Fluorescent Zn(II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture
Abstract
:1. Introduction
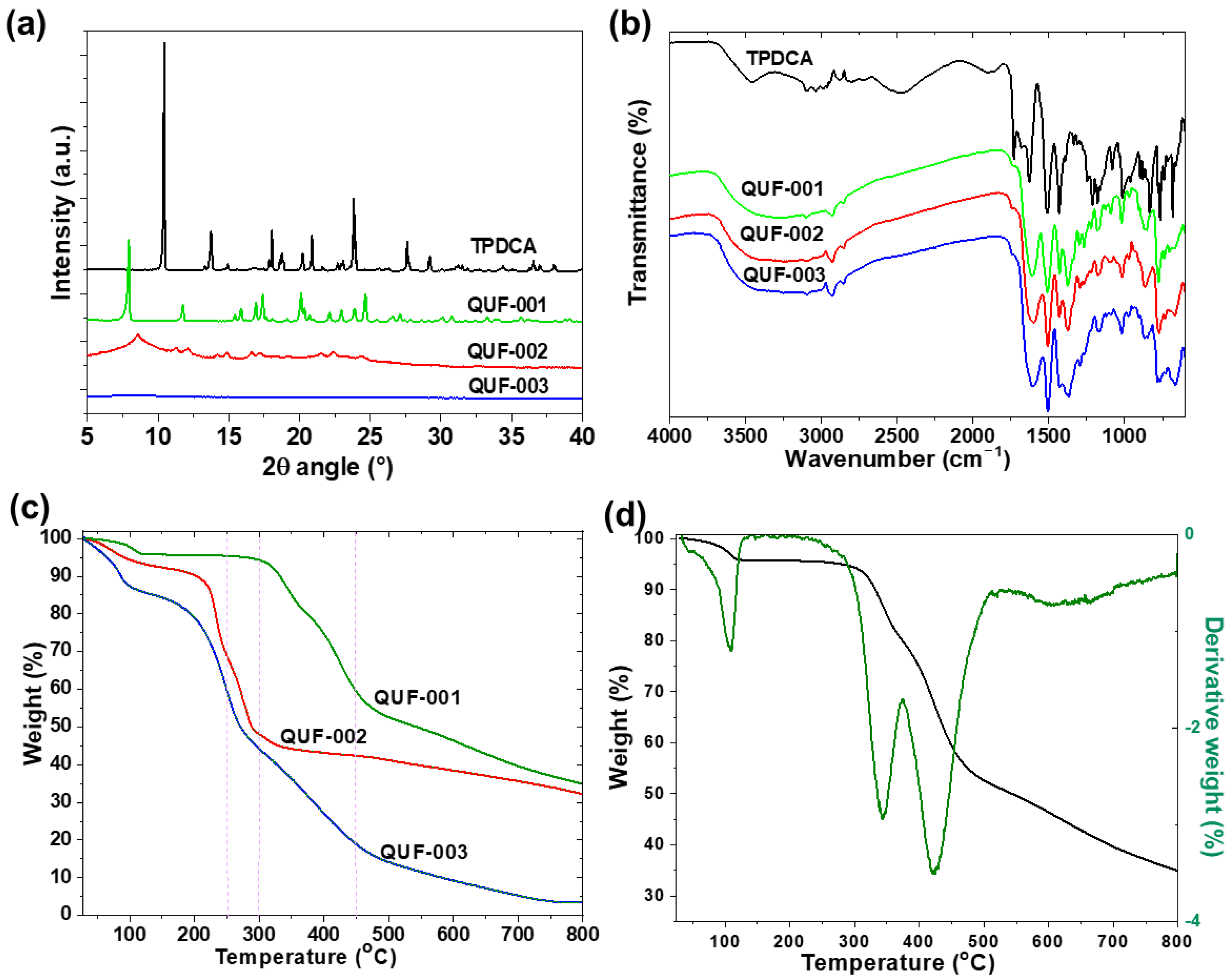
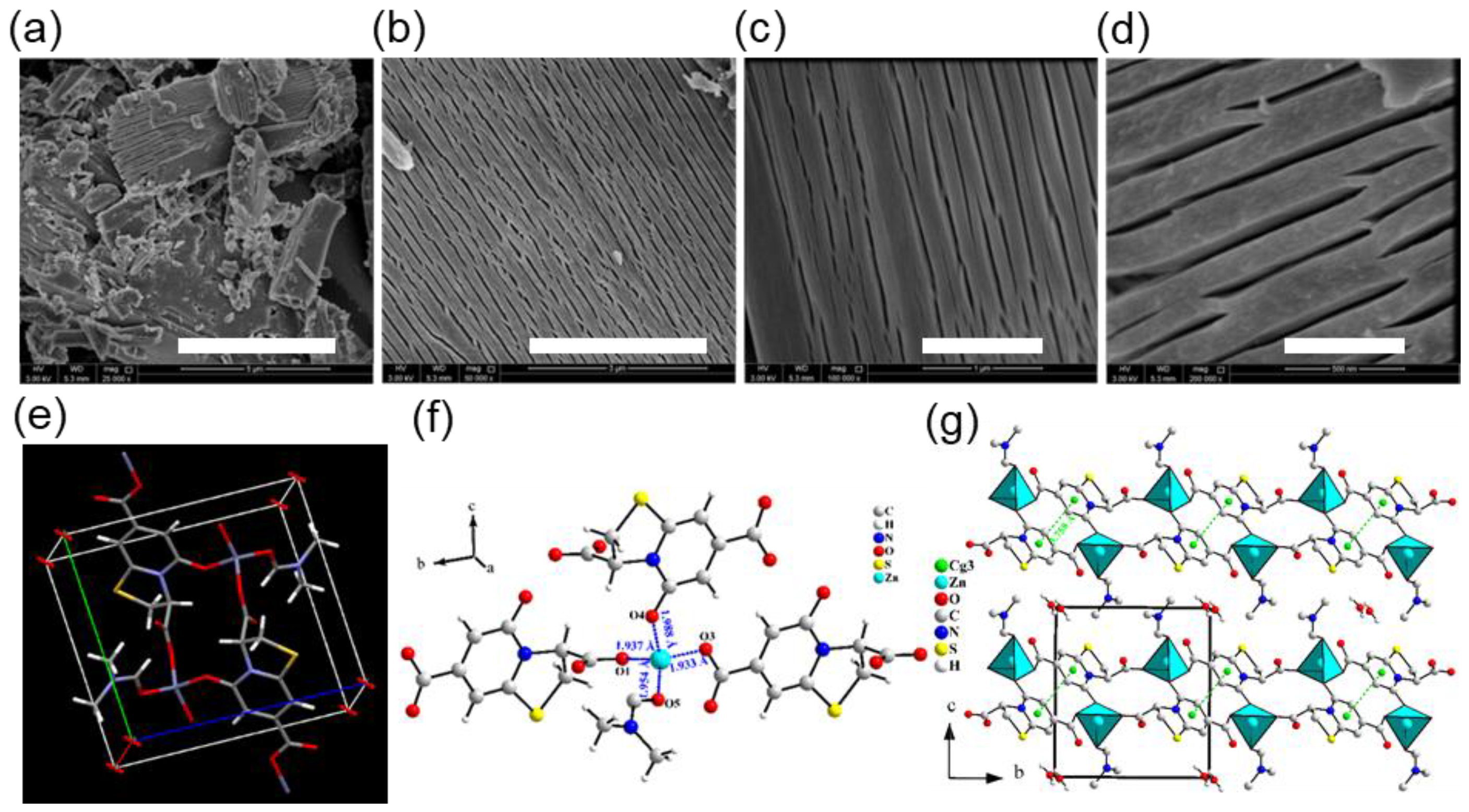
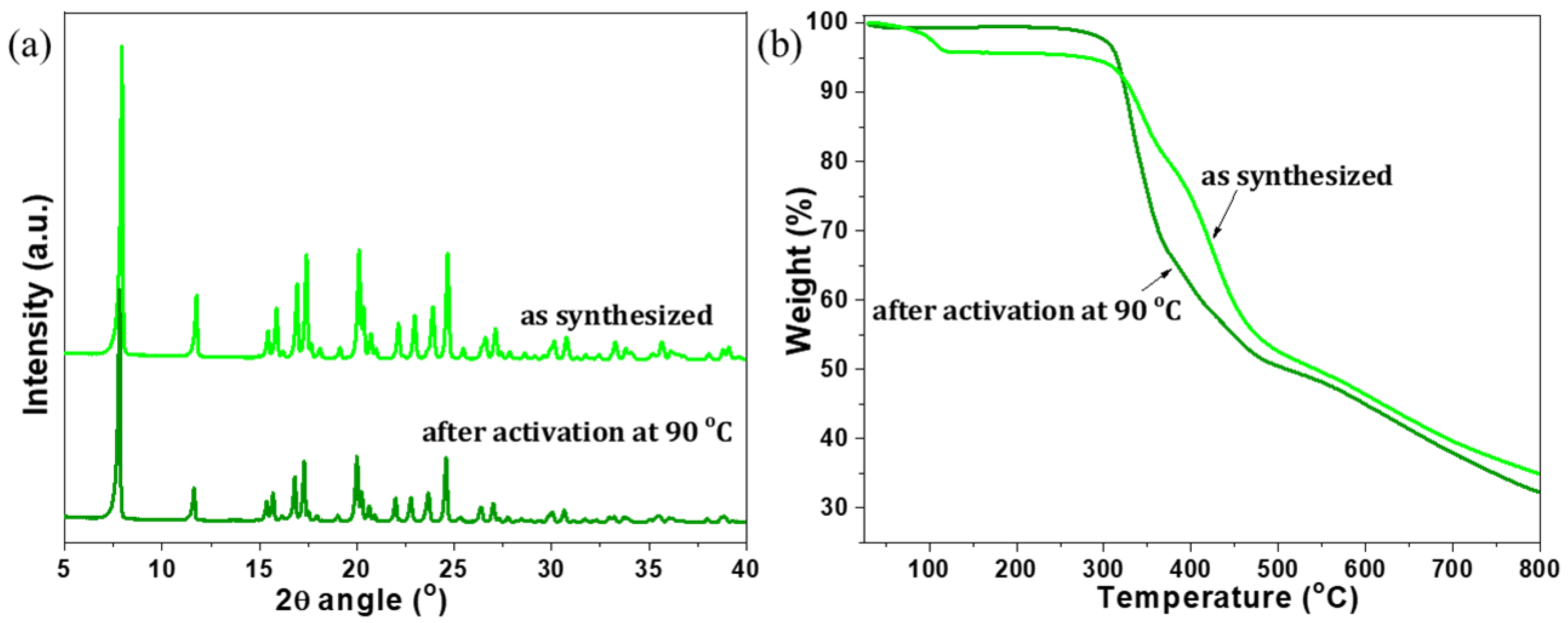
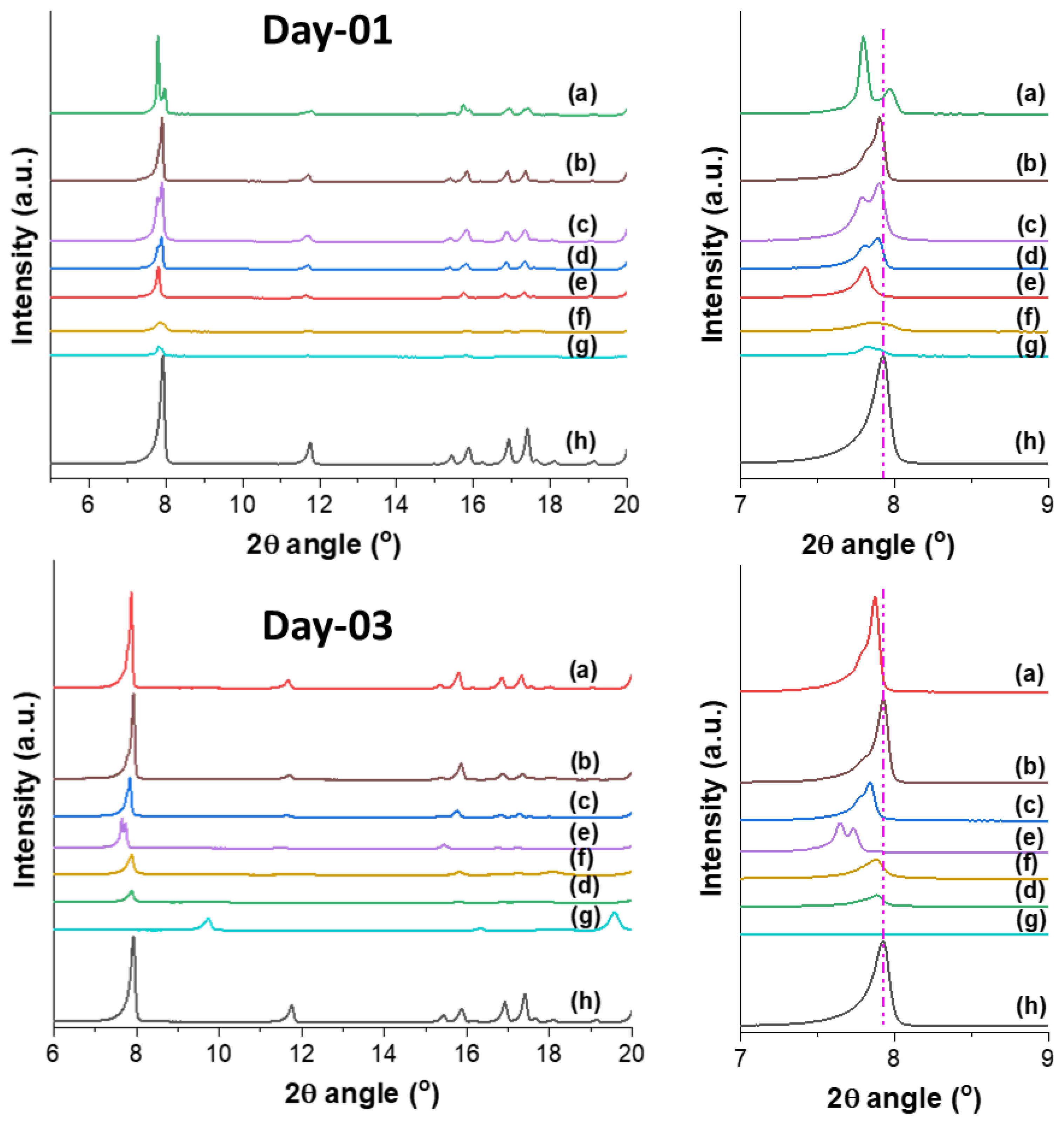
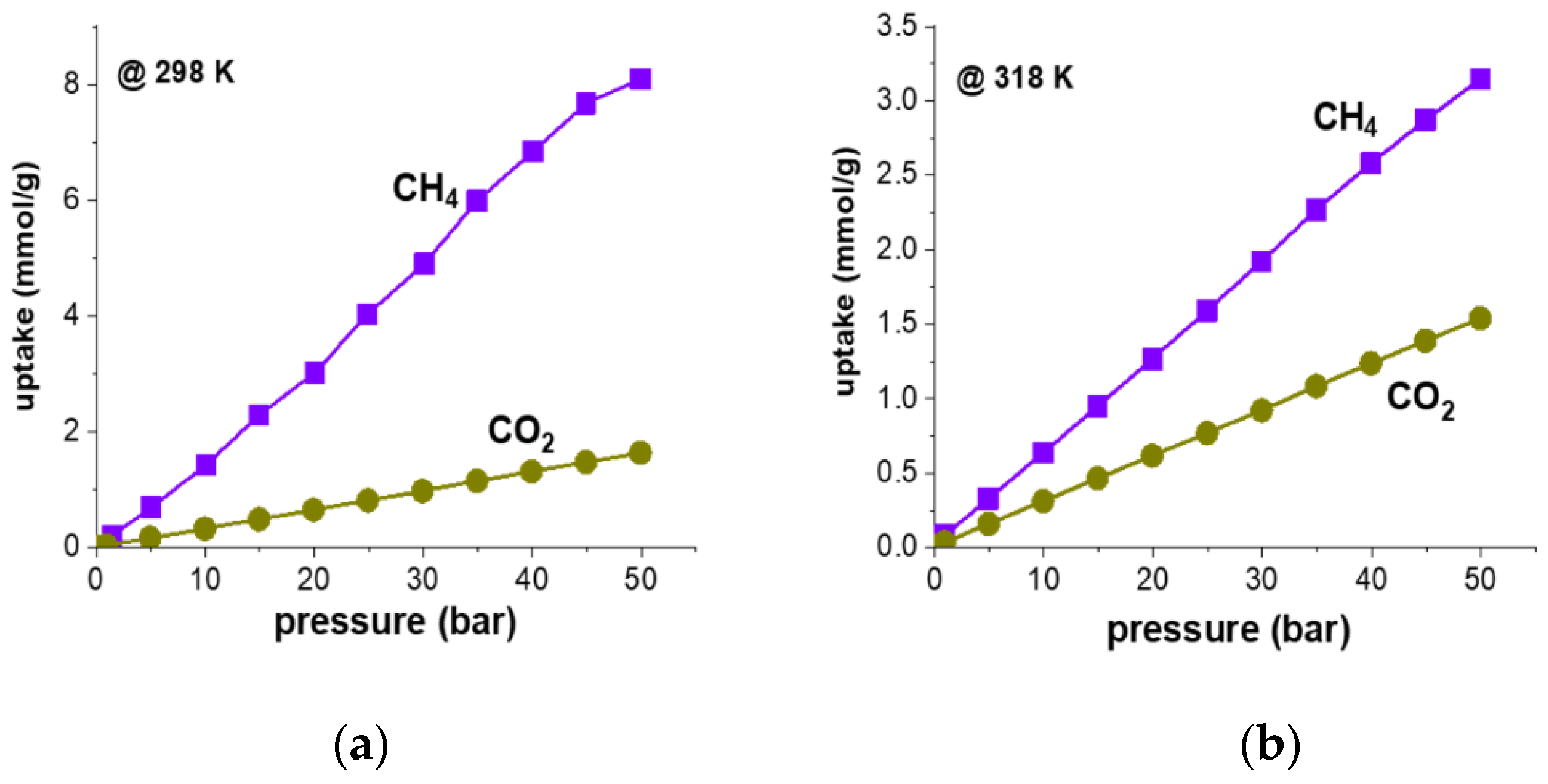
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of MOFs
3.3. Characterization
3.4. Single Crystal X-ray Diffraction Measurements
3.5. Solvent Inclusion
3.6. Gas Sorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, J.E.; Rummukainen, M. Taking science by surprise: The knowledge politics of the IPCC Special Report on 1.5 degrees. Environ. Sci. Policy 2020, 112, 10–16. [Google Scholar] [CrossRef]
- Djalante, R. Key assessments from the IPCC special report on global warming of 1.5 C and the implications for the Sendai framework for disaster risk reduction. Prog. Disaster Sci. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Amhamed, A.; Atilhan, M.; Berdiyorov, G. Permeabilities of CO2, H2S and CH4 through choline-based ionic liquids: Atomistic-scale simulations. Molecules 2019, 24, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tamreh, S.A.; Ibrahim, M.H.; El-Naas, M.H.; Vaes, J.; Pant, D.; Benamor, A.; Amhamed, A. Electroreduction of carbon dioxide into formate: A comprehensive review. ChemElectroChem 2021, 8, 3207–3220. [Google Scholar] [CrossRef]
- Tariq, M.; Soromenho, M.R.; Rebelo, L.P.N.; Esperança, J.M. Insights into CO2 hydrates formation and dissociation at isochoric conditions using a rocking cell apparatus. Chem. Eng. Sci. 2022, 249, 117319. [Google Scholar] [CrossRef]
- Rozyyev, V.; Thirion, D.; Ullah, R.; Lee, J.; Jung, M.; Oh, H.; Atilhan, M.; Yavuz, C.T. High-capacity methane storage in flexible alkane-linked porous aromatic network polymers. Nat. Energy 2019, 4, 604–611. [Google Scholar] [CrossRef]
