Pharmacokinetics Study of Jin-Gu-Lian Prescription and Its Core Drug Pair (Sargentodoxa cuneata (Oliv.) Rehd. et W and Alangium chinense (Lour.) Harms) by UPLC-MS/MS
Abstract
:1. Introduction
2. Results
2.1. Method Validation
2.1.1. Specificity
2.1.2. Linearity and Lower Limits of Quantification
2.1.3. Precision and Accuracy
2.1.4. Extraction Recovery and Matrix Effect
2.1.5. Stability
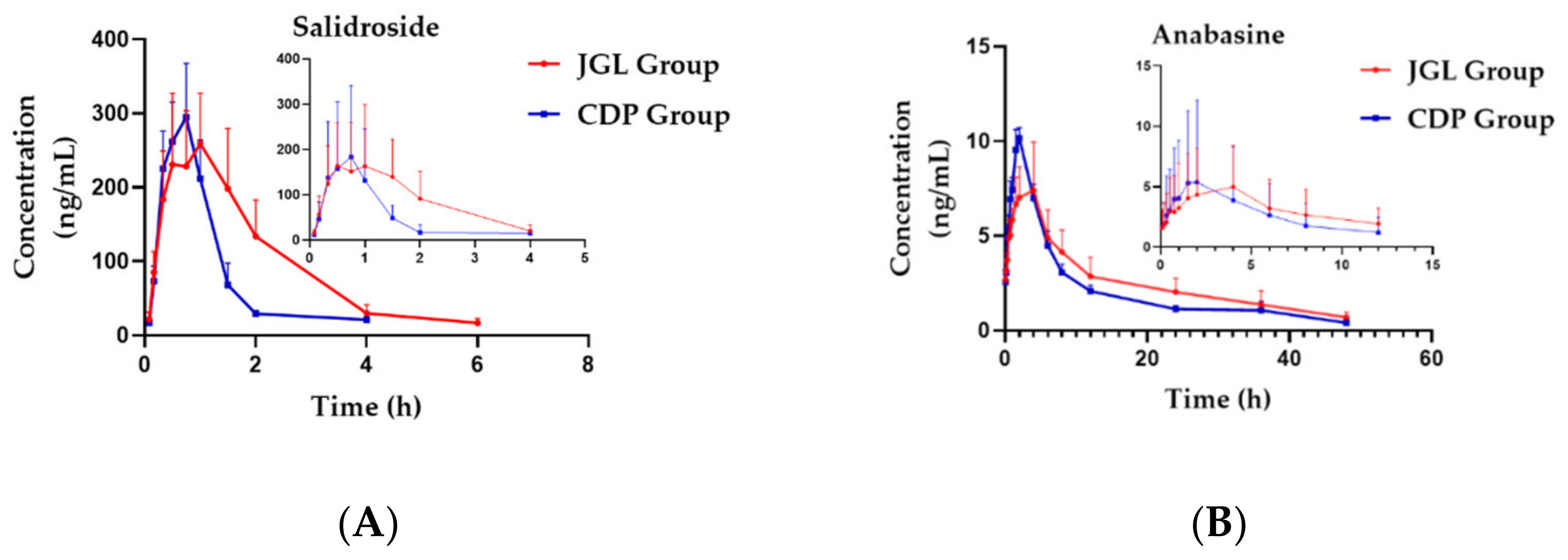
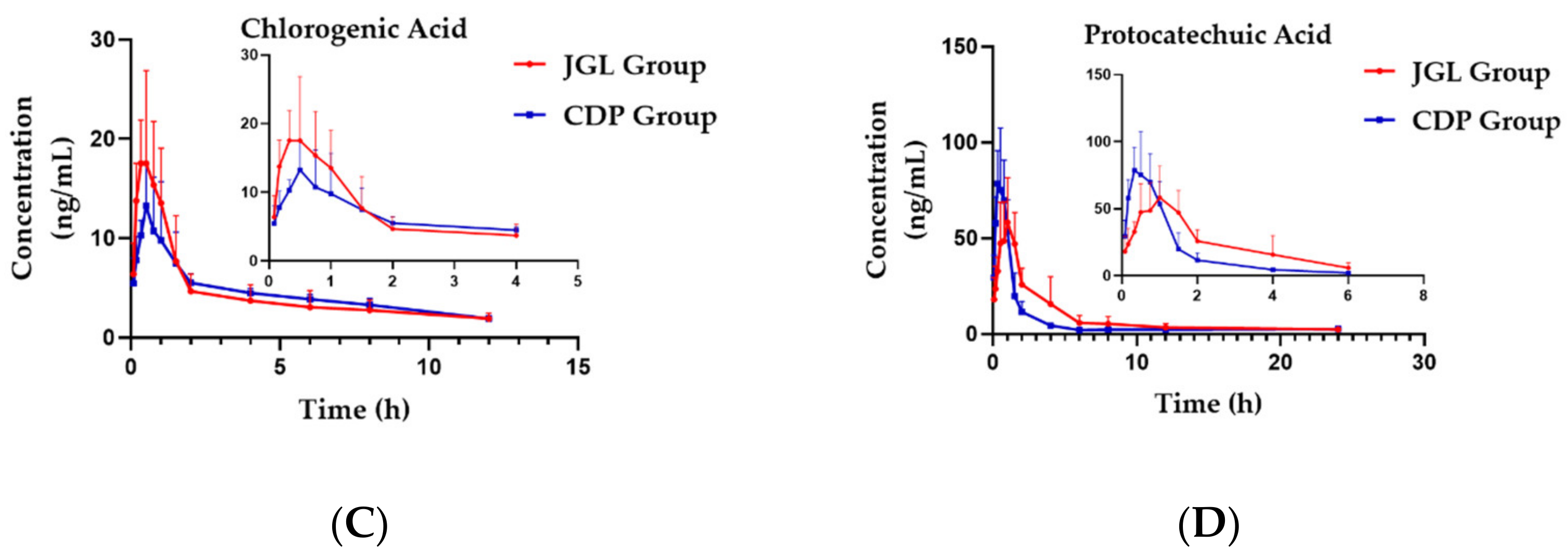
2.2. Pharmacokinetic Study
3. Materials and Methods
3.1. Materials and Reagents
3.2. Animals
3.3. Assay of JGL Extract and the Mixed Extract of SC and AC
3.4. Preparation of Calibration Standards and Quality Control (QC) Solutions
3.5. Plasma Sample Preparation
3.6. Instruments and Analytical Condition
3.7. Method Validation
3.7.1. Specificity
3.7.2. Linearity and Lower Limits of Quantification
3.7.3. Precision and Accuracy
3.7.4. Extraction Recovery and Matrix Effect
3.7.5. Stability
3.8. Pharmacokinetic Study
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jian-ming, L.; Quan, J.; Xiao-po, T.; Xun, G.; Jian, W.; Jia-kang, C.; Zhi-qiang, Z.; Zheng, Z. Correlation between TCM syndrome characteristics of rheumatoid arthritis and the count of platelet and hemoglobin in 1602 cases. World J. Integr. Tradit. West. Med. 2020, 15, 352–356. [Google Scholar]
- Chunyan, G.; Huarong, A.; Mengmeng, W.; Jin, L. Clinical study of Jin Gu Lian capsule in the treatment of rheumatic obstruction syndrome rheumatoid arthritis. Chin. J. Clin. Ration. Drug Use 2018, 11, 18–19. [Google Scholar]
- Ya-qin, L.; Rong, Q. Study on The Compatibility of Radix Bupleuri and Radix Scutellariae. J. Basic Chin. Med. 2018, 24, 282–285. [Google Scholar]
