluorous silica gel catalyzed N-formylation of amines using aqueous formic acid. J. Fluor. Chem. 2013, 146, 11–14. [Google Scholar] [CrossRef]
- Ourida, S.; Mark, J.B.; John, B.; James, L.; Stephen, P.M.; Pawel, P.; Robert, J.W.; Williams, J.M.J. Iridium-catalyzed formylation of amines with paraformaldehyde. Tetrahedron Lett. 2010, 51, 5804–5806. [Google Scholar] [CrossRef]
- Lundberg, H. Group (IV) Metal-Catalyzed Direct Amidation: Synthesis and Mechanistic Considerations. Ph.D. Thesis, University of Stockholm, Stockholm, Sweden, 2015. [Google Scholar]
- Ishida, T.; Haruta, M. N-formylation of amines via the aerobic oxidation of methanol over supported gold nanoparticles. ChemSusChem 2009, 2, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Richter, C.; Glorius, F. N-Formylation of amines by methanol activation. Org. Lett. 2013, 15, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Tumma, H.; Nagaraju, N.; Reddy, K.V. A facile method for the N-formylation of primary and secondary amines by liquid phase oxidation of methanol in the presence of hydrogen peroxide over basic copper hydroxyl salts. J. Mol. Catal. A Chem. 2009, 310, 121–129. [Google Scholar] [CrossRef]
- Han, Y.; Zhikang, W.; Zheyu, W.; Yongyan, Z.; Shi, R.; Qixin, Z.; Jingjing, W.; Sheng, H.; Yongge, W. N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst. Commun. Chem. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Bedi, P.S. Silica supported Brönsted acids as catalyst in organic transformations: A comprehensive review. Cuihua Xuebao/Chinese J. Catal. 2015, 36, 520–549. [Google Scholar] [CrossRef]
- Pramanik, A.; Bhar, S. Silica-sulfuric acid and alumina-sulfuric acid: Versatile supported Brønsted acid catalysts. New J. Chem. 2021, 45, 16355–16388. [Google Scholar] [CrossRef]
- Habibi, D.; Rahmani, P.; Akbaripanah, Z. N-formylation of anilines with silica sulfuric acid under solvent free conditions. J. Org. Chem. 2013, 2013, 268654. [Google Scholar] [CrossRef]
- Hu, D.X.; Grice, P.; Ley, S.V. Rotamers or diastereomers? An overlooked NMR solution. J. Org. Chem. 2012, 77, 5198–5202. [Google Scholar] [CrossRef]
- Lanyon-Hogg, T.; Ritzefeld, M.; Masumoto, N.; Magee, A.I.; Rzepa, H.S.; Tate, E.W. Modulation of Amide Bond Rotamers in 5-Acyl-6,7-dihydrothieno [3,2-c]pyridines. J. Org. Chem. 2015, 80, 4370–4377. [Google Scholar] [CrossRef]