- Altamash, T.; Amhamed, A.I.; Aparicio, S.; Atilhan, M. Combined Experimental and Theoretical Study on High Pressure Methane Solubility in Natural Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2019, 58, 8097–8111. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Qiblawey, H.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J. Mol. Liq. 2018, 256, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Anaya, B.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior of natural deep eutectic solvents (NADES) via combined experimental and molecular simulation techniques. Chem. Select 2017, 2, 7278–7295. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. High pressure methane adsorption in the metal-organic frameworks Cu3(btc)2, Zn2(bdc)2dabco, and Cr3F(H2O)2O(bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Becker, T.M.; Heinen, J.; Dubbeldam, D.; Lin, L.-C.; Vlugt, T.J. Polarizable force fields for CO2 and CH4 adsorption in M-MOF-74. J. Phys. Chem. C 2017, 121, 4659–4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursueguía, D.; Díaz, E.; Ordóñez, S. Metal-Organic Frameworks (MOFs) as methane adsorbents: From storage to diluted coal mining streams concentration. Sci. Total Environ. 2021, 790, 148211. [Google Scholar] [CrossRef] [PubMed]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.; Tome, J.P.; Cavaleiro, J.A.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar]
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore chemistry of metal–organic frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Goetjen, T.A.; Liu, J.; Wu, Y.; Sui, J.; Zhang, X.; Hupp, J.T.; Farha, O.K. Metal–organic framework (MOF) materials as polymerization catalysts: A review and recent advances. Chem. Commun. 2020, 56, 10409–10418. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’ Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Konstas, K.; Osl, T.; Yang, Y.; Batten, M.; Burke, N.; Hill, A.J.; Hill, M.R. Methane storage in metal organic frameworks. J. Mater. Chem. 2012, 22, 16698–16708. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Dou, Z.; Yu, J.; Cui, Y.; Yang, Y.; Wang, Z.; Yang, D.; Qian, G. Luminescent metal–organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef]
