Chemical and Pharmacological Profiling of Wrightia coccinea (Roxb. Ex Hornem.) Sims Focusing Antioxidant, Cytotoxic, Antidiarrheal, Hypoglycemic, and Analgesic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Extraction and Partitioning Process
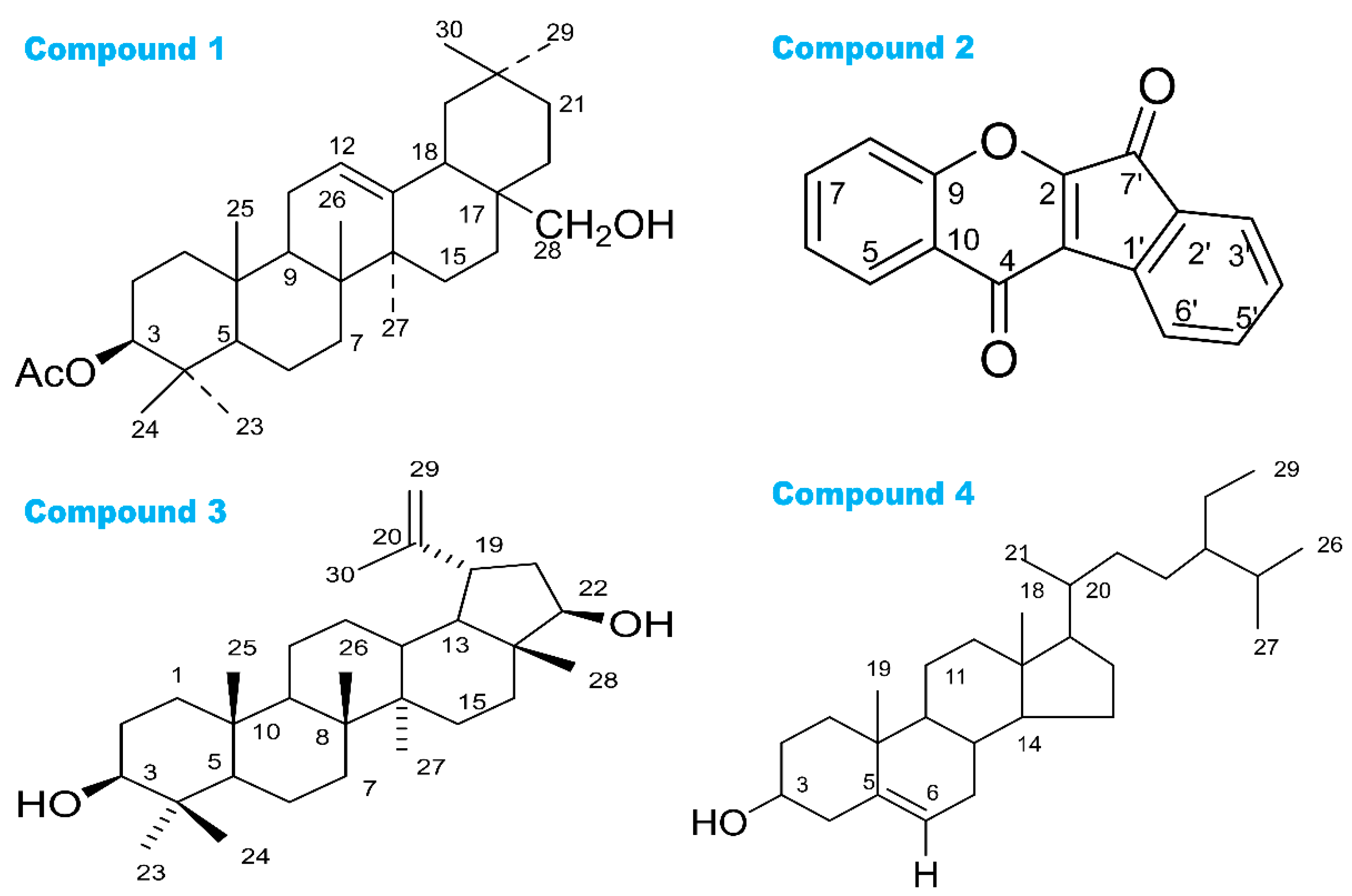
2.4. Isolation of Chemical Compounds
2.5. In Vitro Antioxidant Activity: DPPH Assay
2.6. In Vitro Cytotoxicity
2.7. Experimental Animals
2.8. Preparation of the Loading Doses for In Vivo Study
2.9. In Vivo Study Design
2.10. Anti-Diarrheal Assay
2.11. Hypoglycemic Assay
2.12. Central Analgesic Activity
2.13. Peripheral Analgesic Activity
2.14. Molecular Docking Study
2.14.1. Target Protein Selection
2.14.2. Ligand Preparation
2.14.3. Ligand Protein Interaction
2.15. Statistical Analysis
3. Results
3.1. Phytochemical Studies
3.2. DPPH Free Radical Scavenging Activity
3.3. Cytotoxic Activity
3.4. Anti-Diarrheal Property
3.5. Hypoglycemic Property
3.6. Central Analgesic Activity