- Habibi, D.; Nasrollahzadeh, M.; Sahebekhtiari, H. Green synthesis of formamides using the Natrolite zeolite as a natural, efficient and recyclable catalyst. J. Mol. Catal. A Chem. 2013, 378, 148–155. [Google Scholar] [CrossRef]
- Ma’mani, L.; Sheykhan, M.; Heydari, A.; Faraji, M.; Yamini, Y. Sulfonic acid supported on hydroxyapatite-encapsulated-γ-Fe2O3 nanocrystallites as a magnetically Brønsted acid for N-formylation of amines. Appl. Catal. A Gen. 2010, 377, 64–69. [Google Scholar] [CrossRef]
- Bose, A.K.; Ganguly, S.N.; Manhas, M.S.; Guha, A.; Pombo-Villars, A. Microwave promoted energy-efficient N-formylation with aqueous formic acid. Tetrahedron Lett. 2006, 47, 4605–4607. [Google Scholar] [CrossRef]
- Lygin, A.V.; De Meijere, A. ortho-Lithiophenyl isocyanide: A versatile precursor for 3H-quinazolin-4-ones and 3H-quinazolin-4-thiones. Org. Lett. 2009, 11, 389–392. [Google Scholar] [CrossRef]
- Landquist, J.K. Synthetic antimalarials. Part XLVI. Some 4-[(dialkylaminoalkyl)amino] quinoline derivatives. J. Chem. Soc. 1951, 10, 1038–1048. [Google Scholar] [CrossRef]
- Kim, J.J.; Park, Y.D.; Cho, S.D.; Kim, H.K.; Chung, H.A.; Lee, S.G.; Falck, J.R.; Yoon, Y.J. Efficient N-arylation of pyridazin-3(2H)-ones. Tetrahedron Lett. 2004, 45, 8781–8784. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
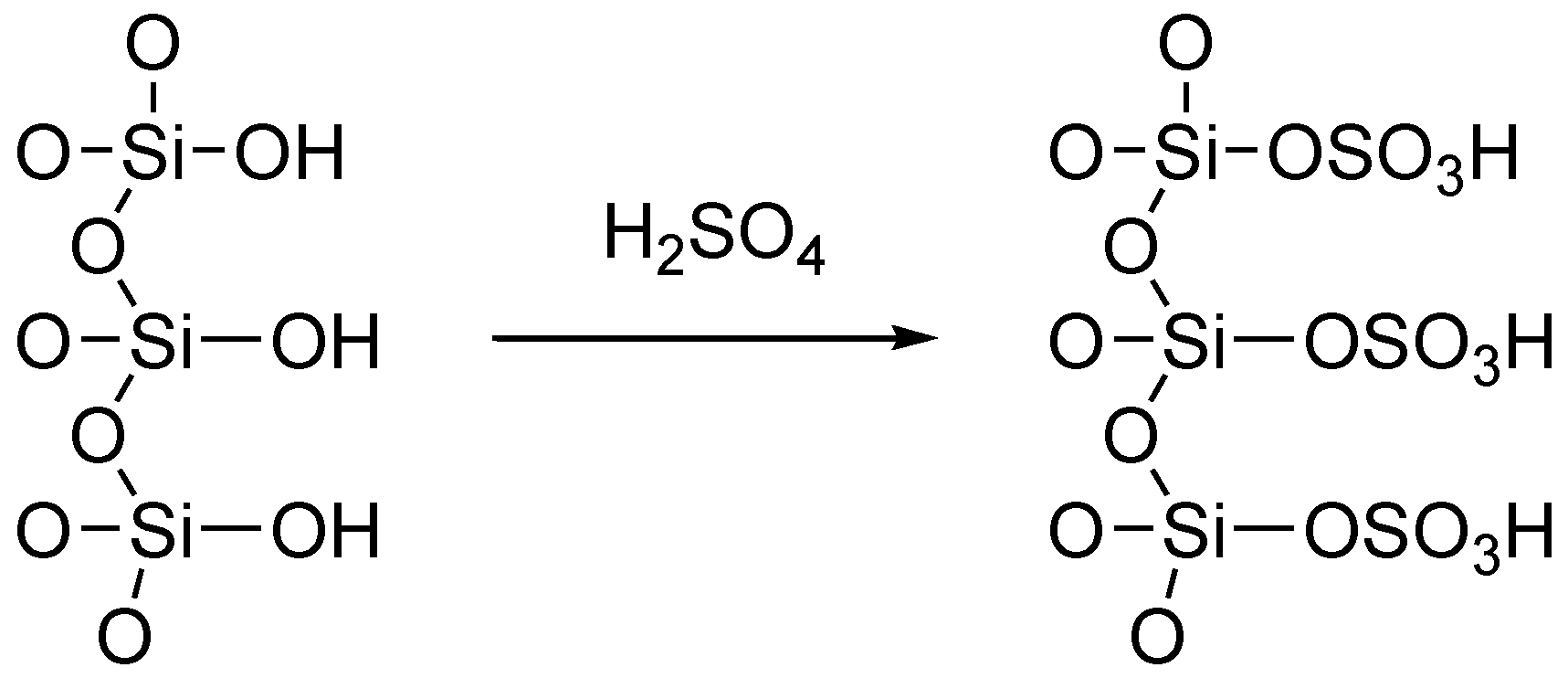
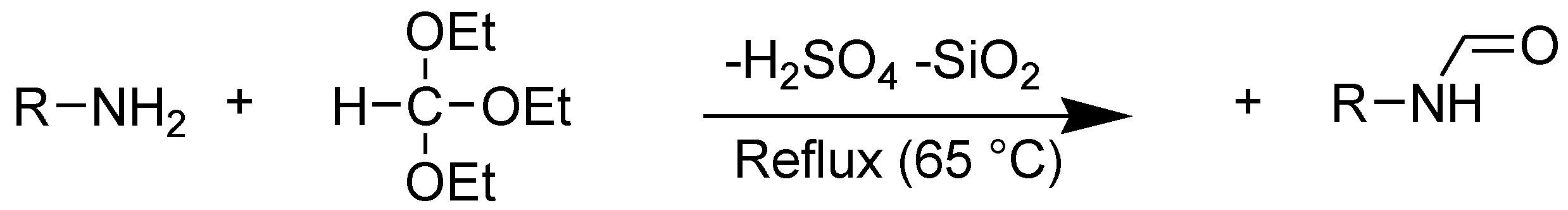
| Entry | Catalyst | Formylating Agent | Reaction Condition | Time | Yield % | Reference |
|---|---|---|---|---|---|---|
| 1 | Sodium formate | Formic acid | Solvent free | >8 h | [31] | |
| 2 | Amberlite IR-120 | Formic acid | Microwave irradiation | 2 min | 90–97 | [23] |
| 3 | Molecular iodine (I2) | Formic acid | Solvent free | 2 h | 60–99 | [32] |
| 4 | Thiamine hydrochloride | Formic acid | Solvent free | 88–96 | [33] | |
| 5 | Fe2O3-Hap-SO3H | Formic acid | Solvent free | 15–60 min | 95–99 | [34] |
| 6 | Sulfated tungstate | Formic acid | Solvent free | 10–45 min | 85–95 | [35] |
| 7 | CDMT II | Formic acid | Microwave irradiation | 3–6 min | 64–94 | [36] |
| 8 | Amidine and Guanidine | Methyl formate | Solvent free | 1–96 h | 65–98 | [37] |
