Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds
Abstract
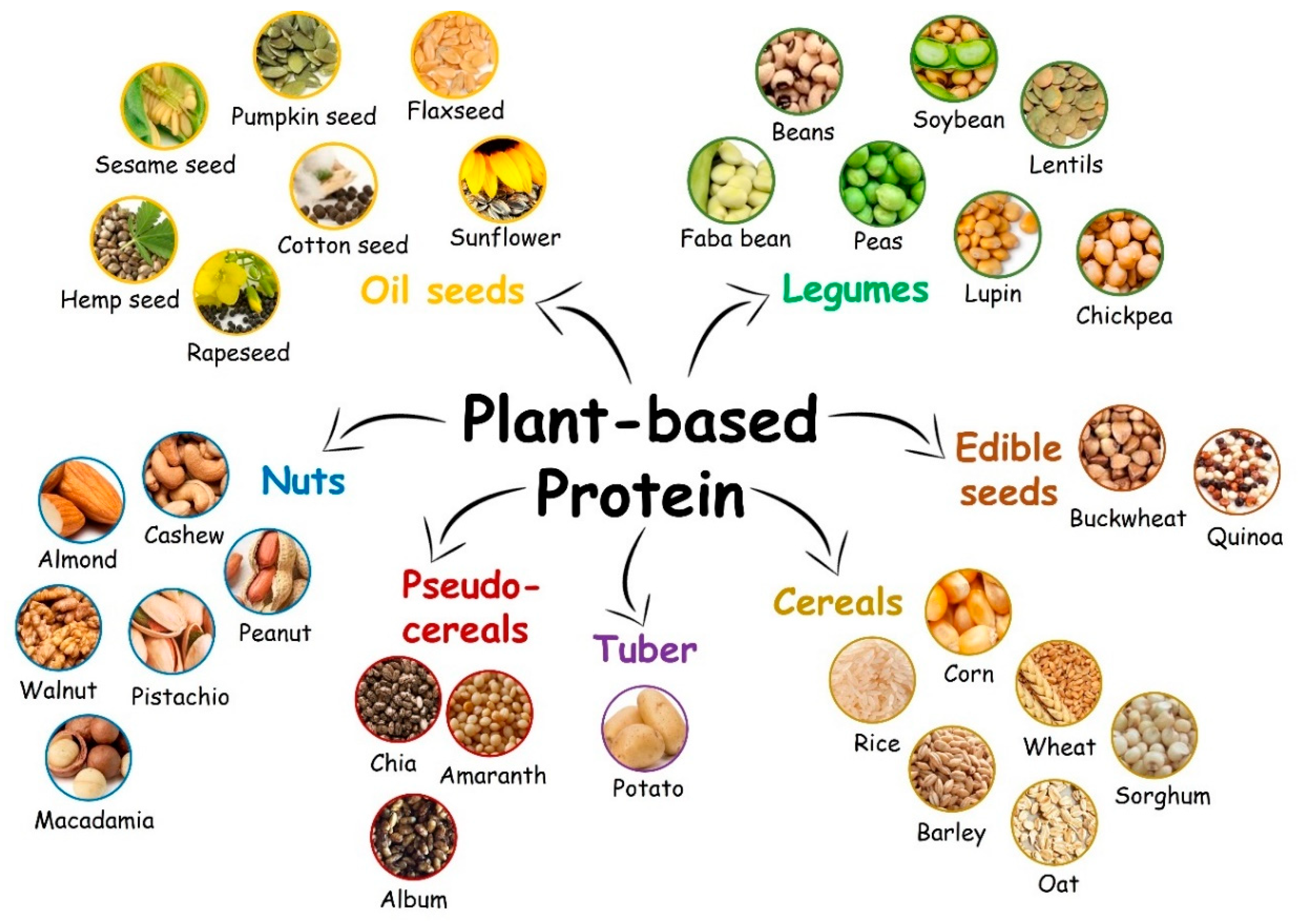
:1. Introduction
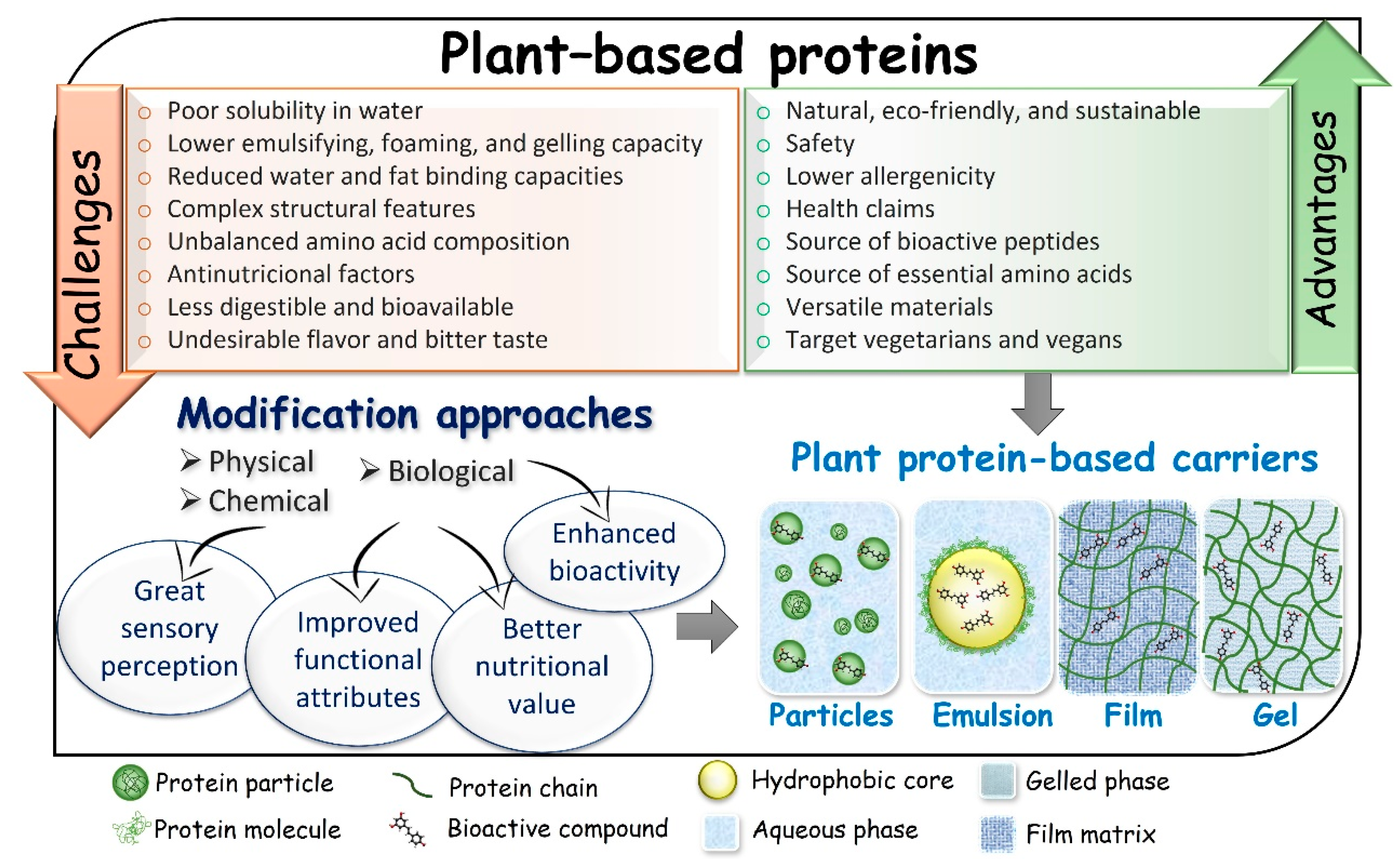
2. Advantages and Challenges of Use of Plant-Based Proteins
3. Effects of the Different Modification Approaches on Nutritional Quality, Bioactivity, and Techno-Functionalities of Plant-Based Proteins
