Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics
Abstract
:1. Instruction
2. Results
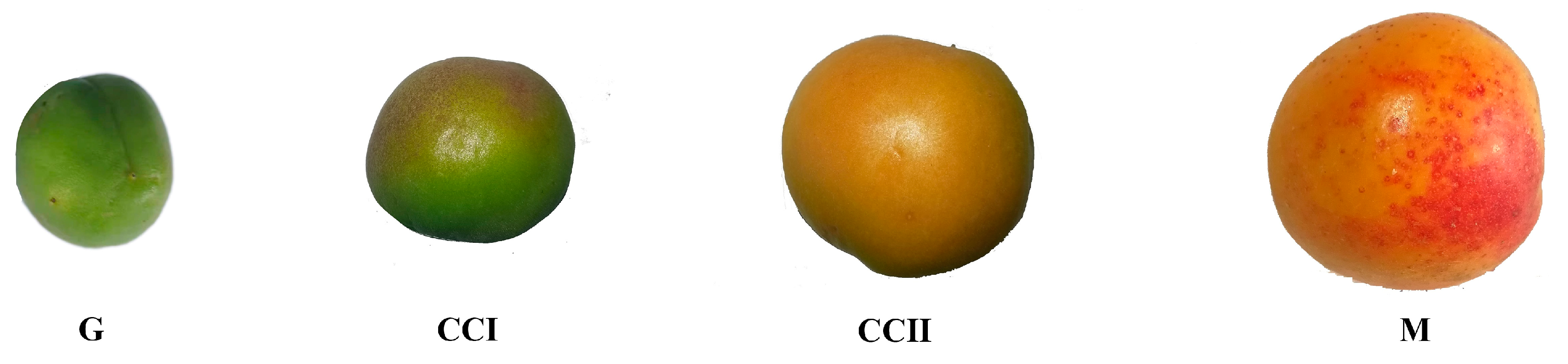
2.1. ‘Shushanggan Apricot’ Fruit Quality Index Analysis
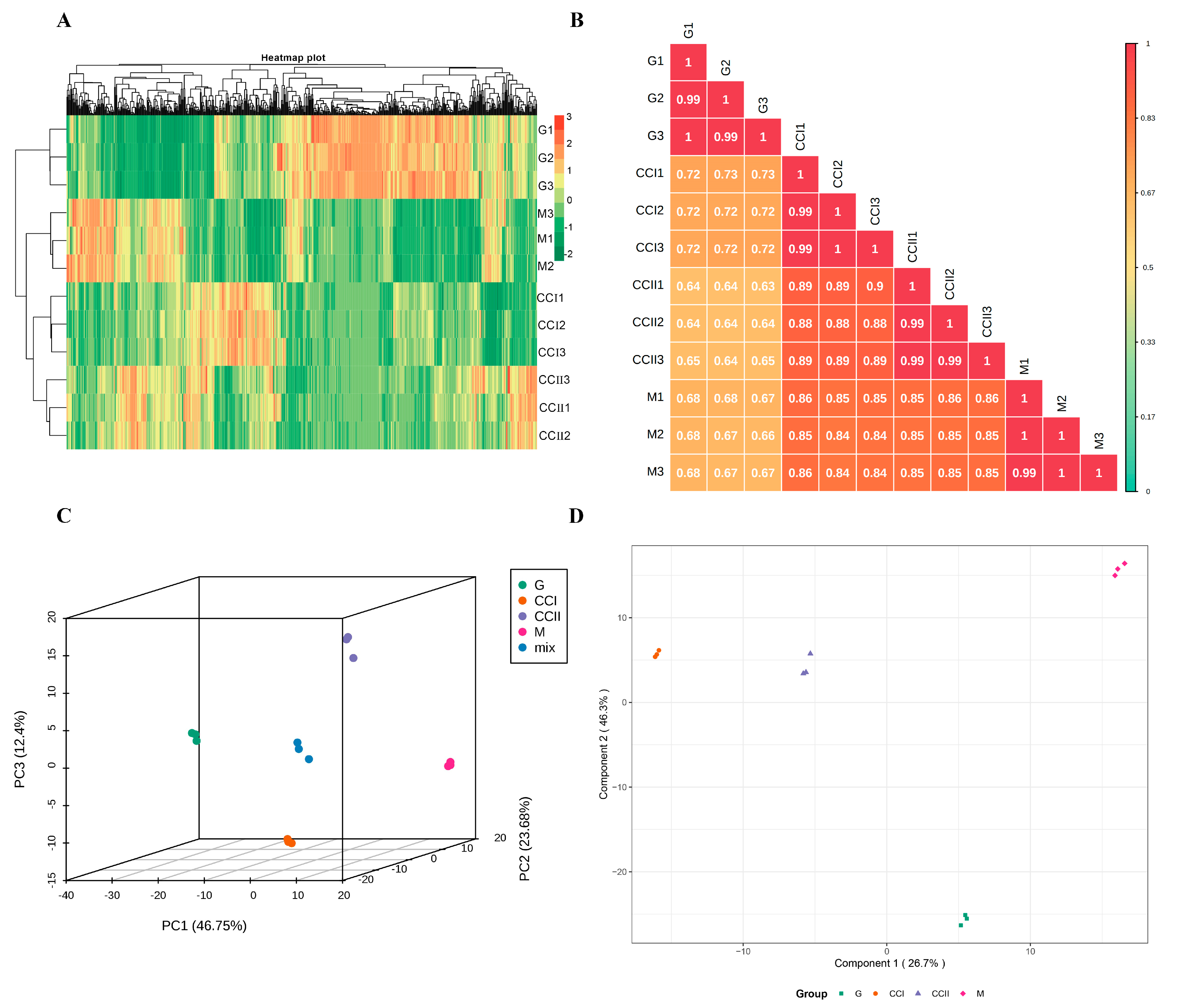
2.2. Metabolome Analysis in ‘Shushanggan Apricot’ Fruits at Developmental Stages
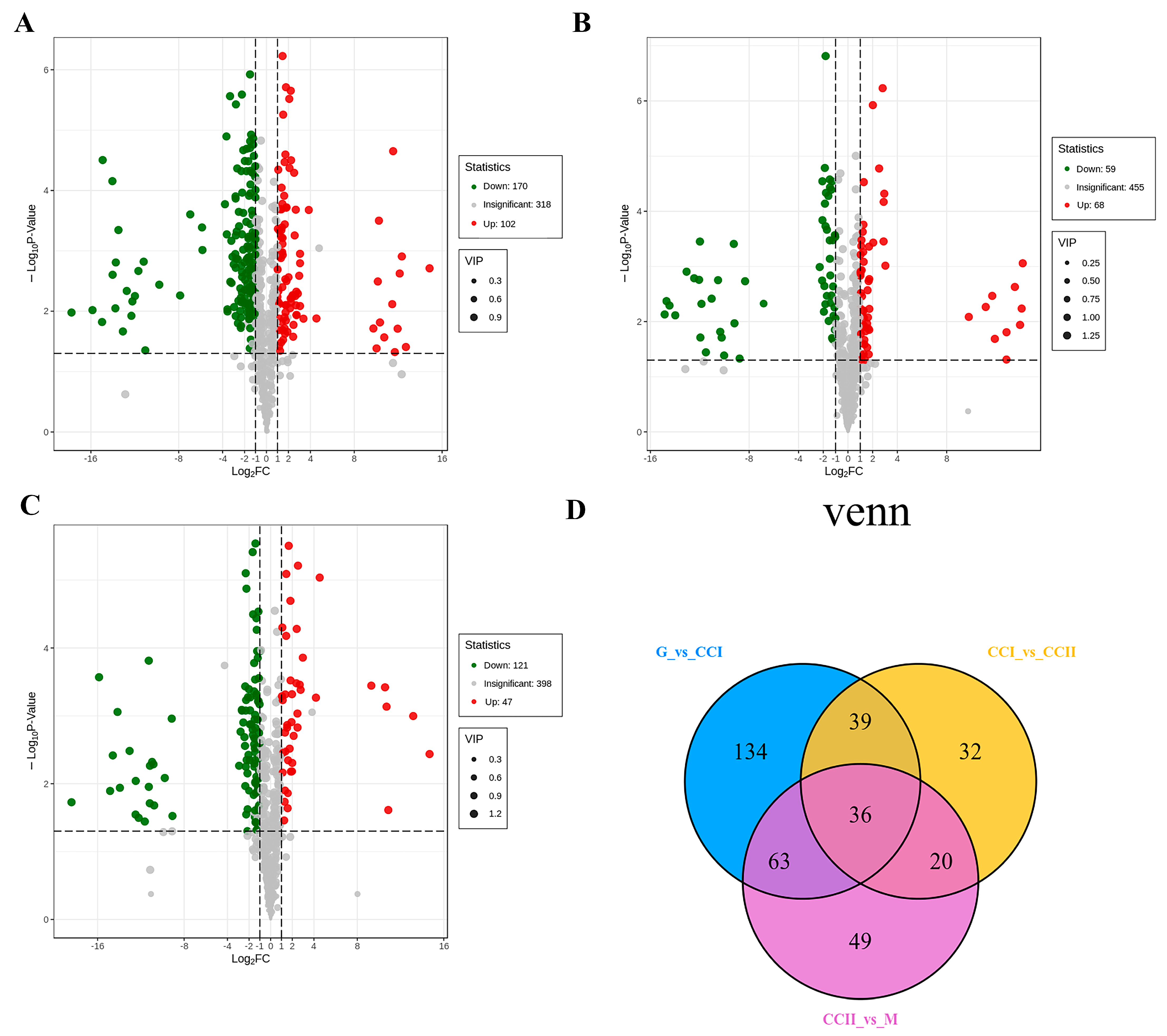
2.3. Differential Metabolites Screening, Identification and Pathway Analysis
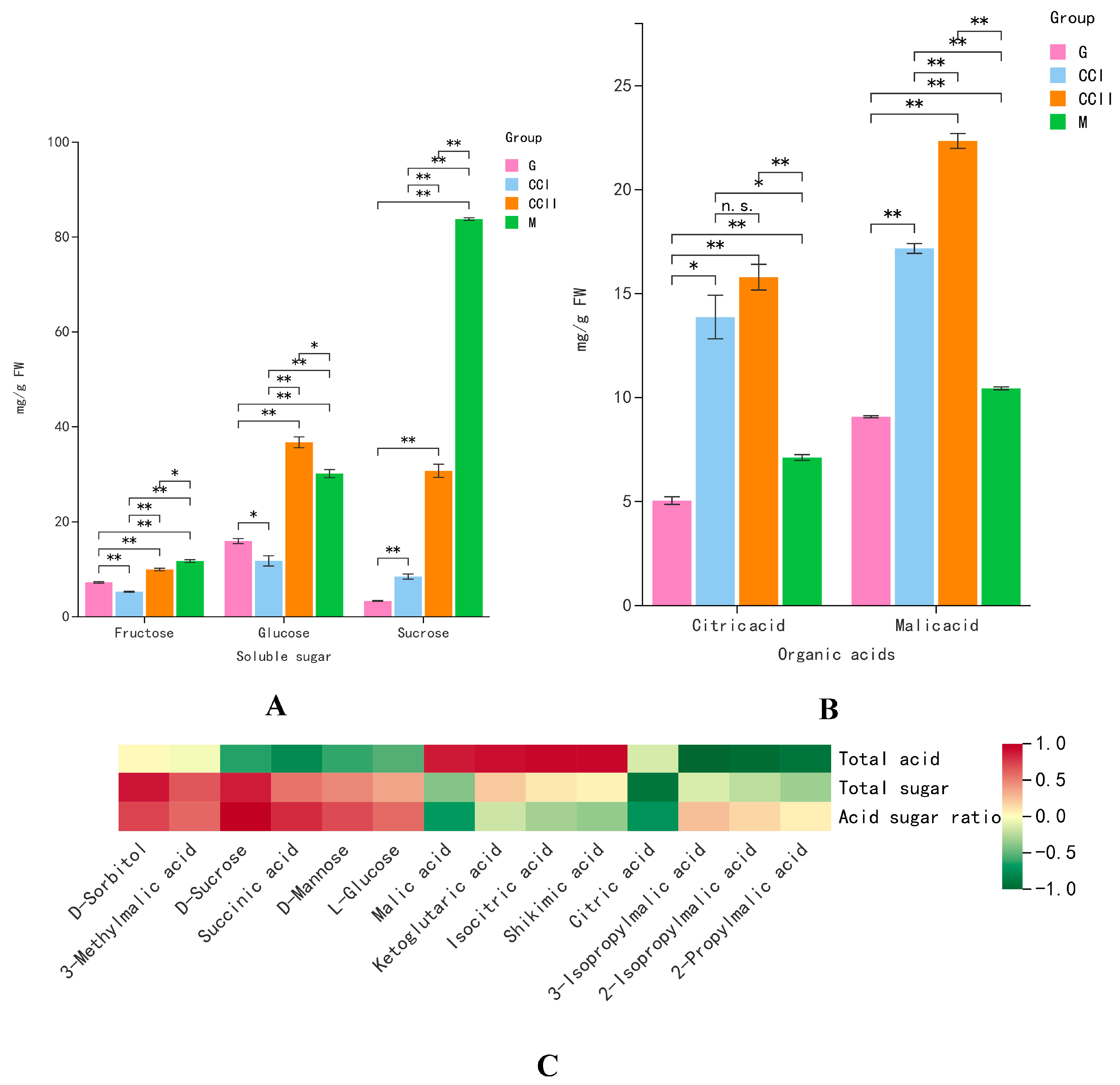
2.4. Sugar, Organic Acids, Vitamin and Amino Acids
2.5. Free Fatty Acids and Phenolic Acids
2.6. Quantitative Detection of Key Taste Compounds by HPLC
2.7. Correlation between Fruit Taste and Sugar and Organic Acids
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Chemicals
4.2. Sample Preparation and Extraction
4.3. Determination of Fruit Quality Indexes
4.4. UPLC-MS/MS Analysis of Taste and Nutritional Compounds
4.5. Quantitative Determination and Verification of Key Taste Compounds by HPLC
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomas-Barberan, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef]
- Xi, W.P.; Zheng, H.W.; Zhang, Q.Y.; Li, W.H. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.W.; Zhang, Q.Y.; Quan, J.P.; Zheng, Q.; Xi, W.P. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Cheong, M.W.; Loke, X.Q.; Liu, S.Q.; Pramudya, K.; Curran, P.; Yu, B. Characterization of volatile compounds and aroma profiles of malaysian pomelo (Citrus grandis (L.) osbeck) blossom and peel. J. Essent. Oil Res. 2011, 23, 34–44. [Google Scholar] [CrossRef]