| 9 | TBD-based ionic liquids | Formic acid | Solvent free | 10–35 min | 75–98 | [38] |
| 10 | Indium | Formic acid | Solvent free | 1.5–24 h | 70–98 | [28] |
| 11 | ZnO | Formic acid | Solvent free | 10–720 min | 65–99 | [24] |
| 12 | ZnCl2 | Formic acid | Solvent free | 10–900 min | 60–98 | [39] |
| 13 | TiO2-P25 or TiO2-SO42− | Formic acid | Solvent free | 30–45 min | 40–99 | [29] |
| 14 | FSG-HF(N(SO2C8F11)2)4 | Formic acid | Solvent free | 1–4 h | 60–88 | [40] |
| 15 | Iridium | Paraformaldehyde | Reflux in H2O | 5–10 h | 41–91 | [41] |
| 16 | Silver and gold surfaces | Formaldehyde | Solvent free | 6 h | 75–97 | [42] |
| 17 | Gold nanoparticles (Au/Al2O3 or Au/NiO) | Methanol | Reflux in H2O | 4 h | 72–97 | [43] |
| 18 | Ruthenium N-heterocyclic catalyst (Ru-NHC) | Methanol | Reflux in toluene (125 °C) | 12–24 h | 27–99 | [44] |
| 19 | Copper salt (CuCl2.H2O) | Methanol | Solvent free | 45–90 min | 63–80 | [45] |
| 20 | Ionic liquid catalyzed formylation | CO | Reflux in methanol (140 °C) | 4 h | 42–99 | [18] |
| 21 | Inorganic ligand-supported chromium (III) catalyst (NH4)3[CrMo6O18(OH)6] | Methanol | Reflux in H2O2 (80 °C) | 12 h | 60–99 | [46] |
| 22 | Lipase | Ethyl formate | Reflux in THF at room temperature | 1–8 h | 29–99 | [14] |
| 23 | No catalyst | Triethyl orthoformate in water | Ultrasound irradiation | 3 h | 35–88 | [1] |
| 24 | Catalyst free | Ammonium formate | Solvent free | 5 min–24 h | 43–98 | [13] |
| Entry | Catalyst | Formylation Agent | Reaction Condition | Time | Yield % | Reference |
|---|---|---|---|---|---|---|
| 1 | HClO4−–SiO2 | Formic acid | Solvent free | 15–90 min | 70–96 | [25] |
| 2 | Fe3O4@SiO2–APTES-TFA | 1,3-dicarbonyl compound | Solvent free | n/a | 68–98 | [34] |
| 3 | H2SO4–SiO2 | Formic acid | Solvent free | 4–46 min | 65–99 | [20] |
| 4 | H2SO4–SiO2 | N,N-dimethyl amide | Solvent free | 6–12 h | 75–95 | [25] |
| Entry | Reaction Condition | Time | Yield |
|---|---|---|---|
| 1 | Aniline (1 mmol)/TEOF (1 mmol), SIS (0.2 g) | 10 min | 44% |
| 2 | Aniline (1 mmol)/TEOF (2 mmol), SIS (0.2 g) | 6 min | 66% |
| 3 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.2 g) | 4 min | 96% |
| 4 | Aniline (1 mmol)/TEOF (4 mmol), SIS (0.2 g) | 4 min | 90% |
| Entry | Catalytic Condition | Time | Yield |
