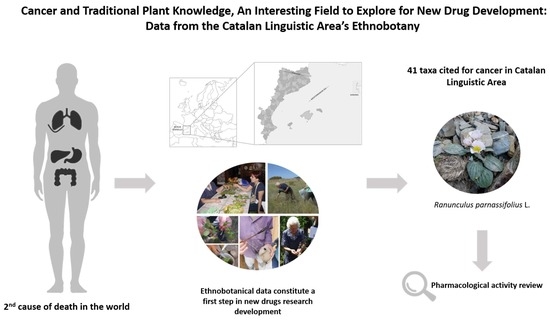
Cancer and Traditional Plant Knowledge, an Interesting Field to Explore: Data from the Catalan Linguistic Area
Abstract
1. Introduction
2. Results and Discussion
2.1. General Data
2.2. Most Recurrent Taxa Used by Informants in Prospected Area
2.3. Wild and Cultivated Vegetables and Their Role against Cancer
2.4. Plants for Dealing with Side Effects of Cancer Treatments
2.5. Pharmacological Activity Review
3. Materials and Methods
3.1. Studied Area
3.2. Databasing and Data Selection
3.3. Pharmacological Activity Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 March 2022).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- The International Agency for Research on Cancer. Available online: https://www.iarc.fr/ (accessed on 22 March 2022).
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Boyd, M.R.; Cardellina, J.H.; Grever, M.R.; Schepartz, S.A.; Snader, K.M.; Suffness, M. Role of plants in the National Cancer Institute drug discovery and development program. In Human Medicinal Agents from Plants. ACS Symposium Series 534; Kinghorn, D.A., Balandrin, M.F., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 80–95. [Google Scholar]
- MPNS Version 9. Medicinal Plant Names Services, the Royal Botanic Gardens, Kew. Available online: http://www.kew.org/mpns (accessed on 22 March 2022).
- Park, E.J.; Pezzuto, J.M. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Gerson-Cwillich, R.; Serrano-Olvera, A.; Villalobos-Prieto, A. Complementary and alternative medicine (CAM) in Mexican patients with cancer. Clin. Transl. Oncol. 2006, 8, 200–207. [Google Scholar] [CrossRef]
- Tascilar, M.; de Jong, F.A.; Verweij, J.; Mathijssen, R.H. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist 2006, 11, 732–741. [Google Scholar] [CrossRef]
- European Parliament. Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. Eur. Union 2004, L136, 85–90. [Google Scholar]
- Heinrich, M.; Jäger, A.K. Ethnopharmacology; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Howes, M.J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.E.; Leaman, D.J.; et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Ethnobotany, phylogeny, and ‘omics’ for human health and food security. Trends Plant Sci. 2017, 22, 187–191. [Google Scholar] [CrossRef]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Reaffirming ‘Ethnobotanical Convergence’. Trends Plant Sci. 2017, 22, 640. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Klitgaard, B.B.; Forest, F.; Francis, L.; Savolainen, V.; Williamson, E.M.; Hawkins, J.A. The use of phylogeny to interpret cross-cultural patterns in plant use and guide medicinal plant discovery: An example from Pterocarpus (Leguminosae). PLoS ONE 2011, 6, e22275. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Savolainen, V.; Williamson, E.M.; Forest, F.; Wagstaff, S.J.; Baral, S.R.; Watson, M.F.; Pendry, C.A.; Hawkins, J.A. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc. Natl. Acad. Sci. USA 2012, 109, 15835–15840. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Saslis-Lagoudakis, C.H.; Carrió, E.; Ernst, M.; Garnatje, T.; Grace, O.M.; Gras, A.; Mumbrú, M.; Vallès, J.; Vitales, D.; et al. A phylogenetic road map to antimalarial Artemisia species. J. Ethnopharmacol. 2018, 225, 1–9. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A. Phytotherapy in chronic prostatitis. Urology 2002, 60, 35–37. [Google Scholar] [CrossRef]
- Tag, H.; Kalita, P.; Dwivedi, P.; Das, A.K.; Namsa, N.D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, Northeast, India. J. Ethnopharmacol. 2012, 141, 786–795. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Pharmaceuticals from plants: Great potential, few funds. Lancet 1994, 343, 1513–1515. [Google Scholar] [CrossRef]
- Fletcher, M.S.; Hamilton, R.; Dressler, W.; Palmer, L. Indigenous knowledge and the shackles of wilderness. Proc. Natl. Acad. Sci. USA 2021, 118, e2022218118. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Ukwuani-Kwaja, A.N.; Garba, A.D.; Singh, D.; Malami, I.; Salihu, T.S.; Muhammad, A.; Yahaya, Y.; Sule, S.M.; Ahmed, S.J. Ethnobotanical study of medicinal plants used for cancer treatment in Kebbi state, North-west Nigeria. Acta Ecol. Sin. 2020, 40, 306–314. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Naqvi, A.A.; Shehzad, A.; Al-Ghamdi, M.S. Role of traditional Islamic and Arabic plants in cancer therapy. J. Tradit. Complement. Med. 2017, 7, 195–204. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Bhatia, A.; Arora, S.; Singh, B.; Kaur, G.; Nagpal, A. Anticancer potential of Himalayan plants. Phytochem. Rev. 2011, 10, 309–323. [Google Scholar] [CrossRef]
- Jacobo-Herrera, N.J.; Jacobo-Herrera, F.E.; Zentella-Dehesa, A.; Andrade-Cetto, A.; Heinrich, M.; Pérez-Plasencia, C. Medicinal plants used in Mexican traditional medicine for the treatment of colorectal cancer. J. Ethnopharmacol. 2016, 179, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Koduru, S.; Grierson, D.S.; Afolayan, A.J. Ethnobotanical information of medicinal plants used for treatment of cancer in the Eastern Cape Province, South Africa. Curr. Sci. 2007, 906–908. [Google Scholar]
- Soladoye, M.O.; Amusa, N.A.; Raji-Esan, S.O.; Chukwuma, E.C.; Taiwo, A.A. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann. Biol. Res. 2010, 1, 261–273. [Google Scholar]
- Rigat, M.; Gras, A.; Vallès, J.; Garnatje, T. Estudis etnobotànics a la comarca del Ripollès (Pirineu, Catalunya, península Ibèrica). Collect. Bot. 2017, 36, e003. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Global documentation of traditionally used medicinal plants in cancer management: A systematic review. S. Afr. J. Bot. 2021, 138, 424–494. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Available online: https://www.ema.europa.eu/en (accessed on 18 March 2022).
