GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae)
Abstract
1. Introduction
2. Results
2.1. Yield and Solubility of Freeze-Dried Extract from P. peltatum
2.2. Phytochemical Profiling by High-Performance Thin Layer Chromatography
2.3. Antioxidant Activity of Freeze-Dried Extract from P. peltatum
2.4. Quantification of Polyphenols, Tannins, and Flavonoids in FDEs from P. peltatum
2.5. Antimicrobial Activity of Freeze-Dried Extract from P. peltatum
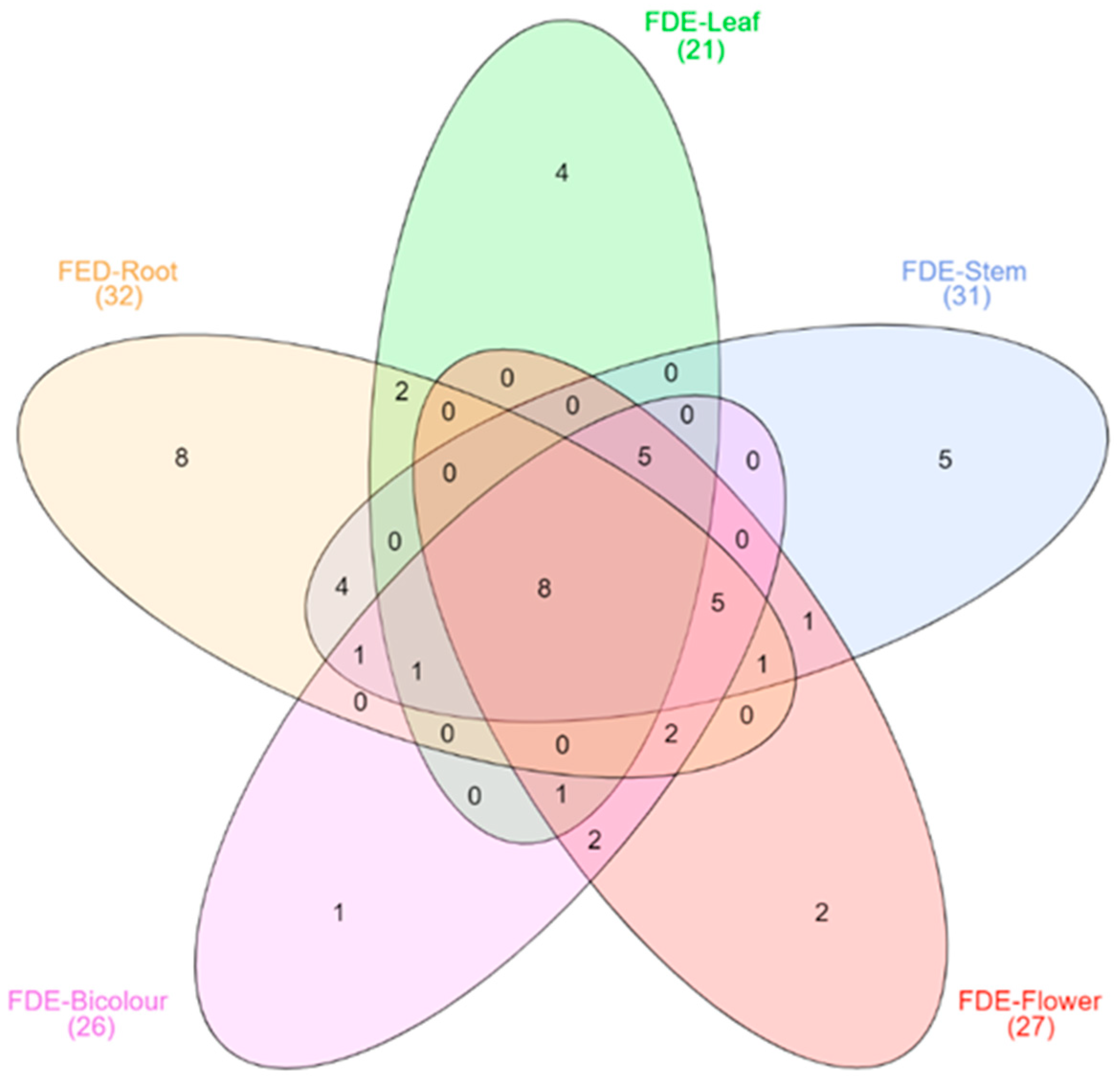
2.6. GC-MS Analysis of FDEs from P. peltatum
2.7. Kupchan Partitioning of FDE-Flower and FDE-Leaf
2.8. Antioxidant Activity of FDE-Flower and FDE-Leaf Partitioned Extracts
2.9. M.I.C. of Lf-EtOAc and Fwr-EtOAc Fractions against Pathogenic Bacteria
2.10. GC-MS Analysis and IC50 of Lf-EtOAC and Fwr-EtOAC Fractions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Extracts
4.2.1. Harvesting and Identification of P. peltatum
4.2.2. Cleaning and Drying of Collected Material
4.2.3. Extracts Preparation
4.2.4. Concentration and Lyophilization of Extracts
4.3. Solubility Test
4.4. Phytochemical Profiling by High-Performance Thin Layer Chromatography
4.5. Antioxidant Activity
4.5.1. 2,2-diphenyl-1-picrylhydrazyl Hydroxyl Radical (DPPH) Activity
4.5.2. 2,2′-azino-bis-(3-ethylbenzothiazolin-6-sulfonic acid) (ABTS) Radical Scavenging Activity
4.5.3. Ferric Ion Reducing Activity (FRAP)
4.5.4. Total Polyphenol Content
4.5.5. Total Tannin Content
4.5.6. Total Flavonoid Content
4.6. Antimicrobial Activity
4.7. Gas Chromatography—Mass Spectrometry Analysis
4.8. Kupchan Partitioning and Fractionation
4.9. Minimum Inhibitory Concentration
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kliebenstein, D.J.; Osbourn, A. Making New Molecules—Evolution of Pathways for Novel Metabolites in Plants. Curr. Opin. Plant Biol. 2012, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated Omics Analysis of Specialized Metabolism in Medicinal Plants. Plant J. 2017, 90, 764–787. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Du, L.; Liu, W. Occurrence, Fate, and Ecotoxicity of Antibiotics in Agro-Ecosystems. A Review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Ara, I.; Shinwari, M.M.A.; Rashed, S.A.; Bakir, M.A. Evaluation of Antimicrobial Properties of Two Different Extracts of Juglans Regia Tree Bark and Search for Their Compounds Using Gas Chromatohraphy-Mass Spectrum. Int. J. Biol. 2013, 5, 92–102. [Google Scholar] [CrossRef]
- Maree, J.; Kamatou, G.; Gibbons, S.; Viljoen, A.; Van Vuuren, S. The Application of GC-MS Combined with Chemometrics for the Identification of Antimicrobial Compounds from Selected Commercial Essential Oils. Chemom. Intell. Lab. Syst. 2014, 130, 172–181. [Google Scholar] [CrossRef]