- Ke, L.; Di, W.; Yue-chen, S.; Xue-lin, Y. An Analysis of Medicine Rules in Miao Medicine Prescriptions for Curing Rheumatology Based on Association Rules. J. Chengdu Univ. TCM 2017, 40, 7–9, 107. [Google Scholar]
- Shu-yong, D.; Jing, L.; Lai-cheng, L. Modern Research Progress of traditional Chinese drug pairs. Lishizhen Med. Mater. Med. Res. 2012, 23, 1003–1005. [Google Scholar]
- Yu-yan, Z.; Hai-tong, W.; Jie-tong, Y.; Hui-fen, Z. Study on attenuating toxicity and synergism of aconite combined with licorice. J. Tradit. Chin. Med. 2012, 53, 1365–1368. [Google Scholar]
- Jin-chao, X.; Hui, L.; Jian, P.; Dan, T. Simultaneous Determination of Nine Components in Jingulian Capsule by HPLC. J. Chin. Med. Mater. 2021, 12, 2885–2890. [Google Scholar]
- Zhou, Z.Y.; Huang, Y.; Xiao, J.C.; Liu, H.; Wang, Y.L.; Gong, Z.P.; Li, Y.T.; Wang, A.M.; Li, Y.J.; Zheng, L. Chemical profiling and quantification of multiple components in Jin-Gu-Lian capsule using a multivariate data processing approach based on UHPLC-Orbitrap Exploris 240 MS and UHPLC-MS/MS. J. Sep. Sci. 2022, 45, 1282–1291. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.L.; Ding, Y.; Du, G.H. Gaultherin, a natural salicylate derivative from Gaultheria yunnanensis: Towards a better non-steroidal anti-inflammatory drug. Eur. J. Pharmacol. 2006, 530, 166–171. [Google Scholar] [CrossRef]
- Yi, D.; Zi-gang, Q.; Wu-lin, L.; Xiong, D.; Hai-feng, C. Anti-inflammatory Activity of Psammosilenes Radix and Total Saponins in Vivo. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 165–170. [Google Scholar]
- Hua, L.; Shu-feng, H.; Chong, D.; Hua-wei, Z. Study on analgesic and anti-inflammatory effects of Sargentodoxae caulis. Shaanxi J. Tradit. Chin. Med. 2013, 34, 1427–1428. [Google Scholar]
- Yu-xia, H.; Fang, H.; Qi-rong, Y.; Bai-li, G. Determination of Anabasine in Radix Alangii Preparata by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 56–58. [Google Scholar]
