Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Bioactive Phytochemicals
2.1.1. Quantification of Bioactive Contents (TPC and TFC)
2.1.2. Characterization of Bioactive Compounds by GCMS Analysis
2.2. Biological Activities
2.2.1. Antioxidant Activities
2.2.2. In Vitro Enzyme Inhibition Activities
2.2.3. Thrombolytic Activity
2.2.4. Antibacterial Activity
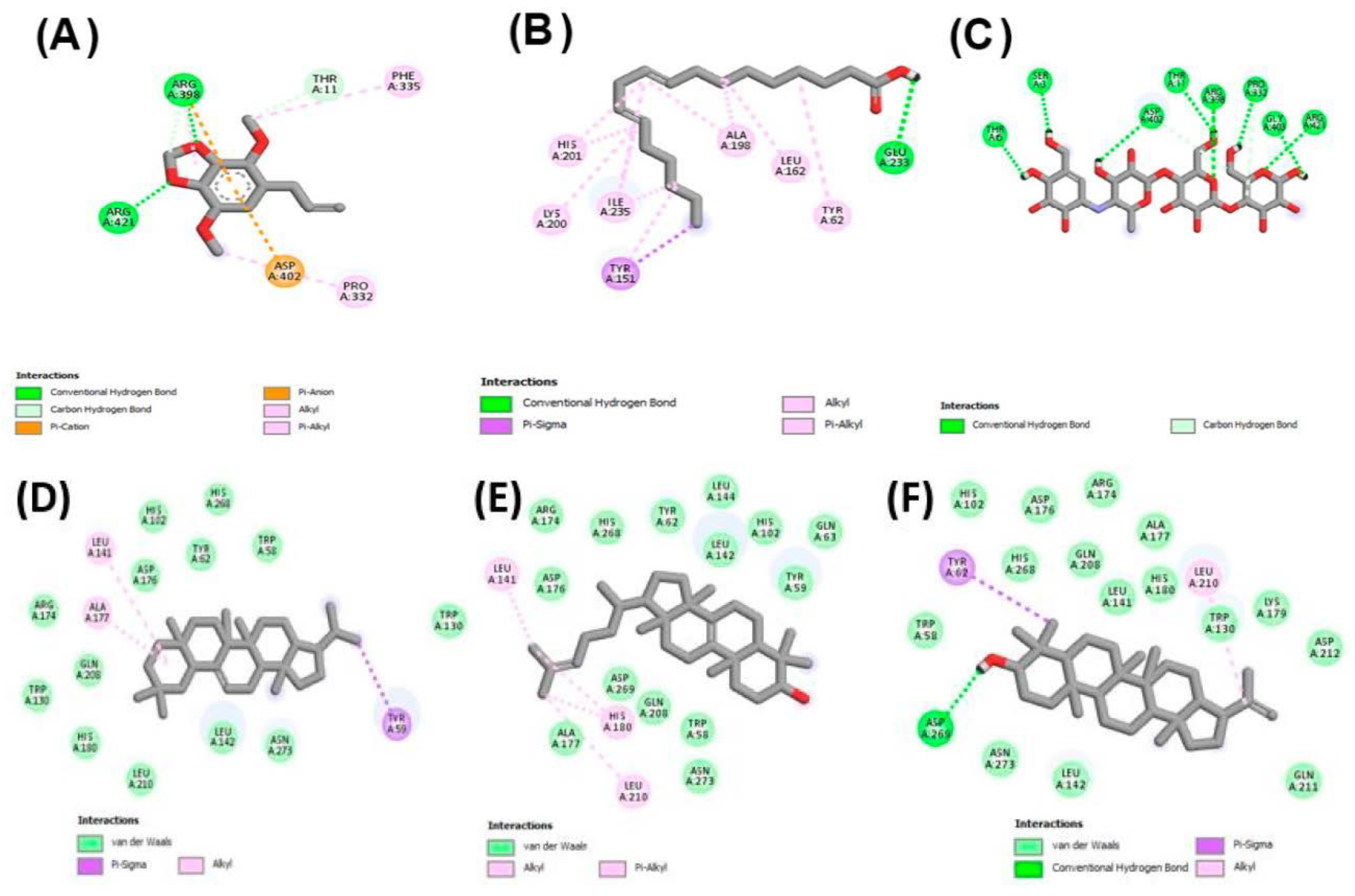
2.3. Molecular Docking Studies
2.3.1. Molecular Docking against Tyrosinase Enzyme
2.3.2. Molecular Docking against α-Amylase, and α-Glucosidase
3. Materials and Methods
3.1. Plant Collection and Authentication
3.2. Extraction and Fractionation
3.3. Determination of Bioactive Phytochemicals
3.3.1. Quantification of Bioactive Contents (TPC and TFC)
3.3.2. Characterization of Bioactive Compounds by GCMS Analysis
3.4. Biological Activities
3.4.1. Antioxidant Activities
3.4.2. In Vitro Enzyme Inhibition Activities
3.4.3. Thrombolytic Activity
3.4.4. Antibacterial Activity
3.5. Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; BT da Rocha, J. Oxidative stress and antioxidant potential of one hundred medicinal plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef] [PubMed]
- Uuh-Narváez, J.J.; González-Tamayo, M.A.; Segura-Campos, M.R. A study on nutritional and functional study properties of Mayan plant foods as a new proposal for type 2 diabetes prevention. Food Chem. 2021, 341, 128247. [Google Scholar] [CrossRef] [PubMed]
- Satil, F.; Selvi, S. Ethnobotanical features of Ziziphora L.(Lamiaceae) taxa in Turkey. Int. J. Nat. Life Sci. 2020, 4, 56–65. [Google Scholar]
- Xie, Y.-G.; Zhao, X.-C.; ul Hassan, S.S.; Zhen, X.-Y.; Muhammad, I.; Yan, S.-K.; Yuan, X.; Li, H.-L.; Jin, H.-Z.J.P.L. One new sesquiterpene and one new iridoid derivative from Valeriana amurensis. Phytochem. Lett. 2019, 32, 6–9. [Google Scholar] [CrossRef]
- ul Hassan, S.S.; Ishaq, M.; Zhang, W.D.; Jin, H.Z. An Overview of the Mechanisms of Marine Fungi-Derived Anti-Inflammatory and Anti-Tumor Agents and their Novel Role in Drug Targeting. Curr. Pharm. Des. 2021, 27, 2605–2614. [Google Scholar] [CrossRef]
- Jideani, A.I.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Veizades, S.; Tso, A.; Nguyen, P.K. Infection, inflammation and thrombosis: A review of potential mechanisms mediating arterial thrombosis associated with influenza and severe acute respiratory syndrome coronavirus 2. Biol. Chem. 2021, 403, 231–241. [Google Scholar] [CrossRef]
- Elegbede, J.; Lateef, A.; Azeez, M.; Asafa, T.; Yekeen, T.; Oladipo, I.; Aina, D.; Beukes, L.; Gueguim-Kana, E.J.W.; Valorization, B. Biofabrication of gold nanoparticles using xylanases through valorization of corncob by Aspergillus niger and Trichoderma longibrachiatum: Antimicrobial, antioxidant, anticoagulant and thrombolytic activities. Waste Biomass Valorization 2020, 11, 781–791. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Sur, P.K. Sanjeevani and Bishalyakarani plants-myth or real! Int. J. Curr. Res. 2015, 7, 19964–19969. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=Ghosh%2C+Swapan+Kr.+%22Pradip+Kr+Sur+Current+Research%2C+7%2C%289%29%2C+19964-19969&btnG= (accessed on 25 May 2022).