- Valle, D.L.; Puzon, J.J.M.; Cabrera, E.C.; Rivera, W.L. Thin Layer Chromatography-Bioautography and Gas Chromatography-Mass Spectrometry of Antimicrobial Leaf Extracts from Philippine Piper Betle L. against Multidrug-Resistant Bacteria. Evidence-Based Complement. Altern. Med. 2016, 4976791, 4976791. [Google Scholar] [CrossRef]
- Wagner, K.; Roth, C.; Willför, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.J.; Sclmabel, T. Identification of Antimicrobial Compounds in Different Hydrophilic Larch Bark Extracts. BioResources 2019, 14, 5807–5815. [Google Scholar] [CrossRef]
- Safdar, M.; Naqvi, S.A.; Anjum, F.; Pasha, I.; Shahid, M.; Waliullah; Jaskani, M.J.; Khan, I.A.; Aadil, R.M. Microbial Biofilm Inhibition, Antioxidants, and Chemical Fingerprints of Afghani Pomegranate Peel Extract Documented by Gas Chromatography–Mass Spectrometry and Fourier Transformation Infrared. J. Food Process. Preserv. 2021, 45, e15657. [Google Scholar] [CrossRef]
- Park, Y.J.; Baskar, T.B.; Yeo, S.K.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, S.S.; Park, S.U. Composition of Volatile Compounds and in Vitro Antimicrobial Activity of Nine Mentha Spp. Springerplus 2016, 5, 1628. [Google Scholar] [CrossRef]
- Taherpour§, A.; Maroofi, H.; Kheradmand, K. Chemical Composition of the Essential Oil of Pelargonium Quercetorum Agnew. of Iran. Nat. Prod. Res. 2007, 21, 24–27. [Google Scholar] [CrossRef]
- Al-Nemari, R.; Al-Senaidy, A.; Semlali, A.; Ismael, M.; Badjah-Hadj-Ahmed, A.Y.; Ben Bacha, A. GC-MS Profiling and Assessment of Antioxidant, Antibacterial, and Anticancer Properties of Extracts of Annona Squamosa L. Leaves. BMC Complement. Med. Ther. 2020, 20, 296. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Van, T.M.; Andriana, Y.; Rayee, R.; Tran, H.-D. Comprehensive Fractionation of Antioxidants and GC-MS and ESI-MS Fingerprints of Celastrus Hindsii Leaves. Medicines 2019, 6, 64. [Google Scholar] [CrossRef]
- Ben ElHadj Ali, I.; Tajini, F.; Boulila, A.; Jebri, M.A.; Boussaid, M.; Messaoud, C.; Sebaï, H. Bioactive Compounds from Tunisian Pelargonium Graveolens (L’Hér.) Essential Oils and Extracts: α-Amylase and Acethylcholinesterase Inhibitory and Antioxidant, Antibacterial and Phytotoxic Activities. Ind. Crops Prod. 2020, 158, 112951. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Paganetto, G.; Tognolini, M.; Muzzoli, M.; Romagnoli, C.; Antognoni, F.; Vertuani, S.; Medici, A.; Bruni, A.; et al. Chemical Characterization (GC/MS and NMR Fingerprinting) and Bioactivities of South-African Pelargonium Capitatum (L.) L’ Her. (Geraniaceae) Essential Oil. Chem. Biodivers. 2011, 8, 624–642. [Google Scholar] [CrossRef]
- Maree, J.E.; Viljoen, A.M. Phytochemical Distinction between Pelargonium Sidoides and Pelargonium Reniforme—A Quality Control Perspective. S. Afr. J. Bot. 2012, 82, 83–91. [Google Scholar] [CrossRef]