- Muchuweti, M.; Zenda, G.; Ndhlala, A.; Kasiyamhuru, A. Sugars, organic acid and phenolic compounds of Ziziphus mauritiana fruit. Eur. Food Res. Technol. 2005, 221, 570–574. [Google Scholar] [CrossRef]
- Desnoues, E.; Gibon, Y.; Baldazzi, V.; Signoret, V.; Genard, M.; Quilot-Turion, B. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios. BMC Plant Biol. 2014, 14, 336. [Google Scholar] [CrossRef] [Green Version]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.L.; Gao, P.; Liu, S.; Zhu, Z.C.; Amanullah, S.; Davis, A.R.; Luan, F.S. Comparative transcriptome analysis of two contrasting watermelon genotypes during fruit development and ripening. BMC Genom. 2017, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Montero, T.M.; Molla, E.M.; Esteban, R.M.; LopezAndreu, F.J. Quality attributes of strawberry during ripening. Sci. Hortic. 1996, 65, 239–250. [Google Scholar] [CrossRef]
- Moing, A.; Renaud, C.; Gaudillere, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oms-Oliu, G.; Hertog, M.L.A.T.M.; Van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.H.; Nicolai, B.M. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, X.; Yu, O.; Tang, J.J.; Gu, X.G.; Wan, X.C.; Fang, C.B. Metabolic profiling of strawberry (Fragariaxananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