3.1. Effects on Properties Related to Hydration Mechanisms
3.2. Effects on Functional Attributes Linked to Structure and Rheology
3.3. Effects on Functionalities Associated with Protein Surface Activity
3.4. Improvement of Perceived Sensory Acceptance
3.5. Enhancement of Nutritional Value
3.6. Positive Effects on the Biological Functions
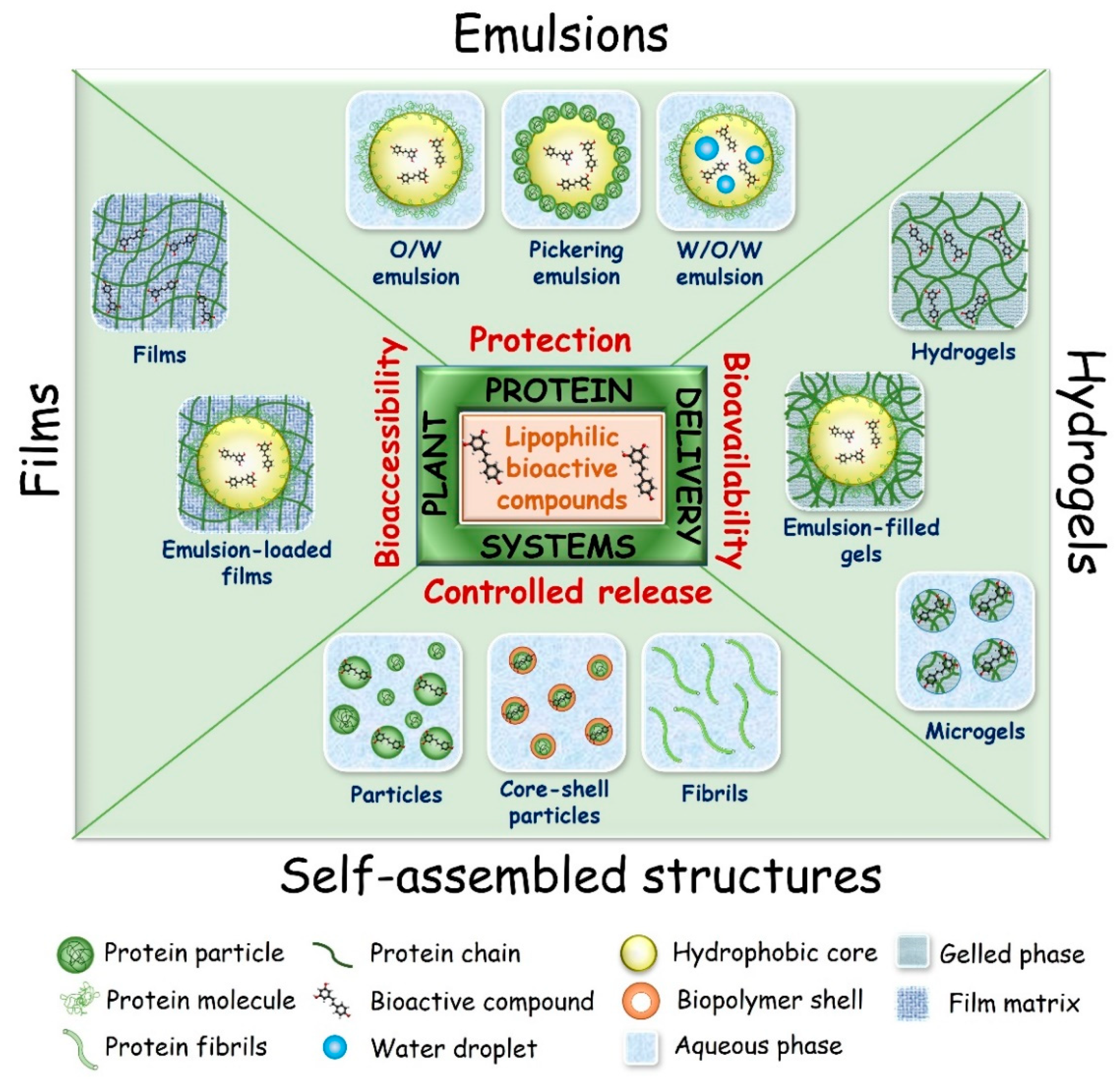
4. Designing Plant Protein-Based Carriers
4.1. Self-Assembly Structures
4.2. Emulsions
4.3. Hydrogels
4.4. Films
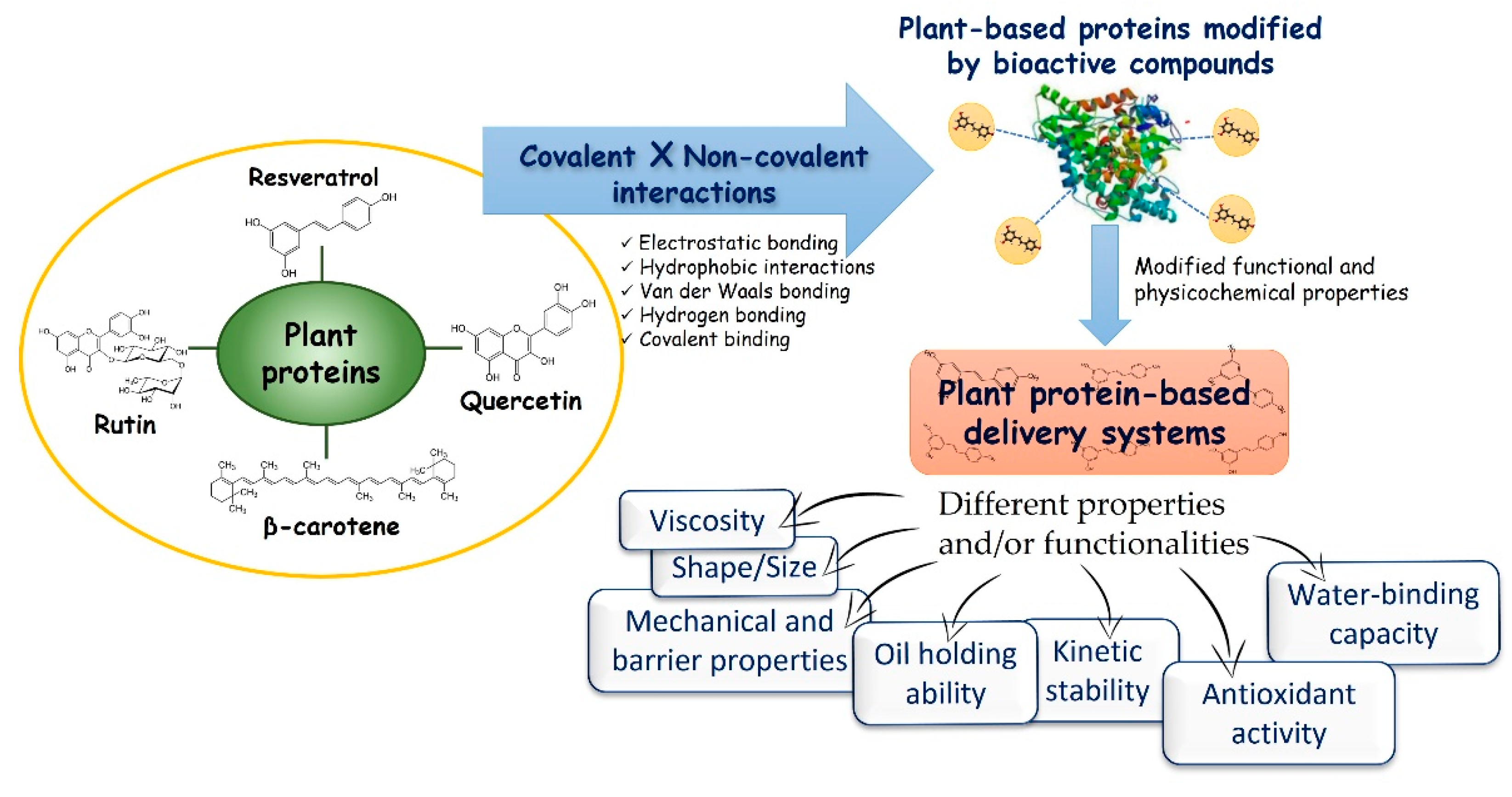
5. Can Lipophilic Bioactive Compounds Influence the Structure/Functionality of the Plant Protein-Based Delivery Systems?
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and functional foods: The foods for the future world. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef]
- Aguiar, L.M.; Geraldi, M.V.; Betim Cazarin, C.B.; Maróstica Junior, M.R. Chapter 11-functional food consumption and its physiological effects. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 205–225. [Google Scholar]
- Schaffer, M.; Schaffer, P.M.; Bar-Sela, G. An update on Curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 605–611. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, I.P.; Bernkop-Schnürch, A. Pre-systemic metabolism of orally administered drugs and strategies to overcome it. J. Control. Release 2014, 192, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Joye, I. Protein digestibility of cereal products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C.; Phillips, S. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Navarro, S.L.; Garrison, C.B.; Randolph, T.W.; Zhang, Y.; Shojaie, A.; Kratz, M.; Hullar, M.A.; Raftery, D.; Neuhouser, M.L.; et al. Urinary enterolactone is associated with plasma proteins related to immunity and cancer development in healthy participants on controlled diets. Hum. Nutr. Metab. 2021, 25, 200128. [Google Scholar] [CrossRef]
- Scott, J.D.; Pawson, T. Cell signaling in space and time: Where proteins come together and when they’re apart. Science 2009, 326, 1220–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, P.; Böcker, L.; Mathys, A.; Fischer, P. Proteins from microalgae for the stabilization of fluid interfaces, emulsions, and foams. Trends Food Sci. Technol. 2021, 108, 326–342. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure–function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.A.; Shah, U.; Hackett, M.J.; Gulzar, M.; Karakyriakos, E.; Johnson, S.K. Technological strategies to improve gelation properties of legume proteins with the focus on lupin. Innov. Food Sci. Emerg. Technol. 2021, 68, 102634. [Google Scholar] [CrossRef]
- Mezzenga, R.; Fischer, P. The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions. Rep. Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef]
- Tang, C.-H. Nano-architectural assembly of soy proteins: A promising strategy to fabricate nutraceutical nanovehicles. Adv. Colloid Interface Sci. 2021, 291, 102402. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Conrad, Z.; Niles, M.T.; Neher, D.A.; Roy, E.D.; Tichenor, N.E.; Jahns, L. Relationship between food waste, diet quality, and environmental sustainability. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Yang, J.; Sagis, L.M. Interfacial behavior of plant proteins—Novel sources and extraction methods. Curr. Opin. Colloid Interface Sci. 2021, 56, 101499. [Google Scholar] [CrossRef]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant proteins from green pea and chickpea: Extraction, fractionation, structural characterization and functional properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Zhu, G.; Luo, S.; Zhang, D.; Liu, F.; Shen, Y. Modification of the structural and functional properties of wheat gluten protein using a planetary ball mill. Food Chem. 2021, 363, 130251. [Google Scholar] [CrossRef]
- Azizi, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Microencapsulation of Lactobacillus rhamnosus using sesame protein isolate: Effect of encapsulation method and transglutaminase. Food Biosci. 2021, 41, 101012. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Gastrointestinal fate of emulsion-based ω-3 oil delivery systems stabilized by plant proteins: Lentil, pea, and faba bean proteins. J. Food Eng. 2017, 207, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Ettoumi, Y.L.; Chibane, M.; Romero, A. Emulsifying properties of legume proteins at acidic conditions: Effect of protein concentration and ionic strength. LWT-Food Sci. Technol. 2016, 66, 260–266. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Chen, L. Intestinal uptake of barley protein-based nanoparticles for β-carotene delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef]
- Witczak, M.; Chmielewska, A.; Ziobro, R.; Korus, J.; Juszczak, L. Rapeseed protein as a novel ingredient of gluten-free dough: Rheological and thermal properties. Food Hydrocoll. 2021, 118, 106813. [Google Scholar] [CrossRef]
- Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and Rheological Properties of Vicia faba L. Protein Isol. 2021, 11, 178. [Google Scholar] [CrossRef]
- Wan, Z.-L.; Guo, J.; Yang, X.-Q. Plant protein-based delivery systems for bioactive ingredients in foods. Food Funct. 2015, 6, 2876–2889. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, M.; Ettelaie, R.; Sarkar, A. Pea protein microgel particles as Pickering stabilisers of oil-in-water emulsions: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 102, 105583. [Google Scholar] [CrossRef]
- Oliveira, T.C.B.; Bispo, M.; Moraes, I.; Campanella, O.H.; Pinho, S.C. Stability of curcumin encapsulated in solid lipid microparticles incorporated in cold-set emulsion filled gels of soy protein isolate and xanthan gum. Food Res. Int. 2017, 102, 759–767. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Mekala, S.; Silva, E.K.; Saldaña, M.D.A. Mechanism, kinetics, and physicochemical properties of ultrasound-produced emulsions stabilized by lentil protein: A non-dairy alternative in food systems. Eur. Food Res. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Malekzad, H.; Mirshekari, H.; Zangabad, P.S.; Basri, S.M.M.; Baniasadi, F.; Aghdam, M.S.; Karimi, M.; Hamblin, M.R. Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems. Crit. Rev. Biotechnol. 2018, 38, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Crosser, N. Plant-Based Meat, Egg and Dairy Products in the U.S. Retail Market; Good Food Institute: Washington, DC, USA, 2021. [Google Scholar]
