The Allelopathic Activity of Extracts and Isolated from Spirulina platensis
Abstract
:1. Introduction
2. Results
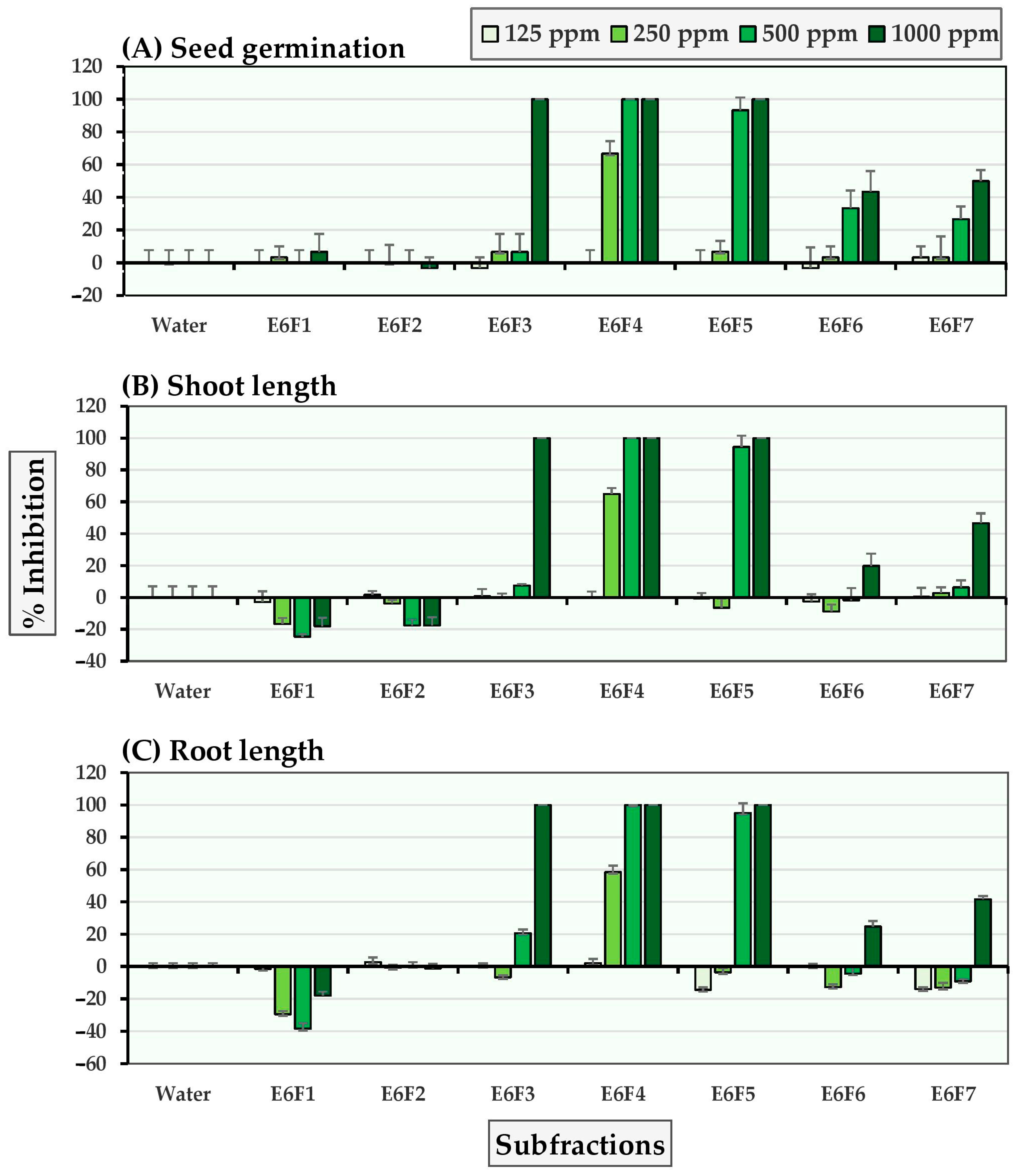
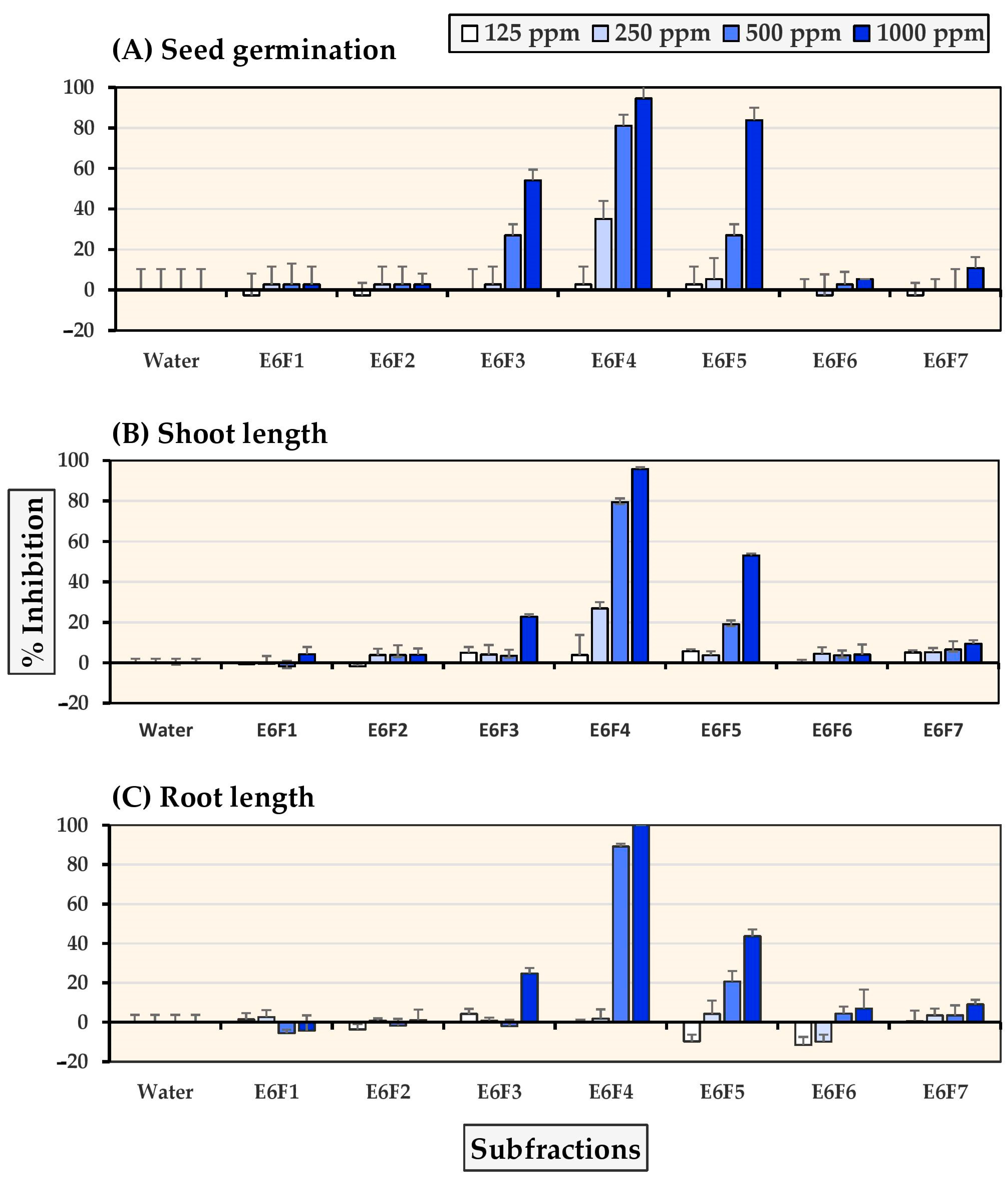
2.1. Allelopathic Effects of the Seven E6 Subfractions
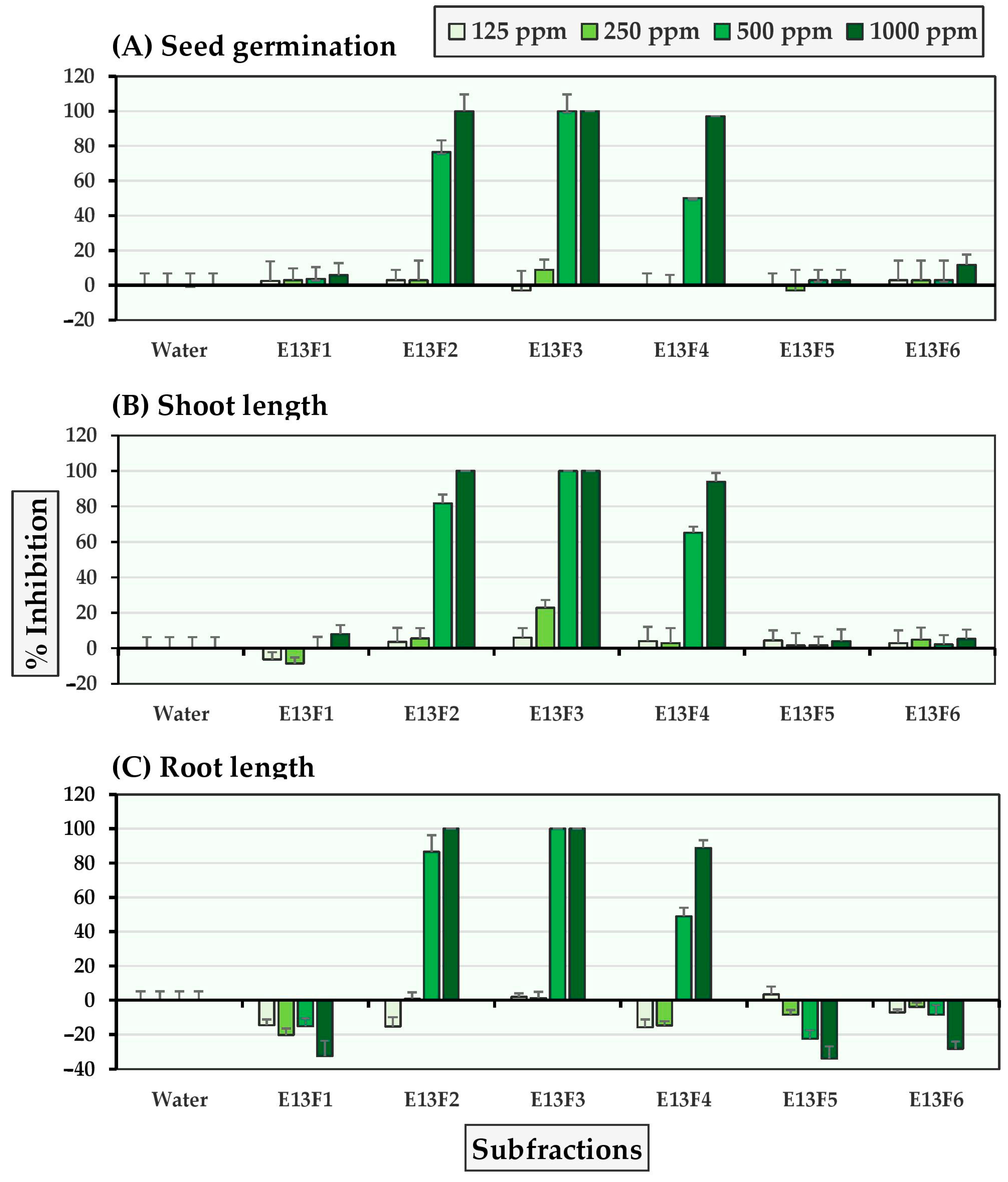
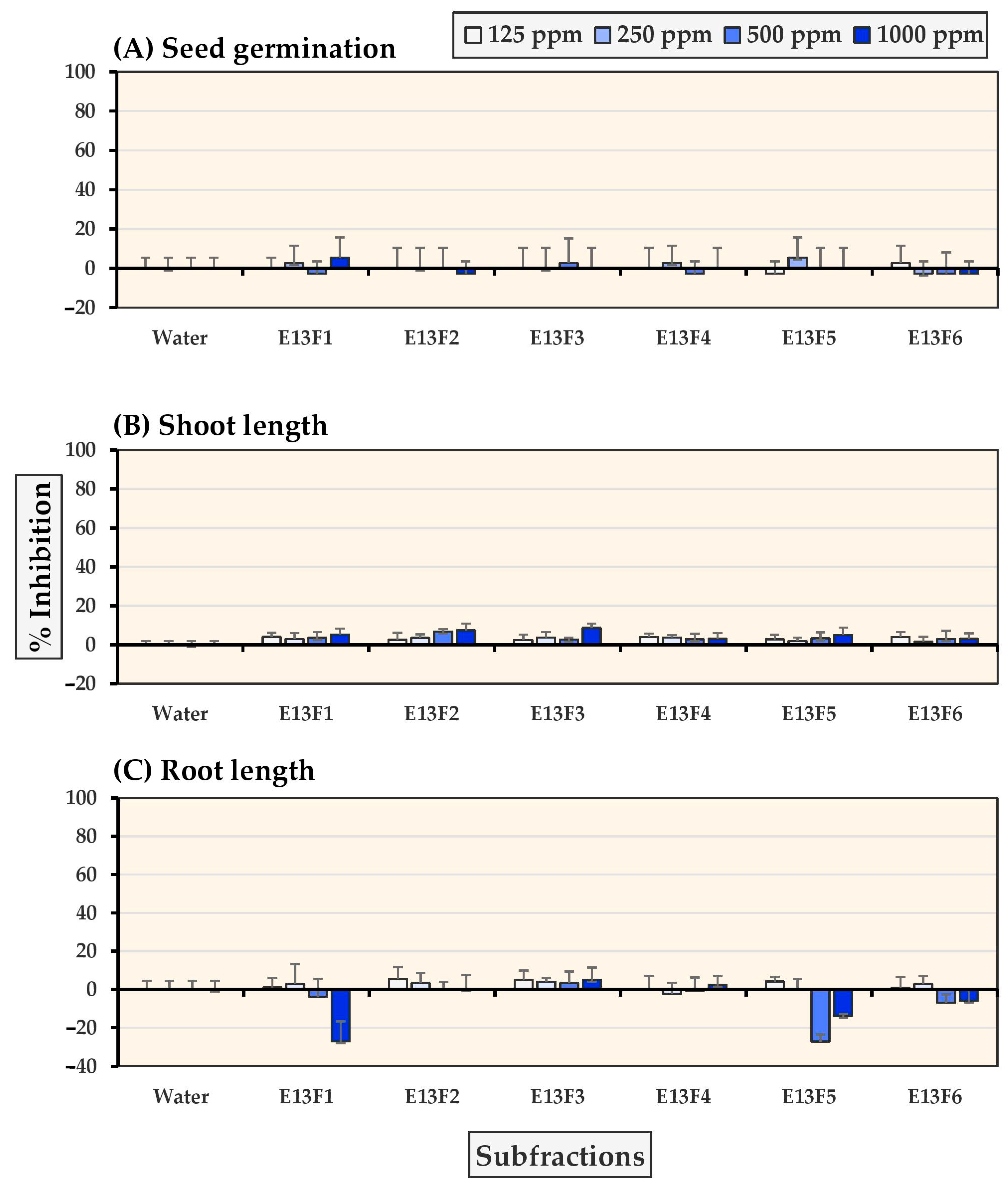
2.2. Allelopathic Effects of Six E13 Subfractions
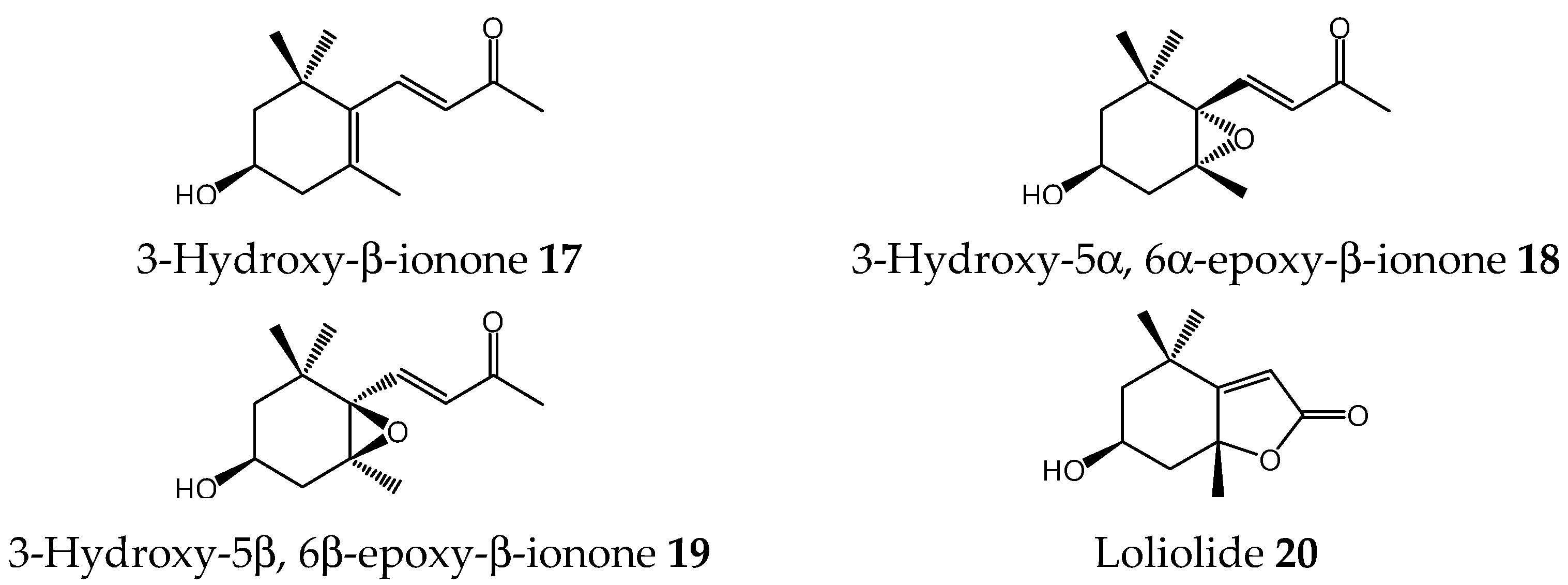
2.3. Isolation and Identification of Compounds
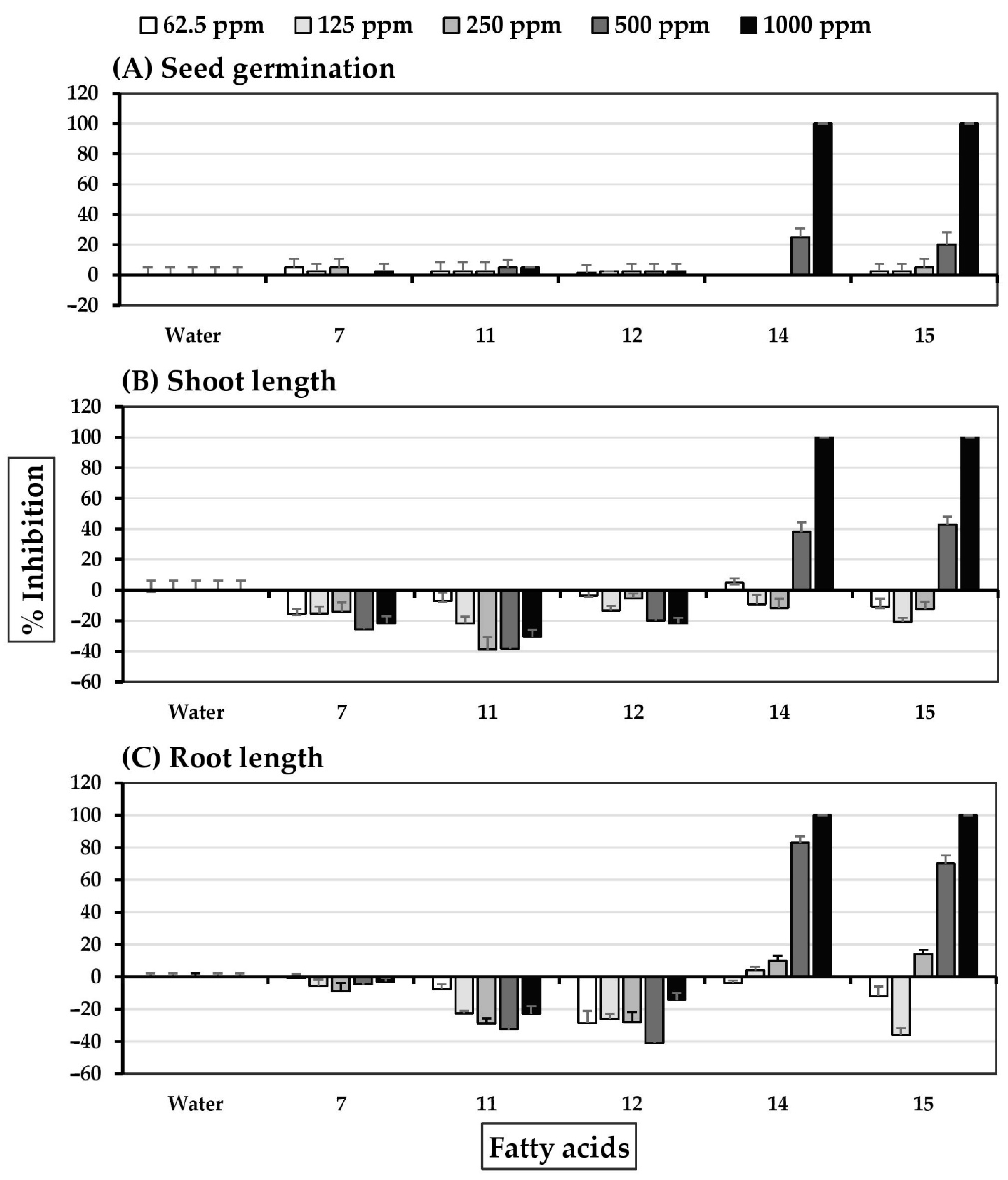
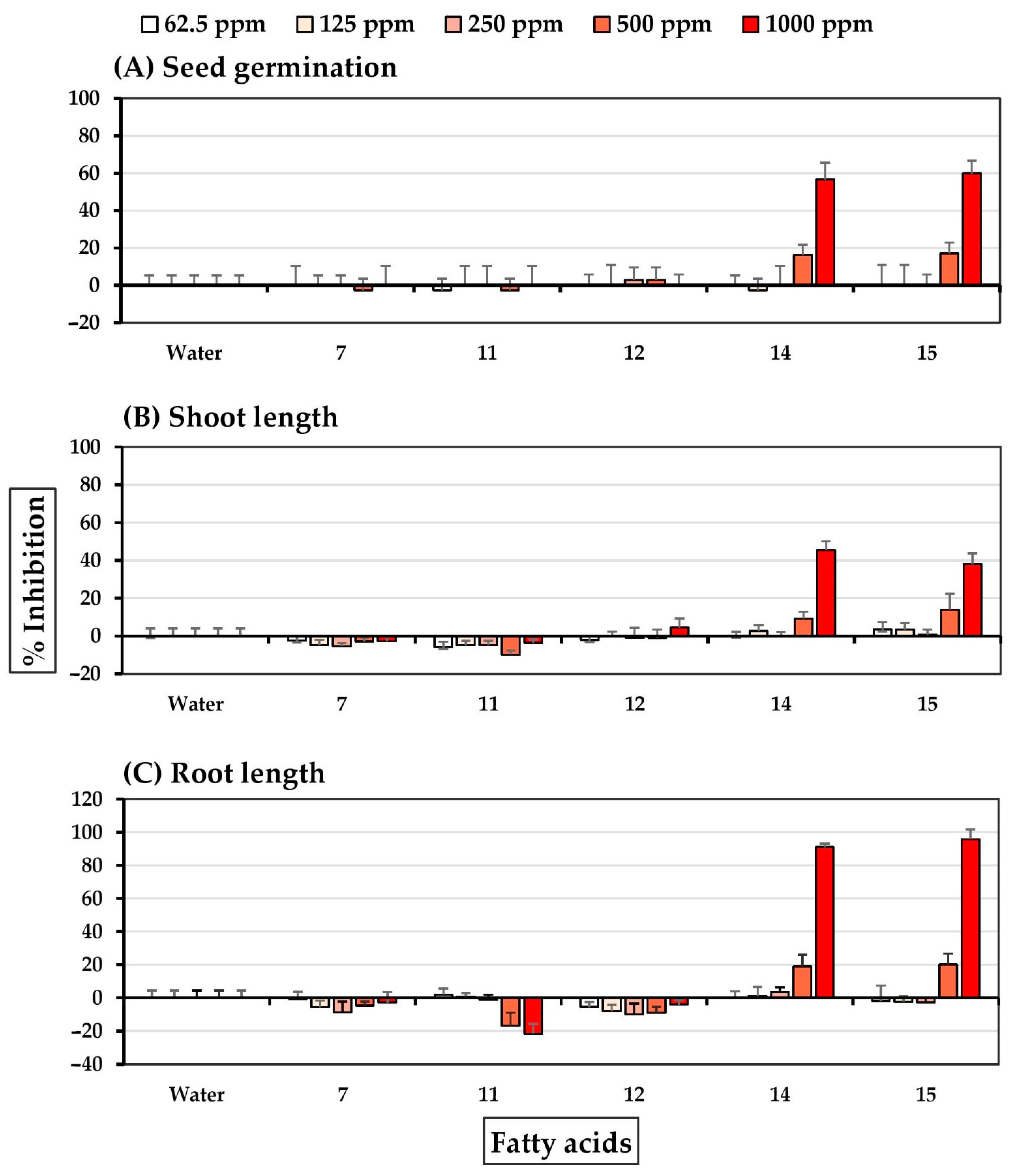
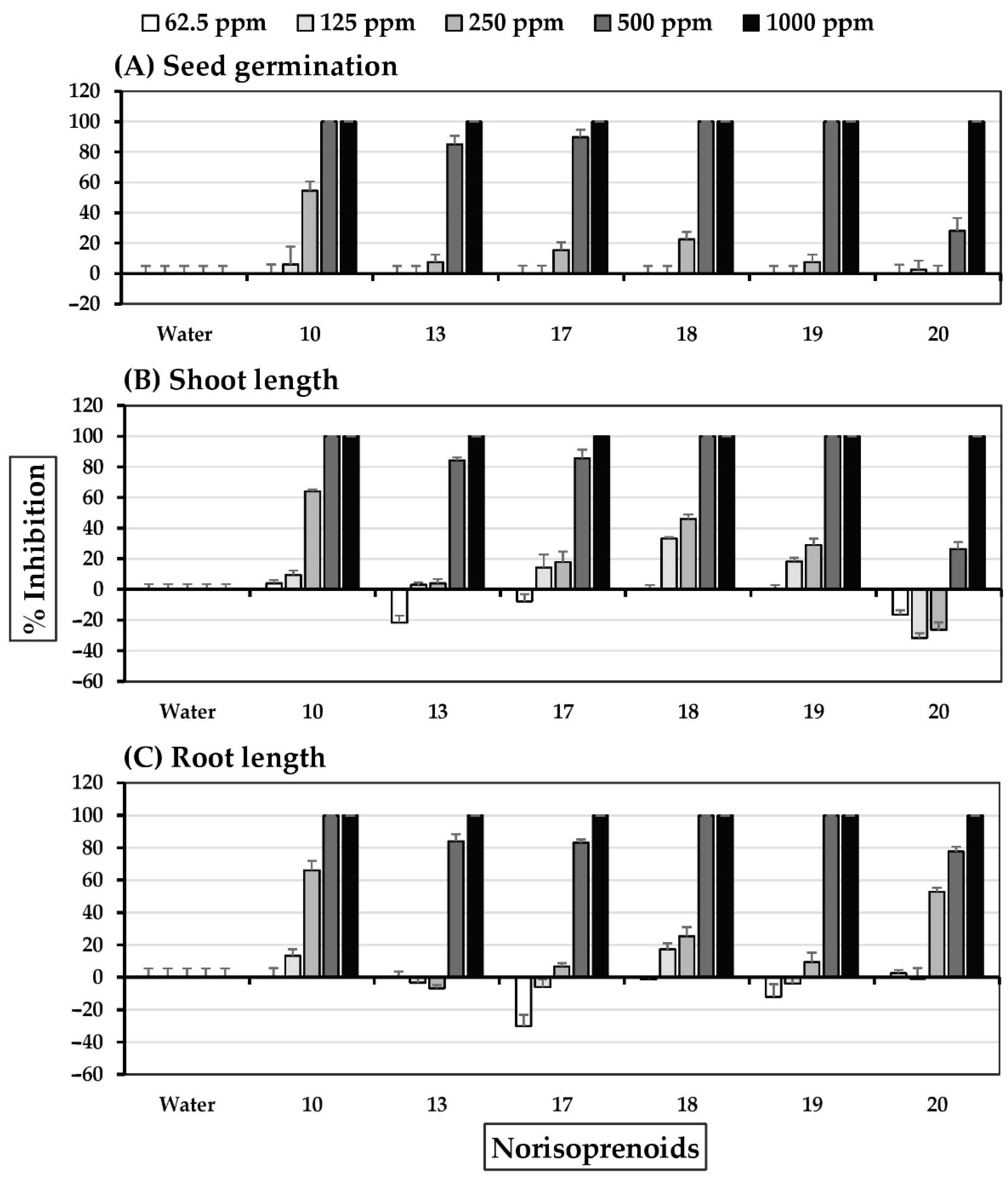
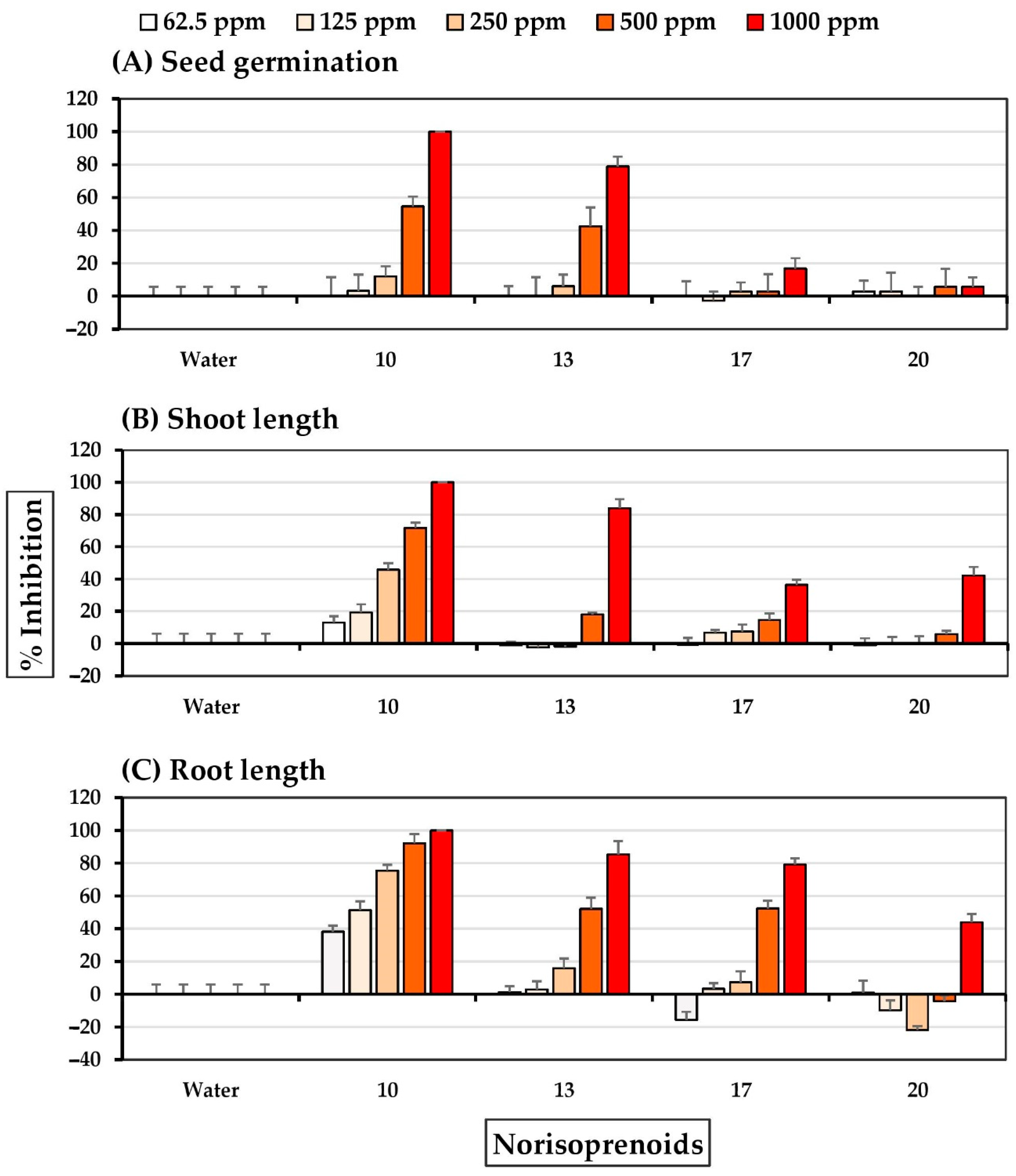
2.4. Allelopathic Effects of Pure Compounds
2.5. Allelopathic Effects of Norisoprenoids
3. Discussion
4. Materials and Methods
4.1. General Experiment Procedures
4.2. Algae Material and Preparation of Crude Extracts
4.3. The Preparation of Fatty Acid Methyl Esters
4.4. GC-MS Conditions
4.5. Tested Plants
4.6. Allelopathic Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patterson, D.T. Effects of environmental stress on weed/crop interactions. Weed Sci. 1995, 43, 483–490. [Google Scholar] [CrossRef]
- Macias, F.A. ACS Symposium Series 582. Allelopathy: Organism, Processes, and Applications; Inderjit, K.M.M.D., Einhelling, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 310–329. [Google Scholar]
- Inderjit, K.M.M.D.; Foy, C.L. (Eds.) Principles and Practices in Plant Ecology: Allelochemical Interactions; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9780849321160. [Google Scholar]
- Dayan, F.E.; Romagni, J.G.; Tellez, M.R.; Rimando, A.M.; Duke, S. Managing Weeds with Natural Products. Pestic. Outlook 1999, 5, 185–188. [Google Scholar]
- Putnam, A.R. Allelopathic Chemicals: Nature’s Herbicides in Action. Chem. Eng. News. 1983, 16, 34–35. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Putnam, A.R.; Tang, C.S. (Eds.) The Science of Allelopathy; John Wiley and Sons: Hoboken, NJ, USA, 1986; pp. 1–22. ISBN 04-718-30275. [Google Scholar]