- Yan-yao, M.; Hong-zhi, D.; Xiao-bo, W.; Wan-ruo, W.; Zi-yu, C. Research process on chemical constituents and pharmacological effects of Alangium chinese (Lour.) Harms. Stud. Trace Elem. Health 2021, 38, 40–43. [Google Scholar]
- Hao, L.; Feng-chun, Z.; Xian-da, Y.; Jing-jing, Z.; Xiao-qian, L.; Hui-min, G.; Liang-mian, C.; Zhi-min, W. Determination of phenols and triterpenoid saponins in stems of Sargentodoxa cuneata. China J. Chin. Mater. Med. 2015, 40, 1865–1871. [Google Scholar]
- Zhili, F.; Jian, P.; Gonglu, L.; Dan, T.; Lin, Z.; Yongjun, L.; Yong, H. Study on Anti-inflammatory Effect and Mechanism of Jingulian Capsule on Inflammatory Model Rats. China Pharm. 2021, 32, 1077–1082. [Google Scholar]
- Yun-dong, N.; Chang-ke, X. Clinical study on Jingulian Capsules combined with glucosamine sulphate and paracetamol in treatment of knee joint osteoarthritis. Drugs Clin. 2021, 36, 961–966. [Google Scholar]
- Wei, L. Observation on treating osteoarthritis with the Jin’gulian capsule. Clin. J. Chin. Med. 2019, 11, 75–77. [Google Scholar]
- Si-han, L.; Dong-yin, L.; Guang-ping, Z.; Zu-guang, Y.; Bo, P.; Ying, C.; Jian-rong, L. Inhibitory Effect and Mechanism of Jingulian Extract on LPS-induced RAW264.7 Cell Inflammatory Response Based on PI3K/Akt Signaling Pathway. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 29–35. [Google Scholar]
- Ashour, M.; Youssef, F.; Gad, H.; Wink, M. Inhibition of Cytochrome P450 (CYP3A4) Activity by Extracts from 57 Plants Used in Traditional Chinese Medicine (TCM). Pharmacogn. Mag. 2017, 13, 300–308. [Google Scholar]
- Dong-yin, L.; Hong-ping, H.; Guang-ping, Z.; Jian-rong, L.; Zu-guang, Y.; Bo, P. The Neurotoxicity and mechanism of Sargentodoxa Cuneata (Oliv.) Rehd. et W. Chin. J. Pharmacol. Toxicol. 2021, 35, 788. [Google Scholar]
