Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands
Abstract
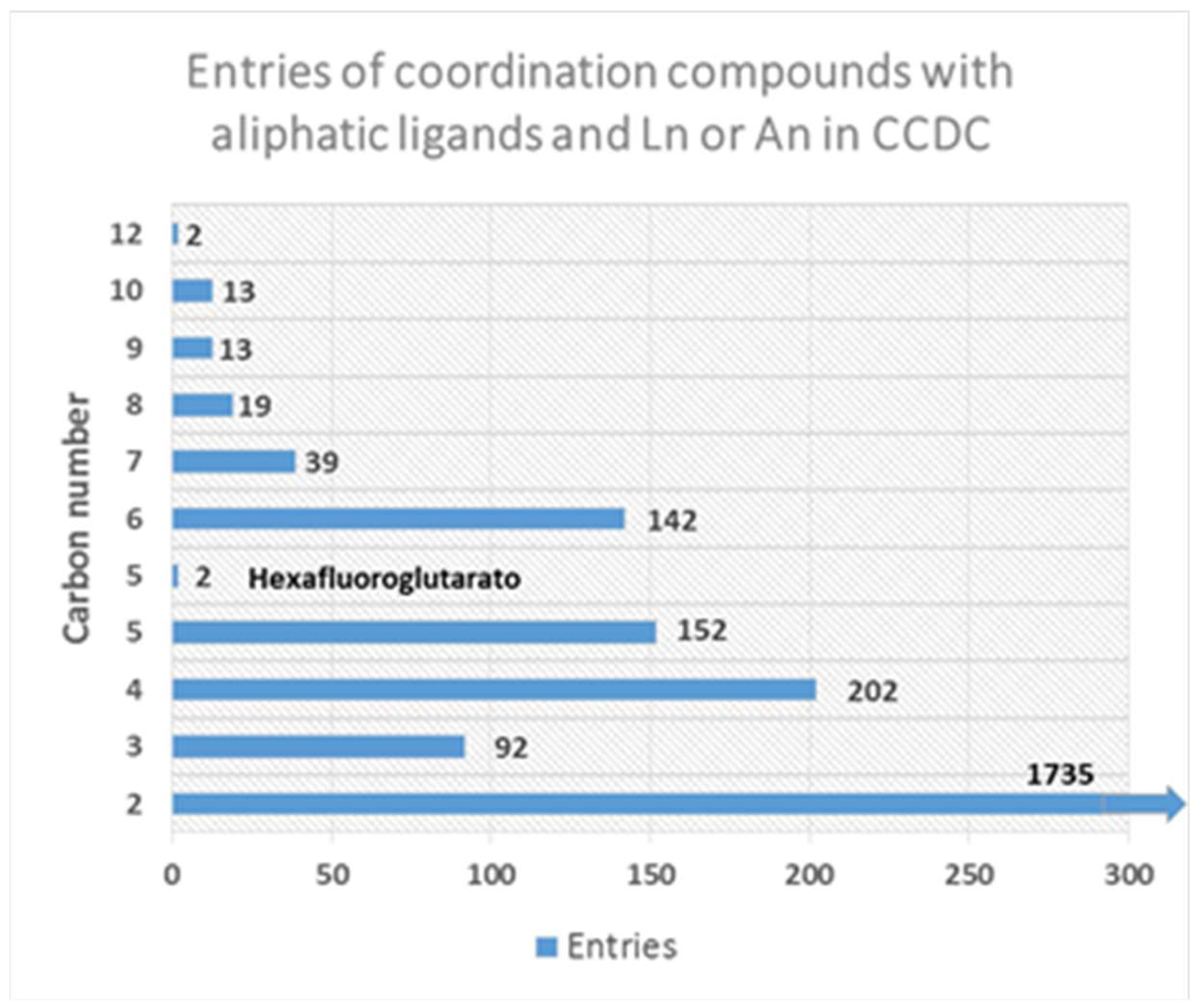
:1. Introduction
2. Discussion
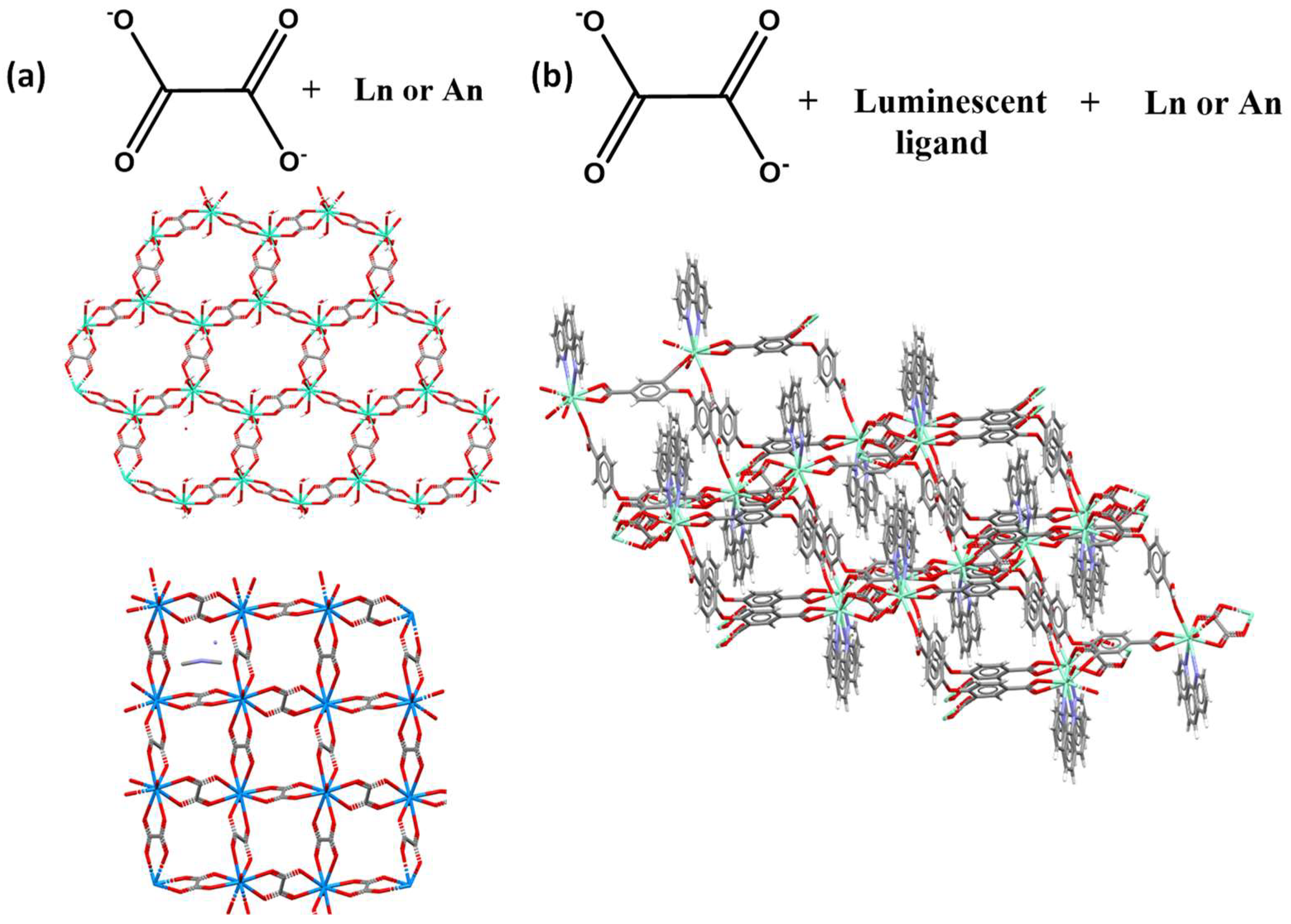
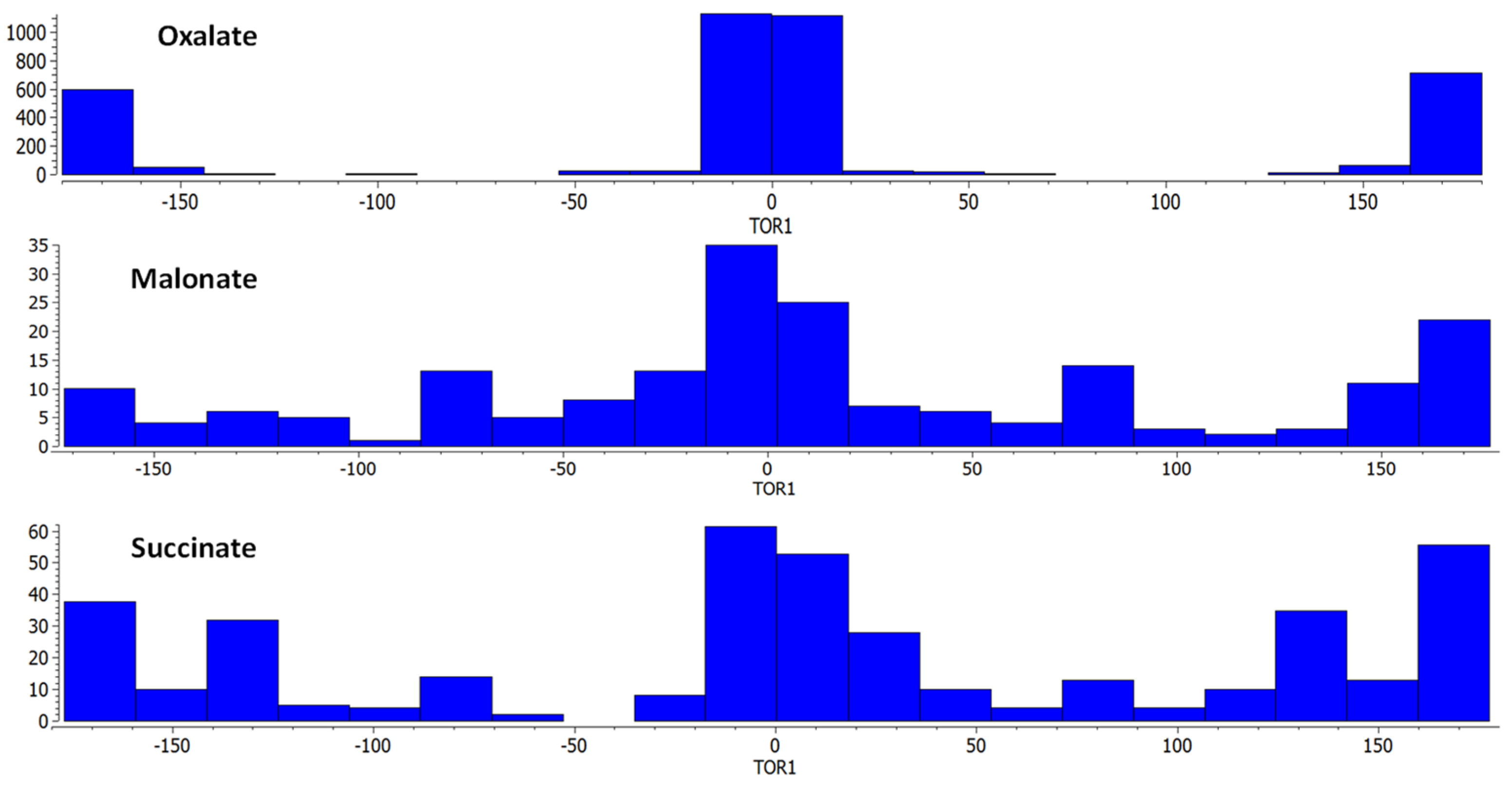
2.1. Coordination Polymers Based on Oxalate Linker
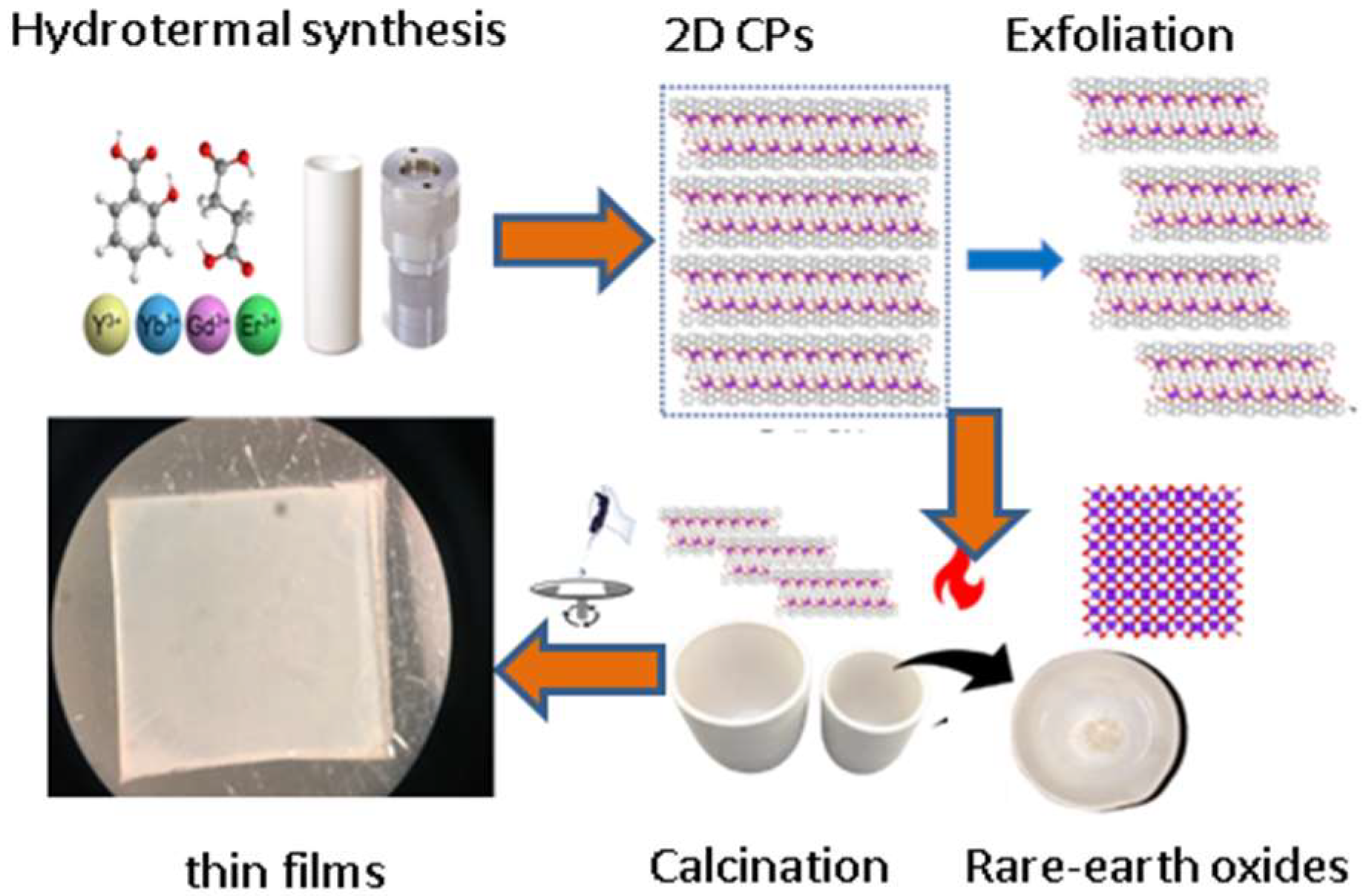
2.2. Flexible Linkers with –CH2– Spacers
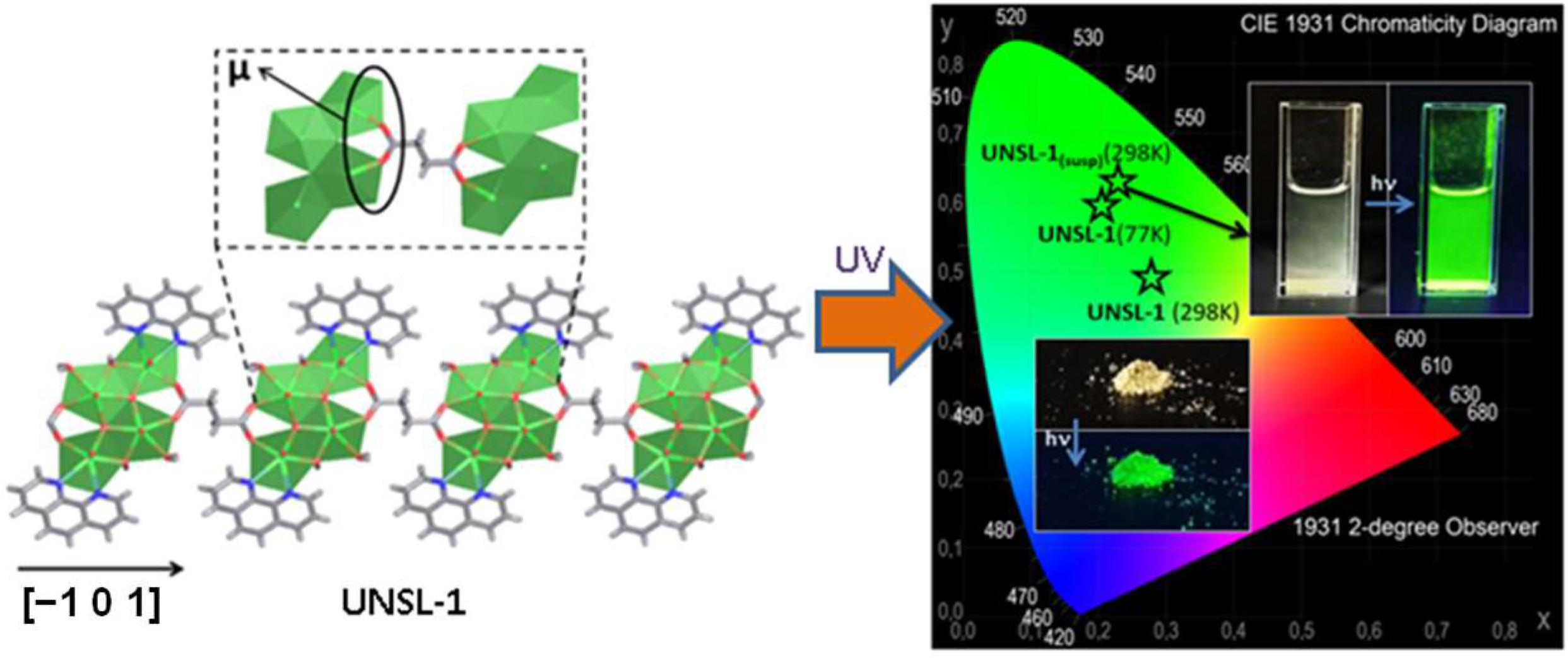
2.3. The Luminescent Properties of 4f-5f Compounds
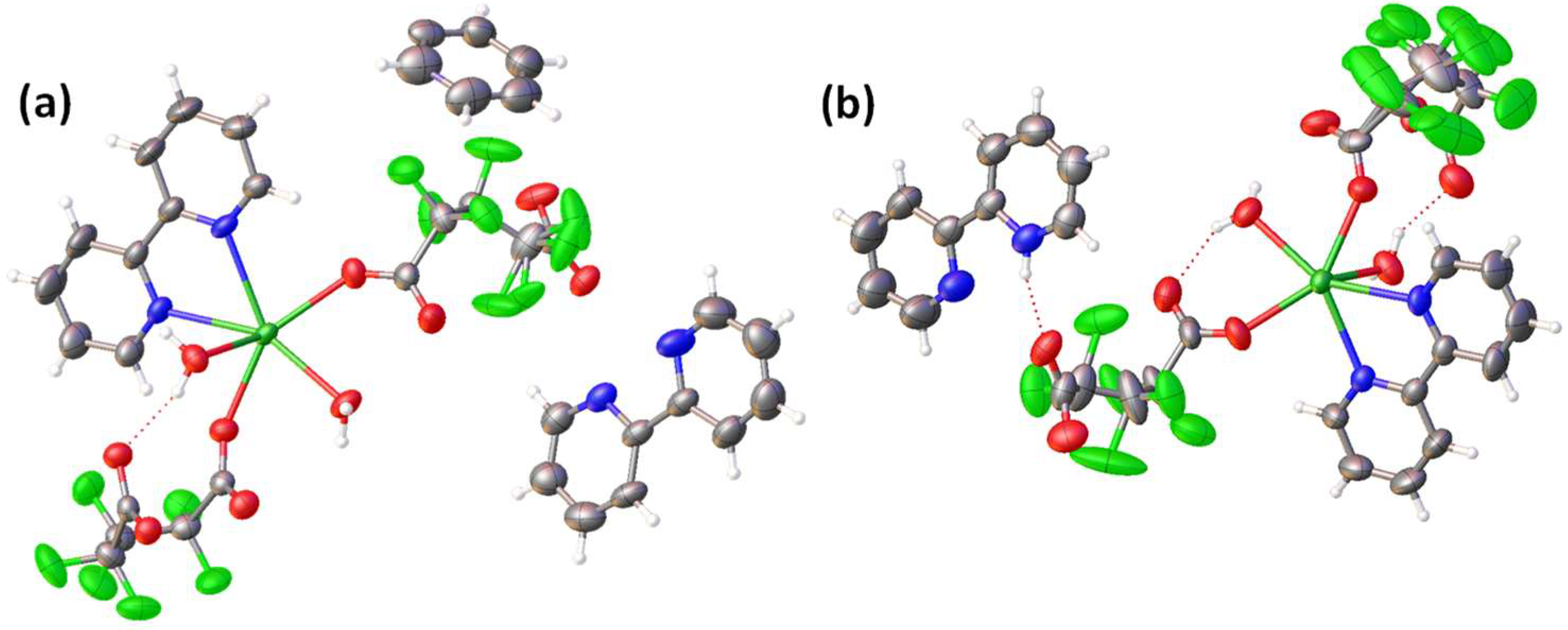
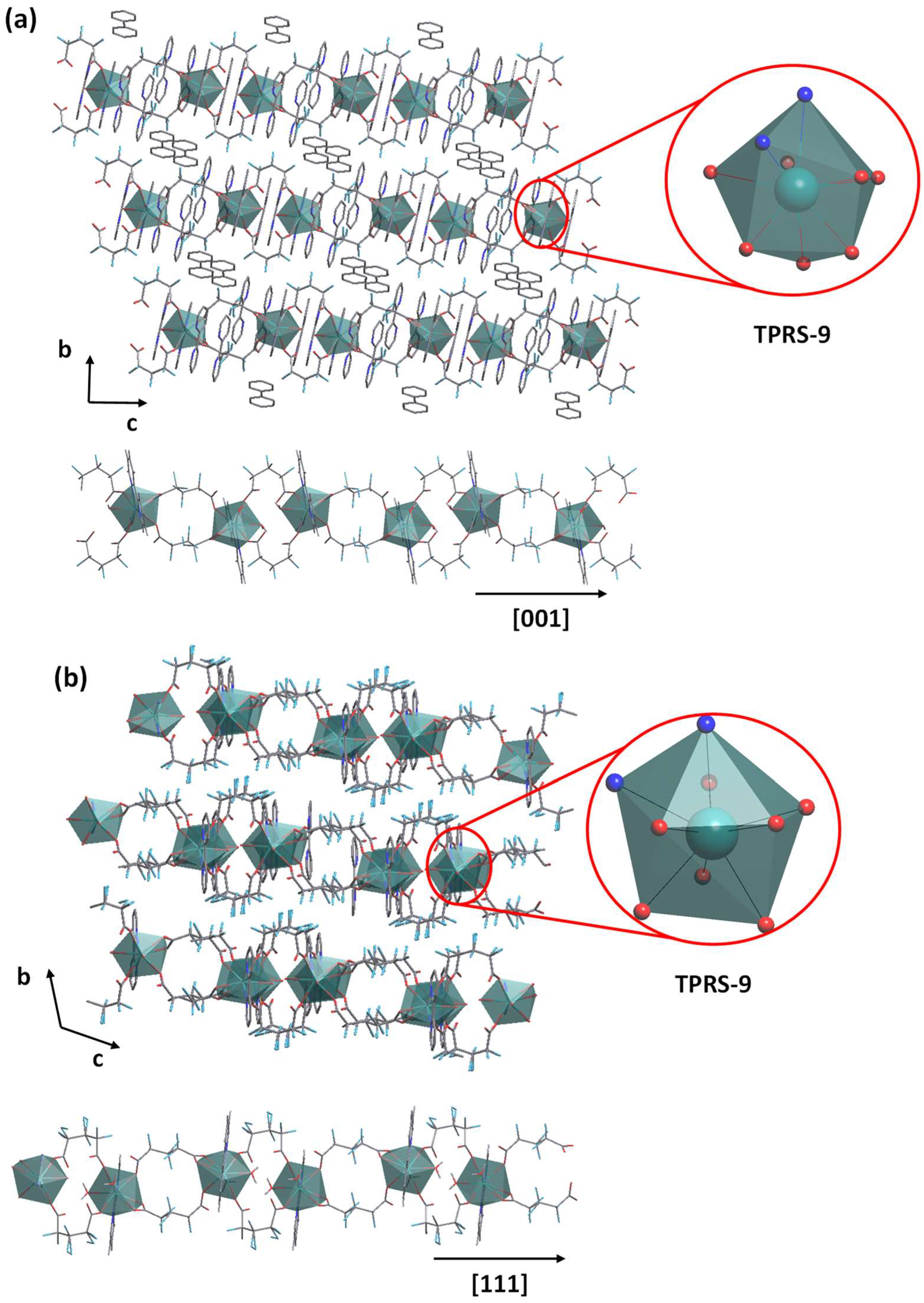
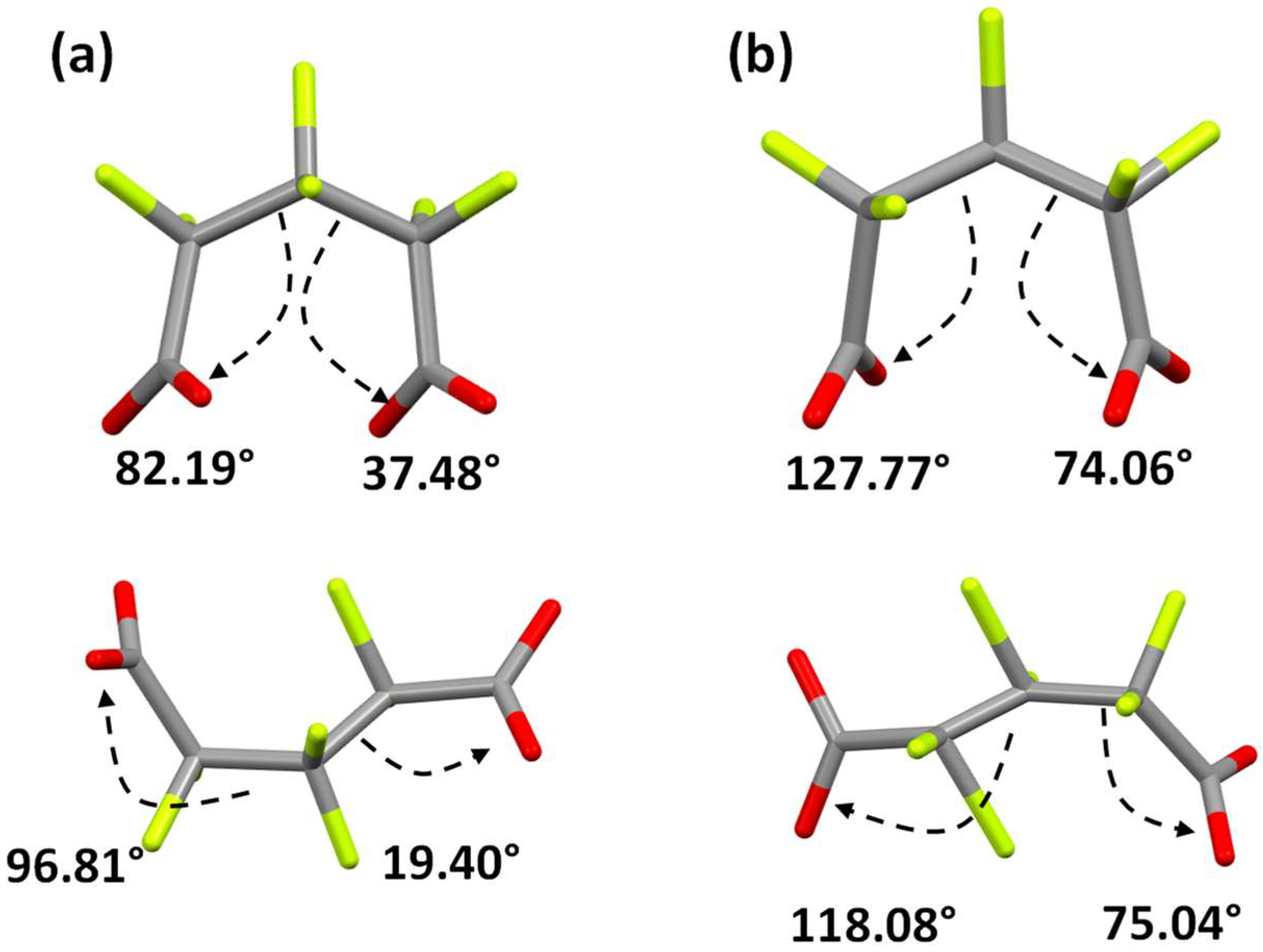
2.4. Sm-Hexafluoroglutarato CPs
3. Materials and Methods
3.1. Synthesis of [2,2′-bipyH][Sm(HFG)2 (2,2′-bipy) (H2O)2]•(2,2-bipy) and [2,2′-bipyH][Sm(HFG)2 (2,2′-bipy) (H2O)2]
3.2. Single-Crystal X-ray Diffraction (SCXRD) for Structure Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Horike, S.; Nagarkar, S.S.; Ogawa, T.; Kitagawa, S. A New Dimension for Coordination Polymers and Metal–Organic Frameworks: Towards Functional Glasses and Liquids. Angew. Chem. Int. Ed. 2020, 59, 6652–6664. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef] [Green Version]
- Bennett, T.D.; Horike, S. Liquid, glass and amorphous solid states of coordination polymers and metal—Organic frameworks. Nat. Rev. Mater. 2018, 3, 431–440. [Google Scholar] [CrossRef]
- Kitagawa, S.; Matsuda, R. Chemistry of coordination space of porous coordination polymers. Coord. Chem. Rev. 2007, 251, 2490–2509. [Google Scholar] [CrossRef]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 14, 2781–2804. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef]
- Bernini, M.C.; Brusau, E.V.; Narda, G.E.; Echeverria, G.E.; Pozzi, C.G.; Punte, G.; Lehmann, C.W. The Effect of Hydrothermal and Non-Hydrothermal Synthesis on the Formation of Holmium(III) Succinate Hydrate Frameworks. Eur. J. Inorg. Chem. 2007, 2007, 684–693. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Iglesias, M.; Snejko, N.; Alvarez-Garcia, S.; Gutierrez-Puebla, E.; Monge, M.A. Mixed lanthanide succinate-sulfate 3D MOFs: Catalysts in nitroaromatic reduction reactions and emitting materials. J. Mater. Chem. 2012, 22, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.C.; Zangrando, E.; Bencini, A.; Benelli, C.; Chaudhuri, N.R. Syntheses, Crystal Structures, and Magnetic Properties of [LnIII2(Succinate)3(H2O)2]·0.5H2O [Ln = Pr, Nd, Sm, Eu, Gd, and Dy] Polymeric Networks: Unusual Ferromagnetic Coupling in Gd Derivative. Inorg. Chem. 2006, 45, 9114–9122. [Google Scholar] [CrossRef]
- Legendziewicz, J.; Keller, B.; Turowska-Tyrk, I.; Wojciechowski, W. Synthesis, optical and magnetic properties of homo- and heteronuclear systems and glasses containing them. New J. Chem. 1999, 23, 1097–1103. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y. Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal–organic frameworks with organic photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Thuéry, P.; Harrowfield, J. Anchoring flexible uranyl dicarboxylate chains through stacking interactions of ancillary ligands on chiral U(vi) centres. CrystEngComm 2016, 18, 3905–3918. [Google Scholar] [CrossRef]