- Einhelling, F.A. Allelopathy Current Status and Future Goals. In Allelopathy: Organism, Process, and Applications; Inderjit, K.M.M.D., Einhelling, F.A., Eds.; ACS Symposium Series 582; American Chemical Society: Washington, DC, USA, 1995; pp. 1–24. [Google Scholar] [CrossRef] [Green Version]
- Einhelling, F.A. Mechanism of Action of Allelochemicals in Allelopathy. In Allelopathy: Organism, Process, and Applications; Inderjit, K.M.M.D., Einhelling, F.A., Eds.; ACS Symposium Series 582; American Chemical Society: Washington, DC, USA, 1995; pp. 96–116. [Google Scholar] [CrossRef]
- Corcuera, L.J. Biochemical basis for the resistance of barley to aphids. Phytochemistry 1993, 33, 741–747. [Google Scholar] [CrossRef]
- Aldrich, R.J. Interference Between Crops and Weeds. In Allelochemicals: Role in Agriculture and Forestry; Waller, G.R., Ed.; American Chemical Society: Washington, DC, USA, 1987; Volume 330, pp. 300–312. ISBN 0-8412-0992-8. [Google Scholar]
- Rice, E.L. Allelopathy: An Overview. In Allelochemicals: Role in Agriculture and Forestry; Waller, G.R., Ed.; American Chemical Society: Washington, DC, USA, 1987; Volume 330, pp. 8–32. ISBN 0-8412-0992-8. [Google Scholar]
- Liu, D.L.; Lovett, J.V. Biologically active secondary metabolites of barley. II. Phytotoxicity of barley allelochemicals. J. Chem. Ecol. 1993, 19, 2231. [Google Scholar] [CrossRef]
- Shamsher, S.N. (Ed.) Allelopathy Update: Basic and Applied Aspects; Science Publishers, Inc.: Enfield, NH, USA, 1999; Volume 2. [Google Scholar]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Teerarak, M.; Charoenying, P.; Laosinwattana, C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hort. 2010, 125, 411–416. [Google Scholar]
- Teerarak, M.; Charoenying, P.; Laosinwattana, C. Physiological and cellular mechanism of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant. 2012, 34, 1277–1285. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. [Google Scholar] [CrossRef] [Green Version]