- Abbas, Q.; Hussain, A.; Khan, S.W.; Hussain, A.; Shinwari, S.; Hussain, A.; Ullah, A.; Zafar, M.; Ali, K. Floristic Diversity, Ethnobotany and Traditional Recipes of Medicinal Plants of Maruk Nallah, Haramosh Valley, District Gilgit, Gilgit Baltistan: Traditional recipes of Maruk Nallah, Haramosh Valley, District Gilgit. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2019, 56, 97–112. [Google Scholar]
- Abbas, Z.; Khan, S.M.; Abbasi, A.M.; Pieroni, A.; Ullah, Z.; Iqbal, M.; Ahmad, Z. Ethnobotany of the balti community, tormik valley, karakorum range, Baltistan, Pakistan. J. Ethnobiol. Ethnomed. 2016, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M. New ethnomedicinal claims from Gujjar and Bakerwals tribes of Rajouri and Poonch districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Mu, Y.; Atif, M.; Hussain, H.; Li, J.; Li, D.; Shabbir, M.; Bankeu, J.J.K.; Cui, L.; Sajjad, S. Separation and anti-inflammatory evaluation of phytochemical constituents from Pleurospermum candollei (Apiaceae) by high-speed countercurrent chromatography with continuous sample load. J. Sep. Sci. 2021, 44, 2663–2673. [Google Scholar] [CrossRef]
- Khan, K.U.; Shah, M.; Ahmad, H.; Ashraf, M.; Rahman, I.U.; Iqbal, Z.; Khan, S.M.; Majid, A. Investigation of traditional veterinary phytomedicines used in Deosai Plateau, Pakistan. Glob. Vet. 2015, 15, 381–388. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Kawarty, A.M.A.; Behçet, L.; Çakilcioğlu, U. An ethnobotanical survey of medicinal plants in Ballakayati (Erbil, North Iraq). Turk. J. Bot. 2020, 44, 345–357. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Fettach, S.; Mrabti, H.; Sayah, K.; Bouyahya, A.; Salhi, N.; Cherrah, Y.; El Abbes, F.M. Phenolic content, acute toxicity of Ajuga iva extracts and assessment of their antioxidant and carbohydrate digestive enzyme inhibitory effects. S. Afr. J. Bot. 2019, 125, 381–385. [Google Scholar] [CrossRef]
- Pieters, L.; Vlietinck, A.J. Bioguided isolation of pharmacologically active plant components, still a valuable strategy for the finding of new lead compounds? J. Ethnopharmacol. 2005, 100, 57–60. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of solvent polarity on the Ultrasound Assisted extraction and antioxidant activity of phenolic compounds from habanero pepper leaves (Capsicum chinense) and its identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef] [PubMed]
- Hapsari, S.; Yohed, I.; Kristianita, R.A.; Jadid, N.; Aparamarta, H.W.; Gunawan, S. Phenolic and flavonoid compounds extraction from Calophyllum inophyllum leaves. Arab. J. Chem. 2022, 15, 103666. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.F.; Al-Sheraideh, M.S.; Mayyas, A.S.; Abu Orabi, S.T.; Al-Qudah, M.A. LC-MS/MS Screening, Total Phenolic, Flavonoid and Antioxidant Contents of Crude Extracts from Three Asclepiadaceae Species Growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Dave, R.; Shah, K. Proximate analysis, preliminary phytochemical screening and characterization of compounds by GC–MS from “Cycas revoluta”. Vegetos 2022, 1–7. [Google Scholar] [CrossRef]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Kamchonwongpaisan, S. GC/GC-MS analysis, isolation and identification of bioactive essential oil components from the Bhutanese medicinal plant, Pleurospermum amabile. Nat. Prod. Commun. 2013, 8, 1934578X1300800930. [Google Scholar] [CrossRef]
- Wang, T.; Xi, M.; Guo, Q.; Wang, L.; Shen, Z. Chemical components and antioxidant activity of volatile oil of a Compositae tea (Coreopsis tinctoria Nutt.) from Mt. Kunlun. Ind. Crops Prod. 2015, 67, 318–323. [Google Scholar] [CrossRef]
- Henneh, I.T.; Huang, B.; Musayev, F.N.; Al Hashimi, R.; Safo, M.K.; Armah, F.A.; Ameyaw, E.O.; Adokoh, C.K.; Ekor, M.; Zhang, Y. Structural elucidation and in vivo anti-arthritic activity of β-amyrin and polpunonic acid isolated from the root bark of Ziziphus abyssinica HochstEx. A Rich (Rhamnaceae). Bioorg. Chem. 2020, 98, 103744. [Google Scholar] [CrossRef]
- Devi, J.A.I.; Muthu, A.K. Gas Chromatography-Mass Spectrometry Analysis of Phytocomponents in the Ethanolic Extract from Whole Plant of Lactuca runcinata DC. GAS 2015, 8, 202–206. [Google Scholar]
- de Castro Jorge, N.; Guedes, L.M.; Aguilera, N.; Becerra, J.; dos Santos Isaias, R.M. Allelopathic potential of the extracts of non-galled stems and globoid stem galls of Eremanthus erythropappus (DC) McLeish (Asteraceae). Biochem. Syst. Ecol. 2022, 100, 104379. [Google Scholar] [CrossRef]
- Adinortey, C.A.; Kwarko, G.B.; Koranteng, R.; Boison, D.; Obuaba, I.; Wilson, M.D.; Kwofie, S.K. Molecular Structure-Based Screening of the Constituents of Calotropis procera Identifies Potential Inhibitors of Diabetes Mellitus Target Alpha Glucosidase. Curr. Issues Mol. Biol. 2022, 44, 963–987. [Google Scholar] [CrossRef]
- Gao, R.; Su, Z.; Yin, Y.; Sun, L.; Li, S. Germplasm, chemical constituents, biological activities, utilization, and control of Chinese tallow (Triadica sebifera (L.) Small). Biol. Invasions 2016, 18, 809–829. [Google Scholar] [CrossRef]
- Aziz, S.S.; El-Zayat, M.M.; El-Khateeb, A.Y. Biological Activity and Composition of the Essential Oil and Fatty Constituents of Petroleum ether Extract of Brassica juncea (L.). J. Plant Prod. 2020, 11, 57–59. [Google Scholar] [CrossRef]
- Jin, J.; Sheraliev, G.; Xie, D.; Zhang, W.; Jin, Q.; Wang, X. Characteristics of Specialty Natural Micronutrients in Certain Oilseeds and Oils: Plastochromanol-8, Resveratrol, 5-Hydroxytryptamine Phenylpropanoid Amides, Lanosterol, Ergosterol and Cyclolinopeptides. J. Am. Oil Chem. Soc. 2016, 93, 155–170. [Google Scholar] [CrossRef]