3.7. Peripheral Analgesic Activity
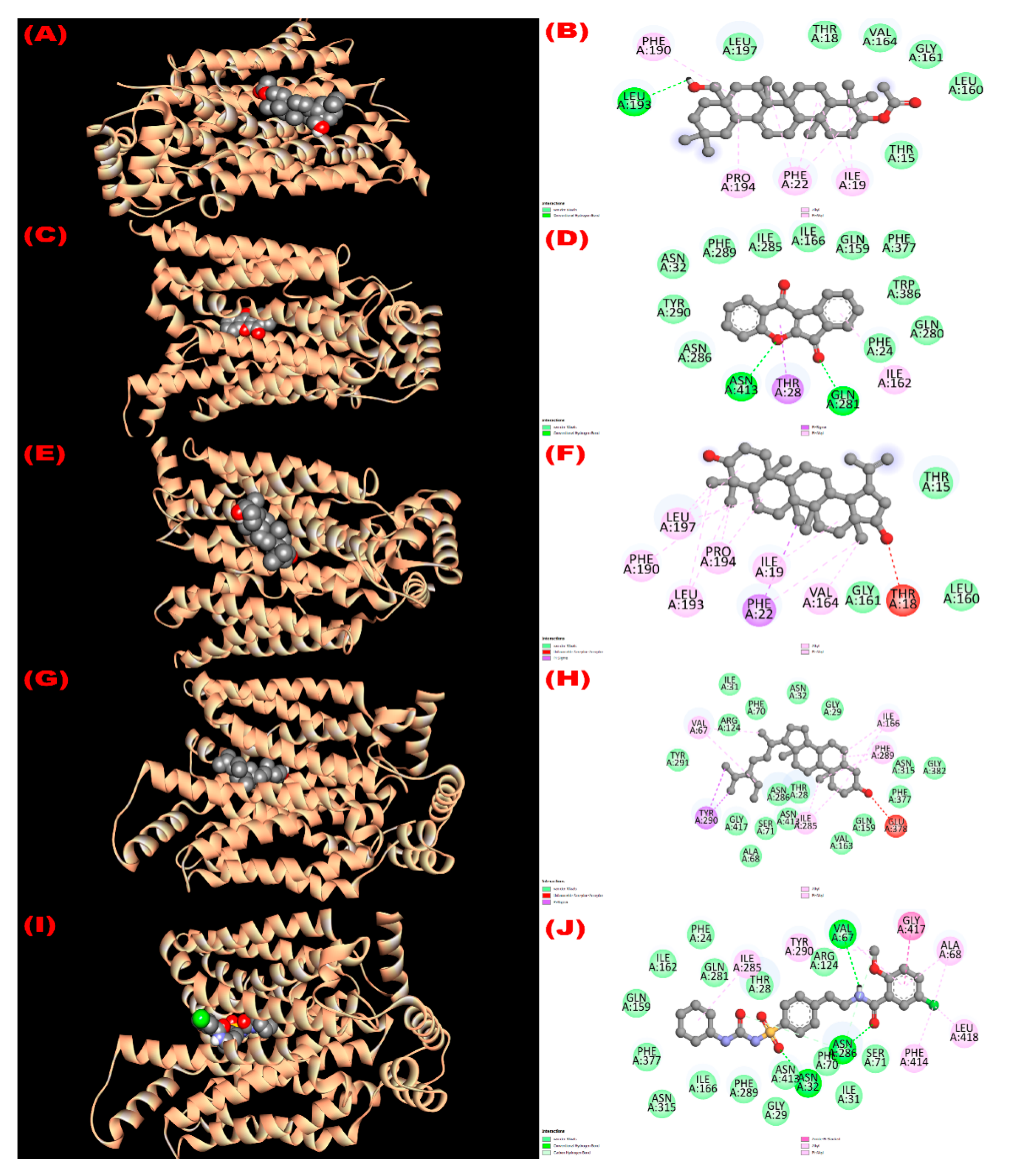
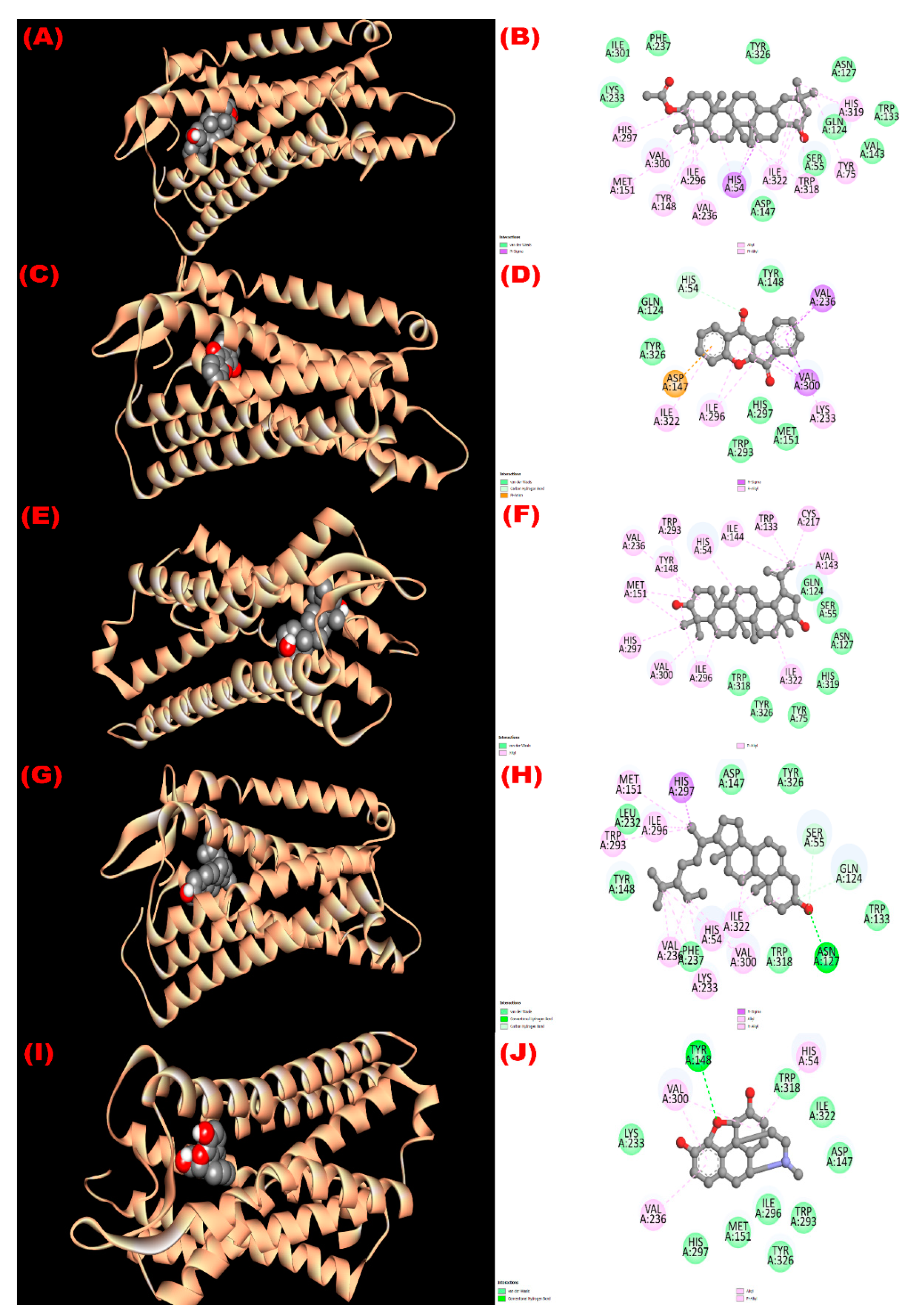
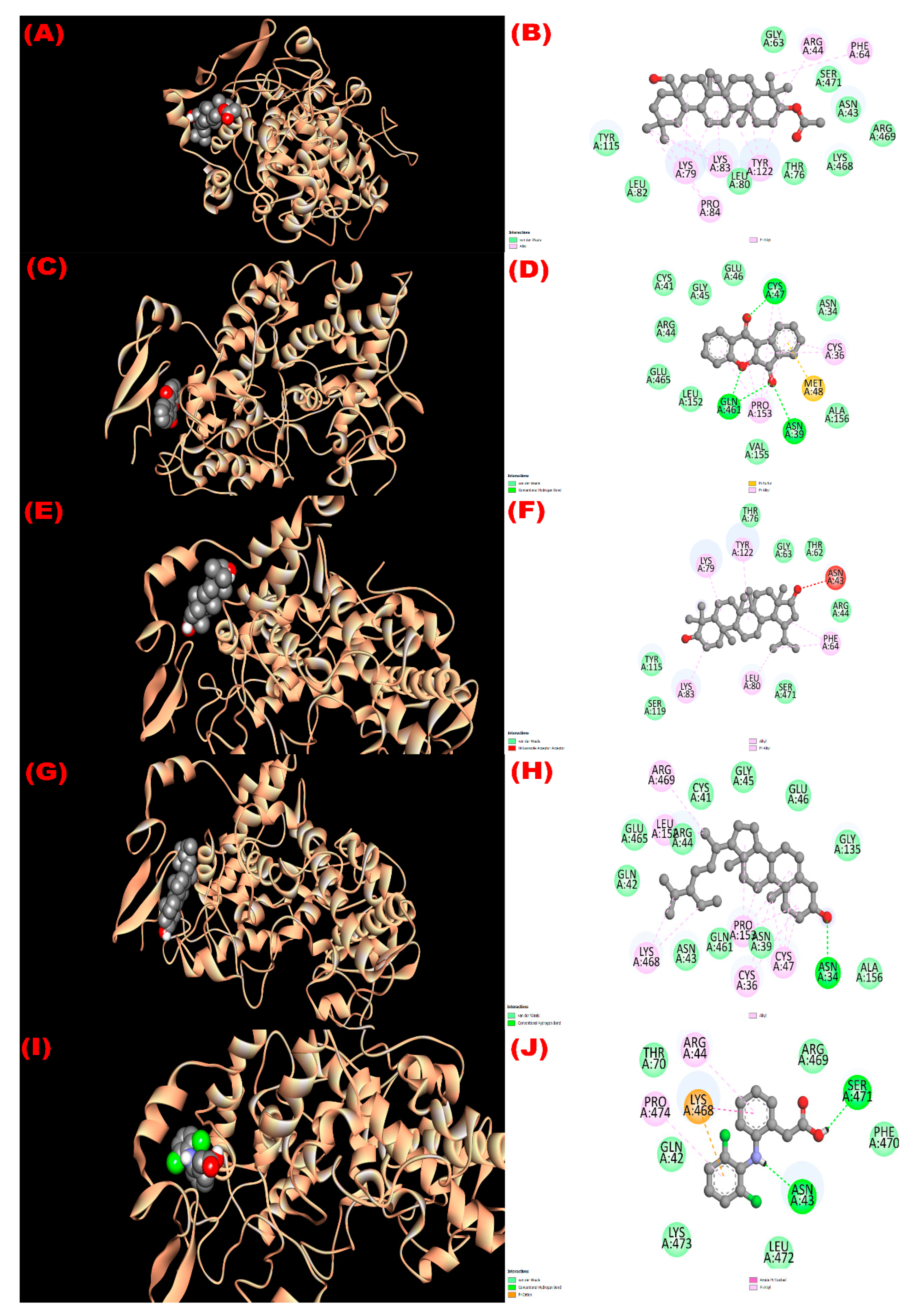
3.8. In Silico Study
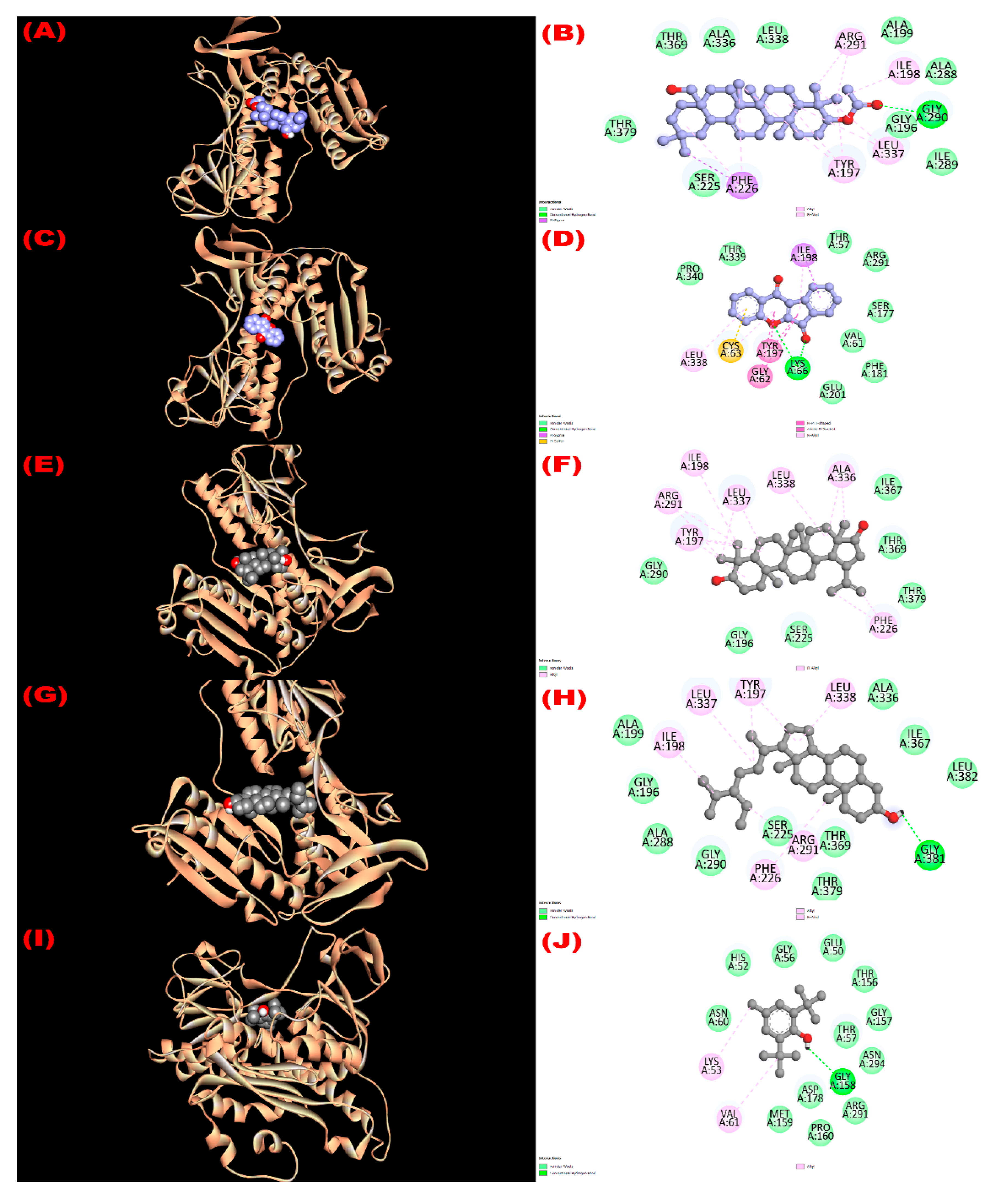
3.8.1. Inhibition of Glutathione Reductase Enzyme: Antioxidant Activity
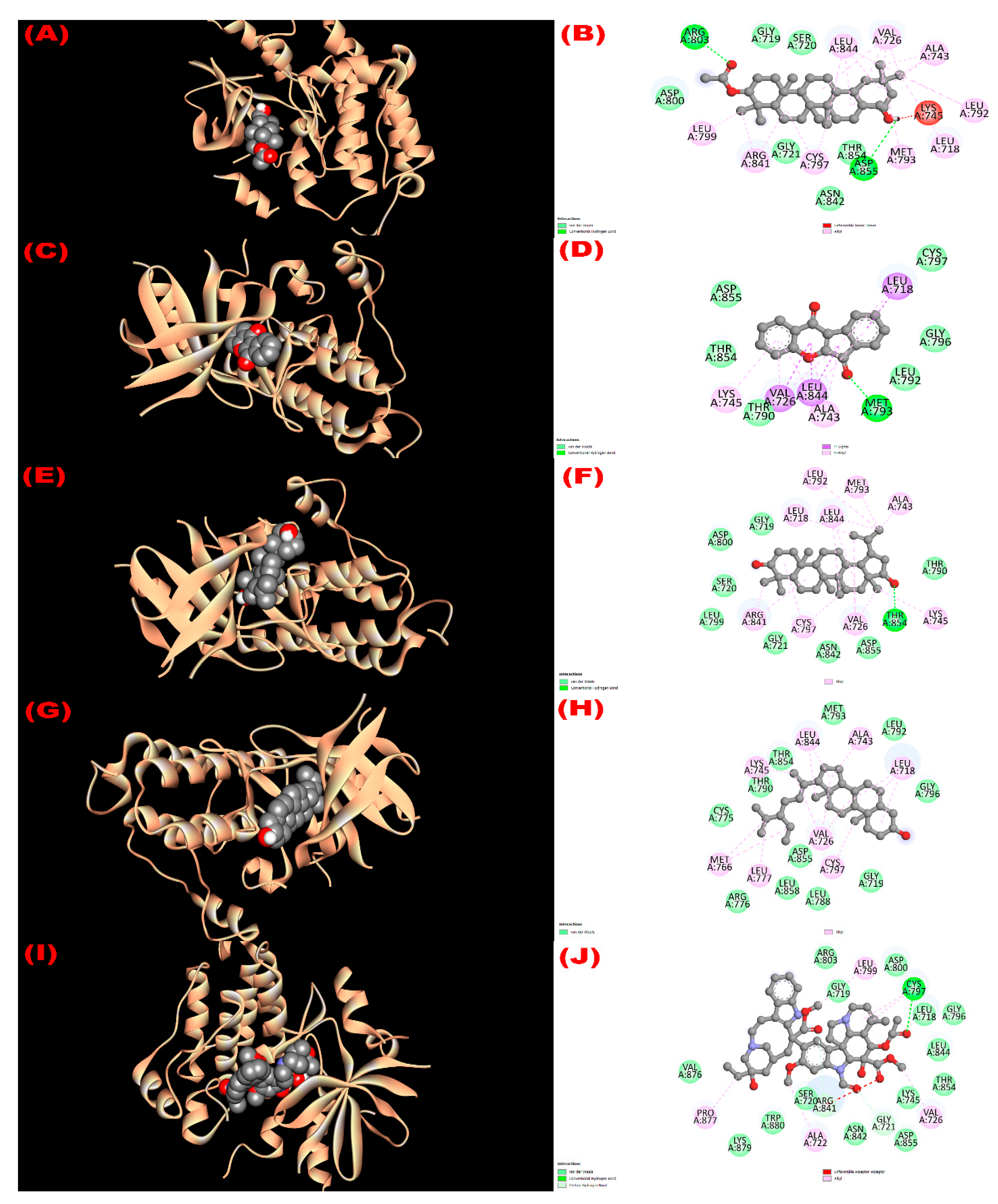
3.8.2. Inhibition of EGFR: Cytotoxicity
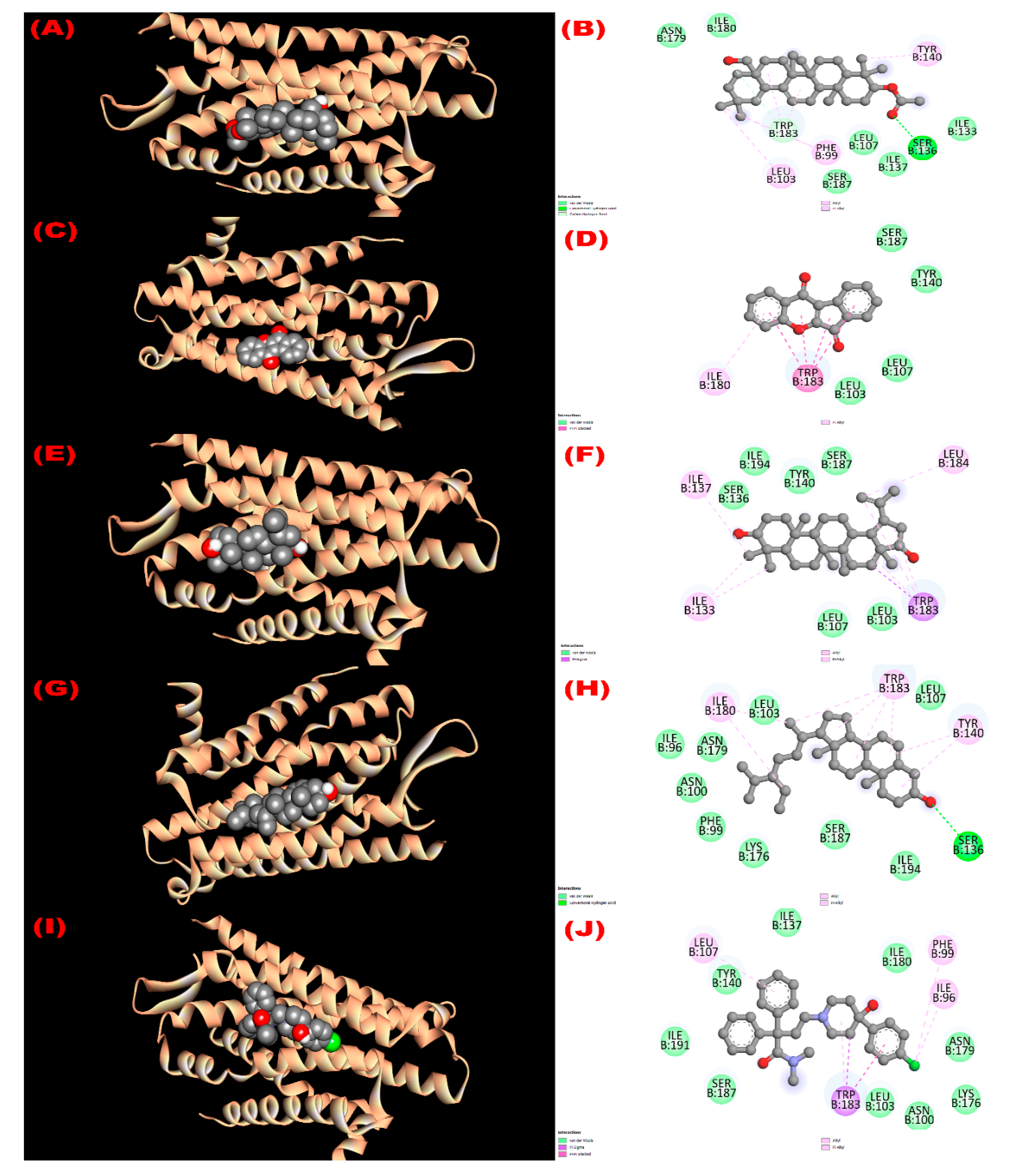
3.8.3. Inhibition of Kappa Opioid Receptor: Antidiarrheal Activity
3.8.4. Inhibition of GLUT 3: Hypoglycemic Activity
3.8.5. Inhibition of Mu-Opioid Receptor and COX-2 Proteins: Analgesic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal plants used against hepatic disorders in Bangladesh: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Uddin, T.M.; Matin Zidan, B.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.J.; Khusro, A.; et al. Allium cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid. Based Complement. Alternat. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Sarwar, S.; Hossain, M.J.; Irfan, N.M.; Ahsan, T.; Arefin, M.S.; Rahman, A.; Alsubaie, A.; Alharthi, B.; Khandaker, M.U.; Bradley, D.A.; et al. Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats. Life 2022, 12, 60. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement. Alternat. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef]
- Baba, S.A.; Vahedi, M.; Ahmad, I.; Rajab, B.S.; Babalghith, A.O.; Irfan, S.; Hossain, M.J. Crocus sativus L. Tepal Extract Induces Apoptosis in Human U87 Glioblastoma Cells. BioMed Res. Int. 2022, 2022, 4740246. [Google Scholar] [CrossRef]
- Anjum, N.; Hossain, M.J.; Haque, M.R.; Chowdhury, A.; Rashid, M.A.; Kuddus, M.R. Phytochemical Investigation of Schleichera oleosa (Lour.) Oken Leaf. Bangladesh Pharm. J. 2021, 24, 33–36. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 28, 1–7. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Kinghorn, A.D.; Farnsworth, N.R. Plant-derived natural products in drug discovery and development: An overview. ACS Symp. 1993, 534, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Rasool, N.; Bukhari, I.H.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In Vitro Antimicrobial, Antioxidant, Cytotoxicity and GC-MS Analysis of Mazus goodenifolius. Molecules 2012, 17, 14275–14287. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine. In Bioorganic Phase in Natural Food: An Overview; Springer: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar]
- Majeed, I.; Rizwan, K.; Ashar, A.; Rasheed, T.; Amarowicz, R.; Kausar, H.; Zia-Ul-Haq, M.; Marceanu, L.G. A Comprehensive Review of the Ethnotraditional Uses and Biological and Pharmacological Potential of the Genus Mimosa. Int. J. Mol. Sci. 2021, 22, 7463. [Google Scholar] [CrossRef]
- Anjum, N.; Hossain, M.J.; Aktar, F.; Haque, M.R.; Rashid, M.A.; Kuddus, M.R. Potential In vitro and In vivo Bioactivities of Schleichera oleosa (Lour.) Oken: A Traditionally Important Medicinal Plant of Bangladesh. Research J. Pharm. Technol. 2022, 15, 113–121. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Endress, M.E.; Bruyns, P.V. A revised classification of the Apocynaceae sl. Bot. Rev. 2000, 66, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Ali, M. Palan, Scarlet wrightia, Wrightia coccinea. Flora of Bangladesh. Available online: www.floraofbangladesh.com/2018/08/palan-scarlet-wrightia-wrightia-coccinea.html (accessed on 22 March 2022).
- Chadha, Y.R. Wealth of India, Raw Material; Chadha, Y.R., Ed.; CSIR: New Delhi, India, 1976; Volume 10, p. 588. [Google Scholar]
- Muruganandam, A.V.; Bhattacharya, S.K.; Ghosal, S. Indole and Flavanoid Constituents of Wrightia tinctoria, W. tomentosa and W. coccinea; NISCAIR-CSIR: New Delhi, India, 2000; pp. 125–131. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants. 1935, Lalit Mohan Basu, Allahbad, India. Available online: https://www.cabdirect.org/cabdirect/abstract/20057004287 (accessed on 1 January 2022).