- Duke, J.A. CRC Handbook of Medicinal Herbs, 2nd ed.; American Botanical Council: Austin, TX, USA, 2003. [Google Scholar]
- Fitoterapia.net. Available online: https://www.fitoterapia.net/index.html (accessed on 18 March 2022).
- Vigo, J. L’alta Muntanya Catalana, Flora i Vegetació, 2nd ed.; Centre Excursionista de Catalunya/Institut d’Estudis Catalans: Barcelona, Spain, 2008. [Google Scholar]
- Brower, V. Back to nature: Extinction of medicinal plants threatens drug discovery. J. Natl. Cancer Inst. 2008, 100, 838–839. [Google Scholar] [CrossRef]
- Hao, D.C.; He, C.N.; Shen, J.; Xiao, P.G. Anticancer chemodiversity of Ranunculaceae medicinal plants: Molecular Mechanisms and Functions. Curr. Genom. 2017, 18, 39–59. [Google Scholar] [CrossRef]
- Hao, D.C.; Xiao, P.G.; Liu, M.; Peng, Y.; He, C.N. Pharmaphylogeny vs. pharmacophylogenomics: Molecular phylogeny, evolution and drug discovery. Acta Pharm. Sin. 2014, 49, 1387–1394. [Google Scholar]
- Sagar, S.M. Future directions for research on Silybum marianum for cancer patients. Integr. Cancer Ther. 2007, 6, 166–173. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Sais, E.; Cañete, M.; Marruecos, J.; Cuyàs, E.; Izquierdo, A.; Porta, R.; Haro, M.; Brunet, J.; Pedraza, S.; et al. Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget 2016, 7, 32006–32014. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Won, S.Y.; Kim, J.S. Insight on Rosaceae family with genome sequencing and functional genomics perspective. BioMed Res. Int. 2019, 2019, 7519687. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Park, Y.; Leitzmann, M.F.; Freedman, N.D.; Dowling, E.C.; Reedy, J.; Schatzkin, A.; Hollenbeck, A.; Subar, A.F. Fruit and vegetable intake and risk of cancer: A prospective cohort study. Am. J. Clin. Nutr. 2008, 89, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J. Fruit and vegetables and cancer risk. Br. J. Cancer 2011, 104, 6–11. [Google Scholar] [CrossRef]
- Vallès, J.; D’Ambrosio, U.; Gras, A.; Parada, M.; Rigat, M.; Serrasolses, G.; Garnatje, T. Medicinal and food plants in ethnobotany and ethnopharmacology: Folk functional foods in Catalonia (Iberian Peninsula). In Recent Advances in Pharmaceutical Sciences VII; Muñoz-Torrero, D., Riu, M., Feliu, C., Eds.; Research Signpost: Trivandra, India, 2017; pp. 1–17. [Google Scholar]
- Poppel, G.; Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A. Brassica vegetables and cancer prevention. Adv. Exp. Med. Biol. 1999, 472, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- ESCOP (European Scientific Cooperative on Phytotherapy). Available online: https://escop.com/online-consultation (accessed on 18 March 2022).