- R&M. Global Plant-Based Protein Market with Focus on Plant-Based Meat: Insights, Trends and Forecast (2020–2024); R&M: Dublin, Ireland, 2020. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P. Plant Protein and Animal Proteins: Do They Differentially Affect Cardiovascular Disease Risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods 2021, 10, 1929. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Sofos, J.N. Safety of Food and Beverages: Meat and Meat Products. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 268–279. [Google Scholar]
- Pantoa, T.; Baricevic-Jones, I.; Suwannaporn, P.; Kadowaki, M.; Kubota, M.; Roytrakul, S.; Mills, E.C. Young rice protein as a new source of low allergenic plant-base protein. J. Cereal Sci. 2020, 93, 102970. [Google Scholar] [CrossRef]
- Aggarwal, A.; Drewnowski, A. Plant- and animal-protein diets in relation to sociodemographic drivers, quality, and cost: Findings from the Seattle Obesity Study. Am. J. Clin. Nutr. 2019, 110, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Tuccillo, F.; Marino, M.G.; Torri, L. Italian consumers’ attitudes towards entomophagy: Influence of human factors and properties of insects and insect-based food. Food Res. Int. 2020, 137, 109619. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.; et al. Plant-based proteins and their multifaceted industrial applications. LWT 2021, 154, 112620. [Google Scholar] [CrossRef]
- Sedlar, T.; Čakarević, J.; Tomić, J.; Popović, L. Vegetable By-Products as New Sources of Functional Proteins. Plant Foods Hum. Nutr. 2021, 76, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Palou, E.; López-Malo, A. Legume proteins, peptides, water extracts, and crude protein extracts as antifungals for food applications. Trends Food Sci. Technol. 2021, 112, 16–24. [Google Scholar] [CrossRef]
- Brion-Espinoza, I.A.; Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Calderón-Chiu, C.; Calderón-Santoyo, M. Edible pectin film added with peptides from jackfruit leaves obtained by high-hydrostatic pressure and pepsin hydrolysis. Food Chem. X 2021, 12, 100170. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Winkler, R.; García-Lara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014, 111, 165–183. [Google Scholar] [CrossRef]
- Shi, Z.; Hao, Y.; Teng, C.; Yao, Y.; Ren, G. Functional properties and adipogenesis inhibitory activity of protein hydrolysates from quinoa (Chenopodium quinoa Willd.). Food Sci. Nutr. 2019, 7, 2103–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Huang, L.; Li, Y.; Feng, Y.; Zhang, Z.; Xu, Z.; Chen, M.-L.; Cheng, Y. New peptides with immunomodulatory activity identified from rice proteins through peptidomic and in silico analysis. Food Chem. 2021, 364, 130357. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Pauls, K.P.; Deng, Z.; Tsao, R. Antioxidant and anti-inflammatory polyphenols and peptides of common bean (Phaseolus vulga L.) milk and yogurt in Caco-2 and HT-29 cell models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Coelho, M.S.; Salas-Mellado, M.D.L.M. How extraction method affects the physicochemical and functional properties of chia proteins. LWT 2018, 96, 26–33. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- da Silva, A.M.M.; Almeida, F.S.; Sato, A.C.K. Functional characterization of commercial plant proteins and their application on stabilization of emulsions. J. Food Eng. 2021, 292, 110277. [Google Scholar] [CrossRef]
- Schutyser, M.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Keivaninahr, F.; Gadkari, P.; Benis, K.Z.; Tulbek, M.; Ghosh, S. Prediction of emulsification behaviour of pea and faba bean protein concentrates and isolates from structure–functionality analysis. RSC Adv. 2021, 11, 12117–12135. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Goli, S.A.H.; Doost, A.S.; Dewettinck, K.; Van der Meeren, P. Bioparticles of flaxseed protein and mucilage enhance the physical and oxidative stability of flaxseed oil emulsions as a potential natural alternative for synthetic surfactants. Coll. Surf. B Biointerfaces 2019, 184, 110489. [Google Scholar] [CrossRef] [PubMed]
- Adal, E.; Sadeghpour, A.; Connell, S.; Rappolt, M.; Ibanoglu, E.; Sarkar, A. Heteroprotein Complex Formation of Bovine Lactoferrin and Pea Protein Isolate: A Multiscale Structural Analysis. Biomacromolecules 2017, 18, 625–635. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Zeeb, B.; Yavuz-Düzgun, M.; Dreher, J.; Evert, J.; Stressler, T.; Fischer, L.; Özcelik, B.; Weiss, J. Modulation of the bitterness of pea and potato proteins by a complex coacervation method. Food Funct. 2018, 9, 2261–2269. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Monnet, A.-F.; Laleg, K.; Michon, C.; Micard, V. Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019, 86, 131–143. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanga, S.K.; Wang, J.; Raghavan, V. Effect of ultrasound and microwave processing on the structure, in-vitro digestibility and trypsin inhibitor activity of soymilk proteins. LWT 2020, 131, 109708. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of Processing Technologies on the Allergenicity of Food Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar] [CrossRef] [Green Version]
- Strieder, M.M.; Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Impact of thermosonication pretreatment on the production of plant protein-based natural blue colorants. J. Food Eng. 2021, 299, 110512. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Ertugrul, U.; Namli, S.; Tas, O.; Kocadagli, T.; Gokmen, V.; Sumnu, S.; Oztop, M.H. Pea protein properties are altered following glycation by microwave heating. LWT 2021, 150, 111939. [Google Scholar] [CrossRef]
- Li, R.; Xiong, Y.L. Ultrasound-induced structural modification and thermal properties of oat protein. LWT 2021, 149, 111861. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Effect of radio frequency treatment on functional, structural and thermal behaviors of protein isolates in rice bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y. Acylation modification and/or guar gum conjugation enhanced functional properties of pea protein isolate. Food Hydrocoll. 2021, 117, 106686. [Google Scholar] [CrossRef]
- Wang, J.; Na, X.; Navicha, W.B.; Wen, C.; Ma, W.; Xu, X.; Wu, C.; Du, M. Concentration-dependent improvement of gelling ability of soy proteins by preheating or ultrasound treatment. LWT 2020, 134, 110170. [Google Scholar] [CrossRef]