- Khyade, M.S.; Vaikos, N.P. Pharmacognostical and physio-chemical standardization of leaves of Wrightia tinctoria R. Br. Int. J. Pharm. Res. Dev. 2009, 8, 1–11. [Google Scholar]
- Nadkarni, K.M. Indian Materia Medica, 3rd ed.; Popular Prakashan Pvt. Ltd: Mumbai, India, 1954; Volume 1, p. 1296. [Google Scholar]
- Chandrashekar, R.; Adake, P.; Rao, S.N.; Santanusaha, S. Wrightia tinctoria: An overview. J. Drug Deliver. Therapeut. 2013, 3, 1–3. [Google Scholar]
- Azwanida, N.N. A review on the extraction methods uses in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015, 4, 2167-0412. [Google Scholar]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of diterpenoid alkaloid mixtures using vacuum liquid chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- Van-Wagenen, B.C.; Larsen, R.; Cardellina, J.H.; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantion, a potent insecticide from the sponge Ulosa ruetzleri. J. Organomet. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Brand-William, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm-Wiss-U-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zubair, M.; Rizwan, K.; Rashid, U.; Saeed, R.; Saeed, A.A.; Rasool, N.; Riaz, M. GC/MS profiling, in vitro antioxidant, antimicrobial and haemolytic activities of Smilax macrophylla leaves. Arab. J. Chem. 2017, 10, S1460–S1468. [Google Scholar] [CrossRef] [Green Version]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Fahad, T.A.; Kuddus, M.R.; Hasan, C.M. Phytochemical and biological studies of bark extract of Miliusa velutina (Dunal) Hook. f. & Thomson. Dhaka Univ. J. Pharm. Sci. 2020, 19, 125–131. [Google Scholar]
- Mahfuz, A.; Salam, F.B.; Deepa, K.N.; Hasan, A.N. Characterization of in-vitro antioxidant, cytotoxic, thrombolytic and membrane stabilizing potential of different extracts of Cheilanthes tenuifolia and Stigmasterol isolation from n-hexane extract. Clin. Phytosci. 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shoba, F.G.; Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol. 2001, 76, 73–76. [Google Scholar] [CrossRef]
- Sisay, M.; Engidawork, E.; Shibeshi, W. Evaluation of the antidiarrheal activity of the leaf extracts of Myrtus communis Linn (Myrtaceae) in mice model. BMC Complement Altern Med. 2017, 17, 103. [Google Scholar] [CrossRef] [Green Version]
- Peungvicha, P.; Thirawarapan, S.S.; Temsiririrkkul, R.; Watanabe, H.; Prasain, J.K.; Kadota, S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J. Ethnopharmacol. 1998, 60, 27–32. [Google Scholar] [CrossRef]
- Ezeja, M.I.; Omeh, Y.S.; Ezeigbo, I.I.; Ekechukwu, A. Evaluation of the analgesic activity of the methanolic stem bark extract of Dialium guineense (Wild). Ann. Med. Health Sci. Res. 2011, 1, 55–62. [Google Scholar]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–415. [Google Scholar]
- Tallei, T.E.; Fatimawali; Adam, A.A.; Elseehy, M.M.; El-Shehawi, A.M.; Mahmoud, E.A.; Tania, A.D.; Niode, N.J.; Kusumawaty, D.; Rahimah, S.; et al. Fruit Bromelain-Derived Peptide Potentially Restrains the Attachment of SARS-CoV-2 Variants to hACE2: A Pharmacoinformatics Approach. Molecules 2022, 27, 260. [Google Scholar] [CrossRef]
- Khatun, M.C.S.; Muhit, M.A.; Hossain, M.J.; Al-Mansur, M.A.; Rahman, S.M.A. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon 2021, 7, e08475. [Google Scholar] [CrossRef]
- El-Mageed, H.R.A.; Abdelrheem, D.A.; Rafi, M.O.; Sarker, M.T.; Al-Khafaji, K.; Hossain, M.J.; Capasso, R.; Emran, T.B. In Silico Evaluation of Different Flavonoids from Medicinal Plants for Their Potency against SARS-CoV-2. Biologics 2021, 1, 416–434. [Google Scholar] [CrossRef]
- Hossain, M.J.; Islam, M.S.; Shahriar, S.; Sanam, S.; Emran, T.B.; Khatun, C.S.; Islam, M.R.; Mitra, S.; Dhama, K. Comedication of rabeprazole sodium causes potential drug-drug interaction with diabetic drug linagliptin: In-vitro and in-silico approaches. J. Exp. Biol. Agric. Sci. 2021, 9, 528–542. [Google Scholar] [CrossRef]
- Hossain, M.J.; Sultan, M.Z.; Rashid, M.A.; Kuddus, M.R. Interactions of linagliptin, rabeprazole sodium, and their formed complex with bovine serum albumin: Computational docking and fluorescence spectroscopic methods. Anal. Sci. Adv. 2021, 2, 480–494. [Google Scholar] [CrossRef]
- Hossain, M.J.; Rashid, M.A.; Sultan, M.Z. Transition Metal Chelation Augments the Half-life of Secnidazole: Molecular Docking and Fluorescence Spectroscopic Approaches. Drug Res. 2020, 70, 583–592. [Google Scholar] [CrossRef]
- El Azab, I.H.; El-Sheshtawy, H.S.; Bakr, R.B.; Elkanzi, N.A.A. New 1,2,3-triazole-containing hybrids as antitumor candidates: Design, click reaction synthesis, DFT calculations, and molecular docking study. Molecules 2021, 26, 708. [Google Scholar] [CrossRef]
- Alam, S.; Rashid, M.A.; Sarker, M.M.R.; Emon, N.U.; Arman, M.; Mohamed, I.N.; Haque, M.R. Antidiarrheal, antimicrobial and antioxidant potentials of methanol extract of Colocasia gigantea Hook. f. leaves: Evidenced from in vivo and in vitro studies along with computer-aided approaches. BMC Complement. Med. Ther. 2021, 21, 119. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods. 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Ahmed, T.; Khan, A.U.; Abbass, M.; Filho, E.R.; Uddin, Z.; Khan, A. Synthesis, characterization, molecular docking, analgesic, antiplatelet and anticoagulant effects of dibenzylidene ketone derivatives. Chem. Cent. J. 2018, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Shrestha, R.L.; Adhikari, A.; Wadood, A.; Khan, H.; Khan, A.Z.; Maione, F.; Mascolo, N.; De Feo, V. First evidence of the analgesic activity of govaniadine, an alkaloid isolated from Corydalis govaniana Wall. Nat. Prod. Res. 2015, 29, 430–437. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminformat. 2009, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, K.; Iraha, R.; Higa, M.; Yogi, S. Studies on constituents from the twigs of Messerschmidia argentea II. Bull. Coll. Sci. Univ. Ryukyus. 1997, 64, 53–59. [Google Scholar]