- Mohan, M.; Essalhi, M.; Durette, D.; Rana, L.K.; Ayevide, F.K.; Maris, T.; Duong, A. A Rational Design of Microporous Nitrogen-Rich Lanthanide Metal–Organic Frameworks for CO2/CH4 Separation. ACS Appl. Mater. Interfaces 2020, 12, 50619–50627. [Google Scholar] [CrossRef]
- Gorai, T.; Schmitt, W.; Gunnlaugsson, T. Highlights of the development and application of luminescent lanthanide based coordination polymers, MOFs and functional nanomaterials. Dalton Trans. 2021, 50, 770–784. [Google Scholar] [CrossRef]
- Loukopoulos, E.; Kostakis, G.E. Review: Recent advances of one-dimensional coordination polymers as catalysts. J. Coord. Chem. 2018, 71, 371–410. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhao, J.; Chen, Q.; Liu, Z. Nanoscale metal-organic frameworks and coordination polymers as theranostic platforms for cancer treatment. Coord. Chem. Rev. 2019, 398, 113009. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Gomez, G.E.; Roncaroli, F. Photofunctional metal-organic framework thin films for sensing, catalysis and device fabrication. Inorg. Chim. Acta 2020, 513, 119926. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.; Qu, X.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. High-energy metal–organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Jiang, W.-J.; Cai, Z.-H.; Li, D.-L.; Liu, Y.-L.; Chen, Z.-Z. Recent Progress in Metal-Organic Framework Based Fluorescent Sensors for Hazardous Materials Detection. Molecules 2022, 27, 2226. [Google Scholar] [CrossRef]
- Gomez, G.E.; Onna, D.; D’Vries, R.F.; Barja, B.C.; Ellena, J.; Narda, G.E.; Soler-Illia, G.J.A.A. Chain-like uranyl-coordination polymer as a bright green light emitter for sensing and sunlight driven photocatalysis. J. Mater. Chem. C 2020, 8, 11102–11109. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Álvarez-García, S.; Snejko, N.; Bausá, L.E.; Gutiérrez-Puebla, E.; de Andrés, A.; Monge, M.Á. Multimetal rare earth MOFs for lighting and thermometry: Tailoring color and optimal temperature range through enhanced disulfobenzoic triplet phosphorescence. J. Mater. Chem. C 2013, 1, 6316–6324. [Google Scholar] [CrossRef] [Green Version]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and CHF2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef]
- Gomez, G.E.; Marin, R.; Carneiro Neto, A.N.; Botas, A.M.P.; Ovens, J.; Kitos, A.A.; Bernini, M.C.; Carlos, L.D.; Soler-Illia, G.J.A.A.; Murugesu, M. Tunable Energy-Transfer Process in Heterometallic MOF Materials Based on 2,6-Naphthalenedicarboxylate: Solid-State Lighting and Near-Infrared Luminescence Thermometry. Chem. Mater. 2020, 32, 7458–7468. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Review: Lanthanide coordination chemistry: From old concepts to coordination polymers. J. Coord. Chem. 2014, 67, 3706–3733. [Google Scholar] [CrossRef]