- Altamash, T.; Ahmed, W.; Rasool, S.; Biswas, K.H. Intracellular Ionic Strength Sensing Using NanoLuc. Int. J. Mol. Sci. 2021, 22, 677. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.; Houk, R. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Amin, N.; Tan, J.-C. A Luminescent Guest@ MOF Nanoconfined Composite System for Solid-State Lighting. Molecules 2021, 26, 7583. [Google Scholar] [CrossRef]
- Salihu, R.; Abd Razak, S.I.; Zawawi, N.A.; Kadir, M.R.A.; Ismail, N.I.; Jusoh, N.; Mohamad, M.R.; Nayan, N.H.M. Citric acid: A green cross-linker of biomaterials for biomedical applications. Eur. Polym. J. 2021, 146, 110271. [Google Scholar] [CrossRef]
- Shan, D.; Hsieh, J.T.; Bai, X.; Yang, J. Citrate-Based Fluorescent Biomaterials. Adv. Healthcare Mater. 2018, 7, 1800532. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Sun, H. Novel properties and applications of carbon nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, J.; Park, C.B. Photonic carbon dots as an emerging nanoagent for biomedical and healthcare applications. ACS Nano 2020, 14, 6470–6497. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Richter, A.F.; Li, Y.; Döring, A.; Kasák, P.; Popelka, A.; Schneider, J.; Kershaw, S.V.; Yoo, S.J. Chemically synthesized carbon nanorods with dual polarized emission. ACS Nano 2019, 13, 12024–12031. [Google Scholar] [CrossRef]
- Liang, T.; Liu, E.; Li, M.; Ushakova, E.V.; Kershaw, S.V.; Rogach, A.L.; Tang, Z.; Qu, S. Morphology control of luminescent carbon nanomaterials: From dots to rolls and belts. ACS Nano 2020, 15, 1579–1586. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z. The formation mechanism and fluorophores of carbon dots synthesized via a bottom-up route. Mater. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Q.; Ni, Y. Facile microwave-assisted solid-phase synthesis of highly fluorescent nitrogen–sulfur-codoped carbon quantum dots for cellular imaging applications. Chem. Eur. J. 2015, 21, 13004–13011. [Google Scholar] [CrossRef]