- Upadhyay, A.; Amanullah, A.; Mishra, R.; Kumar, A.; Mishra, A. Lanosterol suppresses the aggregation and cytotoxicity of misfolded proteins linked with neurodegenerative diseases. Mol. Neurobiol. 2018, 55, 1169–1182. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Chemical constituents and biological activities of essential oils of Hydnora africana thumb used to treat associated infections and diseases in South Africa. Appl. Sci. 2017, 7, 443. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Wang, Q.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. Gleditsia species: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 178, 155–171. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Kassim, H.; Tawang, A. Traditional uses, chemical profile and biological activities of piper hispidum Sw.: A review. Biointerface Res. Appl. Chem. 2021, 11, 13115–13129. [Google Scholar]
- Akbar, M.; Ali, U.; Khalil, T.; Iqbal, M.S.; Amin, A.; Naeem, R.; Nazir, A.; Waqas, H.M.; Aslam, Z.; Jafri, F.I. Cornus macrophylla, the antibacterial activity of organic leaf extracts and the characterization of the more lipophilic components by GC/MS. Molecules 2020, 25, 2395. [Google Scholar] [CrossRef]
- Mahadev, R.; Ramakrishnaiah, H.; Krishna, V.; Kumar, N.N. Chemical Composition of the Essential Oil from the Fruits of Solanum erianthum D. Don. J. Essent. Oil Bear. Plants 2012, 15, 387–391. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, H.; Liu, L. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 141, 206–211. [Google Scholar] [CrossRef]
- Shah, R.; Alabri, S.J.; Ashehi, A.S.A.; Asiyabi, N.S.A.; AlMamari, W.K.A.; AlSabahi, J.N.A.; Al-Ruqaishi, H. Antibacterial Activity and Chemical Composition of Crude Extract and Oil of Zygophyllum (Fagonia) luntii (Baker) 1894 (Family Zygophyllaceae). J. Agric. Mar. Sci. 2020, 25, 58–66. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef] [PubMed]
- PRABHA, M.; Brintha, M. Molecular Profiling and Antioxidant Potential of Citrus limon (L.) Burm. F Fruits. Nveo-Nat. Volatiles Essent. Oils J. 2021, 8, 8360–8373. [Google Scholar]
- Harley, B.K.; Amponsah, I.K.; Ben, I.O.; Adongo, D.W.; Mireku-Gyimah, N.A.; Baah, M.K.; Mensah, A.Y.; Fleischer, T.C. Myrianthus libericus: Possible mechanisms of hypoglycaemic action and in silico prediction of pharmacokinetics and toxicity profile of its bioactive metabolite, friedelan-3-one. Biomed. Pharmacother. 2021, 137, 111379. [Google Scholar] [CrossRef] [PubMed]
- Malik, W.; Ahmed, D.; Izhar, S. Tyrosinase inhibitory activities of Carissa opaca Stapf ex haines roots extracts and their phytochemical analysis. Pharmacogn. Mag. 2017, 13, S544. [Google Scholar] [PubMed]
- Monção, N.B.N.; Araújo, B.Q.; Silva, J.D.N.; Lima, D.J.B.; Ferreira, P.M.P.; Airoldi, F.P.d.S.; Pessoa, C.; Citó, A.M.d.G.L. Assessing chemical constituents of Mimosa caesalpiniifolia stem bark: Possible bioactive components accountable for the cytotoxic effect of M. caesalpiniifolia on human tumour cell lines. Molecules 2015, 20, 4204–4224. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Lin, L. Encompassment of isoeugenol in 2-hydroxypropyl-β-cyclodextrin using ultrasonication: Characterization, antioxidant and antibacterial activities. J. Mol. Liq. 2019, 296, 111777. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.; Lou, J.; Ni, C.; Ashraf, M.A. Molecules and Functions of Rosewood: Dalbergia Cearensis. Caribb. J. Sci. 2018, 51, 458–465. [Google Scholar]
- Prabha, S.; Kumar, J. Gas Chromatographic and Mass Spectroscopic (GC-MS) Analysis of Rhizome of Acorus calamus Linn. for Identification of Potent Antimicrobial Bio-active Compounds. J. Sci. Res. 2021, 13, 263–273. [Google Scholar] [CrossRef]
- Qin, G.-F.; Liang, H.-B.; Liu, W.-X.; Zhu, F.; Li, P.-L.; Li, G.-Q.; Yao, J.-C. Bicyclo [6.3.0] undecane sesquiterpenoids: Structures, biological activities, and syntheses. Molecules 2019, 24, 3912. [Google Scholar] [CrossRef]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L. Synthesis of new dihydroxylated derivatives of ferulic and isoferulic acids. Bull. Georg. Natl. Acad. Sci 2018, 12, 119–124. [Google Scholar]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Tahir, A.A.; Devereux, M.; Kavanagh, K.; Creaven, B.S.; Kellett, A. Silver (I) complexes of 9-anthracenecarboxylic acid and imidazoles: Synthesis, structure and antimicrobial activity. Dalton Trans. 2012, 41, 6516–6527. [Google Scholar] [CrossRef] [PubMed]
- Curran, R.; Lenehan, J.; McCann, M.; Kavanagh, K.; Devereux, M.; Egan, D.A.; Clifford, G.; Keane, K.; Creaven, B.S.; McKee, V. [Ag2(aca)2]n and [Ag4(aca)4(NH3)2] (acaH = 9-anthracenecarboxylic acid): Synthesis, X-ray crystal structures, antimicrobial and anti-cancer activities. Inorg. Chem. Commun. 2007, 10, 1149–1153. [Google Scholar] [CrossRef][Green Version]
- Leal, A.L.A.B.; Machado, A.J.T.; Bezerra, C.F.; Inácio, C.E.S.; Rocha, J.E.; Sales, D.L.; de Freitas, T.S.; de Oliveira Almeida, W.; do Amaral, W.; da Silva, L.E. Chemical identification and antimicrobial potential of essential oil of Piper rivinoides kunth (BETIS-WHITE). Food Chem. Toxicol. 2019, 131, 110559. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.; Lamie, P.F.; Omar, H.A. 3-Methyl-2-phenyl-1-substituted-indole derivatives as indomethacin analogs: Design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Zhou, Z.-Q.; Zou, J.; Yu, Y.; Feng, X.-L.; Chen, G.-D.; He, R.-R.; Yao, X.-S.; Gao, H. Bioactive asarone-derived phenylpropanoids from the rhizome of Acorus tatarinowii Schott. J. Nat. Prod. 2017, 80, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α-and β-Asarone: A review of preclinical evidence. Phytomedicine 2017, 32, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Exploration of some potential bioactive essential oil components as green food preservative. LWT 2021, 137, 110498. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 2017, 106, 241–304. [Google Scholar] [PubMed]