- Ellart, M.; Blanchard, F.; Rivenet, M.; Abraham, F. Structural Variations of 2D and 3D Lanthanide Oxalate Frameworks Hydrothermally Synthesized in the Presence of Hydrazinium Ions. Inorg. Chem. 2020, 59, 491–504. [Google Scholar] [CrossRef]
- Santos, G.C.; de Oliveira, C.A.F.; da Silva, F.F.; Alves, S. Photophysical studies of coordination polymers and composites based on heterometallic lanthanide succinate. J. Mol. Struct. 2020, 1207, 127829. [Google Scholar] [CrossRef]
- Delgado, F.S.; Lorenzo-Luís, P.; Pasán, J.; Cañadillas-Delgado, L.; Fabelo, O.; Hernández-Molina, M.; Lozano-Gorrín, A.D.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Crystal growth and structural remarks on malonate-based lanthanide coordination polymers. CrystEngComm 2016, 18, 7831–7842. [Google Scholar] [CrossRef]
- Kumar, M.; Qiu, C.-Q.; Zaręba, J.K.; Frontera, A.; Jassal, A.K.; Sahoo, S.C.; Liu, S.-J.; Sheikh, H.N. Magnetic, luminescence, topological and theoretical studies of structurally diverse supramolecular lanthanide coordination polymers with flexible glutaric acid as a linker. New J. Chem. 2019, 43, 14546–14564. [Google Scholar] [CrossRef]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nakanishi, T. Luminescent lanthanide coordination polymers for photonic applications. RSC Adv. 2015, 5, 338–353. [Google Scholar] [CrossRef]
- Yang, H.-W.; Xu, P.; Ding, B.; Wang, X.-G.; Liu, Z.-Y.; Zhao, H.-K.; Zhao, X.-J.; Yang, E.-C. Isostructural Lanthanide Coordination Polymers with High Photoluminescent Quantum Yields by Effective Ligand Combination: Crystal Structures, Photophysical Characterizations, Biologically Relevant Molecular Sensing, and Anti-Counterfeiting Ink Application. Cryst. Growth Des. 2020, 20, 7615–7625. [Google Scholar] [CrossRef]
- Culy, C.R.; Clemett, D.; Wiseman, L.R. Oxaliplatin. Drugs 2000, 60, 895–924. [Google Scholar] [CrossRef] [PubMed]
- Piro, O.E.; Baran, E.J. Crystal chemistry of organic minerals—Salts of organic acids: The synthetic approach. Crystallogr. Rev. 2018, 24, 149–175. [Google Scholar] [CrossRef]
- Dazem, C.L.F.; Amombo Noa, F.M.; Nenwa, J.; Öhrström, L. Natural and synthetic metal oxalates—A topology approach. CrystEngComm 2019, 21, 6156–6164. [Google Scholar] [CrossRef] [Green Version]
- Kahwa, I.A.; Fronczek, F.R.; Selbin, J. The Crystal structure and coordination geometry of potassium-catena-μ-oxalato-bis-oxalato aquo lanthanate(III) dihydrates, K3[Ln(Ox)3(OH2)]·2H2O, (Ln = Nd, Sm, Eu, Gd, Tb). Inorg. Chim. Acta 1984, 82, 161–166. [Google Scholar] [CrossRef]