- Zhu, P.; Huang, W.; Guo, X.; Chen, L. Strong and elastic pea protein hydrogels formed through pH-shifting method. Food Hydrocoll. 2021, 117, 106705. [Google Scholar] [CrossRef]
- Zhao, Z.-K.; Mu, T.-H.; Zhang, M.; Richel, A. Effects of high hydrostatic pressure and microbial transglutaminase treatment on structure and gelation properties of sweet potato protein. LWT 2019, 115, 108436. [Google Scholar] [CrossRef]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K. Restructuring of soy protein employing ultrasound: Effect on hydration, gelation, thermal, in-vitro protein digestibility and structural attributes. LWT 2020, 132, 109781. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Z.; Guo, Y.; Li, B.; Yu, W.; Zhou, L.; Jiang, L.; Teng, F.; Wang, Z. Effects of high-pressure homogenization on structural and emulsifying properties of thermally soluble aggregated kidney bean (Phaseolus vulgaris L.) proteins. Food Hydrocoll. 2021, 119, 106835. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effect of heat-induced aggregation of soy protein isolate on protein-glutaminase deamidation and the emulsifying properties of deamidated products. LWT 2022, 154, 112328. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Fang, F.; Chen, D.; Federici, E.; Pan, S.; Jones, O.G. Limited hydrolysis and conjugation of zein with chitosan oligosaccharide by enzymatic reaction to improve functional properties. Food Chem. 2021, 348, 129035. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Geng, H.; Zhang, X.; Chen, Z. Formation, structural characteristics, foaming and emulsifying properties of rice glutelin fibrils. Food Chem. 2021, 354, 129554. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Liu, Y. Effects of partial hydrolysis on the structural, functional and antioxidant properties of oat protein isolate. Food Funct. 2020, 11, 3144–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, Z.; Zhang, J.; Nasiru, M.M.; Wang, Y.; Fu, L. Atmospheric cold plasma treatment of soybean protein isolate: Insights into the structural, physicochemical, and allergenic characteristics. J. Food Sci. 2021, 86, 68–77. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Sun, D.; Li, Y.; Chen, Z. Effect of enzymolysis-assisted electron beam irradiation on structural characteristics and antioxidant activity of rice protein. J. Cereal Sci. 2019, 89, 102789. [Google Scholar] [CrossRef]
- Shi, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. LWT 2021, 150, 111927. [Google Scholar] [CrossRef]
- Schlegel, K.; Leidigkeit, A.; Eisner, P.; Schweiggert-Weisz, U. Technofunctional and Sensory Properties of Fermented Lupin Protein Isolates. Foods 2019, 8, 678. [Google Scholar] [CrossRef] [Green Version]
- Krentz, A.; García-Cano, I.; Ortega-Anaya, J.; Jiménez-Flores, R. Use of casein micelles to improve the solubility of hydrophobic pea proteins in aqueous solutions via low-temperature homogenization. J. Dairy Sci. 2021. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.-H.; Zhao, X.-D.; Zhang, L.; Li, Q.; Wu, C.; Ding, X.; Qian, J.-Y. Assessment of impact of pulsed electric field on functional, rheological and structural properties of vital wheat gluten. LWT 2021, 147, 111536. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.-J.; Jiang, W.; Qian, J.-Y. Effect of pulsed electric field on functional and structural properties of canola protein by pretreating seeds to elevate oil yield. LWT 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, X.; Wang, W.; Wang, L.; Jiang, L.; Liu, T.; Yu, D. Effect of high intensity ultrasound on the structure and solubility of soy protein isolate-pectin complex. Ultrason. Sonochemistry 2021, 80, 105808. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; van der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef]
- Wang, R.; Xu, P.; Chen, Z.; Zhou, X.; Wang, T. Complexation of rice proteins and whey protein isolates by structural interactions to prepare soluble protein composites. LWT 2019, 101, 207–213. [Google Scholar] [CrossRef]
- Wang, T.; Xu, P.; Chen, Z.; Zhou, X.; Wang, R. Alteration of the structure of rice proteins by their interaction with soy protein isolates to design novel protein composites. Food Funct. 2018, 9, 4282–4291. [Google Scholar] [CrossRef]
- Song, T.; Xiong, Z.; Shi, T.; Yuan, L.; Gao, R. Effect of glutamic acid on the preparation and characterization of Pickering emulsions stabilized by zein. Food Chem. 2022, 366, 130598. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, Y. Surface modification of zein colloidal particles with sodium caseinate to stabilize oil-in-water pickering emulsion. Food Hydrocoll. 2016, 56, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Khan, M.A.; Xie, Z.; Tao, S.; Li, Y.; Liang, L. A peppermint oil emulsion stabilized by resveratrol-zein-pectin complex particles: Enhancing the chemical stability and antimicrobial activity in combination with the synergistic effect. Food Hydrocoll. 2020, 103, 105675. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Malcata, F.X.; Vicente, A.A. Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [Green Version]
- Queirós, R.P.; Saraiva, J.A.; Da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Effect of ultraviolet radiation on morphological and physicochemical properties of sesame protein isolate based edible films. Food Hydrocoll. 2018, 85, 136–143. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Kuktaite, R. Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites. Int. J. Mol. Sci. 2019, 20, 58. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT 2020, 129, 109563. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial, Emulsifying and Foaming Properties of Milk Proteins. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer US: Boston, MA, USA, 2003; pp. 1229–1260. [Google Scholar]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M. The role of emergent processing technologies in tailoring plant protein functionality: New insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.M. Exploring the structure-function relationship of Great Northern and navy bean (Phaseolus vulgaris L.) protein hydrolysates: A study on the effect of enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 162, 1516–1525. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Liu, C.; Damodaran, S.; Heinonen, M. Effects of microbial transglutaminase treatment on physiochemical properties and emulsifying functionality of faba bean protein isolate. LWT 2019, 99, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Tan, Y.; Chang, S.; Li, J.; Maleki, S.; Puppala, N. Peanut allergen reduction and functional property improvement by means of enzymatic hydrolysis and transglutaminase crosslinking. Food Chem. 2020, 302, 125186. [Google Scholar] [CrossRef]