- Kakisawa, H.; Asari, F.; Kusumi, T.; Toma, T.; Sakurai, T.; Oohusa, T.; Hara, Y.; Chihara, M. An allelopathic fatty acid from the brown alga Cladosiphon okamuranus. Phytochemistry 1988, 27, 731–735. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Porwoll, J.; Carlson, R.E.; Xavier, A.; Gleason, F.K.; Wood, J.M. Structure of the antibiotic cyanobacterin, a chlorine containing γ-lactone from the freshwater cyanobacterium Scytonema hofmanni. J. Org. Chem. 1983, 48, 4035–4038. [Google Scholar] [CrossRef]
- Mason, C.P.; Edwards, K.R.; Calson, R.E.; Pignatello, J.; Gleason, F.K.; Wood, J.M. Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium Scytonema hofmanni. Science 1982, 215, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Gleason, F.K.; Baxa, C.A. Activity of the natural algicide, cyanobacterin, on eukaryotic microorganisms. FEMS Microbiol. Lett. 1986, 33, 85–88. [Google Scholar] [CrossRef]
- Murakami, N.; Imamura, H.; Sakakibara, J.; Yamada, N. Seven new monogalactosyl diacylglycerols isolated from the axenic cyanobacterium Phormidium tenue Sup. 1 Chem. Pharm. Bull. 1990, 38, 3497–3499. [Google Scholar] [CrossRef] [Green Version]
- Gross, E.M.; Wolk, C.P.; Jüttner, F. Fischerellin, a new allelochemical from the freshwater cyanobacterium Fischerella muscicola. J. Phycol. 1991, 27, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Hagmann, L.; Jüttner, F. Fischerellin A, a noval photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett. 1996, 37, 6539–6542. [Google Scholar] [CrossRef]
- Bagchi, S.N.; Chauhan, V.S.; Marwah, J.B. Effect of an antibiotic from Oscillatoria late-virens on growth, photosynthesis, and toxicity of Microcystis aeroginosa. Curr. Microbiol. 1993, 26, 223–228. [Google Scholar] [CrossRef]
- Bagchi, S.N. Structure and site of action of an algicide from a cyanobacterium, Oscillatoria late-virens. J. Plant. Physiol. 1995, 146, 372–374. [Google Scholar] [CrossRef]