- Mao, J.; Liu, C.; Zhang, Y.; Zhang, Q.; Liu, H. Salidroside inhibits phenotypic transformation of rat pulmonary artery smooth muscle cells induced by hypoxia. China J. Chin. Mater. Med. 2022, 47, 1024–1030. [Google Scholar]
- Qiyong, W.; Yaling, X. Content Determination of L(-)-Anabasine in Different Medicinal Parts of Alangium chinense by HPLC. China Pharm. 2016, 27, 3877–3879. [Google Scholar]
- Jun-zhi, L.; Ding-kun, Z.; Liang, Z.; Jin-hui, Y.; Qu, D. Research on pharmacokinetics of salidroside and tyrosol in rats. Chin. Tradit. Pat. Med. 2014, 36, 1845–1849. [Google Scholar]
- Hao, W.; Liu-qing, D.; Jia-shuang, Z.; Chen, L.; Jin-jun, S.; An, K.; Qiang, Z.; Wei, Y. Development and validation of a UPLC-MS/MS method for determination of salidroside in rat plasma: Application to the pharmacokinetics study. Chin. Tradit. Pat. Med. 2014, 36, 1176–1181. [Google Scholar]
- Ke-yu, L. The Investigation of the Absorption, Metabolism and Excretion Process of Salidroside in Rats. Master’s Thesis, Yanbian University, Yanji, China, 2010. [Google Scholar]
- Tengfei, C.; Dongyin, L.; Yunhang, G.; Ling, S.; Han, L.; Hongping, H.; Jianliang, L.; Bo, P. Study on Pharmacokinetics in Salidroside of Jingulian Capsules in Rats. Chin. J. Inf. TCM 2022, 1–5. [Google Scholar] [CrossRef]
- WS-10093(ZD)-0093-2002; Compilation of National Standard for Traditional Chinese Medicines, Branch Volume of Brain Meridians, Limbs and Brain System [S]. China Food and Drug Administration: Beijing, China, 2002.
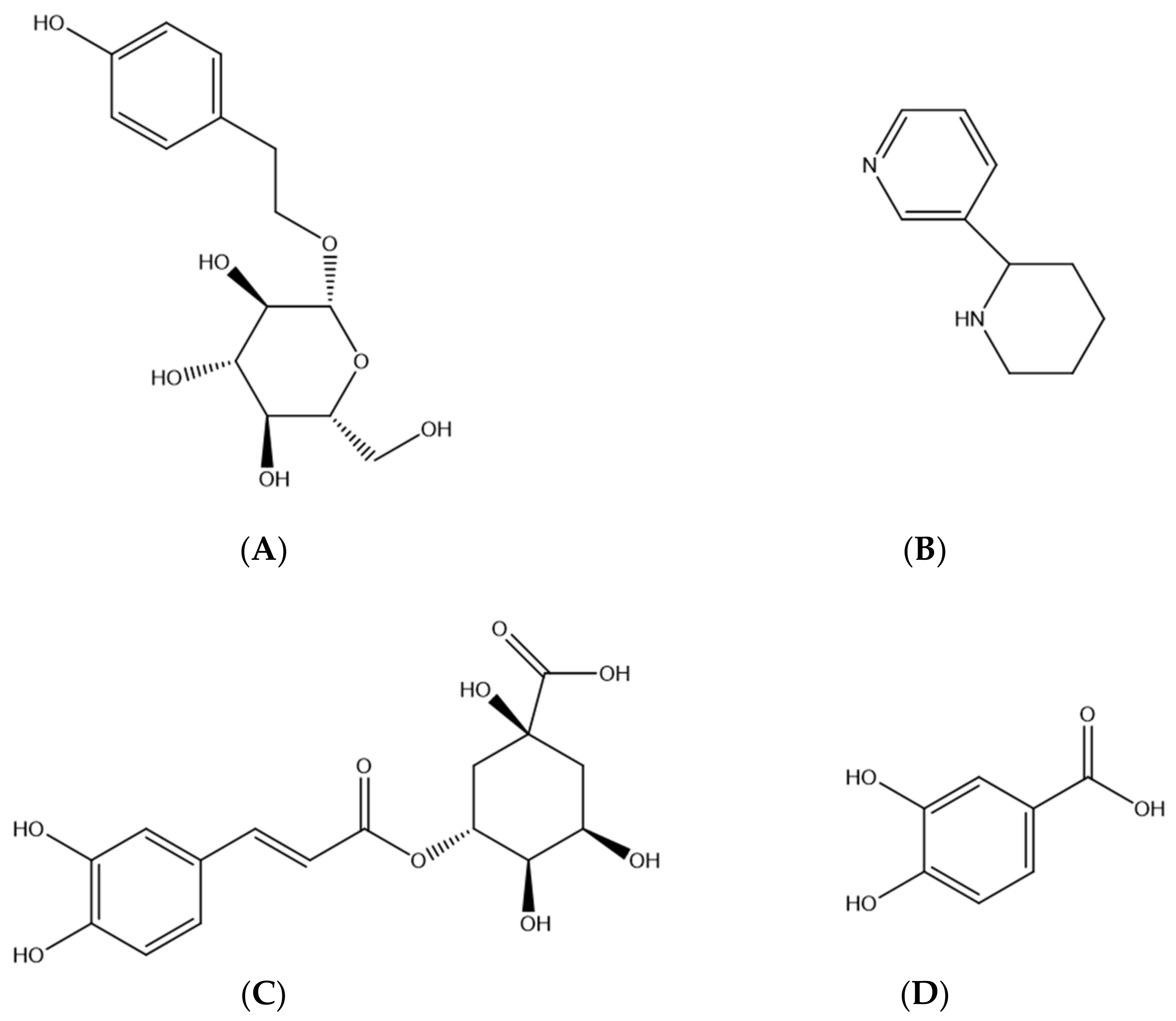
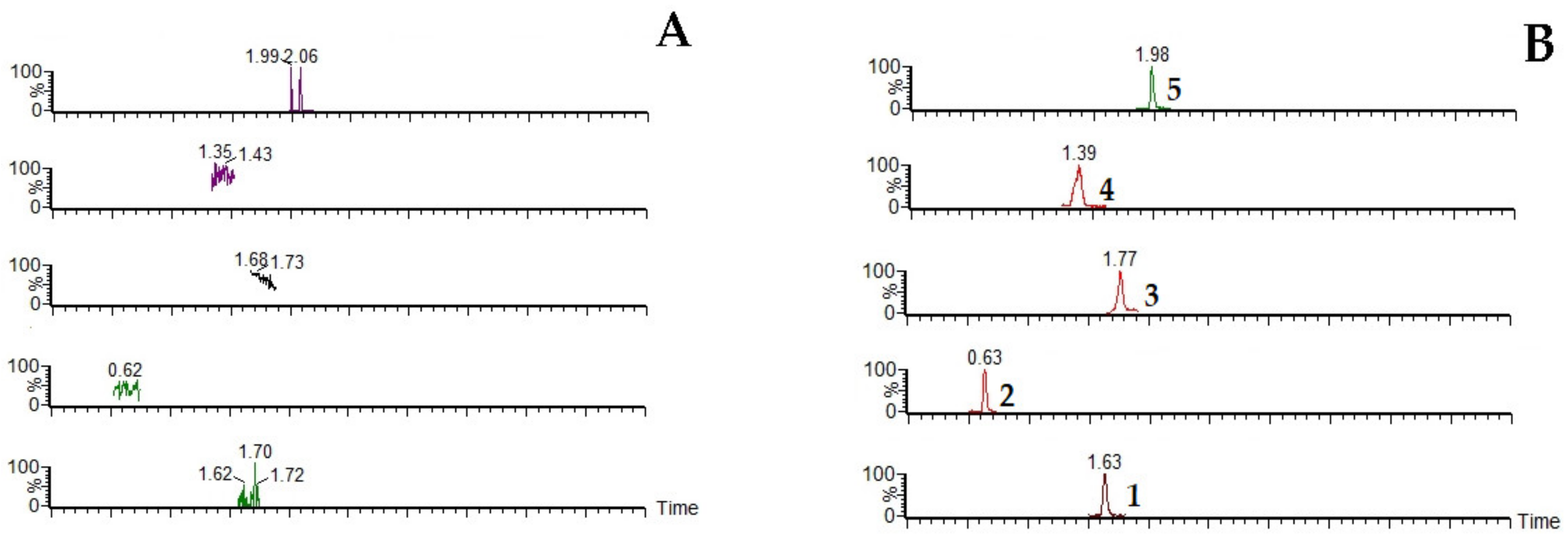
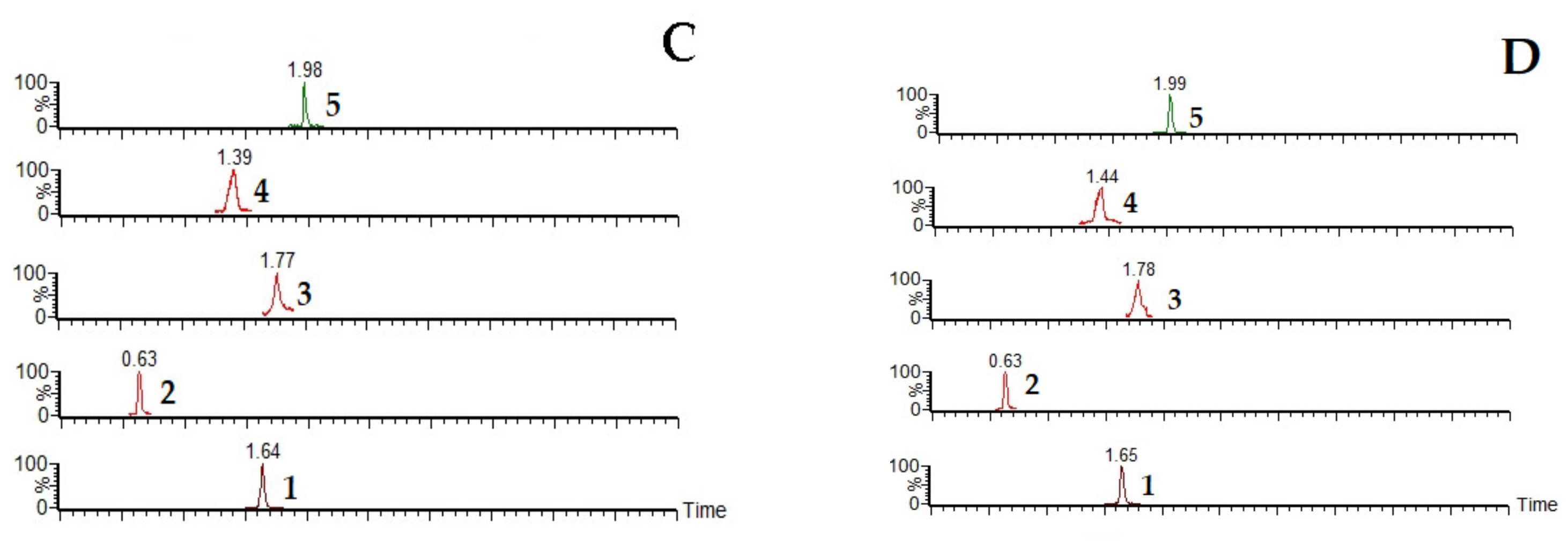
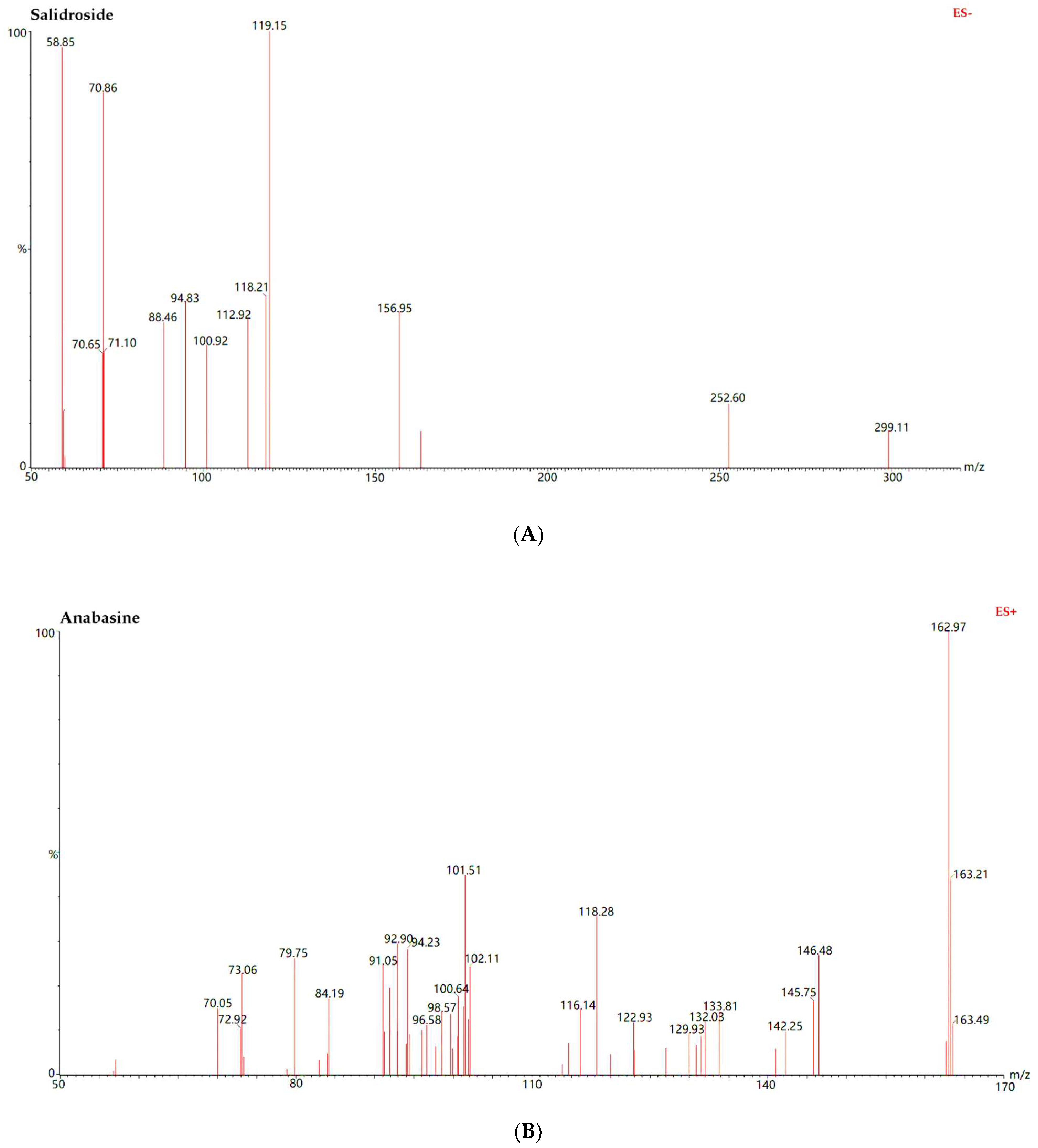
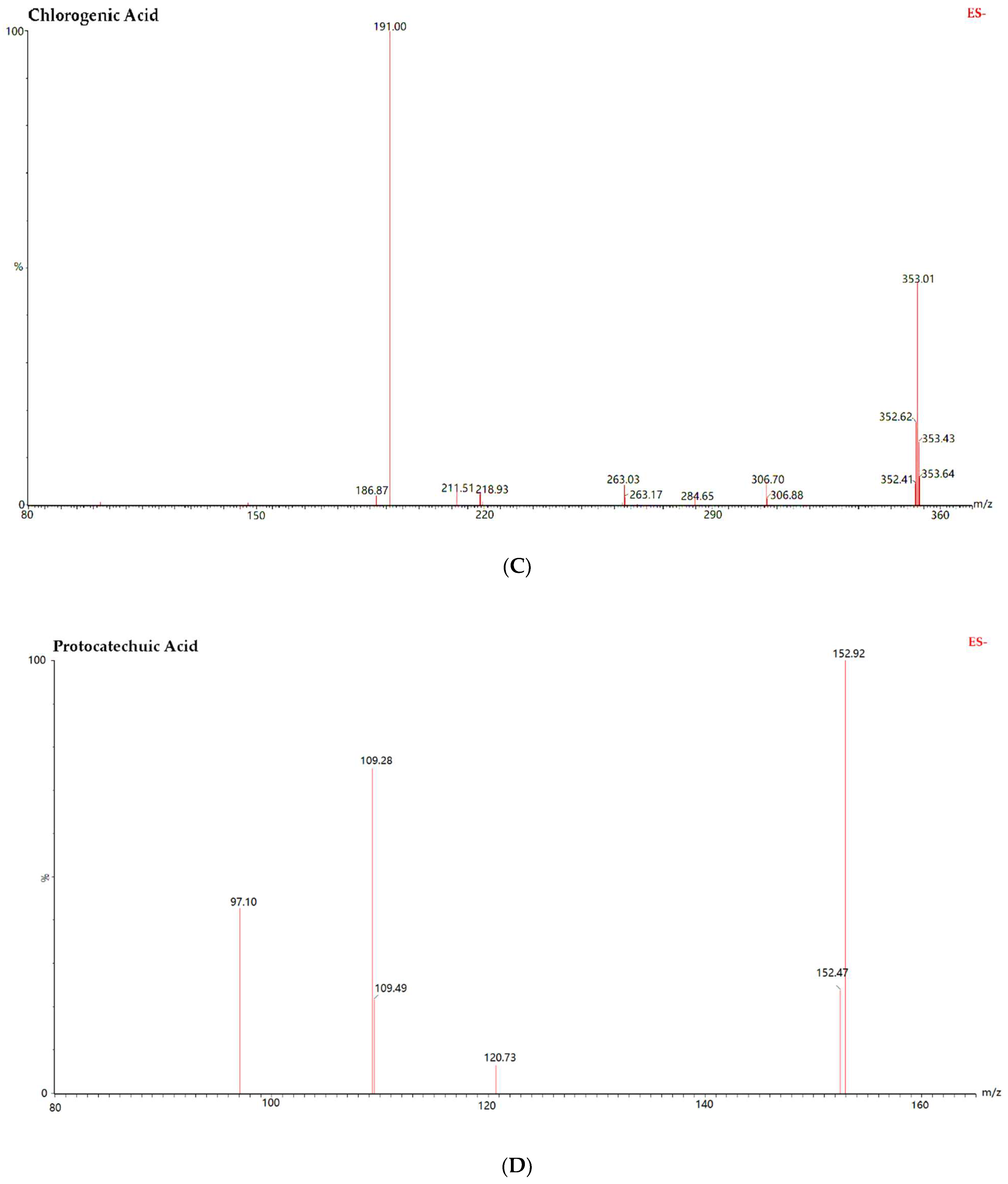