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20 (Suppl. 5), 464S–472S. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A review of the antioxidant activity of celery (Apium graveolens L.). Evid.-Based Complement. Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciąg, A.; Szoka, Ł.; Karna, E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their seed hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Peixoto, V.; Teixeira, A.; Sousa, D.; Barros, L.; Ferreira, I.C.; Vasconcelos, M.H. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cell lines by interfering with cell cycle and inducing apoptosis. Food Chem. Tox. 2018, 118, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Ad’hiah, A.H.; Al-Bederi, O.N.; Al-Sammarrae, K.W. Cytotoxic effects of Agrimonia eupatoria L. against cancer cell lines in vitro. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 14, 87–92. [Google Scholar] [CrossRef][Green Version]
- Fredotović, Ž.; Soldo, B.; Šprung, M.; Marijanović, Z.; Jerković, I.; Puizina, J. Comparison of organosulfur and amino acid composition between triploid onion Allium cornutum Clementi ex Visiani, 1842, and common onion Allium cepa L., and evidences for antiproliferative activity of their extracts. Plants 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Furuta, S.; Ishikawa, H.; Kusuma, I.W.; Shimizu, K.; Kondo, R. Anti-melanogenesis properties of quercetin-and its derivative-rich extract from Allium cepa. Food Chem. 2011, 124, 1024–1028. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, W.S.; Jung, J.H.; Jeong, J.H.; Park, C.; Kim, H.J.; Ryu, C.H.; Shin, S.C.; Hong, S.C.; Choi, Y.H. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem. Toxicol. 2013, 62, 382–389. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Lipowski, J.; Drela, N.; Kowalik, A.; Püssa, T.; Matt, D.; Luik, A.; Gozdowski, D.; Rembiałkowska, E. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: Metabolomics, antioxidant levels and anticancer activity. J. Sci. Food Agric. 2014, 94, 2618–2629. [Google Scholar] [CrossRef]
- Kiani, S.; Akhavan-Niaki, H.; Fattahi, S.; Kavoosian, S.; Jelodar, N.B.; Bagheri, N.; Zarrini, H.N. Purified sulforaphane from broccoli (Brassica oleracea var. italica) leads to alterations of CDX1 and CDX2 expression and changes in miR-9 and miR-326 levels in human gastric cancer cells. Gene 2018, 678, 115–123. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Abdulamir, A.S.; Bakar, F.A.; Jalilian, F.A.; Jahanshiri, F.; Abas, F.; Sekawi, Z. Novel anticancer activity and anticancer mechanisms of Brassica oleracea L. var. capitata f. rubra. Eur. J. Integr. Med. 2013, 5, 450–464. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Liu, C.; Jin, J.; Shao, B.; Shen, L. Inhibition of growth and induction of apoptosis in A549 cells by compounds from oxheart cabbage extract. J. Sci. Food Agric. 2016, 96, 3813–3820. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakashima, S.; Abdel-Halim, O.B.; Morikawa, T.; Yoshikawa, M. Cucurbitane-type triterpenes with anti-proliferative effects on U937 cells from an Egyptian natural medicine, Bryonia cretica: Structures of new triterpene glycosides, bryoniaosides A and B. Chem. Pharm. Bull. 2010, 58, 747–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benarba, B.; Meddah, B.; Aoues, A. Bryonia dioica aqueous extract induces apoptosis through mitochondrial intrinsic pathway in BL41 Burkitt’s lymphoma cells. J. Ethnopharmacol. 2012, 141, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Abudunia, A.M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essani, E.M.; Salama, A.; Khedid, K.; Ibrahimi, A. Anticandidal, antibacterial, cytotoxic and antioxidant activities of Calendula arvensis flowers. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kogan, N.M.; Rabinowitz, R.; Levi, P.; Gibson, D.; Sandor, P.; Schlesinger, M.; Mechoulam, R. Synthesis and antitumor activity of quinonoid derivatives of cannabinoids. J. Med. Chem. 2004, 47, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Mangoato, I.M.; Mahadevappa, C.P.; Matsabisa, M.G. Cannabis sativa L. Extracts can reverse drug resistance in colorectal carcinoma cells in vitro. Synergy 2019, 9, 100056. [Google Scholar] [CrossRef]
- Guesmi, F.; Hmed, M.B.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. In vivo pathogenesis of colon carcinoma and its suppression by hydrophilic fractions of Clematis flammula via activation of TRAIL death machinery (DRs) expression. Biomed. Pharmacother. 2019, 109, 2182–2191. [Google Scholar] [CrossRef]
- Mir, M.A.; Ganai, S.A.; Mansoor, S.; Jan, S.; Mani, P.; Masoodi, K.Z.; Amin, H.; Rehman, M.U.; Ahmad, P. Isolation, purification and characterization of naturally derived Crocetin beta-d-glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER-alpha/HDAC2. Saudi J. Biol. Sci. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Abdullaev, F.I.; Riveron-Negrete, L.; Caballero-Ortega, H.; Hernández, J.M.; Perez-Lopez, I.; Pereda-Miranda, R.; Espinosa-Aguirre, J.J. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.). Toxicol. In Vitro 2003, 17, 731–736. [Google Scholar] [CrossRef]
- Behdani, M.A.; Hoshyar, R. Phytochemical properties of Iranian organic saffron stigma: Antioxidant, anticancer and apoptotic approaches. Cell. Mol. Biol. 2016, 62, 69–73. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, J.; Niu, J.; Huang, Y.F.; Chen, M.; Wang, M.Z.; Shang, Q.; Lu, W.Q.; Peng, L.H.; Jiang, Z.H. Synthesis, characterization and inhibitory effects of crocetin derivative compounds in cancer and inflammation. Biomed. Pharmacother. 2018, 98, 157–164. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, C. Antioxidant activity, cytotoxic activity and metabolic profiling of juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef]