- Jüttner, F.; Todorova, A.K.; Walch, N.; Phillipsborn, W.V. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry 2001, 57, 613–619. [Google Scholar] [CrossRef]
- Martin, D.F.; Kutt, E.C.; Kim, Y.S. Use of a multiple diffusion chamber unit in culture studies. Application to Gomphosphaeria aponina. Environ. Lett. 1974, 7, 39–46. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Pagliarami, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Pardhasaradhi, B.V.V.; Ali, A.M.; Kumari, A.L.; Reddanna, P.; Khar, A. Phycocyanin-mediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol. Cancer Ther. 2003, 2, 1165–1170. [Google Scholar] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A biliprotein with antioxidant, anti-inflammatory, and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, P.; Chotsaeng, P.; Laosinwattana, C. Effects of Spirulina platensis and C-phycocyanin on seed germination and seedling growth of two monocot and dicot plants. Allelopathy J. 2010, 25, 453–464. [Google Scholar]
- Charoenying, P.; Chotsaeng, N.; Laosinwattana, C. Allelopathic potential of subfractions from aqueous extract of Spirulina platensis and denatured C-phycocyanin. Curr. Appl. Sci. Technol. 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Yao, S.; Johannsen, M.; Hazell, R.G.; Jørgensen, K.A. Total synthesis of (R)-Dihydroactinidiolide and (R)-Actinidiolide Using Asymmetric Catalytic Hetero-Diels-Alder Methodology. J. Org. Chem. 1988, 63, 118–121. [Google Scholar] [CrossRef]
- Mori, K.; Khlebnikov, V. Carotenoids and degraded carotenoids, VIII-synthesis of (+)-dihydroactinidiolide, (+)-and (−)-actinidiolide, (+)-and (−)-loliolide as well as (+)-and (−)-epiloliolide. Liebigs Ann. Chem. 1993, 1993, 77–82. [Google Scholar] [CrossRef]
- Eidman, K.F.; MacDougall, B.S. Synthesis of loliolide, actinidiolide, dihydroactinidiolide, and aeginetolide via cerium Enolate Chemistry. J. Org. Chem. 2006, 71, 9513–9516. [Google Scholar] [CrossRef]