- Theisen, L.L.; Gohrbandt, S.; Muller, C.P.; Luetteke, N. EPs® 7630 (Umckaloabo®), an Extract from Pelargonium Sidoides Roots, Exerts Anti-Influenza Virus Activity in Vitro and in Vivo. Int. J. Infect. Dis. 2012, 16, e95. [Google Scholar] [CrossRef][Green Version]
- Kayser, O.; Kolodziej, H. Antibacterial Activity of Extracts and Constituents of Pelargonium Sidoides and Pelargonium Reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar] [CrossRef]
- Lewu, F.B.; Grierson, D.S.; Afolayan, A.J. Extracts from Pelargonium Sidoides Inhibit the Growth of Bacteria and Fungi. Pharm. Biol. 2006, 44, 279–282. [Google Scholar] [CrossRef]
- Papies, J.; Emanuel, J.; Heinemann, N.; Kulić, Ž.; Schroeder, S.; Tenner, B.; Lehner, M.D.; Seifert, G.; Müller, M.A. Antiviral and Immunomodulatory Effects of Pelargonium Sidoides DC. Root Extract EPs® 7630 in SARS-CoV-2-Infected Human Lung Cells. Front. Pharmacol. 2021, 12, 757666. [Google Scholar] [CrossRef]
- Pereira, A.; Bester, M.; Soundy, P.; Apostolides, Z. Anti-Proliferative Properties of Commercial Pelargonium Sidoides Tincture, with Cell-Cycle G0/G1 Arrest and Apoptosis in Jurkat Leukaemia Cells. Pharm. Biol. 2016, 54, 1831–1840. [Google Scholar] [CrossRef]
- Terlizzi, M.; Colarusso, C.; Di Maio, U.; Bagnulo, A.; Pinto, A.; Sorrentino, R. Antioxidant and Antimicrobial Properties of Pelargonium Sidoides DC and Lactoferrin Combination. Biosci. Rep. 2020, 40, BSR20203284. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kayser, O.; Radtke, O.A.; Kiderlen, A.F.; Koch, E. Pharmacological Profile of Extracts of Pelargonium Sidoides and Their Constituents. Phytomedicine 2003, 10, 18–24. [Google Scholar] [CrossRef]
- Jeiter, J.; Hilger, H.H.; Smets, E.F.; Weigend, M. The Relationship between Nectaries and Floral Architecture: A Case Study in Geraniaceae and Hypseocharitaceae. Ann. Bot. 2017, 120, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Bakker, F.T.; Culham, A.; Hettiarachi, P.; Touloumenidou, T.; Gibby, M. Phylogeny of Pelargonium (Geraniaceae) Based on DNA Sequences from Three Genomes. Taxon 2004, 53, 17. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Ghannadi, A.; Bagherinejad, M.R.; Abedi, D.; Jalali, M.; Absalan, B.; Sadeghi, N. Antibacterial Activity and Composition of Essential Oils from Pelargonium Graveolens L’Her and Vitex Agnus-Castus L. Iran. J. Microbiol. 2012, 4, 171–176. [Google Scholar]
- Williams, V.L.; Victor, J.E.; Crouch, N.R. Red Listed Medicinal Plants of South Africa: Status, Trends, and Assessment Challenges. South African J. Bot. 2013, 86, 23–35. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El Ghani, S.A.; El-Moez, S.I.A. Phytochemical Analysis and Antimicrobial Activities of Different Callus Extracts of Pelargonium Sidoides DC. against Food Borne Pathogenic Bacteria. J. Appl. Pharm. Sci. 2018, 8, 109–118. [Google Scholar] [CrossRef][Green Version]