|---|---|---|---|
| 1 | Aniline (1 mmol)/TEOF (3 mmol) without catalyst at 65 °C | 3 h | traces |
| 2 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.1 g), 65 °C | 5 min | 78% |
| 3 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.2 g), 65 °C | 4 min | 96% |
| 4 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.3 g), 65 °C | 4 min | 88% |
| 5 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.4 g), 65 °C | 6 min | 71% |
| 6 | Aniline (1 mmol)/TEOF (3 mmol), SIS (0.5 g), 65 °C | 6 min | 64% |
| Entry | Amines | Time (Min) | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 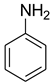 | 4 |  | 96 |
| 2 | 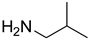 | 4 |  | 81 |
| 3 | 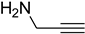 | 4 |  | 78 |
| 4 | 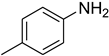 | 9 |  | 95 |
| 5 | 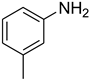 | 4 | 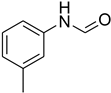 | 90 |
| 6 | 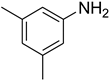 | 4 | 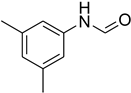 | 97 |
| 7 | 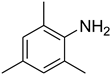 | 10 | 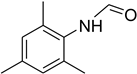 | 83 |
| 8 |  | 10 | 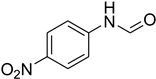 | 97 |
| 9 |  | 10 | 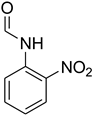 | 90 |
| 10 |  | 10 | 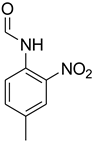 | 96 |
| 11 |  | 15 |  | 90 |
| 12 | 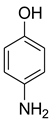 | 13 |  | 75 |
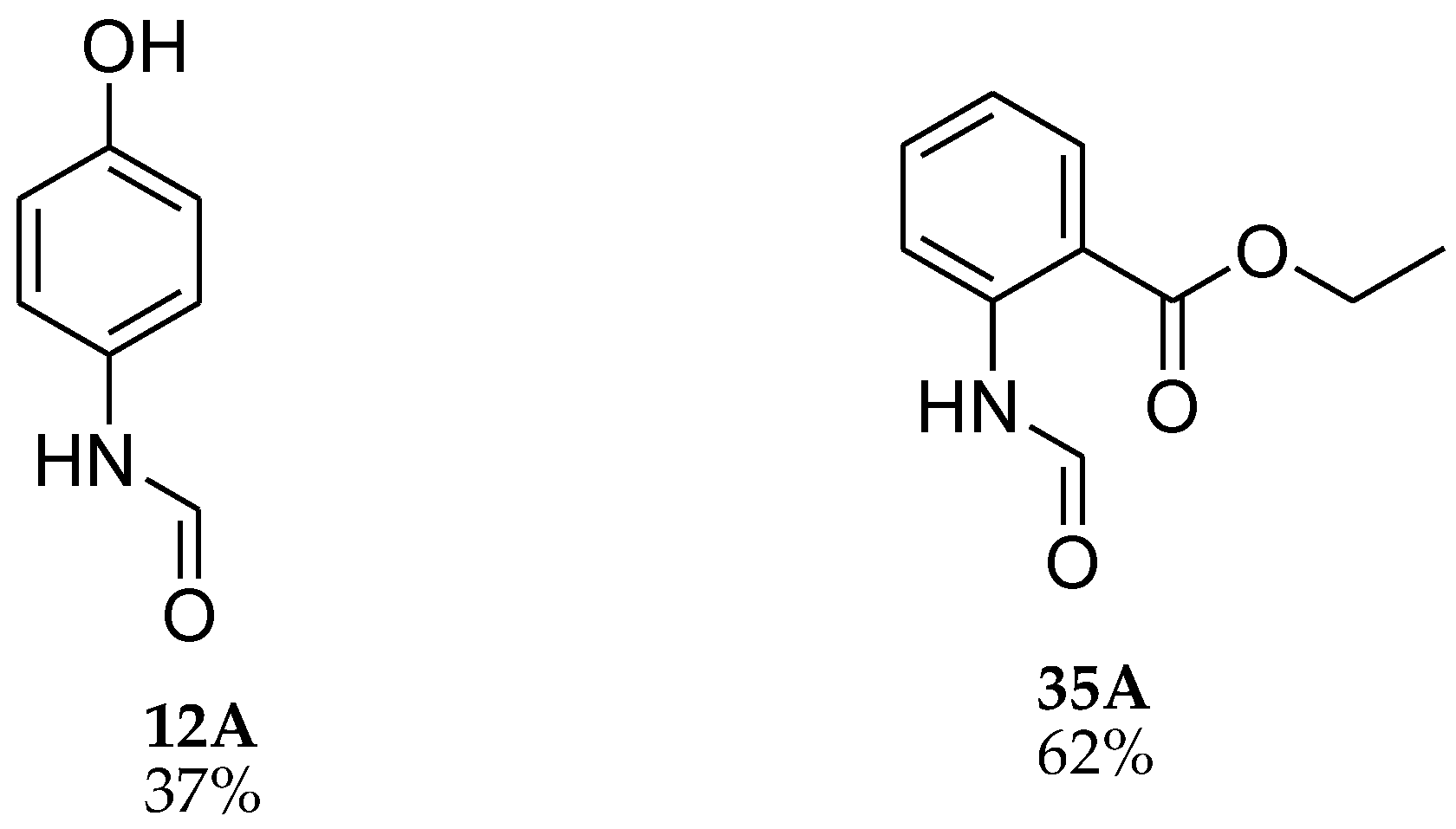
| 13 | 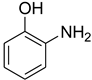 | 13 | 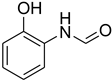 | 81 |
| 14 | 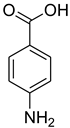 | 5 |  | 86 |
| 15 | 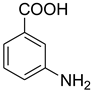 | 5 |  | 94 |
| 16 | 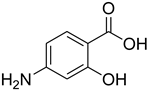 | 20 | 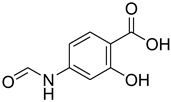 | 75 |
| 17 |  | 12 | 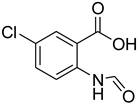 | 73 |
| 18 |  | 20 | 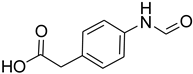 | 85 |
| 19 | 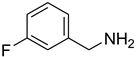 | 6 | 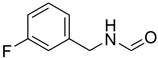 | 97 |
| 20 | 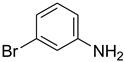 | 6 | 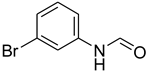 | 78 |
| 21 | 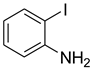 | 5 | 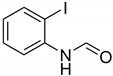 | 94 |
| 22 | 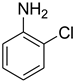 | 6 |  | 78 |