- Tang, R.-R.; Zhou, Y.-P.; Gong, N.-H.; Zhao, Y. Aerobic oxidation behavior of α-ionone catalyzed by N-hydroxyphthalimide combined with acetylacetone cobalt(II). J. Cent. South Univ. Technol. 2008, 15, 474–478. [Google Scholar] [CrossRef]
- Becher, E.; Albrecht, R.; Bernhard, K.; Leuenberger, H.G.W.; Mayer, H.; Müller, R.K.; Schüep, W.; Wangner, H.P. Synthesis of astaxanthin from β-ionone. I. enantiomeric C15-wittig salts by chemical and microbial resolution of (±)-3-acetoxy-4-oxo-β-ionone. Helv. Chim. Acta 1981, 64, 2419–2435. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kosemura, S.; Yamamura, S.; Hasegawa, K. A growth inhibitor, R-(-)-3-hydroxy-β-ionone, from light-growth shoots of a dwarf cultivar of Phaseolus Vulgaris. Phytochemistry 1993, 33, 553–555. [Google Scholar] [CrossRef]
- Gueldner, A.; Winterhalter, P. Structures of two new ionone glycosides from quince fruit (Cydonia oblonga Mill.). J. Agric. Food Chem. 1991, 39, 2142–2146. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Oriano, P.; Temussi, F. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Chin, Y.-W.; Lim, S.W.; Kim, Y.C.; Kim, J. Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Arch. Pharm. Res. 2004, 27, 600–603. [Google Scholar] [CrossRef]
- Mori, K. Synthesis of optically active grasshopper ketone and dehydrovomifoliol as a synthetic support for the revised absolute configuration of (+)-abscisic acid. Tetrahedron 1974, 30, 1065–1072. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliva, R.M.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Allelochemicals from sunflower leaves cv. Peredovick. Phytochemistry 1999, 52, 613–621. [Google Scholar] [CrossRef]
- Kuba, M.; Furuichi, N.; Katsumura, S. Stereocontrolled syntheses of carotenoid oxidative metabolites, (−)-loliolide, (−)-xanthoxin, and their stereoisomers. Chem. Lett. 2002, 31, 1248–1249. [Google Scholar] [CrossRef]