- Geromichalos, G.D.; Papadopoulos, T.; Sahpazidou, D.; Sinakos, Z. Safranal, a Crocus sativus L. constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia. In silico and in vitro study. Food Chem. Toxicol. 2014, 74, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Choi, H.S.; Kim, S.L.; Kim, J.H.; Yun, B.S.; Lee, D.S. 6-Methoxymellein isolated from carrot (Daucus carota L.) targets breast cancer stem cells by regulating NF-κB signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef] [PubMed]
- Jafargholizadeh, N.; Zargar, S.J.; Yassa, N.; Tavakoli, S. Purification of cucurbitacins D, E, and I from Ecballium elaterium (L.) A. rich fruits and study of their cytotoxic effects on the AGS cell line. Asian Pac. J. Cancer Prev. 2016, 17, 4631. [Google Scholar] [CrossRef]
- Touihri, I.; Kallech-Ziri, O.; Boulila, A.; Fatnassi, S.; Marrakchi, N.; Luis, J.; Hanchi, B. Ecballium elaterium (L.) A. Rich. seed oil: Chemical composition and antiproliferative effect on human colonic adenocarcinoma and fibrosarcoma cancer cell lines. Arab. J. Chem. 2019, 12, 2347–2355. [Google Scholar] [CrossRef]
- Jacquot, C.; Rousseau, B.; Carbonnelle, D.; Chinou, I.; Malleter, M.; Tomasoni, C.; Roussakis, C. Cucurbitacin-D-induced CDK1 mRNA up-regulation causes proliferation arrest of a non-small cell lung carcinoma cell line (NSCLC-N6). Anticancer Res. 2014, 34, 4797–4806. [Google Scholar]
- Paun, G.; Neagu, E.; Litescu, S.C.; Rotinberg, P.; Radu, G.L. Application ofmembrane processes for the concentration of Symphytum officinale and Geranium robertianum extracts to obtain compounds with high anti-oxidative activity. J. Serb. Chem. Soc. 2012, 77, 1191–1203. [Google Scholar] [CrossRef]
- Jantaharn, P.; Mongkolthanaruk, W.; Senawong, T.; Jogloy, S.; McCloskey, S. Bioactive compounds from organic extracts of Helianthus tuberosus L. flowers. Ind. Crops Prod. 2018, 119, 57–63. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, M.; Gao, M.; Zhuo, R.; Zhang, L.; Xiao, H. Cytotoxic constituents from the leaves of Jerusalem artichoke (Helianthus tuberosus L.) and their structure–activity relationships. Phytochem. Lett. 2013, 6, 21–25. [Google Scholar] [CrossRef]
- Iguchi, T.; Yokosuka, A.; Kawahata, R.; Andou, M.; Mimaki, Y. Bufadienolides from the whole plants of Helleborus foetidus and their cytotoxicity. Phytochemistry 2020, 172, 112277. [Google Scholar] [CrossRef]
- Alesiani, D.; Pichichero, E.; Canuti, L.; Cicconi, R.; Karou, D.; D’Arcangelo, G.; Canini, A. Identification of phenolic compounds from medicinal and melliferous plants and their cytotoxic activity in cancer cells. Caryologia 2007, 60, 90–95. [Google Scholar] [CrossRef]
- Tang, E.L.H.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum crispum has antioxidant properties, protects against DNA damage and inhibits proliferation and migration of cancer cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.Đ.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT-Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Galvez, M.; Martın-Cordero, C.; Lopez-Lazaro, M.; Cortes, F.; Ayuso, M.J. Cytotoxic effect of Plantago spp. on cancer cell lines. J. Ethnopharmacol. 2003, 88, 125–130. [Google Scholar] [CrossRef]
- Piyaviriyakul, S.; Siripong, P.; Vallisuta, O. HPTLC simultaneous quantification of triterpene acids for quality control of Plantago major L. and evaluation of their cytotoxic and antioxidant activities. Ind. Crops Prod. 2014, 60, 239–246. [Google Scholar] [CrossRef]
- Radovanovic, A.M.; Cupara, S.M.; Popovic, S.L.; Tomovic, M.T.; Slavkovska, V.N.; Jankovic, S.M. Cytotoxic effect of Potentilla reptans L. rhizome and aerial part extracts. Acta Pol. Pharm. 2013, 70, 851–854. [Google Scholar]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Xiao, J.; Fu, X.; You, L.; Liu, R.H. Preparation of Prunella vulgaris polysaccharide-zinc complex and its antiproliferative activity in HepG2 cells. Int. J. Biol. Macromol. 2016, 91, 671–679. [Google Scholar] [CrossRef]
- Dhingra, N.; Kar, A.; Sharma, R.; Bhasin, S. In-vitro antioxidative potential of different fractions from Prunus dulcis seeds: Vis a vis antiproliferative and antibacterial activities of active compounds. S. Afr. J. Bot. 2017, 108, 184–192. [Google Scholar] [CrossRef]
- Eo, H.J.; Park, G.H.; Song, H.M.; Lee, J.W.; Kim, M.K.; Lee, M.H.; Koo, J.S.; Jeong, J.B. Silymarin induces cyclin D1 proteasomal degradation via its phosphorylation of threonine-286 in human colorectal cancer cells. Int. Immunopharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Khoshdel, Z.; Amirghofran, Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: A comparison with desferrioxamine. Eur. J. Pharmacol. 2008, 589, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Sapio, L.; Illiano, M.; Caiafa, I.; Naviglio, S. Chemical analysis and anti-proliferative activity of Campania Thymus vulgaris essential oil. J. Essent. Oil Res. 2017, 29, 461–470. [Google Scholar] [CrossRef]
- Heidari, Z.; Salehzadeh, A.; Sadat Shandiz, S.A.; Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Piccolella, S.; Papale, F.; Nocera, P.; Lettieri, A.; Catauro, M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Encalada, M.A.; Rehecho, S.; Ansorena, D.; Astiasarán, I.; Cavero, R.Y.; Calvo, M.I. Antiproliferative effect of phenylethanoid glycosides from Verbena officinalis L. on colon cancer cell lines. LWT-Food Sci. Technol. 2015, 63, 1016–1022. [Google Scholar] [CrossRef]
- Deffontaines, P. Geografia dels Països Catalans; Editorial Ariel: Barcelona, Spain, 1978. [Google Scholar]
- Riba, O.; de Bolòs, O.; Panareda, J.M.; Nuet, J.; Gosàlbez, J. Geografia física dels Països Catalans; Ketres Editora: Barcelona, Spain, 1984. [Google Scholar]
- De Bolòs, O.; Vigo, J. Flora dels Països Catalans; Editorial Barcino: Barcelona, Spain, 1984. [Google Scholar]
- De Bolòs, O.; Vigo, J.; Masalles, R.M.; Ninot, J. Flora Manual dels Països Catalans, 3rd ed.; Editorial Pòrtic: Barcelona, Spain, 2005. [Google Scholar]
- Folch, R. La Vegetació dels Països Catalans; Ketres Editora: Barcelona, Spain, 1984. [Google Scholar]
- Badia, A.M. Llengua i Cultura als Països Catalans; Edicions 62: Barcelona, Spain, 1966. [Google Scholar]
- Departament d’Estadística del Govern d’Andorra. Available online: https://www.estadistica.ad/ (accessed on 5 March 2022).