- Reckmeier, C.; Schneider, J.; Susha, A.; Rogach, A. Luminescent colloidal carbon dots: Optical properties and effects of doping. Opt. Express 2016, 24, A312–A340. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, M.; Wang, Z.; Schneider, J.; Huang, H.; Kershaw, S.V.; Zhi, C.; Rogach, A.L. A Building Brick Principle to Create Transparent Composite Films with Multicolor Emission and Self-Healing Function. Small 2018, 14, 1800315. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Schneider, J.; Xiong, Y.; Häusler, J.; Kasák, P.; Schnick, W.; Rogach, A.L. Aggregated molecular fluorophores in the ammonothermal synthesis of carbon dots. Chem. Mater. 2017, 29, 10352–10361. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Bednarz, S.; Żmudzki, P.; Galica, M.; Bogdał, D. Novel efficient fluorophores synthesized from citric acid. RSC Adv. 2015, 5, 34795–34799. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.H.; Zeng, H.B.; Chen, Y.M.; Yang, S.C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon dots with high fluorescence quantum yield: The fluorescence originates from organic fluorophores. Nanoscale 2016, 8, 14374–14378. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Gautam, S.; Liu, L.; Dey, J.; Chen, W.; Mason, R.P.; Serrano, C.A.; Schug, K.A.; Tang, L. Development of aliphatic biodegradable photoluminescent polymers. Proc. Natl. Acad. Sci. USA 2009, 106, 10086–10091. [Google Scholar] [CrossRef] [Green Version]
- Kasprzyk, W.; Bednarz, S.; Bogdał, D. Luminescence phenomena of biodegradable photoluminescent poly (diol citrates). Chem. Commun. 2013, 49, 6445–6447. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yang, Z.; Liu, Z.G.; Wan, J.Y.; Xiao, J.; Zhang, H.L. Facile Preparation of Bright-Fluorescent Soft Materials from Small Organic Molecules. Chem. Eur. J. 2016, 22, 8096–8104. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Feng, Q.; Zhao, P.; Xu, X.; Ng, D.H.; Bian, L. Citric acid/cysteine-modified cellulose-based materials: Green preparation and their applications in anticounterfeiting, chemical sensing, and UV shielding. ACS Sustain. Chem. Eng. 2017, 5, 11387–11394. [Google Scholar] [CrossRef]
- Kim, J.P.; Xie, Z.; Creer, M.; Liu, Z.; Yang, J. Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of cystic fibrosis. Chem. Sci. 2017, 8, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kim, J.P.; Creer, M.; Yang, J.; Liu, Z. A smartphone-based chloridometer for point-of-care diagnostics of cystic fibrosis. Biosens. Bioelectron. 2017, 97, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kasak, P.; Danko, M.; Zavahir, S.; Mrlik, M.; Xiong, Y.; Yousaf, A.B.; Lai, W.-F.; Krupa, I.; Tkac, J.; Rogach, A.L. Identification of molecular fluorophore as a component of carbon dots able to induce gelation in a fluorescent multivalent-metal-ion-free alginate hydrogel. Sci. Rep. 2019, 9, 15080. [Google Scholar] [CrossRef] [PubMed]
- Langer, M.; Paloncyova, M.; Medved’, M.; Otyepka, M. Molecular fluorophores self-organize into C-dot seeds and incorporate into C-dot structures. J. Phys. Chem. Lett. 2020, 11, 8252–8258. [Google Scholar] [CrossRef]
- Langer, M.; Hrivnák, T.s.; Medved’, M.; Otyepka, M. Contribution of the molecular fluorophore IPCA to excitation-independent photoluminescence of carbon dots. J. Phys. Chem. C 2021, 125, 12140–12148. [Google Scholar] [CrossRef]
- Mutailipu, M.; Li, F.; Jin, C.; Yang, Z.; Poeppelmeier, K.R.; Pan, S. Strong Nonlinearity Induced by Coaxial Alignment of Polar Chain and Dense [BO3] Units in CaZn2(BO3)2. Angew. Chem. 2022, 134, e202202096. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [Green Version]