| Analytes | Regression Equation | R2 | Calibration Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| Salidroside | y = 0.0007x + 0.0038 | 0.9979 | 13.10~1048 | 13.10 |
| Anabasine | y = 0.0203x + 0.0779 | 0.9986 | 0.1125~28.80 | 0.1125 |
| Chlorogenic Acid | y = 0.0392x − 0.0218 | 0.9976 | 1.850~118.2 | 1.850 |
| Protocatechuic Acid | y = 0.0180x − 0.0079 | 0.9996 | 1.617~258.8 | 1.6172 |
| Analytes | Spiked Conc. (ng/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Measured Conc. a (ng/mL) | Precision | Accuracy | Measured Conc. a (ng/mL) | Precision | Accuracy | ||
| (RSD)% | (%) | (RSD)% | (%) | ||||
| Salidroside | 26.20 | 26.84 ± 1.17 | 4.35 | 102.46 | 25.10 ± 1.81 | 7.23 | 95.80 |
| 104.80 | 96.67 ± 8.36 | 8.65 | 92.25 | 108.42 ± 6.66 | 6.14 | 103.45 | |
| 419.20 | 384.46 ± 38.71 | 10.07 | 91.71 | 392.19 ± 41.64 | 10.62 | 93.56 | |
| Anabasine | 0.23 | 0.20 ± 0.01 | 7.21 | 87.87 | 0.21 ± 0.02 | 9.01 | 91.34 |
| 3.60 | 3.32 ± 0.18 | 5.40 | 92.26 | 3.72 ± 0.12 | 3.11 | 103.28 | |
| 14.40 | 14.09 ± 1.12 | 7.97 | 97.86 | 15.07 ± 1.15 | 7.65 | 104.62 | |
| Chlorogenic Acid | 3.69 | 3.30 ± 0.19 | 5.74 | 89.47 | 3.30 ± 0.20 | 6.10 | 89.52 |
| 14.78 | 13.69 ± 0.60 | 4.40 | 92.64 | 14.60 ± 1.08 | 7.37 | 98.81 | |
| 59.10 | 61.30 ± 4.30 | 7.02 | 103.72 | 63.27 ± 4.14 | 6.54 | 107.06 | |
| Protocatechuic Acid | 3.23 | 3.33 ± 0.11 | 3.43 | 103.25 | 3.34 ± 0.28 | 8.42 | 103.54 |
| 12.94 | 12.25 ± 0.84 | 6.83 | 94.68 | 13.64 ± 0.83 | 6.12 | 105.44 | |
| 103.50 | 94.15 ± 6.01 | 6.38 | 90.96 | 98.26 ± 7.55 | 7.68 | 94.94 | |
| Analytes | Spiked Conc. (ng/mL) | Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD% | Mean ± SD (%) | RSD% | ||
| Salidroside | 26.20 | 85.34 ± 5.66 | 6.63 | 94.14 ± 7.04 | 7.48 |
| 104.80 | 95.10 ± 8.60 | 10.70 | 106.65 ± 7.42 | 6.96 | |
| 419.20 | 104.01 ± 7.41 | 7.12 | 103.96 ± 5.01 | 4.82 | |