- Ennaifer, M.; Bouzaiene, T.; Chouaibi, M.; Hamdi, M. Pelargonium Graveolens Aqueous Decoction: A New Water-Soluble Polysaccharide and Antioxidant-Rich Extract. Biomed Res. Int. 2018, 2018, 2691513. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Göger, F.; Yerer, M.B.; Koşar, M. Chemical Composition and Biological Investigation of Pelargonium Endlicherianum Root Extracts. Pharm. Biol. 2017, 55, 1608–1618. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Asuzu, P.C.; Besong, S.A.; Aryee, A.N. Polyphenols and Other Phytochemicals in Cancer Prevention and Management. FASEB J. 2019, 33, 802.43. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef]
- Abdelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha Pulegium L. Leaves Essential Oil: Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities. Ind. Crops Prod. 2016, 94, 197–205. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Yong-Bing, X.; Gui-Lin, C.; Ming-Quan, G. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa Oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Hsu, F.L.; Huang, W.J.; Wu, T.H.; Lee, M.H.; Chen, L.C.; Lu, H.J.; Hou, W.C.; Lin, M.H. Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia Pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus Precatorius (L). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Cynthia; Florence, I.; Hery, S.; Akhmad, D. Antibacterial and Antioxidant Activities of Pyrogallol and Synthetic Pyrogallol Dimer. Res. J. Chem. Environ. 2018, 22, 39–47. [Google Scholar]
- Kocaçalişkan, I.; Talan, I.; Terzi, I. Antimicrobial Activity of Catechol and Pyrogallol as Allelochemicals. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Shao, J.; Wu, Z.; Yu, G.; Peng, X.; Li, R. Allelopathic Mechanism of Pyrogallol to Microcystis Aeruginosa PCC7806 (Cyanobacteria): From Views of Gene Expression and Antioxidant System. Chemosphere 2009, 75, 924–928. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Shin, M.; Park, E.; Lee, H. Plant-Inspired Pyrogallol-Containing Functional Materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- Johnstone, D.B.; Little, J.E. Bacteriostatic, Bactericidal, and Drug Resistance Studies of Ethyl Gallate on Mycobacterium Tuberculosis. J. Bacteriol. 1953, 66, 320–323. [Google Scholar] [CrossRef]
- Mink, S.N.; Jacobs, H.; Gotes, J.; Kasian, K.; Cheng, Z.Q. Ethyl Gallate, a Scavenger of Hydrogen Peroxide That Inhibits Lysozyme-Induced Hydrogen Peroxide Signaling in Vitro, Reverses Hypotension in Canine Septic Shock. J. Appl. Physiol. 2011, 110, 359–374. [Google Scholar] [CrossRef]
- Oladimeji, O.H.; Igboasoiyi, A. Isolation, Characterization and Antimicrobial Analysis of Ethyl Gallate and Pyrogallol from Acalypha Wilkesiana Var. Lace-Acalypha (Muell &Arg.). Afr. J. Pharmacol. Ther. 2014, 3, 79–84. [Google Scholar]