- Yu, K.Q.; Xu, Q.; Da, X.L.; Guo, F.; Ding, Y.D.; Deng, X.X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.J.; Yuan, P.L.; Zhu, H.J.; Kaseb, M.O.; Anees, M.; Lu, X.Q.; He, N.; et al. Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during fruit development. Sci. Hortic. 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Quilot, B.; Genard, M.; Kervella, J.; Li, S.H. Changes in sugar and organic acid concentrations during fruit maturation in peaches, P. davidiana and hybrids as analyzed by principal component analysis. Sci. Hortic. 2005, 103, 429–439. [Google Scholar] [CrossRef]
- Ernst, M.; Silva, D.B.; Silva, R.R.; Vencio, R.Z.N.; Lopes, N.P. Mass spectrometry in plant metabolomics strategies: From analytical platforms to data acquisition and processing. Nat. Prod. Rep. 2014, 31, 784–806. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.D.; Zhou, S.S.; Gong, X.Q.; Song, Y.; van Nocker, S.; Ma, F.W.; Guan, Q.M. Single-base methylome analysis reveals dynamic epigenomic differences associated with water deficit in apple. Plant Biotechnol. J. 2018, 16, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.T.; Wang, S.C.; Huang, Z.J.; Zhang, S.B.; Liao, Q.G.; Zhang, C.Z.; Lin, T.; Qin, M.; Peng, M.; Yang, C.K.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, J.; Fischer, M.; Amado, R. Changes in sugars, acids, and amino-acids during ripening and storage of apples (Cv Glockenapfel). J. Agric. Food Chem. 1992, 40, 1131–1134. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, S.J.; Lu, X.Q.; He, N.; Zhu, H.J.; Dou, J.L.; Liu, W.G. Comparative transcriptome analysis reveals key genes potentially related to soluble sugar and organic acid accumulation in watermelon. PLoS ONE 2018, 13, e0190096. [Google Scholar] [CrossRef]
- Zheng, T.; Su, K.X.; Gao, M.S.; Zhang, D.L.; Chen, X.Y.; Liu, S.M. Chemotaxonomic variation in volatile component contents and their correlation between climate factors in Chinese prickly ash peels (Zanthoxylum bungeanum Maxim.). Food Chem. X 2021, 12, 100176. [Google Scholar] [CrossRef]
- Zou, S.C.; Wu, J.C.; Shahid, M.Q.; He, Y.H.; Lin, S.Q.; Liu, Z.H.; Yang, X.H. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Zheng, H.W.; Zhang, Q.Y.; Li, W.H. Changes in soluble sugars and organic acids of Xinjiang apricot during fruit development and ripening. Sci. Agric. Sin. 2017, 49, 3981–3992. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Golding, J.B. Changes in sugars, acids and fatty acids in naturally parthenocarpic date plum persimmon (Diospyros lotus L.) fruit during maturation and ripening. Eur. Food Res. Technol. 2005, 221, 113–118. [Google Scholar] [CrossRef]