| Anabasine | 0.23 | 91.64 ± 4.35 | 4.74 | 88.52 ± 6.75 | 7.63 |
| 3.60 | 94.68 ± 5.31 | 5.61 | 102.45 ± 2.57 | 2.51 | |
| 14.40 | 95.98 ± 7.70 | 8.02 | 108.13 ± 8.97 | 8.30 | |
| Chlorogenic Acid | 3.69 | 86.05 ± 5.33 | 6.20 | 105.29 ± 9.25 | 8.79 |
| 14.78 | 94.83 ± 6.79 | 7.16 | 101.86 ± 3.25 | 3.19 | |
| 59.10 | 93.94 ± 4.83 | 5.15 | 94.56 ± 6.74 | 7.13 | |
| Protocatechuic Acid | 3.23 | 90.14 ± 7.87 | 8.73 | 89.93 ± 8.46 | 12.47 |
| 12.94 | 103.10 ± 4.76 | 4.62 | 93.61 ± 6.50 | 6.95 | |
| 103.50 | 106.48 ± 5.20 | 4.88 | 107.84 ± 9.39 | 8.70 | |
| Analytes | Spiked Conc. (ng/mL) | Room Temperature Stability | Refrigeration Stability | Freeze-Thaw Stability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measured Conc. a (ng/mL) | RSD% | Accuracy (%) | Measured Conc. a (ng/mL) | RSD% | Accuracy (%) | Measured Conc. a (ng/mL) | RSD% | Accuracy (%) | ||
| Salidroside | 26.20 | 28.46 ± 1.29 | 4.54 | 108.63 | 27.71 ± 1.32 | 4.77 | 105.75 | 26.77 ± 1.88 | 7.02 | 102.17 |
| 104.80 | 112.08 ± 6.28 | 5.60 | 106.94 | 101.93 ± 8.62 | 8.46 | 97.26 | 110.4 ± 7.36 | 6.66 | 105.35 | |
| 419.20 | 405.56 ± 27.81 | 6.86 | 96.75 | 400.97 ± 22.00 | 5.49 | 95.65 | 402.81 ± 18.34 | 4.55 | 96.09 | |
| Anabasine | 0.23 | 0.23 ± 0.01 | 4.74 | 103.14 | 0.24 ± 0.03 | 12.19 | 108.53 | 0.21 ± 0.02 | 10.62 | 94.57 |
| 3.60 | 3.44 ± 0.28 | 8.23 | 95.43 | 3.48 ± 0.30 | 8.49 | 96.57 | 3.87 ± 0.15 | 3.81 | 107.43 | |
| 14.40 | 12.95 ± 0.85 | 6.53 | 89.91 | 13.67 ± 0.79 | 5.74 | 94.93 | 15.05 ± 0.98 | 6.53 | 104.52 | |
| Chlorogenic Acid | 3.69 | 3.82 ± 0.25 | 6.50 | 103.43 | 3.93 ± 0.22 | 5.64 | 106.41 | 3.41 ± 0.21 | 6.21 | 92.37 |
| 14.78 | 13.82 ± 1.17 | 8.44 | 93.51 | 13.91 ± 0.75 | 5.42 | 94.10 | 15.06 ± 0.84 | 5.59 | 101.87 | |
| 59.10 | 60.75 ± 3.88 | 6.38 | 102.78 | 58.04 ± 5.53 | 9.53 | 98.20 | 61.16 ± 3.38 | 5.53 | 103.49 | |