- IBESTAT (Institut d’Estadística de les Illes Balears). Available online: https://ibestat.caib.es/ibestat/inici (accessed on 5 March 2022).
- IDESCAT (Institut d’Estadística de Catalunya). Available online: https://www.idescat.cat/ (accessed on 5 March 2022).
- ISTAT (Istituto Nazionale di Statistica). Available online: https://www.istat.it/ (accessed on 5 March 2022).
- Portal Estadístic de la Generalitat Valenciana. Available online: https://pegv.gva.es/va/ (accessed on 5 March 2022).
- Sáez, L.; (Universitat Autònoma de Barcelona, Bellaterra, Catalonia, Spain). Personal Communication, 2019.
- Gras, A.; Serrasolses, G.; Vallès, J.; Garnatje, T. Traditional knowledge in semi-rural close to industrial areas: Ethnobotanical studies in western Gironès (Catalonia, Iberian Peninsula). J. Ethnobiol. Ethnomed. 2019, 15, 1–37. [Google Scholar] [CrossRef]
- ISE (International Society of Ethnobiology). International Society of Ethnobiology Code of Ethics (with 2008 Additions). Available online: http://ethnobiology.net/code-of-ethics/ (accessed on 5 March 2022).
- Garnatje, T.; Gras, A.; Parada, J.; Parada, M.; Vallès, J. La web ‘Etnobotànica dels Països Catalans’: Coneixement tradicional al servei de la societat. Collect. Bot. 2021, 40, e006. [Google Scholar] [CrossRef]
- APG (Angiosperm Phylogeny Group). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]

| Taxon (Herbarium Voucher) | Family | Plant Part Used | Pharmaceutical Form | Pharmacological Literature |
|---|---|---|---|---|
| Curative | ||||
| Abies alba Mill. (BCN 24699) | Pinaceae | Resin | Without pharmaceutical form (internal use) | [33] |
| Agrimonia eupatoria L. (BCN 24704) | Rosaceae | Aerial part | Unknown | [33] |
| Allium cepa L. (BCN 27279) | Amaryllidaceae | Bulb | Tisane | [32,33,34] |
| Anemone hepatica L. (BCN 27247) | Ranunculaceae | Leaf | Tisane | |
| Angelica sylvestris L. (BCN 24712) | Apiaceae | Unknown | Unknown | |
| Brassica oleracea L. (BCN 24728) | Brassicaceae | Leaf | Tisane/Without pharmaceutical form (internal use) | [33,34] |
| Bryonia cretica L. (BCN 24730) | Cucurbitaceae | Root | Without pharmaceutical form (topical use) | [33] |
| Clematis flammula L. (BCN 29856) | Ranunculaceae | Aerial part | Poultice | |
| Crocus sativus L. (BCN 32170) | Iridaceae | Styles and stigmas | Poultice | [33,34] |
| Daucus carota L. subsp. sativus (Hoffm.) Arcang. (BCN 46847) | Apiaceae | Root | Tisane | [33,34] |
| Ecballium elaterium (L.) A.Rich (BCN 46846) | Cucurbitaceae | Aerial part | Unknown | |
| Geranium robertianum L. (BCN 24894) | Geraniaceae | Aerial part | Tisane | [33] |
| Helleborus foetidus L. (BCN 29705) | Ranunculaceae | Aerial part | Poultice | |
| Malva sylvestris L. (BCN 24924) | Malvaceae | Aerial part/Flower | Tisane | [33] |
| Plantago lanceolata L. (BCN 24949) | Plantaginaceae | Leaf | Without pharmaceutical form (topical use) | [33] |
| Plantago major L. (BCN 24950) | Plantaginaceae | Aerial part/Leaf | Without pharmaceutical form (internal and topical use) | [33] |
| Potentilla reptans L. (BCN 47660) | Rosaceae | Leaf | Tisane | [33] |
| Prunella vulgaris L. (BCN 29759) | Lamiaceae | Flower | Tisane | [33] |
| Prunus dulcis (Mill.) Weeb. (BCN 46833) | Rosaceae | Resin | Without pharmaceutical form (topical use) | [33] |
| Ranunculus bulbosus L. (BCN 24966) | Ranunculaceae | Root | Without pharmaceutical form (topical use) | [33] |
| Ranunculus parnassifolius L. (BCN 24967) | Ranunculaceae | Root/Whole plant | Tisane/Without pharmaceutical form (topical use) | |
| Rubus ulmifolius Schott (BCN 24978) | Rosaceae | Tender bud | Tisane | |
| Ruta chalepensis L. (BCN 24980) | Rutaceae | Aerial part | Unknown | [33] |
| Scrophularia alpestris J.Gay ex Benth. (BCN 29790) | Scrophulariaceae | Young leaf | Without pharmaceutical form (topical use) | |
| Silybum marianum (L.) Gaertn. (BCN 29958) | Asteraceae | Inflorescence | Unknown | [33,34] |
| Tetragonia tetragonioides (Pall.) Kuntze (BCN 29805) | Aizoaceae | Young aerial part | Without pharmaceutical form (internal use) | |
| Thymus vulgaris L. (BCN 25023) | Lamiaceae | Flowering aerial part | Gargarism | [33] |