- Yadav, D.N.; Mir, N.A.; Wadhwa, R.; Tushir, S.; Sethi, S.; Anurag, R.K.; Oberoi, H.S. Hydrolysis of peanut (Arachis hypogea L) protein concentrate by fungal crude protease extract: Effect on structural, functional and in-vitro protein digestibility. J. Food Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Zheng, S.; Wang, Y.; Fu, L. Combining Alcalase hydrolysis and transglutaminase-cross-linking improved bitterness and techno-functional properties of hypoallergenic soybean protein hydrolysates through structural modifications. LWT 2021, 151, 112096. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Zhu, K.-X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Effect of deamidation-induced modification on umami and bitter taste of wheat gluten hydrolysates. J. Sci. Food Agric. 2017, 97, 3181–3188. [Google Scholar] [CrossRef]
- He, W.; Yang, R.; Zhao, W. Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 2019, 277, 655–663. [Google Scholar] [CrossRef]
- Hassan, A.B.; Mahmoud, N.S.; Elmamoun, K.; Adiamo, O.Q.; Ahmed, I.A.M. Effects of gamma irradiation on the protein characteristics and functional properties of sesame (Sesamum indicum L.) seeds. Radiat. Phys. Chem. 2018, 144, 85–91. [Google Scholar] [CrossRef]
- Jin, J.; Okagu, O.D.; Yagoub, A.E.A.; Udenigwe, C.C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason. Sonochem. 2021, 70, 105348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, A.; Hussain, L.; Jha, P.; Pal, V.; Petwal, V.C.; Dwivedi, J. Impact of Electron Beam on Storage Protein Subunits, In Vitro Protein Digestibility and Trypsin Inhibitor Content in Soybean Seeds. Food Bioprocess Technol. 2017, 10, 407–412. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Effect of High Pressure Processing and heat treatment on in vitro digestibility and trypsin inhibitor activity in lentil and faba bean protein concentrates. LWT 2021, 152, 112342. [Google Scholar] [CrossRef]
- Chandra-Hioe, M.V.; Wong, C.H.M.; Arcot, J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Rui, X.; Huang, J.; Xing, G.; Zhang, Q.; Li, W.; Dong, M. Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains. LWT 2018, 99, 156–165. [Google Scholar] [CrossRef]
- Abe, R.; Matsukaze, N.; Yamaguchi, Y.; Akao, M.; Kumagai, H.; Kumagai, H. Wheat gliadin deamidated by cation-exchange resins induces oral tolerance in a mouse model of wheat allergy. J. Food Bioact. 2018, 2, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, T.; Sun, D.; Tang, M.; Sun, Z.; Chen, L.; Luo, X.; Li, Y.; Wang, R.; Li, Y.; et al. Effect of electron beam irradiation on the functional properties and antioxidant activity of wheat germ protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2019, 54, 192–199. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Effect of high energy electron beam on proteolysis and antioxidant activity of rice proteins. Food Funct. 2020, 11, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Urbizo-Reyes, U.; Martin-González, M.F.S.; Bravo, J.G.; Vigil, A.L.M.; Liceaga, A.M. Physicochemical characteristics of chia seed (Salvia hispanica) protein hydrolysates produced using ultrasonication followed by microwave-assisted hydrolysis. Food Hydrocoll. 2019, 97, 105187. [Google Scholar] [CrossRef]
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009, 113, 196–201. [Google Scholar] [CrossRef]
- Tong, X.; Cao, J.; Tian, T.; Lyu, B.; Miao, L.; Lian, Z.; Cui, W.; Liu, S.; Wang, H.; Jiang, L. Changes in structure, rheological property and antioxidant activity of soy protein isolate fibrils by ultrasound pretreatment and EGCG. Food Hydrocoll. 2022, 122, 107084. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Martinez, M.J.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Comparative behavior of protein or polysaccharide stabilized emulsion under in vitro gastrointestinal conditions. Food Hydrocoll. 2016, 52, 47–56. [Google Scholar] [CrossRef]
- Verkempinck, S.; Salvia-Trujillo, L.; Moens, L.; Carrillo, C.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Mutsokoti, L.; Panozzo, A.; Pallares, A.P.; Jaiswal, S.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Carotenoid bioaccessibility and the relation to lipid digestion: A kinetic study. Food Chem. 2017, 232, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Delphi, L.; Sepehri, H.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Walnut protein–curcumin complexes: Fabrication, structural characterization, antioxidant properties, and in vitro anticancer activity. J. Food Meas. Charact. 2020, 14, 876–885. [Google Scholar] [CrossRef]
- Vijayan, U.K.; Shah, N.N.; Muley, A.B.; Singhal, R.S. Complexation of curcumin using proteins to enhance aqueous solubility and bioaccessibility: Pea protein vis-à-vis whey protein. J. Food Eng. 2021, 292, 110258. [Google Scholar] [CrossRef]
- Fan, Y.; Zeng, X.; Yi, J.; Zhang, Y. Fabrication of pea protein nanoparticles with calcium-induced cross-linking for the stabilization and delivery of antioxidative resveratrol. Int. J. Biol. Macromol. 2020, 152, 189–198. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.-K.; Ling, J. Fabrication of soy protein isolate/cellulose nanocrystal composite nanoparticles for curcumin delivery. Int. J. Biol. Macromol. 2020, 165, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Gastrointestinal fate and antioxidation of β-carotene emulsion prepared by oat protein isolate-Pleurotus ostreatus β-glucan conjugate. Carbohydr. Polym. 2019, 221, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Huang, H.; Liu, Y.; Lu, Y.; Fan, Y.; Zhang, Y. Fabrication of curcumin-loaded pea protein-pectin ternary complex for the stabilization and delivery of β-carotene emulsions. Food Chem. 2020, 313, 126118. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kelly, A.L.; Miao, S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene. Food Chem. 2021, 372, 131262. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-encapsulation of curcumin and β-carotene in Pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, P.; Chen, F.; Yin, L. Effects of emulsion concentration on the physicochemical properties of wheat bran arabinoxylan-soy protein isolate emulsion-filled gels used as β-carotene carriers. LWT 2022, 153, 112498. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Brito-Oliveira, T.C.; Pinho, S.C. Emulsion-filled gels of soy protein isolate for vehiculation of vitamin D3: Effect of protein solubility on their mechanical and rheological characteristics. Food Biosci. 2021, 45, 101455. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-Epigallocatechin-3-gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT 2020, 140, 110809. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, M.; Liu, X.; Chu, R.; Qiao, Z.; Li, C.; Adhikari, B. Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols. Futur. Foods 2021, 3, 100033. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- McManus, J.; Charbonneau, P.; Zaccarelli, E.; Asherie, N. The physics of protein self-assembly. Curr. Opin. Colloid Interface Sci. 2016, 22, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, Y.; Yu, S.; Liu, J. Hierarchical Self-Assembly of Proteins Through Rationally Designed Supramolecular Interfaces. Front. Bioeng. Biotechnol. 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, R.; Xu, X.; Du, M.; Zhu, B.; Wu, C. Structural interplay between curcumin and soy protein to improve the water-solubility and stability of curcumin. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhou, F.; Shen, P.; Zhang, Y.; Lin, L.; Zhao, M. Self-assembled soy protein nanoparticles by partial enzymatic hydrolysis for pH-Driven Encapsulation and Delivery of Hydrophobic Cargo Curcumin. Food Hydrocoll. 2021, 120, 106759. [Google Scholar] [CrossRef]
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.; Lin, K.; Sun, C.; Dai, L.; Yang, S.; Mao, L.; Yuan, F.; Gao, Y. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Q.; McClements, D.J.; Fu, Y.; Xie, H.; Li, T.; Chen, G. Curcumin-loaded core-shell biopolymer nanoparticles produced by the pH-driven method: Physicochemical and release properties. Food Chem. 2021, 355, 129686. [Google Scholar] [CrossRef]
- Xuemei, Z.; Ma, X.-Y.; Gong, W.; Li, X.; Huang, H.-B.; Zhu, X.-M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Walia, N.; Chen, L. Pea protein based vitamin D nanoemulsions: Fabrication, stability and in vitro study using Caco-2 cells. Food Chem. 2020, 305, 125475. [Google Scholar] [CrossRef]
- Almajwal, A.M.; Abulmeaty, M.M.A.; Feng, H.; Alruwaili, N.W.; Dominguez-Uscanga, A.; Andrade, J.E.; Razak, S.; Elsadek, M.F. Stabilization of vitamin d in pea protein isolate nanoemulsions increases its bioefficacy in rats. Nutrients 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Lu, K.; Zhou, S.; Qi, B.; Li, Y. Co-delivery of insulin and quercetin in W/O/W double emulsions stabilized by different hydrophilic emulsifiers. Food Chem. 2022, 369, 130918. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Rao, J.; Chen, B. Plant-based food hydrogels: Constitutive characteristics, formation, and modulation. Curr. Opin. Colloid Interface Sci. 2021, 56, 101505. [Google Scholar] [CrossRef]
- Aliabbasi, N.; Emam-Djomeh, Z.; Askari, G.; Salami, M. Pinto bean protein-based acid-induced cold-set gels as carriers for curcumin delivery: Fabrication and characterization. Food Hydrocoll. Heal. 2021, 1, 100035. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Cazado, C.P.; Cavini, A.C.M.; Santos, L.M.; Moraes, I.C.; Pinho, S.C. Cold-set NaCl-induced gels of soy protein isolate and locust bean gum: How the ageing process affect their microstructure and the stability of incorporated beta-carotene. LWT 2022, 154, 112677. [Google Scholar] [CrossRef]
- Geng, M.; Wang, Z.; Qin, L.; Taha, A.; Du, L.; Xu, X.; Pan, S.; Hu, H. Effect of ultrasound and coagulant types on properties of β-carotene bulk emulsion gels stabilized by soy protein. Food Hydrocoll. 2022, 123, 107146. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Gong, Y.; Li, Z.; Guo, Y.; Yu, D.; Pan, M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochemistry 2021, 79, 105756. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Assad, I.; Bhat, S.U.; Gani, A.; Shah, A. Protein based packaging of plant origin: Fabrication, properties, recent advances and future perspectives. Int. J. Biol. Macromol. 2020, 164, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Luciano, C.G.; Pérez-Córdoba, L.J.; Monteiro, M.L.; Conte-Junior, C.A.; Sobral, P.J.D.A. Advances in biopolymeric active films incorporated with emulsified lipophilic compounds: A review. RSC Adv. 2021, 11, 28148–28168. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef]
- Paglione, I.D.S.; Galindo, M.V.; de Medeiros, J.A.S.; Yamashita, F.; Alvim, I.D.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Characterization and in vitro antimicrobial study of soy protein isolate films incorporating carvacrol. Food Hydrocoll. 2022, 122, 107091. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite- clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated cellulose addition improved the physicochemical and bioactive properties of biodegradable films based on soy protein and clove essential oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Turan, S.; Capanoglu, E. Interaction of lentil protein and onion skin phenolics: Effects on functional properties of proteins and in vitro gastrointestinal digestibility. Food Chem. 2022, 372, 130892. [Google Scholar] [CrossRef]
- Cui, Z.; Kong, X.; Chen, Y.; Zhang, C.; Hua, Y. Effects of rutin incorporation on the physical and oxidative stability of soy protein-stabilized emulsions. Food Hydrocoll. 2014, 41, 1–9. [Google Scholar] [CrossRef]
- Benjakul, S.; Singh, A.; Chotphruethipong, L.; Mittal, A. Protein-polyphenol conjugates: Preparation, functional properties, bioactivities and applications in foods and nutraceuticals. In Advances in Food and Nutrition Research; Academic Press: London, UK, 2021. [Google Scholar]
- Quan, T.H.; Benjakul, S.; Sae-Leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef]