- Alexander, D.; Joy, M.; Thomas, K.; Sisira, S.; Biju, P.R.; Unnikrishnan, N.V.; Sudarsanakumar, C.; Ittyachen, M.A.; Joseph, C. Efficient green luminescence of terbium oxalate crystals: A case study with Judd-Ofelt theory and single crystal structure analysis and the effect of dehydration on luminescence. J. Solid State Chem. 2018, 262, 68–78. [Google Scholar] [CrossRef]
- Xu, Q.-W.; Dong, G.; Cui, R.; Li, X. 3D lanthanide-coordination frameworks constructed by a ternary mixed-ligand: Crystal structure, luminescence and luminescence sensing. CrystEngComm 2020, 22, 740–750. [Google Scholar] [CrossRef]
- Xu, C.; Tian, G.; Teat, S.J.; Rao, L. Complexation of U(VI) with Dipicolinic Acid: Thermodynamics and Coordination Modes. Inorg. Chem. 2013, 52, 2750–2756. [Google Scholar] [CrossRef]
- Masci, B.; Thuéry, P. Uranyl complexes with the pyridine-2,6-dicarboxylato ligand: New dinuclear species with μ-η2,η2-peroxide, μ2-hydroxide or μ2-methoxide bridges. Polyhedron 2005, 24, 229–237. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Lugan, N.; Shahverdizadeh, G.H.; Soudi, A.A.; Thuéry, P. Solid-State Luminescence and π-Stacking in Crystalline Uranyl Dipicolinates. Eur. J. Inorg. Chem. 2006, 2006, 389–396. [Google Scholar] [CrossRef]
- Masci, B.; Thuéry, P. Pyrazinetetracarboxylic Acid as an Assembler Ligand in Uranyl−Organic Frameworks. Cryst. Growth Des. 2008, 8, 1689–1696. [Google Scholar] [CrossRef]
- Thuéry, P.; Masci, B. Uranyl Ion Complexation by Cucurbiturils in the Presence of Perrhenic, Phosphoric, or Polycarboxylic Acids. Novel Mixed-Ligand Uranyl−Organic Frameworks. Cryst. Growth Des. 2010, 10, 716–725. [Google Scholar] [CrossRef]
- Shu, Y.-B.; Xu, C.; Liu, W.-S. A Uranyl Hybrid Compound Designed from Urea-Bearing Dipropionic Acid. Eur. J. Inorg. Chem. 2013, 2013, 3592–3595. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, X.; Liu, W.; Xie, J.; Chen, J.; Silver, M.A.; Sheng, D.; Chen, L.; Diwu, J.; Liu, N.; et al. Emergence of Uranium as a Distinct Metal Center for Building Intrinsic X-ray Scintillators. Angew. Chem. Int. Ed. 2018, 57, 7883–7887. [Google Scholar] [CrossRef]
- Cantos, P.M.; Frisch, M.; Cahill, C.L. Synthesis, structure and fluorescence properties of a uranyl-2,5-pyridinedicarboxylic acid coordination polymer: The missing member of the UO22+-2,n-pyridinedicarboxylic series. Inorg. Chem. Commun. 2010, 13, 1036–1039. [Google Scholar] [CrossRef]
- Frisch, M.; Cahill, C.L. Synthesis, structure and fluorescent studies of novel uranium coordination polymers in the pyridinedicarboxylic acid system. Dalton Trans. 2006, 39, 4679–4690. [Google Scholar] [CrossRef]
- Gomez, G.E.; Ridenour, J.A.; Byrne, N.M.; Shevchenko, A.P.; Cahill, C.L. Novel Heterometallic Uranyl-Transition Metal Materials: Structure, Topology, and Solid State Photoluminescence Properties. Inorg. Chem. 2019, 58, 7243–7254. [Google Scholar] [CrossRef]