| Verbena officinalis L. (BCN 25036) | Verbenaceae | Aerial part | Poultice | [33] |
| Viola sylvestris Lam. (BCN 26791) | Violaceae | Leaf | Tisane | |
| Palliative | ||||
| Cannabis sativa L. (BCN 24735) | Cannabaceae | Leaf/Young aerial part | Tisane/Poultice | [33,34] |
| Papaver somniferum L. (BCN 24941) | Papaveraceae | Latex | Unknown | [33] |
| Plantago sempervirens Crantz (BCN 96761) | Plantaginaceae | Flowering aerial part | Mouthwash | |
| Santolina chamaecyparissus L. (BCN 24986) | Asteraceae | Flowering aerial part | Mouthwash | [33] |
| Preventive | ||||
| Achillea millefolium L. (BCN 24700) | Asteraceae | Inflorescence | Tisane | [33] |
| Angelica sylvestris L. (BCN 24712) | Apiaceae | Unknown | Unknown | |
| Apium graveolens L. (BCN 24714) | Apiaceae | Aerial part | Unknown | [33] |
| Beta vulgaris L. subsp. vulgaris var. conditiva Alef. (BCN 52089) | Amaranthaceae | Root | Tisane/Without pharmaceutical form (internal use) | [33] |
| Brassica oleracea L. (BCN 24728) | Brassicaceae | Leaf | Without pharmaceutical form (internal use) | [33,34] |
| Calendula arvensis L. (BCN 32863) | Asteraceae | Inflorescence | Liniment | |
| Helianthus tuberosus L.(BCN 24898) | Asteraceae | Tuber | Boiled | |
| Petroselinum crispum (Mill.) Hill (BCN 24943) | Apiaceae | Leaf | Poultice | [33] |
| Ranunculus parnassifolius L. (BCN 24967) | Ranunculaceae | Root/Whole plant | Tisane/Without pharmaceutical form (topical use) | |
| Sambucus nigra L. (BCN 24984) | Adoxaceae | Inflorescence | Fumigation | [33] |
| Urtica sp. | Urticaceae | Unknown | Tisane | [33] |
| Taxon | Plant Part Used | Extract | Chemical Compound | Cell Line | Cytotoxic Activity (Key Results) | Reference |
|---|---|---|---|---|---|---|
| Abies alba Mill. | Seed and cone | Aq | - | MCF7 and MDA-MBA-231 | The influence of the essential oils on the cancer cells was weak. The IC50 values were similar to those found towards normal cells (100 µg/mL) | [52] |
| Achillea millefolium L. | - | EtOH | Phenolic acids (3,5-O-dicaffeoylquinic acid, 5-O-caffeoylquinic acid), flavonoids (luteolin-O-acetylhexoside, apigenin-O-acetylhexoside) | NCI-H460 and HCT-15 | The extract showed an inhibitory effect on the growth of NCI-H460 and HCT-15 cell lines with IC50 values 187.3 µg/mL and 70.8 µg/mL, respectively | [53] |
| Agrimonia eupatoria L. | Aerial part | Aq and MeOH | - | RD and HeLa | The extracts showed anti-tumor properties in a concentration-dependent manner, and the MeOH extract recorded better values of percentage of growth inhibition than aqueous extract in HeLa and RD cell lines (IC50: 96 µg/mL) | [54] |
| Allium cepa L. | Bulb | MeOH | - | HeLa, HCT 116 and U2OS | The IC50 values obtained were 24.79, 24.73 and 36.6 µg/mL for HeLa, HCT 116 and U2OS cell lines, respectively | [55] |
| Bulb | MeOH | Quercetin and quercetin 4′-O-β-glucoside | B16 | Quercetin and quercetin 4′-O-β-glucoside compounds showed inhibition in B16 cells with IC50 values of 26.5 and 131 µM, respectively | [56] | |
| Flower | MeOH | Polyphenols | K562, THP-1 and U937 | The results revealed IC50 value less than 40 µg/mL for U937 cells and 60 µg/mL for THP-1 and K562 | [57] | |
| Beta vulgaris L. subsp. vulgaris var. conditiva Alef. | Root | EtOH | - | AGS | The highest concentration of extract (0.05%) induced significantly greater early apoptosis in relation to the other concentrations. At the same time, it activated the lowest level of late apoptosis and necrosis in AGS cells | [58] |
| Brassica oleracea L. | Sprout | Hx | Sulforaphane | AGS and MKN45 | Significant dose-dependent and anti-proliferative effects were observed on AGS and MKN45 cells, with an IC50 value of about 112 and 125 μg/mL, respectively | [59] |
| Leaf | HCl MeOH | - | HeLa and Hep G2 | The IC50 values of the extract were 23.38 and 28.66 mg/mL for HeLa and Hep G2, respectively | [60] | |
| - | - | Sulforaphane, iberin and iberverin | A549 | The IC50 values were 3.53, 4.93 and 7.07 µg/mL for sulforaphane, iberin and iberverin, respectively | [61] | |