- Ragasa, C.Y.; Ng, V.A.; De Los Reyes, M.M.; Mandia, E.H.; Shen, C.C. An isoflavone from Wrightia pubescens. Int. J. Pharmacog. Phytochem. Res. 2015, 7, 353–355. [Google Scholar]
- David, J.P.; Ferrari, J.; David, J.M.; Guimarães, A.G.; Lima, F.W.; de Souza, G.L. New triterpene and antibacterial labdenoic acid derivatives from Moldenhawera nutans. J. Braz. Chem. Soc. 2007, 18, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Morales, G.; Sierra, P.; Mancilla, A.; Paredes, A.; Loyola, L.A.; Gallardo, O.; Borquez, J. Secondary metabolites from four medicinal plants from northern Chile: Antimicrobial activity and biotoxicity against Artemia salina. J. Chil. Chem. Soc. 2003, 48, 13–18. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Srivastava, R. A review on phytochemical, pharmacological, and pharmacognostical profile of Wrightia tinctoria: Adulterant of kurchi. Pharmacogn. Rev. 2014, 8, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Oviya, I.R.; Sharanya, M.; Jeyam, M. Phytochemical and Pharmacological assessment of Wrightia tinctoria R. BR.: A review. World J. Pharm. Res. 2015, 4, 1992–2015. [Google Scholar]
- Garcellano, R.C.; Moinuddin, S.G.A.; Young, R.P.; Zhou, M.; Bowden, M.E.; Renslow, R.S.; Yesiltepe, Y.; Thomas, D.G.; Colby, S.M.; Chouinard, C.D.; et al. Isolation of Tryptanthrin and Reassessment of Evidence for Its Isobaric Isostere Wrightiadione in Plants of the Wrightia Genus. J. Nat. Prod. 2019, 82, 440–448. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, I.; Zubair, M.; Rizwan, K.; Rasool, N.; Jamil, M.; Khan, S.A.; Tareen, R.B.; Ahmad, V.U.; Mahmood, A.; Riaz, M.; et al. Chemical composition, antioxidant and antimicrobial potential of essential oils from different parts of Daphne mucronata Royle. Chem. Cent. J. 2018, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and biological studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef]
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U.A.; Nafady, A.; Jaafar, H.Z. Chemical composition and Biological studies of Ficus benjamina. Chem. Cent. J. 2014, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.N.; Zubair, M.; Rizwan, K.; Tareen, R.B.; Rasool, N.; Zia-Ul-Haq, M.; Ercisli, S. Compositional studies and Biological activities of Perovskia abrotanoides Kar. oils. Biol. Res. 2014, 47, 12. [Google Scholar] [CrossRef] [Green Version]
- Tantary, S.; Masood, A.; Bhat, A.H.; Dar, K.B.; Zargar, M.A.; Ganie, S.A. In vitro antioxidant and RBC membrane stabilization activity of Euphorbia wallichii. Free Rad. Antiox. 2017, 7, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.A.; Mayor, A.B. Lupeol: An antioxidant triterpene in Ficus pseudopalma Blanco (Moraceae). Asian Pac. J. Trop. Biomed. 2014, 4, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Sudhahar, V.; Kumar, S.A.; Varalakshmi, P. Role of lupeol and lupeol linoleate on lipemic-oxidative stress in experimental hypercholesterolemia. Life Sci. 2006, 78, 1329–1335. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes. 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; León, M.M.; Zamora, R. Effect of β-sitosterol in the antioxidative activity of oxidized lipid–amine reaction products. Food Res. Int. 2009, 42, 1215–1222. [Google Scholar] [CrossRef]
- Omeke, J.N.; Anaga, A.O.; Okoye, J.A. Brine shrimp lethality and acute toxicity tests of different hydro-methanol extracts of Anacardium occidentale using in vitro and in vivo models: A preliminary study. Comp. Clin. Path. 2018, 27, 1717–1721. [Google Scholar] [CrossRef]
- Logarto Parra, A.; Silva Yhebra, R.; Guerra Sardiñas, I.; Iglesias Buela, L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001, 8, 395–400. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; Mc Laughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sultana, R.; Emran, T.B.; Islam, M.S.; Rahman, M.A.; Chakma, J.S.; Rashid, H.U.; Hasan, C.M. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement. Altern. Med. 2013, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Gschwind, A.; Zwick, E.; Prenzel, N.; Leserer, M.; Ullrich, A. Cell communication networks: Epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 2001, 20, 1594–1600. [Google Scholar] [CrossRef] [Green Version]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer. 2001, 37, 3–8. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; Alkhoja, O.A.; Mohamed, W.R. Design, synthesis and antitumor activity of novel pyrazolo [3,4-d]pyrimidine derivatives as EGFR-TK inhibitors. Bioorg. Chem. 2016, 66, 88–96. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, S.M.; Hong, S. Discovery of wrightiadione as a novel template for the TrkA kinase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 5186–5189. [Google Scholar] [CrossRef]
- Wang, T.; Yu, D.; Lamb, M.L. Trk kinase inhibitors as new treatments for cancer and pain. Expert Opin. Ther. Pat. 2009, 19, 305–319. [Google Scholar] [CrossRef]
- Zewdie, K.A.; Bhoumik, D.; Wondafrash, D.Z.; Tuem, K.B. Evaluation of in vivo antidiarrhoeal and in vitro antibacterial activities of the root extract of Brucea antidysenterica JF Mill (Simaroubaceae). BMC Complement. Med. Ther. 2020, 20, 201. [Google Scholar] [CrossRef]
- Naher, S.; Aziz, M.A.; Akter, M.I.; Rahman, S.M.; Sajon, S.R.; Mazumder, K. Anti-diarrheal activity and brine shrimp lethality bioassay of methanolic extract of Cordyline fruticosa (L.) A. Chev. leaves. Clin. Phytosci. 2019, 5, 15. [Google Scholar] [CrossRef]
- Jyoti, M.A.; Barua, N.; Hossain, M.S.; Hoque, M.; Bristy, T.A.; Mahmud, S.; Kamruzzaman; Adnan, M.; Chy, M.N.U.; Paul, A.; et al. Unravelling the Biological Activities of the Byttneria pilosa Leaves Using Experimental and Computational Approaches. Molecules 2020, 25, 4737. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, T.B.; Hymete, A.; Mekonnen, Y.; Tadesse, M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, R.A.; Kumar, A.S.; Gandhimathi, R. Hypoglycemic and hypolipidemic activity of Wrightia tinctoria L. in alloxan induced diabetes in albino wistar rats. Pharmacologyonline 2009, 3, 550–559. [Google Scholar]