- Ooshiro, A.; Hiradate, S.; Kawano, S.; Takushi, T.; Fujii, Y.; Natsume, M.; Abe, H. Identification and Activity of Ethyl Gallate as an Antimicrobial Compound Produced by Geranium Carolinianum. Weed Biol. Manag. 2009, 9, 169–172. [Google Scholar] [CrossRef]
- Hall, G.; Le, T.T.T.; Stanford, J.B.; Sugden, J.K. Hydroxyl Radical Scavenging by Ethyl Gallate and Related Compounds: A Method for Rapid Evaluation. Pharm. Acta Helv. 1996, 71, 221–224. [Google Scholar] [CrossRef]
- Hausen, B.M.; Beyer, W. The Sensitizing Capacity of the Antioxidants Propyl, Octyl, and Dodecyl Gallate and Some Related Gallic Acid Esters. Contact Dermatitis 1992, 26, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Hamed, A.; Mohamed, S.; Ayoub, H. Chemical Composition and Antimicrobial Activity of Sudanese Lupinus Termis L. root extracts. Pharma Innov. J. 2015, 4, 1–4. [Google Scholar]
- Driscoll, W.J.; Chaturvedi, S.; Mueller, G.P. Oleamide Synthesizing Activity from Rat Kidney: Identification as Cytochrome C. J. Biol. Chem. 2007, 282, 22353–22363. [Google Scholar] [CrossRef] [PubMed]
- Nischitha, R.; Shivanna, M.B. Antimicrobial Activity and Metabolite Profiling of Endophytic Fungi in Digitaria Bicornis (Lam) Roem. and Schult. and Paspalidium Flavidum (Retz.) A. Camus. 3 Biotech 2021, 11, 53. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant Properties in Vitro and Total Phenolic Contents in Methanol Extracts from Medicinal Plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified Vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A Retention Index Calculator Simplifies Identification of Plant Volatile Organic Compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Reza, A.S.M.A.; Haque, M.A.; Sarker, J.; Nasrin, M.S.; Rahman, M.M.; Tareq, A.M.; Khan, Z.; Rashid, M.; Sadik, M.G.; Tsukahara, T.; et al. Antiproliferative and Antioxidant Potentials of Bioactive Edible Vegetable Fraction of Achyranthes Ferruginea Roxb. in Cancer Cell Line. Food Sci. Nutr. 2021, 9, 3777–3805. [Google Scholar] [CrossRef]


| Sample | Dry Weight (g) | Lyophilized Weight (g) | Yield (%) |
|---|---|---|---|
| FDE-Root | 150 | 18.16 | 12.10 |
| FDE-Stem | 290 | 19.3 | 6.65 |
| FDE-Leaf | 200 | 46.04 | 23.01 |
| FDE-Flower | 158 | 37.22 | 23.55 |
| FDE-Bicolour | 152 | 43.24 | 28.47 |
| Compounds | FDE-Root | FDE-Stem | FDE-Leaf | FDE-Flower | FDE-Flower (Bicolour) |
|---|---|---|---|---|---|
| 0.015 | 0.015 | 0.015 | |||
| 0.015 | 0.036 | 0.036 | 0.036 | ||
| Tannins | 0.015 | 0.036 | 0.64 | 0.64 | 0.64 |
| 0.036 | 0.83 | 0.66 | 0.82 | 0.82 | |
| 0.85 | |||||
| 0.07 | |||||
| 0.21 | 0.21 | ||||
| 0.26 | 0.26 | ||||
| 0.07 | 0.27 | 0.32 | |||
| 0.08 | 0.30 | 0.34 | |||
| 0.07 | 0.07 | 0.36 | 0.4 | 0.43 | |
| Flavonoids | 0.10 | 0.72 | 0.52 | 0.42 | 0.45 |
| 0.73 | 0.73 | 0.54 | 0.45 | 0.56 | |
| 0.87 | 0.69 | 0.53 | 0.65 | ||
| 0.78 | 0.55 | 0.7 | |||
| 0.64 | |||||