- Hua, X.; Yang, Q.; Zhang, W.; Dong, Z.; Yu, S.; Schwarz, S.; Liu, S. Antibacterial activity and mechanism of action of aspidinol against multi-drug-resistant methicillin-resistant Staphylococcus aureus. Front. Pharmacol. 2018, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.S.d.; Xavier, J.d.C.; Pereira, R.L.; Rocha, J.E.; Muniz, D.F.; da Silva, P.T.; da Hora, J.P.; Dos Santos, H.S.; Bandeira, P.N.; Nogueira, C.E. Direct antibacterial and antibiotic resistance modulatory activity of chalcones synthesized from the natural product 2-hydroxy-3, 4, 6-trimethoxyacetophenone. FEMS Microbiol. Lett. 2020, 367, fnaa124. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food Chem. 2020, 309, 125649. [Google Scholar] [CrossRef]
- Dhivya, K.; Vengateswari, G.; Arunthirumeni, M.; Karthi, S.; Senthil-Nathan, S.; Shivakumar, M.S. Bioprospecting of Prosopis juliflora (Sw.) DC seed pod extract effect on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Physiol. Mol. Plant Pathol. 2018, 101, 45–53. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mahmoud, A.Z.; Farshori, N.N.; Alfaraj, R.; Al-Sheddi, E.S.; Alsarra, I.A. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J. Biol. Sci. 2019, 26, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.M.; Martelli, L.S.; Corrêa, A.G. Asymmetric organocatalyzed synthesis of coumarin derivatives. Beilstein J. Org. Chem. 2021, 17, 1952–1980. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Für Nat. C 2008, 63, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Yadav, S.S.; Singh, V.; Dwivedi, L. Phytochemical screening and GC-MS studies of the methanolic extract of Tridax procumbens. Int. J. Pharm. Sci. Res. 2019, 10, 2492–2496. [Google Scholar]
- Abubakar, M.N.; Majinda, R.R. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahmad, S.; Mohamed, M.; Ab Rahman, M. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014, 2014, 635240. [Google Scholar] [CrossRef]
- Yu, F.-R.; Lian, X.-Z.; Guo, H.-Y.; McGuire, P.M.; Li, R.-D.; Wang, R.; Yu, F.-H. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci. 2005, 8, 528–535. [Google Scholar] [PubMed]
- El-Naggar, M.E.; Soliman, R.A.; Morsy, O.M.; Abdel-Aziz, M.S. Nanoemulsion of Capsicum fruit extract as an eco-friendly antimicrobial agent for production of medical bandages. Biocatal. Agric. Biotechnol. 2020, 23, 101516. [Google Scholar] [CrossRef]
- Kurashov, E.A.; Fedorova, E.V.; Krylova, J.V.; Mitrukova, G.G. Assessment of the potential biological activity of low molecular weight metabolites of freshwater macrophytes with QSAR. Scientifica 2016, 2016, 1205680. [Google Scholar] [CrossRef]
- Wanyama, A.W. Evaluation of Phytoconstituents, Antioxidants Potential, Cytotoxic, Antimicrobial Activities and Mineral Composition of Vigna subterranea (L) Verdic. Extracts. Ph.D. Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2018. [Google Scholar]
- Sui, G.; Li, T.; Zhang, B.; Wang, R.; Hao, H.; Zhou, W. Recent advances on synthesis and biological activities of aurones. Bioorg. Med. Chem. 2021, 29, 115895. [Google Scholar] [CrossRef]
- Abbasi, M.; Omrani, M.; Raiatparvar Malieki, L.; Sonboli, A.; Nejad Ebrahimi, S. Phytochemical studies of Tetrataenium nephrophyllum and anti-acetylcholinesterase activities. Trends Phytochem. Res. 2021, 5, 210–217. [Google Scholar]
- Yang, J.; Liu, C.; Cai, H.; Gu, D.; Ji, Z.; Guo, X.; Tian, J.; Meng, J.; Yang, Y. Identification and theoretical explanation of chemical composition against α-amylase in the n-hexane extract from Sargassum fusiforme. Algal Res. 2019, 43, 101642. [Google Scholar] [CrossRef]
- Abuabaker, M.; Guo, H.; Shi, J.; Farah, A.; Zhang, J. Gas Chromatography-Mass spectrum and Infra-Red spectral analysis of Fixed Oil from Sudanese Adansonia digitata Seeds. Chem. Methodol. 2021, 5, 240–249. [Google Scholar]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological Properties, Health Benefits and Enzymatic Modifications of Dietary Methoxylated Derivatives of Cinnamic Acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef]
- Al-Mudhafr, A.W.H. Detection of Active Compounds By Gc/Ms in Extract of Red Cabbage (Brassica oleracea) and Their Effect in the Preservation of Raw Milk. Plant Arch. 2020, 20, 4–8. [Google Scholar]
- Mohamed, S.S.; Saber, A.A. Antifungal potential of the bioactive constituents in extracts of the mostly untapped brown seaweed Hormophysa cuneiformis from the Egyptian coastal waters. Egypt. J. Bot. 2019, 59, 695–708. [Google Scholar] [CrossRef]
- Naik, B.; Maurya, V.K.; Kumar, V.; Kumar, V.; Upadhyay, S.; Gupta, S. Phytochemical analysis of Diplazium esculentum reveals the presence of medically important components. Curr. Nutr. Food Sci. 2021, 17, 210–215. [Google Scholar] [CrossRef]
- Suresh, P.S.; Bhatt, V.; Singh, P.P.; Sharma, U. Steroidal sapogenins from genus Trillium: Chemistry, synthesis, and opportunities in neuro-active steroids designing. Stud. Nat. Prod. Chem. 2021, 68, 67–95. [Google Scholar]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free. Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Singh, A.; Dixit, A.K.; Shrivastava, B.; Kondalkar, S.A.; Subhose, V. Determination of phytochemical, antioxidant, antimicrobial, and protein binding qualities of hydroethanolic extract of Celastrus paniculatus. J. Appl. Biol. Biotechnol. 2018, 6, 11–17. [Google Scholar]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M.J.R.C. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.; Ali, H.; Sarwat, M.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Shousha, W.G.; Aboulthana, W.M.; Salama, A.H.; Saleh, M.H.; Essawy, E.A. Evaluation of the biological activity of Moringa oleifera leaves extract after incorporating silver nanoparticles, in vitro study. Bull. Natl. Res. Cent. 2019, 43, 1–13. [Google Scholar] [CrossRef]
- Dias, W.; do Vale Junior, E.; de Oliveira, M.d.D.A.; Barbosa, Y.; do Nascimento Silva, J.; da Costa Júnior, J.; de Almeida, P.; Martins, F. Cytogenotoxic effect, phytochemical screening and antioxidant potential of Jatropha mollissima (Pohl) Baill leaves. S. Afr. J. Bot. 2019, 123, 30–35. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and therapeutic potential of selected Australian plants: A review. J. Ethnopharmacol. 2021, 268, 113580. [Google Scholar] [CrossRef]