- Ye, J.; Deng, L.; Wang, Y.; McClements, D.J.; Luo, S.; Liu, C. Impact of rutin on the foaming properties of soybean protein: Formation and characterization of flavonoid-protein complexes. Food Chem. 2021, 362, 130238. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sun, Y.; Yang, Y.; Zhou, X.; Cen, K.; Yu, C.; Xu, T.; Tang, X. Zein nanoparticle stabilized Pickering emulsion enriched with cinnamon oil and its effects on pound cakes. LWT 2020, 122, 109025. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.-M.; Wang, L.-Y.; Yuan, Y.; Yang, X.-Q. Complexation of resveratrol with soy protein and its improvement on oxidative stability of corn oil/water emulsions. Food Chem. 2014, 161, 324–331. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Zhang, Y.; Gao, Y.; Mao, L. Facile synthesis of zein-based emulsion gels with adjustable texture, rheology and stability by adding β-carotene in different phases. Food Hydrocoll. 2021, 124, 107178. [Google Scholar] [CrossRef]
- Gomes, A.; Furtado, G.D.F.; Cunha, R.L. Bioaccessibility of Lipophilic Compounds Vehiculated in Emulsions: Choice of Lipids and Emulsifiers. J. Agric. Food Chem. 2019, 67, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Cardoso, D.D.; Náthia-Neves, G.; Meireles, M.A.A.; Cunha, R.L. Interactions of β-carotene with WPI/Tween 80 mixture and oil phase: Effect on the behavior of O/W emulsions during in vitro digestion. Food Chem. 2021, 341, 128155. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Sun-Waterhouse, D.; Jiang, Y.; Li, F.; Waterhouse, G.I.; Li, D. Phenolic-protein interactions in foods and post ingestion: Switches empowering health outcomes. Trends Food Sci. Technol. 2021, 118, 71–86. [Google Scholar] [CrossRef]
- Marze, S. Bioaccessibility of lipophilic micro-constituents from a lipid emulsion. Food Funct. 2015, 6, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) leaf as a new source to obtain protein hydrolysates: Physicochemical characterization, techno-functional properties and antioxidant capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Özdemir, E.E.; Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Physicochemical, functional and emulsifying properties of plant protein powder from industrial sesame processing waste as affected by spray and freeze drying. LWT 2022, 154, 112646. [Google Scholar] [CrossRef]
| Modified Characteristics | Protein Type | Modification Approach | Mechanism | Reference |
|---|---|---|---|---|
| Increased solubility and water hydration ability | Pea protein | Microwave heating-assisted glycation—Maillard reaction (glucose, fructose, and allulose) | Heat and covalent bond between protein and polysaccharides | [82] |
| Reduced particle size, increased solubility, and improved thermal stability and gelling ability | Oat protein | Ultrasound treatment | Cavitation and heat | [83] |
| Improved water binding capacity, oil holding capacity, and emulsifying properties | Rice bran protein | Radio frequency treatment | Heat | [84] |
| Better water binding capacity and oil holding capacity, emulsifying and gelation properties | Pea protein | Acylation (acetylation and succinylation) and/or conjugation (guar gum) | Introduction of an acyl group to the protein and covalent bond between protein and polysaccharide | [85] |
| Improved gelling capacity | Soy proteins | Conventional heat treatment or ultrasound treatment | Cavitation and heat | [86] |
| Improved gelling capacity | Pea protein | pH-shifting method | pH (alkaline treatment) | [87] |
| Enhanced thermal stability of protein, strengthened formation of gels, and improved textural properties (hardness, gumminess, and chewiness) | Sweet potato protein | High hydrostatic pressure and/or microbial transglutaminase | Pressure and covalent crosslinking (ε-(γ-glutaminyl) lysine isopeptide bonds) | [88] |
| Better water absorption capacity, water solubility, thermal stability, gel strength, gelation capacity, and in-vitro digestibility | Soy protein | Ultrasound treatment | Cavitation and heat | [89] |
| Improved emulsifying properties | Kidney bean protein | Conventional heat treatment/high-pressure homogenization | Heat and pressure | [90] |
| Enhanced emulsifying activity | Soy protein | Heat treatment and/or glutaminase deamidation | Heat and transformation of amide groups of glutamine and asparagine residues into carboxyl groups | [91] |
| Increased solubility and improved emulsifying and foaming capacity | Zein | Hydrolysis-glycation by transglutaminase (chitosan) | Breakdown into smaller peptides enzymatically and covalent bond between protein and polysaccharides | [92] |
| Improved foaming and emulsifying properties | Rice glutelin protein | Amyloid fibrilization | Heat, acidic condition, hydrolysis, and aggregation | [93] |
| Improved emulsifying activity and in-vitro antioxidant activity | Oat protein | Enzymatic hydrolysis | Breakdown into smaller peptides enzymatically | [94] |
| Improved emulsifying and foaming properties, solubility in water, andreduced allergenicity | Soy protein | Cold atmospheric plasma | Temperature and pressure (combination of thermal, mechanical, nuclear, and electrical energy sources) | [95] |
| Enhanced antioxidant activity | Rice protein | Hydrolysis-assisted electron beam irradiation | Breakdown into smaller peptides enzymatically and ionizing irradiation | [96] |
| Reduced off-flavors andimproved taste | Pea protein | Fermentation | Reduction of pH (lactic acid) and removal of aldehyde and ketone with low alcohol production | [97] |
| Decreased bitterness and improved aroma impression | Lupin protein | Fermentation | Degradation of glucose and production of lactic acid (pH acidification) | [98] |
| Improved solubility | Pea protein | Protein–protein blend/low-temperature homogenization | Cold temperatures and sodium citrate addition (disruption of the micelle structure of casein), hydrophobic interactions (casein core and hydrophobic amino acids of pea proteins), and pressure | [99] |
| Carrier Material | Lipophilic Bioactive Compound | Colloidal Carriers | Encapsulation Efficiency | Bioaccessibility/ Bioavailability | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Walnut protein | Curcumin | Complexes | 60.7% | - | Encapsulation in walnut proteins improved water solubility of curcumin and promoted its sustained release under simulated gastrointestinal conditions. | [144] |
| Pea protein | Curcumin | Pea protein-curcumin complex | 98.6% | 72% | Complexed curcumin showed improved water solubility (1.02 mg/g), bioavailability, and storage stability under physiological conditions compared to the free curcumin. | [145] |
| Pea protein | Resveratrol | Nanoparticles Nanocomplexes | 74.08% | - | Water solubility, chemical stability, and antioxidant capacity of resveratrol were improved in pea protein nanoparticles and nano complexes compared to free resveratrol. | [146] |