| 0.67 | |||||
| 0.76 |
| Sample | ABTS IC50 (µg/mL) | DPPH IC50 (µg/mL) | FRAP µM TE |
|---|---|---|---|
| FDE-Root | 77.47 ± 6.92 b | 209.50 ± 26.20 b | 44.03 ± 3.79 a |
| FDE-Stem | 75.02 ± 10.57 b | 293.40 ± 24.75 c | 120.08 ± 3.79 b |
| FDE-Leaf | 46.32 ± 1.84 a | 132.50 ± 19.15 a | 274.00 ± 3.70 d |
| FDE-Flower | 48.90 ± 3.36 a | 103.90 ± 19.20 a | 258.70 ± 2.46 c |
| FDE-Bicolour | 55.36 ± 1.96 a | 215.60 ± 21.05 b | 249.29 ± 1.19 c |
| % Area | ||||||||||
| # | Name | MF | MW | FDE-Roots | FDE- Leaf | FDE- Stem | FDE-Flower | FDE-Bicolour | KIexp (1) | KIlit (2) |
| 1 | 2-Hydroxy-propanoic acid, ethyl ester | C5H10O3 | 118.1 | 4.11 | 821.7 | 821 | ||||
| 2 | Furfural | C5H4O2 | 96.0 | 0.41 | 0.14 | 0.15 | 856.5 | 852 | ||
| 3 | 2-Furanmethanol | C5H6O2 | 98.0 | 0.27 | 0.09 | 874.7 | 875 | |||
| 4 | Cyclopent-4-ene-1,3-dione | C5H4O2 | 96.0 | 0.10 | 894.5 | 880 | ||||
| 5 | 2-Acetylfuran | C6H6O2 | 110.0 | 0.56 | 0.05 | 0.28 | 0.26 | 0.31 | 921.4 | 921 |
| 6 | 5-Methyl-2-furancarboxaldehyde | C6H6O2 | 110.0 | 0.23 | 0.01 | 0.07 | 0.09 | 0.11 | 972.5 | 970 |
| 7 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | 144.0 | 0.71 | 0.06 | 0.49 | 0.20 | 986.5 | 989 | |
| 8 | 2-methyl-1,3-Cyclopentanedione | C6H8O2 | 112.0 | 0.51 | 0.29 | 0.09 | 1003.6 | 1003 | ||
| 9 | Furaneol | C6H8O3 | 128.1 | 0.15 | 0.05 | 0.20 | 0.07 | 0.10 | 1071.6 | 1073 |
| 10 | 2-Furancarboxylic acid | C5H4O3 | 112.0 | 0.31 | 0.35 | 1091.0 | 1088 | |||
| 11 | Phenylethyl alcohol | C8H10O | 122.1 | 0.05 | 1120.8 | 1117 | ||||
| 12 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | C6H8O4 | 144.0 | 10.60 | 0.26 | 3.03 | 1.35 | 1.42 | 1153.9 | 1153 |
| 13 | Benzoic acid | C7H6O2 | 122.1 | 0.72 | 0.29 | 1175.2 | 1178 | |||
| 14 | Catechol | C6H6O2 | 110.1 | 0.02 | 1215.3 | 1219 | ||||
| 15 | 2,3-dihydro-Benzofuran | C8H8O | 120.1 | 0.30 | 0.38 | 3.63 | 3.31 | 1226.7 | 1226 | |
| 16 | 5-Hydroxymethylfurfural | C6H6O3 | 126.0 | 1.48 | 1235.2 | 1234 | ||||
| 17 | Benzeneacetic acid | C8H8O2 | 136.1 | 0.23 | 1259.5 | 1257 | ||||
| 18 | Malic acid | C4H6O5 | 134.0 | 1.03 | 1.19 | 1.52 | 1266.8 | MS (3) | ||
| 19 | Indole | C8H7N | 117.0 | 0.03 | 1301.6 | 1300 | ||||
| 20 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.1 | 0.80 | 0.18 | 0.08 | 0.05 | 1319.9 | 1317 | |
| 21 | Eugenol | C10H12O2 | 164.1 | 0.30 | 0.20 | 0.38 | 0.37 | 1362.9 | 1362 | |
| 22 | 1,2,3-Benzenetriol | C6H6O3 | 126.0 | 23.87 | 13.45 | 28.31 | 27.15 | 1398.5 | 1386 | |
| 23 | 3-hydroxy-4-methoxy-Benzaldehyde | C8H8O3 | 152.0 | 0.64 | 1404.6 | 1401 | ||||
| 24 | 2-methoxyphenyl-Ethanol | C9H12O2 | 152.1 | 0.31 | 1439.5 | 1421 | ||||
| 25 | 2,4-di-tert-butylphenol | C14H22O | 206.2 | 0.52 | 1512.1 | 1513 | ||||
| 26 | 4-hydroxy-Benzoic acid | C7H6O3 | 138.0 | 0.56 | 0.22 | 0.59 | 0.58 | 1537.3 | 1538 | |