| Protocatechuic Acid | 3.23 | 3.37 ± 0.11 | 3.16 | 104.13 | 3.43 ± 0.25 | 7.36 | 106.25 | 3.08 ± 0.20 | 6.38 | 95.35 |
| 12.94 | 12.39 ± 0.26 | 2.06 | 95.79 | 11.89 ± 1.07 | 9.02 | 91.90 | 13.43 ± 0.54 | 3.99 | 103.82 | |
| 103.50 | 105.03 ± 5.94 | 5.66 | 101.48 | 105.06 ± 12.80 | 12.18 | 101.50 | 94.97 ± 5.29 | 5.57 | 91.75 | |
| Parameters | Group | Sal | Ana | CA | PCA |
|---|---|---|---|---|---|
| Tmax (h) | JGL | 0.72 ± 0.31 | 2.83 ± 1.33 | 0.49 ± 0.15 | 0.92 ± 0.38 * |
| CDP | 0.71 ± 0.10 | 1.83 ± 0.26 | 0.63 ± 0.21 | 0.49 ± 0.15 | |
| Cmax (ng/mL) | JGL | 285.08 ± 76.24 | 7.76 ± 2.31 * | 21.93 ± 6.81 * | 63.45 ± 21.21 |
| CDP | 298.98 ± 67.05 | 10.42 ± 0.44 | 14.52 ± 3.68 | 87.42 ± 21.68 | |
| AUC(0-t) (hr × ng/mL) | JGL | 588.27 ± 180.86 * | 122.57 ± 38.72 | 54.09 ± 17.76 | 210.88 ± 82.57 |
| CDP | 347.83 ± 68.20 | 102.16 ± 5.53 | 53.24 ± 7.21 | 154.45 ± 33.25 | |
| AUC(0-∞) (hr × ng/mL) | JGL | 620.33 ± 187.46 * | 140.39 ± 46.21 | 81.34 ± 26.26 | 238.54 ± 85.62 |
| CDP | 404.08 ± 88.52 | 111.83 ± 11.39 | 72.65 ± 10.19 | 201.88 ± 32.51 | |
| CLz/F (L/h/kg) | JGL | 3.73 ± 1.20 * | 0.15 ± 0.04 ** | 107.87 ± 36.04 ** | 14.89 ± 5.00 |
| CDP | 2.13 ± 0.48 | 0.20 ± 0.02 | 32.24 ± 4.21 | 11.28 ± 1.98 | |
| Vz/F (L/kg) | JGL | 6.98 ± 2.75 | 3.72 ± 1.33 ** | 1129.06 ± 433.16 ** | 203.82 ± 145.87 |
| CDP | 5.01 ± 2.69 | 4.21 ± 1.06 | 318.18 ± 67.43 | 261.77 ± 219.30 | |
| MRT(0-t) (h) | JGL | 1.68 ± 0.14 ** | 15.15 ± 1.52 * | 4.04 ± 0.37 | 5.42 ± 0.58 |
| CDP | 1.10 ± 0.09 | 12.93 ± 1.26 | 4.41 ± 0.25 | 5.20 ± 1.14 | |
| MRT(0-∞) (h) | JGL | 2.01 ± 0.26 | 22.49 ± 4.52 | 10.61 ± 3.98 | 9.42 ± 1.61 |
| CDP | 1.92 ± 0.84 | 17.86 ± 4.68 | 9.23 ± 1.73 | 16.50 ± 9.41 |
| Time (min) | %A | %B | Curve |
|---|---|---|---|
| Initial | 95 | 5 | Initial |
| 0.5 | 95 | 5 | 6 |
| 2.5 | 60 | 40 | 6 |