- Susan, A.; Rajendran, K.; Sathyasivam, K.; Krishnan, U.M. An overview of plant-based interventions to ameliorate arsenic toxicity. Biomed. Pharmacother. 2019, 109, 838–852. [Google Scholar] [CrossRef]
- Mehrandish, R.; Rahimian, A.; Shahriary, A. Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J. Herbmed Pharmacol. 2019, 8, 69–77. [Google Scholar] [CrossRef]
- Karim, F.A.; Suleiman, M.; Rahmat, A.; Bakar, M.A. Phytochemicals, antioxidant and antiproliferative properties of five moss species from Sabah, Malaysia. Int. J. Pharm. Pharm. Sci 2014, 6, 292–297. [Google Scholar]
- Mohandas, G.G.; Kumaraswamy, M. Antioxidant activities of terpenoids from Thuidium tamariscellum (C. muell.) Bosch. and Sande-Lac. a Moss. Pharmacogn. J. 2018, 10, 645–649. [Google Scholar] [CrossRef]
- Khan, S.; Nazir, M.; Raiz, N.; Saleem, M.; Zengin, G.; Fazal, G.; Saleem, H.; Mukhtar, M.; Tousif, M.I.; Tareen, R.B. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): A comprehensive approach. Ind. Crops Prod. 2019, 131, 117–124. [Google Scholar] [CrossRef]
- Khan, S.; Nazir, M.; Saleem, H.; Raiz, N.; Saleem, M.; Anjum, S.M.M.; Zengin, G.; Mukhtar, M.; Tousif, M.I.; Mahomoodally, F.M. Valorization of the antioxidant, enzyme inhibition and phytochemical propensities of Berberis calliobotrys Bien. ex Koehne: A multifunctional approach to probe for bioactive natural products. Ind. Crops Prod. 2019, 141, 111693. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Zuo, A.-R.; Dong, H.-H.; Yu, Y.-Y.; Shu, Q.-L.; Zheng, L.-X.; Yu, X.-Y.; Cao, S.-W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef]
- Corradi, I.; De Souza, E.; Sande, D.; Takahashi, J.A. Correlation between phenolic compounds contents, anti-tyrosinase and antioxidant activities of plant extracts. Chem. Eng. Trans. 2018, 64, 109–114. [Google Scholar]
- Kumar, S.C.; Ramesh, N.; Sreevatsan, S.; Joseph, B.; Alle, P.; Belani, K.G.; Osterholm, M.T. Knowledge, attitudes, and poultry-handling practices of poultry workers in relation to avian influenza in India. Indian J. Occup. Environ. Med. 2013, 17, 16. [Google Scholar] [CrossRef]
- Ling, J.; Kim, Y.-B.; Kim, J.-H.; Shin, J.-D.; Yoon, S.-A.; Shim, M.-W.; Kim, E.-K. Screening of depigmenting agents from Philippine plants. 한국생물공학회 학술대회 2008, 183. [Google Scholar] [CrossRef]
- Ya, W.; Chun-Meng, Z.; Tao, G.; Yi-Lin, Z.; Ping, Z. Preliminary screening of 44 plant extracts for anti-tyrosinase and antioxidant activities. Pak. J. Pharm. Sci. 2015, 28, 1737–1744. [Google Scholar] [PubMed]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Khanna, V.G.; Thiruvengadam, M. Insights on the current status and advancement of diabetes mellitus type 2 and to avert complications: An overview. Biotechnol. Appl. Biochem. 2020, 67, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120. [Google Scholar] [CrossRef]
- Salazar, M.O.; Osella, M.I.; Arcusin, D.E.; Lescano, L.E.; Furlan, R.L. New α-glucosidase inhibitors from a chemically engineered essential oil of Origanum vulgare L. Ind. Crops Prod. 2020, 156, 112855. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L.; Wang, H.; Chang, X.; Ren, S.; Lai, H.; Liu, L. Phytochemical profiles and antioxidant, anticholinergic, and antidiabetic activities of Odontites serotina (Lam.) dum. Eur. J. Integr. Med. 2021, 44, 101340. [Google Scholar] [CrossRef]
- Sen, A.; Kurkcuoglu, M.; Senkardes, I.; Bitis, L.; Baser, K.H.C. Chemical composition, antidiabetic, anti-inflammatory and antioxidant activity of Inula ensifolia L. essential oil. J. Essent. Oil Bear. Plants 2019, 22, 1048–1057. [Google Scholar] [CrossRef]
- Bothon, F.T.; Debiton, E.; Avlessi, F.; Forestier, C.; Teulade, J.-C.; Sohounhloue, D.K. In vitro biological effects of two anti-diabetic medicinal plants used in Benin as folk medicine. BMC Complementary Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Pandey, B.P.; Pradhan, S.P. Chemical composition, in vitro antioxidant, and enzymes inhibitory potential of three medicinally important plants from Nepal (Lepisorus mehrae, Pleurospermum benthamii, and Roscoea auriculata). Adv. Tradit. Med. 2020, 22, 75–90. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Shaikh, T.G.; Waseem, S.; Qadir, N.A.; Yousaf, Z.; Ullah, I. Vaccine-induced thrombotic thrombocytopenia following coronavirus vaccine: A narrative review. Ann. Med. Surg. 2022, 73, 102988. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus structural composition in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Severac, F.; Merdji, H.; Schenck, M.; Clere-Jehl, R.; Baldacini, M.; Ohana, M.; Grunebaum, L.; Castelain, V.; Anglés-Cano, E. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: Bi-center cohort study. Ann. Intensive Care 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vani, M.; Rani, P.V.; Madhuri, O.; Sree, M.V.S.; Ramya, L.S.; Chandrika, M.G.; Padmalatha, K.; Supriya, J. Phytochemical and in vitro thrombolytic activity evaluation of Cassia siamea L., Leguminosae leaf extracts, and pyrogallol. Int. J. Green Pharm. 2019, 13, 213–217. [Google Scholar]
- Maqsood, M.; Mushtaq, Z.; Rasheed, T.; Nisa, Z.U.; Sher, F. Thrombolytic and cytotoxic activity of different bioactive extracts of E. coli. Case Stud. Chem. Environ. Eng. 2021, 3, 100080. [Google Scholar] [CrossRef]
- Manju, P.; Pushpa, D.A. A Study On Thrombolytic and Cytotoxic Activity of Methanolic Extract of Zingiber officinale. Int. J. Life Sci. Pharma. Res. 2020, 10, 1–5. [Google Scholar]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-derivatives small molecules with antibacterial activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, S.; Wu, G.; Xie, Y.; Ishaq, M.; Sun, Y.; Yan, S.-K.; Qian, X.-P.; Jin, H.-Z. Chemical Constituents of Vernonia parishii. Chem. Nat. Compd. 2020, 56, 134–136. [Google Scholar] [CrossRef]