| 27 | Dodecanoic acid | C12H24O2 | 200.1 | 0.17 | 1557.9 | 1557 | ||||
| 28 | 3-Methoxy-4-Hydroxybenzoic acid (Vanillic acid) | C8H8O4 | 168.0 | 1.38 | 0.27 | 0.30 | 0.54 | 1572.5 | 1570 | |
| 29 | 4-hydroxy-3-methoxy-Benzoic acid, ethyl ester | C10H12O4 | 196.1 | 0.03 | 1590.1 | 1589 | ||||
| 30 | Tridecanoic acid | C13H26O2 | 214.2 | 17.76 | 1652.4 | 1662 | ||||
| 31 | Tetradecanoic acid | C14H28O2 | 228.2 | 0.51 | 0.36 | 1755.3 | 1761 | |||
| 32 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (Loliolide) | C11H16O3 | 196.1 | 0.21 | 1784.8 | 1784 | ||||
| 33 | 4-Acetoxycinnamic acid | C11H10O4 | 206.1 | 0.74 | 0.22 | 1805.9 | MS | |||
| 34 | Pentadecanoic acid | C15H30O2 | 242.2 | 0.20 | 1854.0 | 1857 | ||||
| 35 | 2-methyl-Undecanoic acid, methyl ester | C13H26O2 | 214.2 | 0.06 | 1884.9 | MS (3) | ||||
| 36 | Methyl gallate | C8H8O5 | 184.0 | 1.79 | 3.24 | 1914.6 | MS (3) | |||
| 37 | n-Hexadecanoic acid | C16H32O2 | 256.2 | 10.21 | 0.31 | 6.38 | 1.40 | 3.01 | 1960.3 | 1957 |
| 38 | Ethyl gallate | C9H10O5 | 198.2 | 9.84 | 60.25 | 24.49 | 38.63 | 36.68 | 1978.0 | MS (3) |
| 39 | Hexadecanoic acid, ethyl ester (Ethyl palmitate) | C18H36O2 | 284.3 | 5.23 | 1985.0 | 1984 | ||||
| 40 | Heptadecanoic acid | C17H34O2 | 270.3 | 0.23 | 2054.3 | 2065 | ||||
| 41 | Phytol | C20H40O | 296.3 | 0.18 | 1.80 | 0.25 | 0.52 | 2108.3 | 2112 | |
| 42 | (Z,Z)-9,12-Octadecadienoic acid | C18H32O2 | 280.2 | 3.07 | 2.80 | 0.71 | 2131.4 | 2130 | ||
| 43 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid | C18H30O2 | 278.2 | 1.38 | 2135.4 | 2143 | ||||
| 44 | 9-Octadecenoic acid | C18H34O2 | 282.3 | 2.91 | 3.60 | 2139.7 | 2141 | |||
| 45 | Linoleic acid ethyl ester | C20H36O2 | 308.3 | 3.38 | 4.26 | 1.06 | 2.17 | 2155.7 | 2155 | |
| 46 | 9-Octadecenoic acid, ethyl ester | C20H38O2 | 310.3 | 3.22 | 2.35 | 2160.9 | 2171 | |||
| 47 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid, ethyl ester | C20H34O2 | 306.3 | 0.59 | 1.64 | 0.40 | 0.77 | 2163.2 | 2169 | |
| 48 | Octadecanoic acid | C18H36O2 | 284.3 | 0.68 | 0.19 | 0.30 | 0.55 | 0.95 | 2174.5 | 2172 |
| 49 | Octadecanoic acid, ethyl ester | C20H40O2 | 312.3 | 0.74 | 0.60 | 2183.1 | 2188 | |||
| 50 | (Z)-9-Octadecenamide | C18H35NO | 281.3 | 9.00 | 1.65 | 3.52 | 5.57 | 9.33 | 2364.0 | 2375 |
| 51 | Eicosanoic acid, ethyl ester | C22H44O2 | 340.3 | 1.30 | 0.57 | 0.20 | 0.41 | 2382.2 | 2394 | |
| 52 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C19H38O4 | 330.3 | 3.25 | 0.73 | 0.49 | 2501.6 | 2498 | ||
| 53 | Docosanoic acid, ethyl ester | C24H48O2 | 368.6 | 0.51 | 2586.3 | 2594 | ||||
| Solvent | ||||
|---|---|---|---|---|
| Strain | Chl | EtOAc | MeOH | FDE |
| S. aureus | 250 µg/mL | 31.2 µg/mL | >1000 µg/mL | Leaf |
| 1000 µg/mL | 31.2 µg/mL | 1000 µg/mL | Flower | |
| S. enterica | 1000 µg/mL | 62.5 µg/mL | >1000 µg/mL | Leaf |