- Liu, D.-D.; Wang, Y.-L.; Luo, F.; Liu, Q.-Y. Rare Three-Dimensional Uranyl–Biphenyl-3,3′-disulfonyl-4,4′-dicarboxylate Frameworks: Crystal Structures, Proton Conductivity, and Luminescence. Inorg. Chem. 2020, 59, 2952–2960. [Google Scholar] [CrossRef]
- Yang, W.; Parker, T.G.; Sun, Z.-M. Structural chemistry of uranium phosphonates. Coord. Chem. Rev. 2015, 303, 86–109. [Google Scholar] [CrossRef]
- Andrews, M.B.; Cahill, C.L. Uranyl Bearing Hybrid Materials: Synthesis, Speciation, and Solid-State Structures. Chem. Rev. 2013, 113, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-X.; Chen, J.-S. Extended Structures and Physicochemical Properties of Uranyl–Organic Compounds. Acc. Chem. Res. 2011, 44, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Mihalcea, I.; Henry, N.; Volkringer, C. The crystal chemistry of uranium carboxylates. Coord. Chem. Rev. 2014, 266, 69–109. [Google Scholar] [CrossRef]
- Gao, J.; Ye, K.; He, M.; Xiong, W.-W.; Cao, W.; Lee, Z.Y.; Wang, Y.; Wu, T.; Huo, F.; Liu, X.; et al. Tuning metal–carboxylate coordination in crystalline metal–organic frameworks through surfactant media. J. Solid State Chem. 2013, 206, 27–31. [Google Scholar] [CrossRef]
- Lv, K.; Fichter, S.; Gu, M.; März, J.; Schmidt, M. An updated status and trends in actinide metal-organic frameworks (An-MOFs): From synthesis to application. Coord. Chem. Rev. 2021, 446, 214011. [Google Scholar] [CrossRef]
- Duvieubourg-Garela, L.; Vigier, N.; Abraham, F.; Grandjean, S. Adaptable coordination of U(IV) in the 2D-(4,4) uranium oxalate network: From 8 to 10 coordinations in the uranium (IV) oxalate hydrates. J. Solid State Chem. 2008, 181, 1899–1908. [Google Scholar] [CrossRef]
- Cañadillas-Delgado, L.; Pasán, J.; Fabelo, O.; Hernández-Molina, M.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Two- and Three-Dimensional Networks of Gadolinium(III) with Dicarboxylate Ligands: Synthesis, Crystal Structure, and Magnetic Properties. Inorg. Chem. 2006, 45, 10585–10594. [Google Scholar] [CrossRef]
- Chrysomallidou, K.E.; Perlepes, S.P.; Terzis, A.; Raptopoulou, C.P. Synthesis, crystal structures and spectroscopic studies of praseodymium(III) malonate complexes. Polyhedron 2010, 29, 3118–3124. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Gomez, G.E.; Hodak, J.H.; Soler-Illia, G.J.A.A.; Ellena, J. Tuning the structure, dimensionality and luminescent properties of lanthanide metal–organic frameworks under ancillary ligand influence. Dalton Trans. 2016, 45, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.L.; Chen, X.M. Chapter 8—Synthesis of Coordination Compounds and Coordination Polymers. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 189–217. [Google Scholar] [CrossRef]
- Darwish, S.; Wang, S.-Q.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Comparison of Mechanochemistry vs Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Polymers. ACS Sustain. Chem. Eng. 2019, 7, 19505–19512. [Google Scholar] [CrossRef]