| Zein-chitosan | Resveratrol | Particles | 91% | 47% | Encapsulation of resveratrol in zein-chitosan particles decreased ABTS but increased the DPPH scavenging capacity. The chitosan coating improved the storage stability of resveratrol and allowed its sustained in-vitro release. | [147] |
| Soy protein -cellulose nanocrystal | Curcumin | Nanoparticles | 88.3% | 81% | Nanoparticles produced with soy protein isolate: cellulose ratio of 6:1 showed good stability under a wide range of salt, heat, and pH conditions. The soy protein-cellulose nanocrystal composite nanoparticles reduced the release of curcumin in the stomach and allowed its highly controlled release in the intestine. | [148] |
| Oat protein-Pleurotus ostreatus β-glucan | β-carotene | Emulsion stabilized by Maillard conjugates | - | 16–36% (bioaccessibility) 2–11% (bioavailability) | Oat protein isolate—Pleurotus ostreatus β-glucan conjugates protected emulsion against environmental stresses, facilitated its complete lipidic digestion, and improved the bioaccessibility and oral bioavailability of β-carotene. | [149] |
| Pea protein/curcumin/pectin | β-carotene | Emulsion stabilized by curcumin-protein-polysaccharide complex | 76.15% | - | The pea protein/curcumin/pectin complex improved the physical stability of emulsions and chemical stability of β-carotene when exposed to UV light (76.15%, 8 h) and/or heat treatment at 25 (91.50%) and 50 °C (74.35%) for 30 days. | [150] |
| Soy protein-alginate | Lycopene | Emulsion gel beads | 97% | 0–12% | Emulsion gel beads at pH 3.0 showed lower mechanical strength, higher storage stability, and higher bioaccessibility of lycopene than those produced at pH 7.0 and 5.0. | [151] |
| Zein | Curcumin/ β-carotene | Pickering emulsions stabilized by curcumin-zein nanoparticles | 47–96% (curcumin) 73–97% (β-carotene) | 5–75% (curcumin) 13–28% (β-carotene) | Co-encapsulation improved the chemical stability of β-carotene and curcumin synergistically. The higher particle concentration and heating temperature retarded the free fat acid release, with lower bioaccessibility of nutraceuticals. Conversely, the lower pressure (≤100 MPa) promoted lipolysis and enhanced the bioaccessibility of nutraceuticals. | [152] |
| Zein-pectin | Cinnamon essential oil | Pickering emulsion stabilized by zein-pectin composite nanoparticles | - | - | Zein-pectin-based Pickering emulsions showed good dispersibility and sustained-release ability, the cinnamon essential oil improving its antibacterial performance compared to pure essential oil. | [153] |
| Zein-pectin | Peppermint oil and resveratrol | Emulsion stabilized by resveratrol-loaded zein-pectin complex particles | 88% (peppermint oil) 99% (resveratrol) | - | Emulsions stabilized by resveratrol-loaded zein-pectin complex particles showed improved antimicrobial activity, physical and chemical stability, and prolonged antimicrobial efficiency of peppermint oil and resveratrol against Staphylococcus aureus and Salmonella Typhimurium. | [109] |
| Soy protein-wheat bran arabinoxylan | β-carotene | Emulsion-filled gels | - | 76% | The soy protein (SPI)-wheat bran arabinoxylan (WBAX) emulsion-filled gels showed superior strengths and stabilities to those of the SPI-WBAX hydrogels and the WBAX or SPI emulsion-filled gels. The SPI-WBAX emulsion-filled gels improved the sustained release of β-carotene during digestion compared to the WBAX emulsion and SPI emulsion-filled gels. | [154] |
| Soy protein | Vitamin D3 | Emulsion-filled gels | 103–152 μg of vitamin D3/g gel | - | The application of mechanical stirring (800 rpm; 10–30 min) increased the solubility and decreased the particle size of soy protein (11–15%), affecting the microstructure and rheological properties of the heat-set gels. The gels of soy protein filled with Brazil nut oil emulsion were effective in protecting vitamin D3, presenting good retention over 30 days of storage (around 75% for gel produced with 15% of protein pretreated at 800 rpm for 30 min). | [155] |
| Wheat gliadin | Quercetin | W/O/W emulsion-filled gels | 97.2% | - | The emulsion gels improved the quercetin solubility under simulated gastrointestinal conditions, which led to a four-fold increase in their effective bioaccessibility. | [156] |
| Zein-chitosan | Cinnamal-dehyde | Chitosan/zein-cinnamaldehyde nano-cellulose composite film | - | - | The addition of cinnamaldehyde increased water resistance of the film and contributed to a more flexible and dense film structure. Furthermore, coating with the chitosan/zein-cinnamaldehyde nano-cellulose composite film delayed yellowing and maintained the quality of mango during storage at ambient temperature, and the respiration rate and weight loss of mangoes were significantly inhibited. | [157] |
| Zein-chitosan | Oregano essential oil | Emulsion-based active films | - | - | The control film, composed of zein chitosan and oregano essential oil, presented good antimicrobial and antioxidant activity. However, the addition of phenolics (tea polyphenols, propolis flavones or grape proanthocyanidins) increased inhibition zone for E. coli and Bacillus subtilis, and these films had considerable potential for extending the shelf-life of fresh pork by delaying spoilage. | [158] |
| Soy protein- cellulose nanocrystals | Curcumin | pH-responsive films | - | - | The nanocomposite films were responsible for delaying the release of curcumin from the film matrix. The film composed of cellulose nanocrystals and curcumin nanocapsules displayed higher antiradical scavenging activity than that with free curcumin. Moreover, cellulose nanocrystals/curcumin nanocapsules film decreased the total volatile basic nitrogen of stored shrimp and visually monitored shrimp freshness in real-time. | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.; Sobral, P.J.d.A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2022, 27, 60. https://doi.org/10.3390/molecules27010060
Gomes A, Sobral PJdA. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules. 2022; 27(1):60. https://doi.org/10.3390/molecules27010060
Chicago/Turabian StyleGomes, Andresa, and Paulo José do Amaral Sobral. 2022. "Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds" Molecules 27, no. 1: 60. https://doi.org/10.3390/molecules27010060
APA StyleGomes, A., & Sobral, P. J. d. A. (2022). Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules, 27(1), 60. https://doi.org/10.3390/molecules27010060