- Moing, A.; Svanella, L.; Rolin, D.; Gaudillere, M.; Gaudillere, J.P.; Monet, R. Compositional changes during the fruit development of two peach cultivars differing in juice acidity. J. Am. Soc. Hortic. Sci. 1998, 123, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Kotlega, D.; Peda, B.; Palma, J.; Zembron-Lacny, A.; Golab-Janowska, M.; Masztalewicz, M.; Nowacki, P.; Szczuko, M. Free fatty acids are associated with the cognitive functions in stroke survivors. Int. J. Environ. Res. Public Health 2021, 18, 6500. [Google Scholar] [CrossRef]
- Fadavi, A.; Barzegar, M.; Azizi, M.H. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J. Food Compos. Anal. 2006, 19, 676–680. [Google Scholar] [CrossRef]
- Bacchetta, L.; Aramini, M.; Zini, A.; Di Giammatteo, V.; Spera, D.; Drogoudi, P.; Rovira, M.; Silva, A.P.; Solar, A.; Botta, R. Fatty acids and alpha-tocopherol composition in hazelnut (Corylus avellana L.): A chemometric approach to emphasize the quality of European germplasm. Euphytica 2013, 191, 57–73. [Google Scholar] [CrossRef]
- Kale, A.; Kale, E. Are alanine, cysteine, glycine and valine amino acids the cause of non-immune hydrops fetalis? Clin. Exp. Obstet. Gynecol. 2012, 39, 341–342. [Google Scholar] [CrossRef]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C. Study of vitamin C degradation in acerola pulp during ohmicand conventional heat treatment. LWT-Food Sci. Technol. 2012, 47, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.W.; Li, W.; You, B.Y.; Yang, S.Y.; Xian, W.Y.; Deng, Y.; Huang, W.; Yang, R.L. Phenolic profiles, bioaccessibility and antioxidant activity of plum (Prunus Salicina Lindl). Food Res. Int. 2021, 143, 110300. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]