- Wangchuk, P.; Pyne, S.G.; Keller, P.A.; Taweechotipatr, M.; Kamchonwongpaisan, S. Phenylpropanoids and furanocoumarins as antibacterial and antimalarial constituents of the Bhutanese medicinal plant Pleurospermum amabile. Nat. Prod. Commun. 2014, 9, 1934578X1400900719. [Google Scholar] [CrossRef]
- Khan, I.; Abbas, T.; Anjum, K.; Abbas, S.Q.; Shagufta, B.I.; Ali Shah, S.A.; Akhter, N.; Hassan, S.S. Antimicrobial potential of aqueous extract of Camellia sinensis against representative microbes. Pak. J. Pharm. Sci. 2019, 32, 631–636. [Google Scholar] [PubMed]
- Aziz, M.; Ahmad, S.; Iqbal, M.N.; Khurshid, U.; Saleem, H.; Alamri, A.; Anwar, S.; Alamri, A.S.; Chohan, T.A. Phytochemical, pharmacological, and In-silico molecular docking studies of Strobilanthes glutinosus Nees: An unexplored source of bioactive compounds. S. Afr. J. Bot. 2022, 147, 618–627. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Zhang, W.-D.; Jin, H.-Z.; Basha, S.H.; Priya, S.V.S.S. In-silico anti-inflammatory potential of guaiane dimers from Xylopia vielana targeting COX-2. J. Biomol. Struct. Dyn. 2022, 40, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.P.; Kinghorn, A.D. Extraction of plant secondary metabolites. In Natural Products Isolation; Springer: Berlin/Heidelberg, Germany, 2006; pp. 323–351. [Google Scholar]
- Chang, X.; Ye, Y.; Pan, J.; Lin, Z.; Qiu, J.; Peng, C.; Guo, X.; Lu, Y. Comparative Analysis of Phytochemical Profiles and Antioxidant Activities between Sweet and Sour Wampee (Clausena lansium) Fruits. Foods 2022, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.u.; Tabassum, F.; Khursheed, A.; Zaman, Q.u.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical characterization and antioxidant properties of ethanolic extract and its fractions from sweet potato (Ipomoea batatas L.) leaves. Foods 2019, 9, 15. [Google Scholar] [CrossRef]
- Saleem, M.; Shazmeen, N.; Nazir, M.; Riaz, N.; Zengin, G.; Ataullah, H.M.; Nisar, F.; Mukhtar, M.; Tousif, M.I.J.C. Investigation on the Phytochemical Composition, Antioxidant and Enzyme Inhibition Potential of Polygonum Plebeium R. Br: A Comprehensive Approach to Disclose New Nutraceutical and Functional Food Ingredients. Chem. Biodivers 2021, 18, e2100706. [Google Scholar] [CrossRef]
- Ghalloo, B.A.; Khan, K.-u.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical Profiling, In Vitro Biological Activities, and In Silico Molecular Docking Studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef]
- Shoibe, M.; Chy, M.; Uddin, N.; Alam, M.; Adnan, M.; Islam, M.; Nihar, S.W.; Rahman, N.; Suez, E. In vitro and in vivo biological activities of Cissus adnata (Roxb.). Biomedicines 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Palmeira, A.; Ramos, I.I.; Carneiro, C.; Afonso, C.; Tiritan, M.E.; Cidade, H.; Pinto, P.C.; Saraiva, M.L.M.; Reis, S.J.P. Chiral derivatives of xanthones: Investigation of the effect of enantioselectivity on inhibition of cyclooxygenases (COX-1 and COX-2) and binding interaction with human serum albumin. Pharmaceuticals 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]





| Sample Codes | TPC (mg GAE/g Dried wt.) | TFC (mg QE/g Dried wt.) |
|---|---|---|
| PCME | 240.69 ± 2.94 | 167.59 ± 3.47 |
| PCHF | 57.02 ± 1.31 | 48.21 ± 0.75 |
| PCCF | 97.02 ± 1.83 | 88.32 ± 1.45 |
| PCBF | 144.02 ± 2.11 | 96.58 ± 2.30 |
| Sr.no. | RT | Tentative Identification of Compounds | Molecular Formula | Molecular Weight | Chemical Class | Area % | Reported Activities from Literature |
|---|---|---|---|---|---|---|---|
| 1 | 15.13 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.5 | Fatty acid ester | 0.05 | Antioxidant, antimicrobial [27] |
| 2 | 17.34 | β-Amyrin | C30H50O | 426.70 | Terpenoid | 2.77 | Antioxidant, anti-inflammatory, antibacterial, antiulcer, antiarthritic, antidiahreal [28] |
| 3 | 17.42 | 2(1H)Naphthalenone, 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)- | C15H22O | 218.33 | Terpenoid | 0.45 | Anticancer, antioxidant ani-inflammatory, analgesic, sedative [29] |
| 4 | 18.46 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo[5.1.0.0(2,4)]octane | C15H22O | 218.33 | Terpenoid | 9.20 | Antioxidant [27] |
| 5 | 19.21 | A-Neooleana-3(5),12-diene | C30H48 | 408.7 | Terpenoid | 9.92 | Anti-inflammatory, antimicrobial [30], antidiabetic [31] |
| 6 | 19.31 | 3-Epimoretenol | C30H50O | 426.7 | Terpenoid | 3.37 | Anti-inflammatory, analgesic [32] |
| 7 | 19.77 | 9,19-Cyclolanost-24-en-3-ol, acetate, (3beta)- | C32H52O2 | 468.8 | Steroid ester | 8.00 | Antibacterial, antioxidant [33] |
| 8 | 20.27 | Lanosterol | C30H50O | 426.7 | Steroid | 1.97 | Antioxidant [34], cytoprotective, neuroprotective, anti-inflammatory [35] |
| 9 | 23.27 | Phenanthrene, 7-ethenyl-1,2,3,4,4a,4b,5,6,7,8,8a,9-dodecahydro-1,1,4b,7-tetramethyl- | C20H32 | 272.5 | Terpenoid | 0.58 | Antioxidant, antibacterial [36] |
| 10 | 25.21 | D:C-Friedours-7-en-3-one | C30H48O | 424.7 | Terpenoid | 1.93 | Antioxidant, anti-inflammatory, antibacterial [37] |
| 11 | 25.33 | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate, (3β,4α,5α)- | C32H52O2 | 468.75 | Steroid ester | 0.66 | Anti-inflammatory, antibacterial [32] |
| 12 | 25.49 | 2′-Hydroxy-3,4,4′,6′-tetramethoxychalcone | C19H20O6 | 344.4 | Flavonoid | 1.93 | Antioxidant, antibacterial, antidiabetic [38] |
| 13 | 25.63 | A′-Neogammacer-22(29)-en-3-one | C30H48O | 424.7 | Terpenoid | 0.47 | Antibacterial, antioxidant [39] |
| 14 | 26.22 | Cedran-diol, 8S,14- | C15H26O2 | 238.37 | Terpenoid | 9.22 | Anti-inflammatory, anticancer [40]- |
| 15 | 26.64 | Taraxasterol | C30H50O | 426.7 | Terpenoid | 19.00 | Antidiabetic [31], anti-inflammatory, analgesic [41] |
| 16 | 26.93 | Hop-22(29)-en-3.beta.-ol | C30H50O | 426.7 | Terpenoid | 12.13 | Antibacterial, antioxidant [42] |
| 17 | 27.18 | Lupeol | C30H50O | 426.7 | Terpenoid | 10.40 | Antimicrobial, antioxidant, anticancer, anti-inflammatory [43] |