| Bryonia cretica L. | Root | EtOH | Cucurbitacin B and E | U937 | The cucurbitacin B and E showed great effects with IC50 values of 9.2 and 16 nM | [62] |
| Root | Aq | - | BL41 | The IC50 of extract was estimated to be approximately 15.63 µg/mL | [63] | |
| Calendula arvensis L. | Inflorescence | Aq and MeOH | - | AML | The extracts exhibited activity against AML (IC50: 31 mg/mL) | [64] |
| Cannabis sativa L. | - | - | Cannabidiol (1), tetrahydrocannabinol (2) and cannabinol (3) para-quinones | Raji, Jurkat E6-1, SNB-19, MCF7, DU 145, NCI-H-226 and HT-29 | The three compounds displayed antiproliferative activity in all cell lines | [65] |
| Aerial part | Aq, Hx, DCM, DCM:MeOH and MeOH | - | Caco-2, HCT-15, HT-29, LS513 | Aq and DCM:MeOH extracts moderately inhibited the growth in HCT-15 and LS513 cells (IC50: 20–100 µg/mL). Aq and DCM extracts potently inhibited HT-29 cell growth (IC50: 7.52–10.06 µg/mL). Hx and DCM extracts slightly stimulated growth in Caco-2 cells (IC50: 100 µg/mL) | [66] | |
| Clematis flammula L. | Aerial part | - | - | HCT 116 | The extract showed apoptosis in HCT 116 cell lines | [67] |
| Crocus sativus L. | Leaf | PET | Crocetin (β-D-glucosyl) ester | MCF7 | The antiproliferative activity of the compound against MCF7 cell line has showed inhibitory effect in a dose-dependent way with IC50 value of 628.36 µg/mL | [68] |
| Stigma | - | - | HeLa, A-204 and Hep G2 | All tested cell lines showed a good response to the effect of the saffron extract (50–400 µg/mL), but the A-204 cells showed a higher sensitivity to the inhibitory effect | [69] | |
| Stigma | MeOH | - | AGS, MDA-MB-468 and U-87 | The IC50 are between 0.8 and 4.5 mg/mL | [70] | |
| Stigma | - | Crocetin | A549, B16-F10, MCF7 and SK-OV-3 | The IC50 were 79.79, 55.39, 270.13 and 559.0 µg/mL for MCF7, A549, B16-F10 and SK-OV-3, respectively | [71] | |
| Stigma | - | Phenols | Caco-2 | A significant 32% decrease in Caco-2 cell viability was observed, but only at a concentration of 50 µL/mL | [72] | |
| - | - | Crocin and safranal | K-562 | Drug cytotoxicity experiments showed a dose-dependent cell growth inhibition after exposure of cells to crocin and safranal withIC50 values of 160.00 μM and 241.00 μM, respectively | [73] | |
| Daucus carota L. subsp. sativus (Hoffm.) Arcang. | Root | MeOH | 6-Methoxymellein | MCF7 and MDA-MB-231 | The compound induced suppression of proliferation at >0.8 mM (MDA-MB-231) and >0.5 nM (MCF7) | [74] |
| Ecballium elaterium (L.) A.Rich | Fruit | MeOH | Cucurbitacin D, E and I | AGS | The cytotoxic effects on AGS gastric cancer cell line showed that cucurbitacin E has greater cytotoxicity in comparison with cucurbitacins D and I. The IC50 values were 0.3, 0.1, and 0.5 μg/mL for cucurbitacins D, E, and I, respectively. | [75] |
| Seed | Hx | - | HT-29 and HT-1080 | The extract showed a potent antiproliferative HT-29 AND HT-1080 cell lines and the IC50 values were 4.86 µg/mL and 4.16 µg/mL, respectively | [76] | |
| - | - | Cucurbitacin D | NSCLC-N6 | The treatment with cucurbitacin D inhibited NSCLC-N6 proliferation (IC50: 2.5 µg/mL) | [77] | |
| Geranium robertianum L. | - | Aq and EtOH | - | Hep-2p | The extracts showed cytotoxic effect on Hep-2p cancer cells (6.1–25.39% in the EtOH extracts; 0.9–32.5% in the Aq extracts) | [78] |
| Helianthus tuberosus L. | Flower | Hx | - | HT-29 and HCT 116 | Feradiol exhibited a significant growth inhibitory effect against HT-29 and HCT 116 cell lines (IC50 values of 3.93 and 6.02 μg/mL, respectively) | [79] |
| Leaf | EtOAc | 4,15-iso-Atripliciolide tiglate | A549, HeLa and MCF7 | The compound exhibited significant activity against MCF7, A549 and HeLa (1.97, 7.79, 9.87 µg/mL, respectively) | [80] | |
| Helleborus foetidus L. | Whole plant | MeOH | Bufadienolide glucosides | A549 and HL-60 | The isolated compounds were cytotoxic to A549 and HL-60 cells, with the IC50 values ranging from 0.019 to 3.0 μM | [81] |
| Malva sylvestris L. | Leaf and flower | MeOH | Phenols | A-375 and B16 | This extract showed a cytotoxic effect for B16 and A-375 cells, an antiproliferative activity of 97% and 85% with respect to the control | [82] |