| 23 |  | 6 | 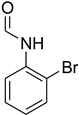 | 84 |
| 24 | 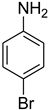 | 5 | 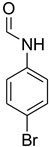 | 81 |
| 25 | 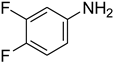 | 10 | 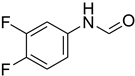 | 56 |
| 26 | 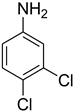 | 10 |  | 81 |
| 27 | 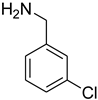 | 12 | 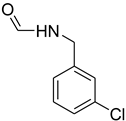 | 82 |
| 28 | 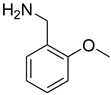 | 15 | 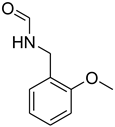 | 85 |
| 29 | 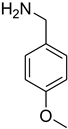 | 15 | 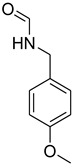 | 96 |
| 30 | 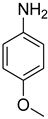 | 8 | 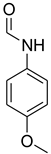 | 93 |
| 31 | 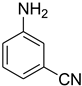 | 6 | 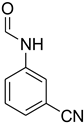 | 94 |
| 32 | 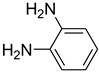 | 20 | 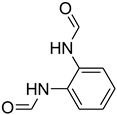 | 96 |
| 33 | 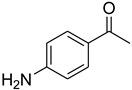 | 18 | 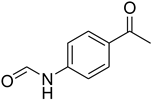 | 95 |
| 34 | 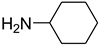 | 5 | 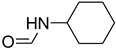 | 86 |
| 35 |  | 12 | 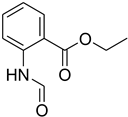 | 93 |
| 36 | 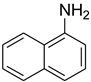 | 12 | 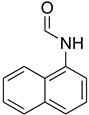 | 98 |
| 37 | 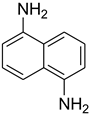 | 15 | 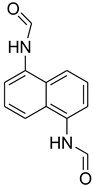 | 80 |
| 38 | 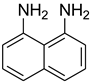 | 20 |  | 91 |
| 39 | 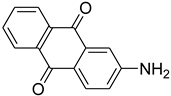 | 24 |  | 93 |
| 40 | 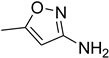 | 15 | 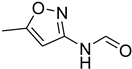 | 95 |
| 41 | 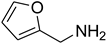 | 13 | 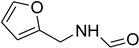 | 92 |
| 42 | 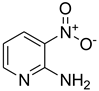 | 25 | 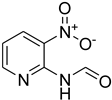 | 77 |
| 43 | 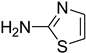 | 30 |  | 67 |
| 44 | 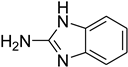 | 54 |  | 76 |
| 45 | 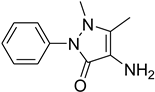 | 45 | 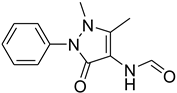 | 79 |
| 46 | 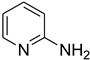 | 45 | 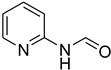 | 71 |
| 47 | 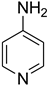 | 60 | 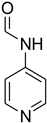 | 94 |