| 18 | 27.55 | Urs-12-en-3-ol, acetate, (3beta)- | C32H52O2 | 468.8 | Ester | 3.31 | Antioxidant, antimicrobial, anticancer [44] |
| 19 | 28.21 | Friedelan-3-one | C30H50O | 426.7 | Terpenoid | 0.79 | Antioxidant, antidiabetic, antimicrobial [45] |
| 20 | 29.40 | 2,4,6-Cycloheptatrien-1-one, 3-hydroxy- | C7H6O2 | 300.31 | Alcohol | 0.38 | - |
| 21 | 30.69 | Lup-20(29)-en-3-ol, acetate, (3β)- | C32H52O2 | 468.8 | Terpenoid | 2.38 | Anti-inflammatory, analgesic, antibacterial [32], tyrosinase-inhibitory effect [46] |
| 22 | 31.12 | Olean-18-en-28-oic acid, 3-oxo-, methyl ester | C31H48O3 | 468.7 | Fatty acid ester | 1.09 | Antimicrobial, antioxidant [47] |
| Sr.no. | RT | Tentative Identification of Compounds | Molecular Formula | Molecular Weight | Chemical Class | Area % | Reported Activities from Literature |
|---|---|---|---|---|---|---|---|
| 1 | 10.28 | Methyl iso-eugenol 2 | C11H14O2 | 178.22 | Phenolic | 0.55 | Antibacterial, antioxidant. [48] |
| 2 | 10.94 | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | C12H16O3 | 208.25 | Phenolic | 0.06 | Anti-inflammatory, antioxidant [49] antifungal, antimicrobial [50] |
| 3 | 11.15 | Bicyclo[6.3.0]undec-1(8)-en-3-ol, 2,2,5,5-tetramethyl- | C15H26O | 222.19 | Terpenoid | 0.17 | Cytotoxic, antiplasmodial, antiviral, anti-inflammatory [51] |
| 4 | 11.55 | 3-Hydroxy-4-methoxycinnamic acid | C10H10O4 | 194.18 | Phenolic | 0.28 | Antioxidant, anti-inflammatory [52] |
| 5 | 11.80 | 9-Anthracenecarboxylic acid | C15H10O2 | 222.24 | Aromatic carboxylic acid | 0.24 | Antimicrobial, antifungal [53], antibacterial, anticancer [54] |
| 6 | 11.97 | Diepi-.alpha.-cedrene epoxide | C15H24O | 220.35 | Terpenoid | 0.06 | Cytotoxic, antibacterial [54] |
| 7 | 12.08 | Isoelemicin | C12H16O3 | 208.25 | Phenolic | 3.35 | Antimicrobial [55] |
| 8 | 12.20 | 1,3-Benzodioxole, 4,5-dimethoxy-7-(2-propenyl)- | C12H14O4 | 222.24 | Phenolic | 0.14 | Antimicrobial, antioxidant, anticancer [56] |
| 9 | 12.43 | Asarone | C12H16O3 | 208.25 | Phenolic | 6.30 | Hypoglycemic, antimicrobial, anti-Alzheimer’s disease, anticonvulsive, antiepileptic and antioxidant properties [57,58] |
| 10 | 12.87 | Apiol | C12H14O4 | 222.23 | Phenolic | 3.97 | Antioxidant, antimicrobial [59] |
| 11 | 13.07 | 2H-1-Benzopyran-2-one, 7-methoxy- | C10H8O3 | 176.17 | Coumarin | 0.12 | Antioxidant, analgesic, anticoagulant, anti-inflammatory, antimicrobial [60]. |
| 12 | 13.14 | Aspidinol | C13H18O4 | 238.28 | Phenolic | 0.59 | Antibacterial [61] |
| 13 | 13.22 | Tetradecanoic acid | C14H28O2 | 228.37 | Fatty acid | 0.14 | - |
| 14 | 13.88 | Phenol,2-[[(4-methylphenyl)imino]methyl]- | C14H13NO | 211.26 | Phenolic | 0.15 | _ |
| 15 | 13.97 | 2,4,6-Trimethoxyacetophenone | C11H14O4 | 210.23 | Phenolic | 0.14 | Antibacterial and synergistic effect with antibiotics [62] |
| 16 | 14.06 | 1-Methoxy-3-(2-hydroxyethyl)nonane | C12H26O2 | 202.33 | Alcohol | 0.31 | Antifungal, antioxidant [63] |
| 17 | 14.34 | 9-Octadecyne | C18H34 | 250.5 | Alkyne | 0.34 | Larvicidal, antioxidant [64] |
| 18 | 14.42 | Tricyclo[7.2.0.0(2,6)]undecan-5-ol | C15H26O | 222.37 | Terpenoid | 0.18 | - |
| 19 | 14.79 | 7,10,13-Hexadecatrienoic acid, methyl ester | C17H28O2 | 264.40 | Fatty acid ester | 0.17 | Antioxidant, anti-inflammatory, antimicrobial [65] |
| 20 | 15.03 | 3,4-dihydrocoumarin | C9H8O2 | 148.16 | Coumarin | 0.12 | Anticoagulant, antifungal, anticancer, antibacterial [66] |
| 21 | 15.08 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.45 | Fatty acid ester | 0.20 | Antibacterial, antifungal [67] |
| 22 | 15.48 | 11,14,17-Eicosatrienoic acid, methyl ester | C20H34O2 | 306.5 | Fatty acid | 1.52 | Anti-microbial, anti-cancer, anti-hair fall, CNS depressant, analgesic, anti-inflammatory, antipyretic, anti-arthritic, anti-coronary [68] |
| 23 | 15.92 | n-Hexadecanoic acid | C16H32O2 | 256.42 | Fatty acid | 9.40 | Antioxidant, antibacterial, anti-inflammatory [69] |
| 24 | 17.16 | 7H-Furo[3,2-g][1]benzopyran-7-one | C11H6O5 | 218.16 | Coumarin | 2.42 | - |
| 25 | 17.32 | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 294.5 | Fatty acid ester | 0.84 | Antioxidant, anti-inflammatory, antimicrobial [65] |
| 26 | 17.42 | 9,12,15-Octadecatrienoic acid, methyl ester | C19H32O2 | 278.4 | Fatty acid ester | 0.92 | Antimicrobial [70] Antioxidant, anti-inflammatory, antimicrobial [65] |
| 27 | 17.58 | Phytol | C20H40O | 296.5 | Terpenoid | 1.14 | Antioxidant, anticancer [71] |
| 28 | 18.05 | Z,Z-10,12-Hexadecadien-1-ol acetate | C18H32O2 | 280.4 | Ester of fatty alcohol | 2.55 | Antimicrobial [72] |
| 29 | 18.15 | 9,12-Octadecadienoic acid (Z,Z) | C18H32O2 | 280.4 | Fatty acid | 3.55 | Antibacterial, antifungal, anti-inflammatory, antineoplastic [73] |
| 30 | 18.69 | 2-Chloroethyl linoleate | C20H35ClO2 | 342.9 | Fatty acid ester | 39.69 | Cytotoxic, antioxidant, antimicrobial [74] |
| 31 | 19.31 | Flavone | C15H10O2 | 222.24 | Flavonoid | 1.26 | Antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory [75] |
| 32 | 19.59 | Pimpinellin | C13H10O5 | 246.21 | Furocoumarin | 2.57 | Strong antibacterial [76] |
| 34 | 22.05 | Phenol, 2,2’-methylenebis[6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 340.49 | Phenolic | 0.16 | α-amylase inhibitor [77] |
| 35 | 22.52 | 1,3,14,16-Nonadecatetraene | C19H32 | 260.45 | Alkene | 0.23 | - |
| 36 | 22.77 | (R)-(-)-14-Methyl-8-hexadecyn-1-ol | C17H32O | 252.4 | Alcohol | 0.21 | - |
| 37 | 22.92 | 1,5,9,13-Tetradecatetraene | C14H22 | 190.32 | Alkene | 0.14 | - |
| 38 | 23.04 | 9-Tricosene, (Z)- | C23H46 | 322.6 | Alkene | 0.35 | - |
| 39 | 23.55 | 4-(3-Methyl-2-oxobutoxy)-7H-furo[3,2-g][1]benzopyran-7-one | C16H14O5 | 286.28 | Coumarin | 1.20 | Antibacterial [76] |
| 40 | 24.57 | 7H-Furo(3,2-g)(1)benzopyran-7-one, 4,9-dihydroxy- | C11H6O5 | 218.16 | Coumarin | 1.47 | Antibacterial, antiacetyl, and butyrylcholinesterase [76] |