- Fernandez, I.; Pedro, J.R.; Vidal, R. Norisoprenoids from Centaurea aspera and C. salmantica. Phytochemistry 1993, 34, 733–736. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the Brown Alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- Bowers, W.S.; Hoch, H.C.; Evans, P.H.; Katayama, M. Thallphytic Allelopathy: Isolation and identification of laetisaric acid. Science 1985, 32, 105–106. [Google Scholar]
- Gallardo-Williams, M.T.; Geiger, C.L.; Pidala, J.A.; Martin, D.F. Essential fatty acids and phenolic acids from extracts and leachates of southern cattail (Typha domingensis P.). Phytochemistry 2002, 59, 305–308. [Google Scholar] [CrossRef]
- Jin, Z.H.; Zhuang, Y.Y.; Dai, S.G.; Li, T.L. Isolation and identification of extracts of Eichhornia crassipes and their allelopathic effects on algae. Bull. Environ. Contam. Toxicol. 2003, 71, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Eom, S.H.; Kim, M.J.; Yu, C.Y.; Lee, Y.S. Allelopathy of rice husk on barnyardgrass. J. Agron. 2005, 4, 288–292. [Google Scholar]
- Alamsjah, M.A.; Hirao, S.; Ishibashi, F.; Fujita, Y. Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci. Biotechnol. Biochem. 2005, 69, 2186–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, T.D.; Chung III, M.; Khanh, T.D.; Tawata, S. Identification of phytotoxic substances from early growth of barnyard grass (Echinochloa crusgalli) root exudates. J. Chem. Ecol. 2006, 32, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Spruell, J.A. Response of algae and zooplankton to C18 fatty acids of Chlamydomonas reinhardtii. Hydrobiologia 1984, 114, 9–12. [Google Scholar] [CrossRef]
- Aliotta, G.; Della Greca, M.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L. In vitro algal growth inhibition by phytotoxins of Typha latifolia L. J. Chem. Ecol. 1990, 16, 2637–2646. [Google Scholar] [CrossRef]