- Flynn Jr, C.M. Hydrolysis of inorganic iron (III) salts. Chem. Rev. 1984, 84, 31–41. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Burla, M.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Polidori, G.; Spagna, R.T.; Viterbo, D. SIR88–a direct-methods program for the automatic solution of crystal structures. J. Appl. Crystallogr. 1989, 22, 389–393. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.W.; Cheng, Y.; Zheng, L.Y.; Cao, Q.E. Reversible phase transition of porous coordination polymers. Chem. A Eur. J. 2020, 26, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Fan, W.W.; Lu, Z.X.; Qin, Y.; Yang, S.X.; Li, Y.; Liu, Y.X.; Zheng, L.Y.; Cao, Q.E. Solvent-Driven Reversible Phase Transition of a Pillared Metal–Organic Framework. Chem. A Eur. J. 2019, 25, 5787–5792. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bartolome, E.; Martinez-Martinez, A.; Resines-Urien, E.; Piñeiro-Lopez, L.; Costa, J.S. Reversible single-crystal-to-single-crystal transformations in coordination compounds induced by external stimuli. Coord. Chem. Rev. 2022, 452, 214281. [Google Scholar] [CrossRef]
- Essehli, R.; Sabri, S.; El-Mellouhi, F.; Aïssa, B.; Ben Yahia, H.; Altamash, T.; Khraisheh, M.; Amhamed, A.; El Bali, B. Single crystal structure, vibrational spectroscopy, gas sorption and antimicrobial properties of a new inorganic acidic diphosphates material (NH4)2Mg (H2P2O7)2•2H2O. Sci. Rep. 2020, 10, 8909. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, A.; El-Naas, M.H.; Amhamed, A. Enhancing gas loading and reducing energy consumption in acid gas removal systems: A simulation study based on real NGL plant data. J. Nat. Gas Sci. 2018, 55, 565–574. [Google Scholar] [CrossRef]
- Deng, L.; Dong, X.; An, D.-L.; Weng, W.-Z.; Zhou, Z.-H. Gas Adsorption of Mixed-Valence Trinuclear Oxothiomolybdenum Glycolates. Inorg. Chem. 2020, 59, 4874–4881. [Google Scholar] [CrossRef] [PubMed]
- Qazvini, O.T.; Babarao, R.; Telfer, S.G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 2021, 12, 197. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Esteves, I.A.; Mota, J.P. Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn (dcpa) Metal-Organic Framework. Energies 2021, 14, 5598. [Google Scholar] [CrossRef]