- Malik, A.; Jamil, U.; Butt, T.T.; Waquar, S.; Gan, S.H.; Shafique, H.; Jafar, T.H. In silico and in vitro studies of lupeol and iso-orientin as potential antidiabetic agents in a rat model. Drug Des. Devel. Ther. 2019, 13, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods. 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Ahmad, G.; Rasool, N.; Rizwan, K.; Imran, I.; Zahoor, A.F.; Zubair, M.; Sadiq, A.; Rashid, U. Synthesis, in-vitro cholinesterase inhibition, in-vivo anticonvulsant activity and in-silico exploration of N-(4-methylpyridin-2-yl)thiophene-2-carboxamide analogs. Bioorg. Chem. 2019, 92, 103216. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Lisa, S.R.; Islam, M.K.; Qais, N. Plants and plant constituents with analgesic and anti-inflammatory activities: A systematic review. Dhaka Univ. J. Pharm. Sci. 2020, 19, 207–224. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Sarmento-Neto, J.F.; Do Nascimento, L.G.; Felipe, C.F.; De Sousa, D.P. Analgesic potential of essential oils. Molecules 2016, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. InflammoPharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
| Sample Code | IC50 (µg/mL) from DPPH Assay | LC50 (µg/mL) from Brine Shrimp Lethality Assay | ||
|---|---|---|---|---|
| Bark | Fruit Coat | Bark | Fruit Coat | |
| ME | 90.86 | 31.6 | 161.50 | 160.91 |
| PE | 79.95 | 118.9 | 32.65 | 10.67 |
| DCM | 10.91 | 337.05 | 41.05 | 41.08 |
| CF | 26.88 | 4.55 | 40.39 | 41.51 |
| AQ | 7.22 | 35.25 | 51.09 | 70.47 |
| BHT | 4.3 | 4.3 | ||
| AA | 17.45 | 17.45 | ||
| VS | 0.451 | 0.451 | ||
| Group | Treatment | % Reduction in Diarrheal Feces | |||
|---|---|---|---|---|---|
| Time after Loading the Plant Sample/Drug | |||||
| 1 h | 2 h | 3 h | 4 h | ||
| Positive control | Loperamide 50 mg/kg | 100 ± 0.0 ** | 88.89 ± 0.33 ** | 82.95 ± 0.20 * | 80.40 ± 0.61 * |
| I | MEB 200 mg/kg | 71.43 ± 0.33 * | 66.67 ± 0.58 | 64.19 ± 0.67 | 48.18 ± 1.0 |
| II | MEB 400 mg/kg | 85.71 ± 0.33 ** | 87.78 ± 0.67 ** | 80.2 ± 0.33 * | 74.55 ± 0.67 |
| III | MEF 200 mg/kg | 69.35 ± 0.33 | 58.67 ± 0.58 | 50.0 ± 0.67 | 53.33 ± 1.0 |
| IV | MEF 400 mg/kg | 88.69 ± 0.33 ** | 80.40 ± 0.67 * | 79.21 ± 0.78 | 77.78 ± 1.5 |
| Group | Treatment | % Reduction in Blood Glucose Level of Mice | ||
|---|---|---|---|---|
| Time after Loading the Plant Sample/Drug | ||||
| 1 h | 2 h | 3 h | ||
| Positive control | Glibenclamide 10 mg/kg | 29.1 ± 0.79 | 62.1 ± 0.21 * | 66.7 ± 0.61 ** |
| I | MEB 200 mg/kg | 30.0 ± 0.36 | 53.1 ± 0.63 | 60.1 ± 0.87 * |
| II | MEB 400 mg/kg | 43.8 ± 1.14 | 46.2 ± 1.20 | 74.7 ± 0.19 ** |
| III | MEF 200 mg/kg | 30.0 ± 0.36 | 53.1 ± 0.63 | 60.1 ± 0.78 |
| IV | MEF 400 mg/kg | 26.6 ± 1.47 | 43.3 ± 0.56 | 70.6 ± 0.30 * |
| Group | Treatment | Average Time of Tail Immersion of Mice | ||
|---|---|---|---|---|
| Time (in Sec) after Loading the Plant Sample/Drug | ||||
| 30 min | 60 min | 90 min | ||
| Negative control | Tween 80 solution | 2.50 ± 0.05 ** | 2.41 ± 0.11 ** | 2.18 ± 0.13 ** |
| Positive control | Morphine 2 mg/kg | 5.42 ± 0.21 ** | 10.20 ± 0.66 ** | 12.06 ± 0.53 ** |
| I | MEB 200 mg/kg | 3.67 ± 0.16 * | 5.69 ± 0.08 ** | 6.92 ± 0.39 ** |
| II | MEB 400 mg/kg | 4.16 ± 0.06 ** | 6.58 ± 0.40 ** | 8.50 ± 0.28 ** |
| III | MEF 200 mg/kg | 3.99 ± 0.14 ** | 5.94 ± 0.23 ** | 7.24 ± 0.46 ** |
| IV | MEF 400 mg/kg | 4.28 ± 0.24 * | 6.20 ± 0.10 ** | 8.57 ± 0.19 ** |
| Group | Treatment | % Inhibition of Writhing |
|---|---|---|
| Negative control | Tween 80 solution | -- |
| Positive control | DS 50 mg/kg | 76.79 ± 0.33 ** |
| I | MEB 200 mg/kg | 42.86 ± 0.88 * |
| II | MEB 400 mg/kg | 66.07 ± 0.88 ** |
| III | MEF 200 mg/kg | 45.61 ± 1.20 ** |
| IV | MEF 400 mg/kg | 54.39 ± 1.20 ** |
| Com. No. | Name of Compounds/Drugs | PubChem ID | Binding Affinity towards Corresponding Receptors/Macromolecules (kcal/mol) | |||||
|---|---|---|---|---|---|---|---|---|
| 3GRS (Antioxidant) | 1XKK (Cytotoxicity) | 6VI4 (Antidiarrheal) | 4ZWB (Hypoglycemic) | 5C1M (Central Analgesic) | 1CX2 (Peripheral Analgesic) | |||
| 1 | 3β-acetyloxy-olean-12-en-28-ol | 14010964 | −9.0 | −8.1 | −7.7 | −8.6 | −6.2 | −8.6 |
| 2 | Wrightiadione | 10422105 | −8.4 | −9.4 | −7.1 | −9.5 | −9.1 | −9.4 |
| 3 | 22β-Hydroxylupeol | 24786642 | −9.3 | −9.2 | −7.0 | −8.6 | −6.8 | −8.5 |
| 4 | β-Sitosterol | 222284 | −8.4 | −9.2 | −7.7 | −9.4 | −9.7 | −9.7 |
| Standard drugs | Butylated hydroxy toluene (BHT) | 31404 | −5.8 | |||||
| Vincristine | 5978 | −6.3 | ||||||
| Loperamide | 3955 | −7.3 | ||||||
| Glibenclamide | 3488 | −10.2 | ||||||
| Morphine | 5288826 | −8.0 | ||||||
| Diclofenac | 3033 | −7.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannat, T.; Hossain, M.J.; El-Shehawi, A.M.; Kuddus, M.R.; Rashid, M.A.; Albogami, S.; Jafri, I.; El-Shazly, M.; Haque, M.R. Chemical and Pharmacological Profiling of Wrightia coccinea (Roxb. Ex Hornem.) Sims Focusing Antioxidant, Cytotoxic, Antidiarrheal, Hypoglycemic, and Analgesic Properties. Molecules 2022, 27, 4024. https://doi.org/10.3390/molecules27134024
Jannat T, Hossain MJ, El-Shehawi AM, Kuddus MR, Rashid MA, Albogami S, Jafri I, El-Shazly M, Haque MR. Chemical and Pharmacological Profiling of Wrightia coccinea (Roxb. Ex Hornem.) Sims Focusing Antioxidant, Cytotoxic, Antidiarrheal, Hypoglycemic, and Analgesic Properties. Molecules. 2022; 27(13):4024. https://doi.org/10.3390/molecules27134024
Chicago/Turabian StyleJannat, Tabassum, Md. Jamal Hossain, Ahmed M. El-Shehawi, Md. Ruhul Kuddus, Mohammad A. Rashid, Sarah Albogami, Ibrahim Jafri, Mohamed El-Shazly, and Mohammad Rashedul Haque. 2022. "Chemical and Pharmacological Profiling of Wrightia coccinea (Roxb. Ex Hornem.) Sims Focusing Antioxidant, Cytotoxic, Antidiarrheal, Hypoglycemic, and Analgesic Properties" Molecules 27, no. 13: 4024. https://doi.org/10.3390/molecules27134024