- Murakami, N.; Yamada, N.; Sakakibara, J. An autolytic substance in a freshwater cyanobacterium Phormidium tenue. Chem. Pharm. Bull. 1990, 38, 812–814. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Wakana, I.; Denboh, T.; Tatewaki, M. An allelopathic polyunsaturated fatty acid from red algae. Phytochemistry 1996, 43, 63–65. [Google Scholar] [CrossRef]
- Alamsjah, M.A.; Ishibe, K.; Kim, D.; Yamaguchi, K.; Ishibashi, F.; Fujita, Y.; Oda, T. Selective toxic effects of polyunsaturated fatty acids derived from Ulva fasciata on red tide phytoplanktor species. Biosci. Biotechnol. Biochem. 2007, 71, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Stevens, K.L.; Merrill, G.B. Growth inhibitors from Spikerush. J. Agric. Food Chem. 1980, 28, 644–646. [Google Scholar] [CrossRef]
- Macías, F.A.; Simonet, A.M.; Galindo, J.C.G.; Castellano, D. Bioactive phenolics and polar compounds from Melitotus messanensis. Phytochemistry 1999, 50, 35–46. [Google Scholar]
- Xian, Q.; Chen, H.; Liu, H.; Zou, H.; Yin, D. Isolation and identification of antialgal compounds from the leaves of Vallisneria spiralis L. by activity-guided fractionation. Environ. Sci. Pollut. Res. 2006, 13, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.M.; Emeterio, L.S.; Peralta, J. Autotoxicity in Lolium rigidum: Analyzing the role of chemically mediated interactions in annual plant populations. J. Theor. Biol. 2005, 235, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Burgos, N.R.; Talbert, R.E. Differential activity of allelochemicals from Secale cereale in seedling bioassays. Weed Sci. 2000, 48, 302–310. [Google Scholar] [CrossRef]
- Khanh, T.D.; Hong, N.H.; Nhan, D.Q.; Kim, S.L.; Chung, I.M.; Xuan, T.D. Herbicidal activity of Stylosanthes guianensis and its phytotoxic components. J. Agron. Crop Sci. 2006, 192, 427–433. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Mycrocystis aeruginosas. Wat. Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Kato, T.; Saito, N.; Kashimura, K.; Shinohara, M.; Kurahashi, T.; Taniguchi, K. Germination and growth inhibitors from wheat (Triticum aestivum L.) Husks. J. Agric. Food Chem. 2002, 50, 6307–6312. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à l’étude dune Cyanophycée. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina Maxima. Ph.D. Thesis, University of Paris, Paris, France, 1966; p. 45. (In French). [Google Scholar]