- Jiang, J.; Furukawa, H.; Zhang, Y.-B.; Yaghi, O.M. High methane storage working capacity in metal–organic frameworks with acrylate links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Karadas, F.; Zulfiqar, S.; Deniz, E.; Aparicio, S.; Atilhan, M.; Yavuz, C.T.; Han, S.M. Limitations and high pressure behavior of MOF-5 for CO2 capture. Phys. Chem. Chem. Phys. 2013, 15, 14319–14327. [Google Scholar] [CrossRef]
- Deniz, E.; Karadas, F.; Patel, H.A.; Aparicio, S.; Yavuz, C.T.; Atilhan, M. A combined computational and experimental study of high pressure and supercritical CO2 adsorption on Basolite MOFs. Microporous Mesoporous Mater. 2013, 175, 34–42. [Google Scholar] [CrossRef]
- Karadas, F.; Yavuz, C.T.; Zulfiqar, S.; Aparicio, S.; Stucky, G.D.; Atilhan, M. CO2 adsorption studies on hydroxy metal carbonates M (CO3) x (OH) y (M= Zn, Zn–Mg, Mg, Mg–Cu, Cu, Ni, and Pb) at high pressures up to 175 bar. Langmuir 2011, 27, 10642–10647. [Google Scholar] [CrossRef]
- Caddeo, F.; Loche, D.; Casula, M.F.; Corrias, A.J.S.r. Evidence of a cubic iron sub-lattice in t-CuFe2O4 demonstrated by X-ray Absorption Fine Structure. Sci. Rep. 2018, 8, 797. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Li, Z.; Li, M.-Q.; Diao, Y.; Lin, F.; Liu, T.; Zhang, J.; Tieu, P.; Gao, W.; Qi, F. 2D metal–organic framework for stable perovskite solar cells with minimized lead leakage. Nat. Nanotechnol. 2020, 15, 934–940. [Google Scholar] [CrossRef]
- Altamash, T.; Khraisheh, M.; Qureshi, M.F. Investigating the effects of mixing ionic liquids on their density, decomposition temperature, and gas absorption. Chem. Eng. Res. Des. 2019, 148, 251–259. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Thai, T.Q.; Saitoh, S.; Wu, B.; Saitoh, Y.; Shimo, S.; Fujitani, H.; Otobe, H.; Ohno, N. Conductive resins improve charging and resolution of acquired images in electron microscopic volume imaging. Sci. Rep. 2016, 6, 23721. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS version 2014/5; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]