- Mirtamizdoust, B. Sonochemical synthesis of nano lead(II) metal-organic coordination polymer; New precursor for the preparation of nano-materials. Ultrason. Sonochem. 2017, 35, 263–269. [Google Scholar] [CrossRef]
- Rizzato, S.; Moret, M.; Merlini, M.; Albinati, A.; Beghi, F. Crystal growth in gelled solution: Applications to coordination polymers. CrystEngComm 2016, 18, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Amiaud, T.; Stephant, N.; Dessapt, R.; Serier-Brault, H. Microwave-assisted synthesis of anhydrous lanthanide-based coordination polymers built upon benzene-1,2,4,5-tetracarboxylic acid. Polyhedron 2021, 204, 115261. [Google Scholar] [CrossRef]
- Lin, Z.; Slawin, A.M.Z.; Morris, R.E. Chiral Induction in the Ionothermal Synthesis of a 3-D Coordination Polymer. J. Am. Chem. Soc. 2007, 129, 4880–4881. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- D’Vries, R.F.; de la Peña-O’Shea, V.A.; Benito Hernández, Á.; Snejko, N.; Gutiérrez-Puebla, E.; Monge, M.A. Enhancing Metal–Organic Framework Net Robustness by Successive Linker Coordination Increase: From a Hydrogen-Bonded Two-Dimensional Supramolecular Net to a Covalent One Keeping the Topology. Cryst. Growth Des. 2014, 14, 5227–5233. [Google Scholar] [CrossRef]
- Tanaka, D.; Kitagawa, S. Template Effects in Porous Coordination Polymers. Chem. Mater. 2008, 20, 922–931. [Google Scholar] [CrossRef]
- Xu, N.; Shi, W.; Liao, D.-Z.; Yan, S.-P.; Cheng, P. Template Synthesis of Lanthanide (Pr, Nd, Gd) Coordination Polymers with 2-Hydroxynicotinic Acid Exhibiting Ferro-/Antiferromagnetic Interaction. Inorg. Chem. 2008, 47, 8748–8756. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y. The organic ligands as template: The synthesis, structures and properties of a series of the layered structure rare-earth coordination polymers. J. Mol. Struct. 2004, 690, 137–143. [Google Scholar] [CrossRef]
- Bernini, M.C.; Snejko, N.; Gutierrez-Puebla, E.; Brusau, E.V.; Narda, G.E.; Monge, M.Á. Structure-Directing and Template Roles of Aromatic Molecules in the Self-Assembly Formation Process of 3D Holmium–Succinate MOFs. Inorg. Chem. 2011, 50, 5958–5968. [Google Scholar] [CrossRef] [PubMed]
- D’Vries, R.F.; Camps, I.; Ellena, J. Exploring the System Lanthanide/Succinate in the Formation of Porous Metal–Organic Frameworks: Experimental and Theoretical Study. Cryst. Growth Des. 2015, 15, 3015–3023. [Google Scholar] [CrossRef]
- Bernini, M.C.; Gomez, G.E.; Brusau, E.V.; Narda, G.E. Reviewing Rare Earth Succinate Frameworks from the Reticular Chemistry Point of View: Structures, Nets, Catalytic and Photoluminescence Applications. Isr. J. Chem. 2018, 58, 1044–1061. [Google Scholar] [CrossRef]