| 48 | 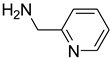 | 50 | 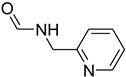 | 94 |
| 49 | 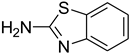 | 40 | 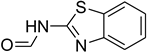 | 87 |
| 50 | 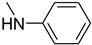 | 40 | 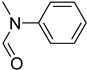 | 78 |
| 51 | 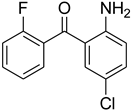 | 50 |  | 73 |
| 52 | 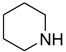 | 40 | 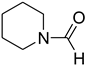 | 85 |
| 53 | 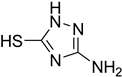 | 40 |  | 75 |
| 54 | 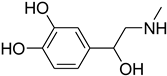 | 60 |  | 85 |
| 55 |  | 35 | 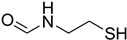 | 93 |
| 56 | 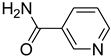 | 60 | 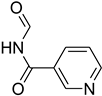 | 93 |
| Entry | Turn | Yield % |
|---|---|---|
| 1 | 1 | 96 |
| 2 | 2 | 89 |
| 3 | 3 | 83 |
| Entry | Conditions | Time | Yield | References |
|---|---|---|---|---|
| 1 | Triethyl orthoformate in H2O under ultrasound irradiation. | 3 h | 88% | [1] |
| 2 | Solid-supported formate, DMSO, 70–80 °C | 4 h | 60% | [15] |
| 3 | SSA, HCOOH, 50–60 °C, solvent-free | 7 min | 99% | [49] |
| 4 | SA on activated charcoal, ethylformate, 54 °C | 4 min | 95% | [21] |
| 5 | Triethyl orthoformate in H2O under neutral condition. Microwave irradiation, 90 °C | 2 h | 87% | [2] |
| 6 | SIS, triethyl orthoformate, 60–65 °C, solvent-free | 3 min | 96% | Present protocol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salami, S.A.; Siwe-Noundou, X.; Krause, R.W.M. Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate. Molecules 2022, 27, 4213. https://doi.org/10.3390/molecules27134213
Salami SA, Siwe-Noundou X, Krause RWM. Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate. Molecules. 2022; 27(13):4213. https://doi.org/10.3390/molecules27134213
Chicago/Turabian StyleSalami, Sodeeq Aderotimi, Xavier Siwe-Noundou, and Rui W. M. Krause. 2022. "Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate" Molecules 27, no. 13: 4213. https://doi.org/10.3390/molecules27134213
APA StyleSalami, S. A., Siwe-Noundou, X., & Krause, R. W. M. (2022). Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate. Molecules, 27(13), 4213. https://doi.org/10.3390/molecules27134213