| 3.5 | 5 | 90 | 6 |
| 4.5 | 5 | 95 | 6 |
| 5 | 95 | 95 | 1 |
| 5.5 | 95 | 95 | 1 |
| Analyte | Polarity | Parent Ion (Da) | Daughter Ion (Da) | CV (V) | CE (eV) |
|---|---|---|---|---|---|
| Salidroside | ESI− | 299.1 | 119.0 | 35 | 20 |
| Anabasine | ESI+ | 163.4 | 94.2 | 30 | 15 |
| Chlorogenic Acid | ESI− | 353.0 | 191.0 | 30 | 15 |
| Protocatechuic Acid | ESI− | 152.9 | 109.0 | 30 | 15 |
| Levofloxacin (IS) | ESI− | 362.2 | 261.1 | 20 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhou, T.; Liu, H.; Zhou, Z.; Chi, M.; Li, Y.; Gong, Z.; Huang, Y. Pharmacokinetics Study of Jin-Gu-Lian Prescription and Its Core Drug Pair (Sargentodoxa cuneata (Oliv.) Rehd. et W and Alangium chinense (Lour.) Harms) by UPLC-MS/MS. Molecules 2022, 27, 4025. https://doi.org/10.3390/molecules27134025
Zheng L, Zhou T, Liu H, Zhou Z, Chi M, Li Y, Gong Z, Huang Y. Pharmacokinetics Study of Jin-Gu-Lian Prescription and Its Core Drug Pair (Sargentodoxa cuneata (Oliv.) Rehd. et W and Alangium chinense (Lour.) Harms) by UPLC-MS/MS. Molecules. 2022; 27(13):4025. https://doi.org/10.3390/molecules27134025
Chicago/Turabian StyleZheng, Lin, Ting Zhou, Hui Liu, Zuying Zhou, Mingyan Chi, Yueting Li, Zipeng Gong, and Yong Huang. 2022. "Pharmacokinetics Study of Jin-Gu-Lian Prescription and Its Core Drug Pair (Sargentodoxa cuneata (Oliv.) Rehd. et W and Alangium chinense (Lour.) Harms) by UPLC-MS/MS" Molecules 27, no. 13: 4025. https://doi.org/10.3390/molecules27134025
APA StyleZheng, L., Zhou, T., Liu, H., Zhou, Z., Chi, M., Li, Y., Gong, Z., & Huang, Y. (2022). Pharmacokinetics Study of Jin-Gu-Lian Prescription and Its Core Drug Pair (Sargentodoxa cuneata (Oliv.) Rehd. et W and Alangium chinense (Lour.) Harms) by UPLC-MS/MS. Molecules, 27(13), 4025. https://doi.org/10.3390/molecules27134025