| 41 | 24.84 | 6-Acetylchrysene | C19H14 | 242.3 | Phenanthrene | 1.27 | - |
| 42 | 25.76 | 13-Tetradecen-1-ol acetate | C16H30O2 | 254.41 | Ester fatty alcohol | 0.17 | Antibacterial, antioxidant [78] |
| 43 | 27.22 | 3,4-Dimethoxycinnamic acid | C11H12O4 | 208.21 | Cinnamic acid | 0.09 | Neuroprotactive, antioxidant, anticancer [79] |
| 44 | 28.40 | N-hydroxy-N’-[2-(trifluoromethyl)phenyl]pyridine-3-carboximidamide | C13H10F3N3O | 281.23 | Pyridine derivative | 0.49 | Antioxidant, anti-inflammatory, antimicrobial [80] |
| 45 | 28.69 | Stigmastan-6,22-dien, 3,5-dedihydro- | C29H46 | 394.7 | Steroid | 0.18 | Antifungal [81] |
| 46 | 29.66 | Stigmastane-3,6-dione | C29H48O2 | 428.7 | Steroid | 0.23 | - |
| 47 | 30.53 | Stigmasta-5,22-dien-3-ol, acetate | C31H50O2 | 454.7 | Steroid | 1.16 | Antimicrobial, antioxidant [82] |
| 48 | 30.88 | Ergosta-4,6,22-trien-3.beta.-ol | C28H44O | 396.6 | Steroid | 0.29 | - |
| 49 | 31.51 | Clionasterol acetate | C31H52O2 | 456.7 | Steroid | 2.51 | - |
| 50 | 31.75 | 3β-acetoxy-pregna-5,16-dien-20-one | C23H32O3 | 298.5 | Steroid | 0.27 | Anti-inflammatory, antibacterial [83] |
| 51 | 32.14 | Vitamin E | C29H50O2 | 430.7 | Chromanol derivative | 0.46 | Antioxidant, anticancer, anti-inflammatory [84]. |
| Sample Codes | Radical Scavenging Assay | Reducing Power Assay | Reducing/Metal-Chelating Assay | |||
|---|---|---|---|---|---|---|
| DPPH (IC50 mg/mL) | ABTS (IC50 mg/mL) | CUPRAC (EC50 mg/mL) | FRAP (EC50 mg/mL) | TAC (EC50 mM/mL) | MCE (IC50 mg/mL) | |
| PCME | 3.64 ± 0.73 a | 2.49 ± 0.84 a | 1.20 ± 0.45 a | 1.67 ± 0.68 a | 0.45 ± 0.06 a | 2.16 ± 0.48 a |
| PCHF | 10.34 ± 1.41 d | 5.31 ± 1.04 d | 2.67 ± 0.80 d | 4.20 ± 1.06 d | 0.24 ± 0.02 d | 7.00 ± 0.86 d |
| PCCF | 9.23 ± 1.95 c | 4.14 ± 1.21 c | 1.93 ± 0.79 c | 3.17 ± 1.10 c | 0.29 ± 0.08 c | 4.38 ± 0.62 c |
| PCBF | 5.90 ± 1.06 b | 3.32 ± 0.95 b | 1.59 ± 0.16 b | 2.36 ± 0.75 b | 0.30 ± 0.04 b | 3.28 ± 0.35 b |
| Sample Codes | Tyrosinase (mg KAE/g Dried wt.) | α-Amylase (mmol ACAE/g Dried wt.) | α-Glucosidase (mmol ACAE/g Dried wt.) |
|---|---|---|---|
| PCME | 112.29 ± 2.79 | 0.93 ± 0.07 | 1.88 ± 0.15 |
| PCHF | 52.61 ± 1.26 | 0.53 ± 0.08 | 0.46 ± 0.01 |
| PCCF | 82.91 ± 1.79 | 0.69 ± 0.06 | 0.78 ± 0.04 |
| PCBF | 90.15 ± 2.10 | 0.81 ± 0.05 | 0.95 ± 0.09 |
| Sample Codes | Subject A | Subject B | Subject C | Subject D | Subject E |
|---|---|---|---|---|---|
| PCME | 55.38 ± 1.51 | 58.16 ± 1.9 | 55.45 ± 1.18 | 58.65 ± 1.25 | 59.85 ± 1.51 |
| PCHF | 40.18 ± 1.80 | 43.1 ± 1.69 | 42.51 ± 0.98 | 43.8 ± 0.82 | 42.63 ± 1.35 |
| PCCF | 41.54 ± 0.95 | 48.15 ± 1.41 | 47.15 ± 1.11 | 50.14 ± 1.61 | 47.85 ± 1.80 |
| PCBF | 52.15 ± 0.68 | 57.25 ± 0.94 | 55.10 ± 1.12 | 56.95 ± 1.70 | 57.15 ± 1.10 |
| Streptokinase | 78.5 ± 1.53 | 80.14 ± 0.91 | 81.43 ± 1.39 | 82.34 ± 1.25 | 79.12 ± 2.3 |
| Strain Name | Zone of Inhibition (mm) of Standard (Co-Amoxiclav) (Concentration = 1 mg/mL) | Concentration (mg/mL) | Zone of Inhibition of PCME Extract (mm) | Zone of Inhibition of PCHF Fraction (mm) | Zone of Inhibition of PCCF Extract (mm) | Zone of Inhibition of PCBF Extract (mm) |
|---|---|---|---|---|---|---|
| Bacillus subtilis | 23 | 10 | 7 | - | - | 7 |
| 20 | 13 | 10 | 12 | 12 | ||
| 40 | 18 | 16 | 16.5 | 18 | ||
| Micrococcus luteus | 20 | 10 | 7 | 6 | 6 | 6 |
| 20 | 13 | 10 | 11 | 13 | ||
| 40 | 17 | 16 | 17 | 17 | ||
| Staphylococcus epidermidis | 24 | 10 | 5 | - | - | - |
| 20 | 10 | 8 | 8 | 10 | ||
| 40 | 12 | 10 | 13 | 15 | ||
| Bacillus pumilus | 22 | 10 | 6 | - | - | - |
| 20 | 11 | 7 | 9 | 9 | ||
| 40 | 15 | 12 | 14 | 14.5 | ||
| Staphylococcus aureus | 23 | 10 | 7 | - | 6 | 6 |
| 20 | 13 | 10 | 11 | 12 | ||
| 40 | 15.5 | 12 | 12.5 | 14 | ||
| Escherichia coli | 25 | 10 | - | - | - | - |
| 20 | 8 | - | 7 | 7.5 | ||
| 40 | 10 | 6 | 8 | 9 | ||
| Bordetella bronchiseptica | 26 | 10 | - | - | - | - |
| 20 | 8 | - | 6 | 8 | ||
| 40 | 9 | 10 | 11 | 11.5 | ||
| Pseudomonas aeruginosa | 18 | 10 | - | - | - | - |
| 20 | - | - | - | - | ||
| 40 | 7 | - | - | 6 |
| Name of Compound | Binding Energy of Ligand with Tyrosinase (kcal/mol) | Binding Energy of Ligand with α-Amylase (kcal/mol) | Binding Energy of Ligand with α-Glucosidase (kcal/mol) |
|---|---|---|---|
| Taraxasterol | −8.6 | −9.5 | −8.5 |
| beta-Amyrin | −8.3 | −9.1 | −8.8 |
| Hopa-22(29)-ene-3alpha-ol | −8.0 | −8.9 | −9.0 |
| A-Neooleana-3(5),12-dien | −7.9 | −10.9 | −8.7 |
| Urs-12-en-3-ol, acetate, (3beta)- | −7.7 | −8.8 | −8.4 |
| Lupeol | −7.4 | −8.8 | −9.1 |
| Lanosterol | −7.3 | −9.7 | −8.7 |
| Lup-20(29)-en-3-ol, (3beta)- | −7.3 | −8.7 | −9.0 |
| 3-Epimoretenol | −7.3 | −8.9 | −8.6 |
| 9,19-Cyclolanost-24-en-3-ol, acetate, (3beta)- | −7.2 | −7.9 | −8.5 |
| Standad | −6.0 1 | −7.7 2 | −7.0 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Khan, K.-u.-R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.-u.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R.; et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules 2022, 27, 4113. https://doi.org/10.3390/molecules27134113
Ahmed M, Khan K-u-R, Ahmad S, Aati HY, Ovatlarnporn C, Rehman MS-u, Javed T, Khursheed A, Ghalloo BA, Dilshad R, et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules. 2022; 27(13):4113. https://doi.org/10.3390/molecules27134113
Chicago/Turabian StyleAhmed, Maqsood, Kashif-ur-Rehman Khan, Saeed Ahmad, Hanan Y. Aati, Chitchamai Ovatlarnporn, Muhammad Sajid-ur Rehman, Tariq Javed, Anjum Khursheed, Bilal Ahmad Ghalloo, Rizwana Dilshad, and et al. 2022. "Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development" Molecules 27, no. 13: 4113. https://doi.org/10.3390/molecules27134113
APA StyleAhmed, M., Khan, K.-u.-R., Ahmad, S., Aati, H. Y., Ovatlarnporn, C., Rehman, M. S.-u., Javed, T., Khursheed, A., Ghalloo, B. A., Dilshad, R., & Anwar, M. (2022). Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules, 27(13), 4113. https://doi.org/10.3390/molecules27134113