| Development Stage | TSS (%) | TS (%) | TA (%) | VC (mg/100 g) | Pr (g/100 g) | ASR |
|---|---|---|---|---|---|---|
| G | 15.56 ± 0.25 d | 6.25 ± 0.04 d | 0.89 ± 0.01 c | 11.42 ± 0.03 b | 7.23 ± 0.19 d | 7.00 ± 0.02 d |
| CC I | 20.21 ± 0.12 c | 8.58 ± 0.05 c | 1.06 ± 0.74 a | 9.23 ± 0.02 c | 11.27 ± 0.40 c | 7.18 ± 0.04 c |
| CC II | 25.95 ± 0.25 b | 10.61 ± 0.06 b | 1.20 ± 0.52 b | 20.45 ± 0.03 b | 15.13 ± 0.18 b | 10.10 ± 0.02 b |
| M | 29.14 ± 0.35 a | 12.59 ± 0.42 a | 0.80 ± 0.01 d | 25.56 ± 0.03 a | 18.63 ± 0.51 a | 16.84 ± 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Liu, S.-M.; Zheng, T. Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics. Molecules 2022, 27, 3870. https://doi.org/10.3390/molecules27123870
Cui B, Liu S-M, Zheng T. Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics. Molecules. 2022; 27(12):3870. https://doi.org/10.3390/molecules27123870
Chicago/Turabian StyleCui, Bei, Shu-Ming Liu, and Tao Zheng. 2022. "Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics" Molecules 27, no. 12: 3870. https://doi.org/10.3390/molecules27123870
APA StyleCui, B., Liu, S.-M., & Zheng, T. (2022). Chemotaxonomic Identification of Key Taste and Nutritional Components in ‘Shushanggan Apricot’ Fruits by Widely Targeted Metabolomics. Molecules, 27(12), 3870. https://doi.org/10.3390/molecules27123870