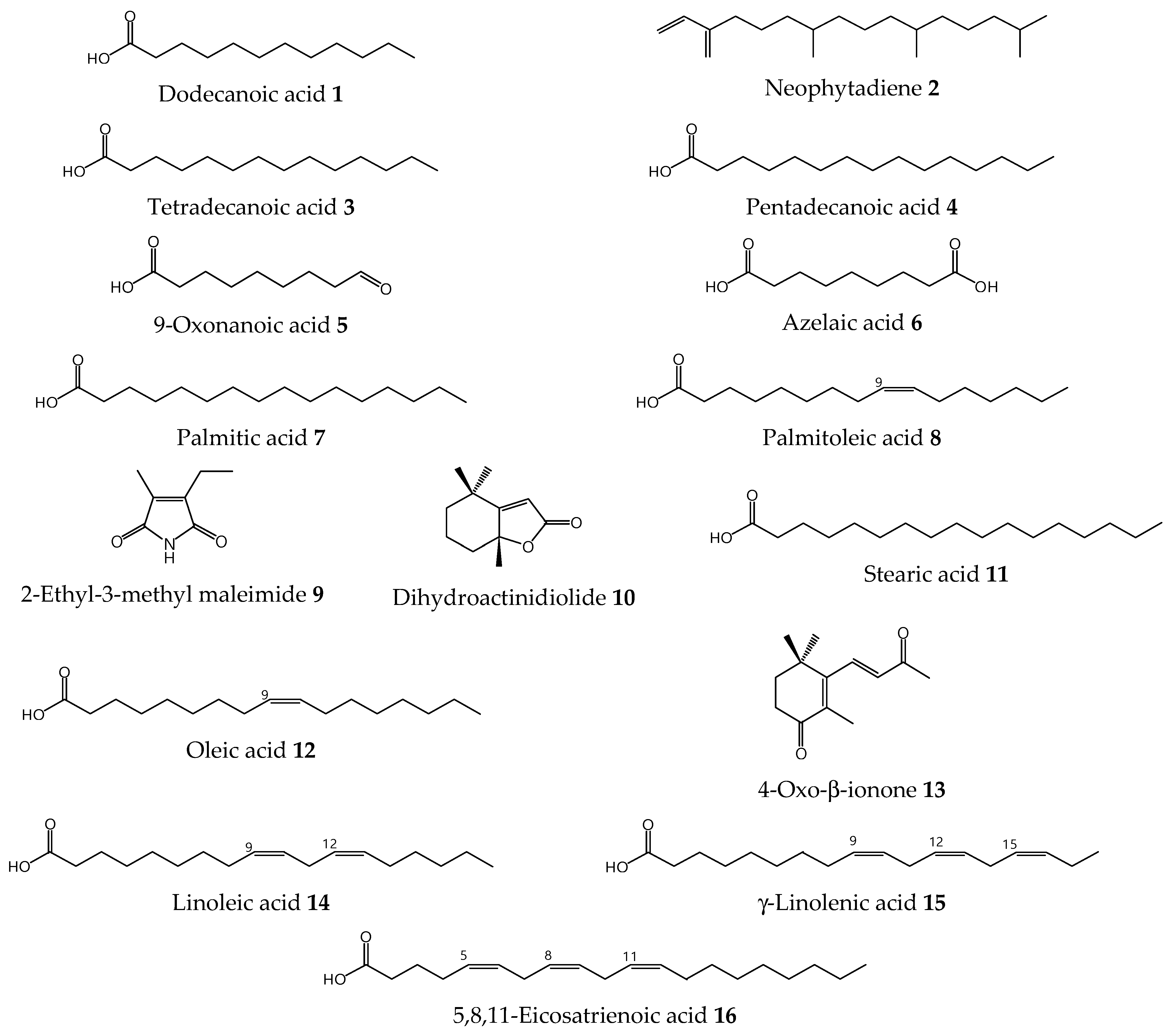
| Compounds | GC Peak Area % a | ||
|---|---|---|---|
| E6F3 | E6F4 | E6F5 | |
| Dodecanoic acid 1 | 0.55 | - | - |
| Neophytadiene 2 | - | - | 1.04 |
| Tetradecanoic acid 3 | 3.97 | - | 0.41 |
| Pentadecanoic acid 4 | 2.16 | - | - |
| 9-Oxonanoic acid 5 | 2.04 | 0.46 | - |
| Azelaic acid 6 | 1.60 | - | - |
| Palmitic acid 7 | 58.71 | 2.29 | 31.41 |
| Palmitoleic acid 8 | 12.82 | 4.55 | 2.71 |
| 2-Ethyl-3-methylmelaimide 9 | - | 2.90 | - |
| Dihydroactinidiolide 10 | 1.27 | 8.32 | - |
| Stearic acid 11 | 1.69 | 0.42 | 1.87 |
| Oleic acid 12 | 11.76 | 1.17 | 3.16 |
| 4-Oxo-β-ionone 13 | 1.66 | 2.15 | 1.91 |
| Linoleic acid 14 | 1.76 | 72.19 | 31.24 |
| γ-Linolenic acid 15 | - | 3.02 | 23.46 |
| 5,8,11-Eicosatrienoic acid 16 | - | 2.53 | 2.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenying, P.; Laosinwattana, C.; Chotsaeng, N. The Allelopathic Activity of Extracts and Isolated from Spirulina platensis. Molecules 2022, 27, 3852. https://doi.org/10.3390/molecules27123852
Charoenying P, Laosinwattana C, Chotsaeng N. The Allelopathic Activity of Extracts and Isolated from Spirulina platensis. Molecules. 2022; 27(12):3852. https://doi.org/10.3390/molecules27123852
Chicago/Turabian StyleCharoenying, Patchanee, Chamroon Laosinwattana, and Nawasit Chotsaeng. 2022. "The Allelopathic Activity of Extracts and Isolated from Spirulina platensis" Molecules 27, no. 12: 3852. https://doi.org/10.3390/molecules27123852
APA StyleCharoenying, P., Laosinwattana, C., & Chotsaeng, N. (2022). The Allelopathic Activity of Extracts and Isolated from Spirulina platensis. Molecules, 27(12), 3852. https://doi.org/10.3390/molecules27123852