| Petroselinum crispum (Mill.) Hill | Leaf and stem | Hx | Phenols | MCF7 | The extract tested at 500 μg/mL showed a percentage inhibition of 48.4%, 25.5% and 49.9% on MCF7, MDA-MB-231 and HT-29 cells, respectively | [83] |
| Plantago lanceolata L. | Aerial part | MeOH | Phenols | HeLa, HT-29 and MCF7 | The inhibition of cell growth exerted a stronger effect with IC50: 172.3, 142.8, 405.5 and 551.7 µg/mL for HeLa, MCF7, HT-29 and MRC-5 cell lines, respectively | [84] |
| Leaf | MeOH | Flavonoids | MCF7 and UACC-62 | The extracts showed good values for IC50 (47.16 and 50.58 µg/mL for MCF7 and UACC-62, respectively) | [85] | |
| Plantago major L. | Leaf | MeOH | Flavonoids | MCF7 and UACC-62 | The extracts showed good values of IC50 (46.5 µg/mL for MCF7 and UACC-62) | [85] |
| Seed | MeOH | Triterpene acids | SiHa and Hep G2 | The extract exhibited cytotoxic activity for SiHa and Hep-G2 (IC50: 174.42 and 246.38 µg/mL, respectively) | [86] | |
| Potentilla reptans L. | Aerial part and rhizome | Aq | - | 4T1 | IC50 values were 280.51 μg/mL for rhizome extract and 310.79 μg/mL for aerial parts extract | [87] |
| Aerial part | Aq | - | A549 and MCF7 | Extract exhibited cytotoxic activity for A549 and MCF7 cells (IC50 < 130 µg/mL) | [88] | |
| Prunella vulgaris L. | - | Aq | Polysaccharide–zinc complex | Hep G2 | The polysaccharide–zinc complex inhibits the proliferation (98.4% inhibition rate at 500 μg/mL) of Hep G2 cells | [89] |
| Prunus dulcis (Mill.) Weeb. | Seed | Ace | Gallic acid and pyrogallol | MCF7 and MDA-MB-468 | For MCF7, both compounds showed cytocidal effect at 10 μg/mL, whereas for MDA-MB-468, both compounds showed cytocidal effect at >20 μg/mL | [90] |
| Silybum marianum (L.) Gaertn. | - | - | Silymarin | HCT 116 and SW480 | A HCT 116 cells treated with 50, 100, and 200 μM of silymarin reduced the cell growth by 11%, 22% and 48%, respectively. A SW480 cells treated with 50, 100, and 200 μM of silymarin reduced the cell growth by 13%, 28% and 47% | [91] |
| - | - | Silybin | Jurkat E6-1 | Silybin increased the reduction in Jurkat E6-1 cells in the concentration range of 50–200 μM | [92] | |
| Thymus vulgaris L. | Aerial part | Hx | - | U2OS and PANC-1 | The essential oil causes a very strong inhibition (60%) of cell viability in PANC-1 cells, compared to 40% of reduction observed in U2OS cells at 10 μg/mL | [93] |
| Leaf | EtOH | - | T47D | The extract inhibited 75% of T47D cells at 200 μg/mL | [94] | |
| Leaf | CHCl3 | Polyphenol complex | SH-SY5Y and SK-N-BE(2)-C | The extract showed strong levels of cytotoxicity towards SH-SY5Y and SK-N-BE(2)-C cell lines at the highest tested dose level (125.0 µg/mL) | [95] | |
| Verbena officinalis L. | Aerial part | Aq | Diacetyl-phenylethanoids | DHD/K12/PROb and HCT 116 | Four diacetyl-phenylethanoid compounds exhibited extremely high antiproliferative activity against HCT 116 and DHD/K12/PROb. The IC50 values were similar to vinblastine sulfate (1.28 µg/mL) | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gras, A.; Parada, M.; Pellicer, J.; Vallès, J.; Garnatje, T. Cancer and Traditional Plant Knowledge, an Interesting Field to Explore: Data from the Catalan Linguistic Area. Molecules 2022, 27, 4070. https://doi.org/10.3390/molecules27134070
Gras A, Parada M, Pellicer J, Vallès J, Garnatje T. Cancer and Traditional Plant Knowledge, an Interesting Field to Explore: Data from the Catalan Linguistic Area. Molecules. 2022; 27(13):4070. https://doi.org/10.3390/molecules27134070
Chicago/Turabian StyleGras, Airy, Montse Parada, Jaume Pellicer, Joan Vallès, and Teresa Garnatje. 2022. "Cancer and Traditional Plant Knowledge, an Interesting Field to Explore: Data from the Catalan Linguistic Area" Molecules 27, no. 13: 4070. https://doi.org/10.3390/molecules27134070
APA StyleGras, A., Parada, M., Pellicer, J., Vallès, J., & Garnatje, T. (2022). Cancer and Traditional Plant Knowledge, an Interesting Field to Explore: Data from the Catalan Linguistic Area. Molecules, 27(13), 4070. https://doi.org/10.3390/molecules27134070