| Crystal Data | |
| Chemical formula | C12H12N2O6SZn × 0.25H2O |
| Mr | 382.2 |
| Crystal system, space group | Triclinic, P-1 |
| Temperature (K) | 293 |
| a, b, c (Å) | 6.4459 (3), 10.4427 (5), 11.2947 (5) |
| α, β, γ (°) | 88.693 (2), 83.751 (3), 77.741 (2) |
| V (Å3) | 738.52 (6) |
| Z | 2 |
| Radiation type | Mo Kα |
| µ (mm−1) | 1.84 |
| Crystal size (mm) | 0.06 × 0.03 × 0.01 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.042, 0.106, 1.09 |
| No. of reflections | 3269 |
| No. of parameters | 203 |
| No. of restraints | 0 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.93, −0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavahir, S.; Ben Yahia, H.; Schneider, J.; Han, D.; Krupa, I.; Altamash, T.; Atilhan, M.; Amhamed, A.; Kasak, P. Fluorescent Zn(II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture. Molecules 2022, 27, 3845. https://doi.org/10.3390/molecules27123845
Zavahir S, Ben Yahia H, Schneider J, Han D, Krupa I, Altamash T, Atilhan M, Amhamed A, Kasak P. Fluorescent Zn(II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture. Molecules. 2022; 27(12):3845. https://doi.org/10.3390/molecules27123845
Chicago/Turabian StyleZavahir, Sifani, Hamdi Ben Yahia, Julian Schneider, DongSuk Han, Igor Krupa, Tausif Altamash, Mert Atilhan, Abdulkarem Amhamed, and Peter Kasak. 2022. "Fluorescent Zn(II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture" Molecules 27, no. 12: 3845. https://doi.org/10.3390/molecules27123845
APA StyleZavahir, S., Ben Yahia, H., Schneider, J., Han, D., Krupa, I., Altamash, T., Atilhan, M., Amhamed, A., & Kasak, P. (2022). Fluorescent Zn(II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture. Molecules, 27(12), 3845. https://doi.org/10.3390/molecules27123845