- Gomez, G.E.; Brusau, E.V.; Kaczmarek, A.M.; Mellot-Draznieks, C.; Sacanell, J.; Rousse, G.; Van Deun, R.; Sanchez, C.; Narda, G.E.; Soler Illia, G.J.A.A. Flexible Ligand-Based Lanthanide Three-Dimensional Metal–Organic Frameworks with Tunable Solid-State Photoluminescence and OH-Solvent-Sensing Properties. Eur. J. Inorg. Chem. 2017, 2017, 2321–2331. [Google Scholar] [CrossRef]
- Gomez, G.E.; Bernini, M.C.; Brusau, E.V.; Narda, G.E.; Vega, D.; Kaczmarek, A.M.; Van Deun, R.; Nazzarro, M. Layered exfoliable crystalline materials based on Sm-, Eu- and Eu/Gd-2-phenylsuccinate frameworks. Crystal structure, topology and luminescence properties. Dalton Trans. 2015, 44, 3417–3429. [Google Scholar] [CrossRef] [Green Version]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of rare earth elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E.B. Luminescent Open Metal Sites within a Metal–Organic Framework for Sensing Small Molecules. Adv. Mater. 2007, 19, 1693–1696. [Google Scholar] [CrossRef]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; de Sousa, A.S.; Gareth Williams, J.A.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Amin, N.; Tan, J.-C. A Luminescent Guest@MOF Nanoconfined Composite System for Solid-State Lighting. Molecules 2021, 26, 7583. [Google Scholar] [CrossRef]
- Sun, G.; Xie, Y.; Sun, L.; Zhang, H. Lanthanide upconversion and downshifting luminescence for biomolecules detection. Nanoscale Horiz. 2021, 6, 766–780. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Chuasaard, T.; Ngamjarurojana, A.; Surinwong, S.; Konno, T.; Bureekaew, S.; Rujiwatra, A. Lanthanide Coordination Polymers of Mixed Phthalate/Adipate for Ratiometric Temperature Sensing in the Upper-Intermediate Temperature Range. Inorg. Chem. 2018, 57, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Bernini, M.C.; Funes, M.D.; Sortino, M.; Collins, S.E.; Narda, G.E. ROS-generating rare-earth coordination networks for photodynamic inactivation of Candida albicans. Dalton Trans. 2021, 50, 5853–5864. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Gomez, G.E.; Miranda, C.D.; Illescas, M.; Barja, B.C.; Vega, D.; Bernini, M.C.; Narda, G.E. Strong Red Up-Conversion Emission in Thin Film Devices Based on Rare-Earth Oxides Obtained from Templating 2D Coordination Networks. Eur. J. Inorg. Chem. 2022, 9, e202101025. [Google Scholar] [CrossRef]
- Denning, R.G. Electronic Structure and Bonding in Actinyl Ions and their Analogs. J. Phys. Chem. A 2007, 111, 4125–4143. [Google Scholar] [CrossRef] [PubMed]
- Thuéry, P.; Harrowfield, J. Uranyl–Organic Frameworks with Polycarboxylates: Unusual Effects of a Coordinating Solvent. Cryst. Growth Des. 2014, 14, 1314–1323. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Natarajan, S.; Rao, C.N.R. A chiral mixed carboxylate, [Nd4(H2O)2(OOC(CH2)3COO)4(C2O4)2], exhibiting NLO properties. J. Solid State Chem. 2004, 177, 1444–1448. [Google Scholar] [CrossRef]