| 250 µg/mL | 62.5 µg/mL | 1000 µg/mL | Flower | |
| E. faecalis | 1000 µg/mL | 62.5 µg/mL | >1000 µg/mL | Leaf |
| 500 µg/mL | 31.2 µg/mL | 1000 µg/mL | Flower | |
| S. marcescens | >1000 µg/mL | 500 µg/mL | >1000 µg/mL | Leaf |
| 1000 µg/mL | 500 µg/mL | >1000 µg/mL | Flower | |
| # | Name | MF | MW | % Area | KIexp (1) | KIlit (2) | |
|---|---|---|---|---|---|---|---|
| Leaf | Flower | ||||||
| 1 | Furfural | C5H4O2 | 96.0 | 0.11 | 855.1 | 852 | |
| 2 | 3-methyl-2,5-furandione | C5H4O3 | 112.0 | 0.02 | 0.03 | 952.0 | 949 |
| 3 | Acetophenone | C8H8O | 120.0 | 0.04 | 1070.4 | 1066 | |
| 4 | 2-methoxy-Phenol | C7H8O2 | 124.1 | 0.06 | 1091.5 | 1090 | |
| 5 | Nonanal | C9H18O | 142.1 | 0.07 | 1114.7 | 1112 | |
| 6 | Catechol | C6H6O2 | 110.0 | 0.13 | 1200.2 | 1208 | |
| 7 | 2,3-dihydro-Benzofuran | C8H8O | 120.1 | 0.04 | 1.59 | 1222.4 | 1226 |
| 8 | 5-Hydroxymethylfurfural | C6H6O3 | 126.0 | 0.10 | 1256.4 | 1261 | |
| 9 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.1 | 0.02 | 0.02 | 1314.5 | 1317 |
| 10 | Eugenol | C10H12O2 | 164.1 | 0.05 | 1358.1 | 1362 | |
| 11 | 1,2,3-Benzenetriol | C6H6O3 | 126.0 | 77.38 | 71.24 | 1387.4 | 1386 |
| 12 | 2,4-di-tert-butylphenol | C14H22O | 206.2 | 0.09 | 1505.9 | 1513 | |
| 13 | Ethyl gallate | C9H10O5 | 198.1 | 13.10 | 1951.3 | MS | |
| 14 | Octadecanoic acid | C18H36O2 | 284.3 | 1.44 | 0.54 | 2167.2 | 2172 |
| 15 | (Z)-9-Octadecenamide | C18H35NO | 281.3 | 13.63 | 6.75 | 2364.0 | 2375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, A.-M.; Reyes-Maldonado, O.K.; Puebla-Pérez, A.M.; Arreola, M.P.G.; Velasco-Ramírez, S.F.; Zúñiga-Mayo, V.; Sánchez-Fernández, R.E.; Delgado-Saucedo, J.-I.; Velázquez-Juárez, G. GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae). Molecules 2022, 27, 3436. https://doi.org/10.3390/molecules27113436
Alonso A-M, Reyes-Maldonado OK, Puebla-Pérez AM, Arreola MPG, Velasco-Ramírez SF, Zúñiga-Mayo V, Sánchez-Fernández RE, Delgado-Saucedo J-I, Velázquez-Juárez G. GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae). Molecules. 2022; 27(11):3436. https://doi.org/10.3390/molecules27113436
Chicago/Turabian StyleAlonso, Alan-Misael, Oscar Kevin Reyes-Maldonado, Ana María Puebla-Pérez, Martha Patricia Gallegos Arreola, Sandra Fabiola Velasco-Ramírez, Victor Zúñiga-Mayo, Rosa E. Sánchez-Fernández, Jorge-Iván Delgado-Saucedo, and Gilberto Velázquez-Juárez. 2022. "GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae)" Molecules 27, no. 11: 3436. https://doi.org/10.3390/molecules27113436
APA StyleAlonso, A.-M., Reyes-Maldonado, O. K., Puebla-Pérez, A. M., Arreola, M. P. G., Velasco-Ramírez, S. F., Zúñiga-Mayo, V., Sánchez-Fernández, R. E., Delgado-Saucedo, J.-I., & Velázquez-Juárez, G. (2022). GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae). Molecules, 27(11), 3436. https://doi.org/10.3390/molecules27113436