- Maouche, R.; Belaid, S.; Benmerad, B.; Bouacida, S.; Daiguebonne, C.; Suffren, Y.; Freslon, S.; Bernot, K.; Guillou, O. Highly Luminescent Europium-Based Heteroleptic Coordination Polymers with Phenantroline and Glutarate Ligands. Inorg. Chem. 2021, 60, 3707–3718. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, I.U.; Harrison, W.T.A.; Tahir, M.N. Crystal structures and characterization of two rare-earth-glutarate coordination networks: One-dimensional [Nd(C5H6O4)(H2O)4]•Cl and three-dimensional [Pr(C5H6O4)(C5H7O4)(H2O)]•H2O. J. Struct. Chem. 2015, 56, 934–941. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, Y. Hydrothermal syntheses, crystal structures and luminescence properties of two lanthanide dinuclear complexes with hexafluoroglutarate. J. Coord. Chem. 2009, 62, 583–592. [Google Scholar] [CrossRef]
- CrysAlisPro. CrysAlisPro; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014. [Google Scholar]
- Hooft, R.W.W. COLLECT; Nonius BV: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. In Methods Enzymol; Academic Press: Cambridge, MA, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]

| α-Sm | β-Sm | |
|---|---|---|
| Empirical formula | C35H24F12N5O10Sm | C30H21F12N4O10Sm |
| Formula weight (g/mol) | 1052.94 | 975.86 |
| Crystal system | triclinic | triclinic |
| Space group | Pī | Pī |
| a/Å | 10.5754(4) | 10.7751(10) |
| b/Å | 12.8624(5) | 13.3601(15) |
| c/Å | 15.5452(7) | 14.5413(17) |
| α(°) | 70.371(4) | 105.535(5) |
| β(°) | 77.786(3) | 98.863(5) |
| γ(°) | 76.070(3) | 114.104(5) |
| Volume/Å3 | 1913.23(13) | 1756.4(3) |
| Z | 2 | 2 |
| ρcalc mg/mm3 | 1.828 | 1.845 |
| μ/mm−1 | 1.658 | 1.797 |
| F(000) | 1038 | 958 |
| 2θ range for data collection/° | 5.04 to 69.18 | 6.31 to 51 |
| Reflections collected | 49,281 | 11,855 |
| Independent reflections | 15,269 | 6533 |
| Data/restraints/parameters | 15,269/12/570 | 6533/676/561 |
| Goodness of fit on F2 | 1.161 | 0.969 |
| Final R index [I>2σ(I)] | 0.0550 | 0.0522 |
| Largest diff. Peak/hole/e.Å−3 | 1.75/−1.66 | 1.13/−1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Vries, R.F.; Gomez, G.E.; Ellena, J. Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands. Molecules 2022, 27, 3830. https://doi.org/10.3390/molecules27123830
D’Vries RF, Gomez GE, Ellena J. Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands. Molecules. 2022; 27(12):3830. https://doi.org/10.3390/molecules27123830
Chicago/Turabian StyleD’Vries, Richard F., Germán E. Gomez, and Javier Ellena. 2022. "Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands" Molecules 27, no. 12: 3830. https://doi.org/10.3390/molecules27123830
APA StyleD’Vries, R. F., Gomez, G. E., & Ellena, J. (2022). Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands. Molecules, 27(12), 3830. https://doi.org/10.3390/molecules27123830







