Plant-Based Colloidal Delivery Systems for Bioactives
Abstract
:1. Introduction
2. Plant-Based Components
2.1. Lipids
2.2. Proteins
2.3. Polysaccharides
2.4. Other Plant-Based Ingredients
3. Ingredient Functionality
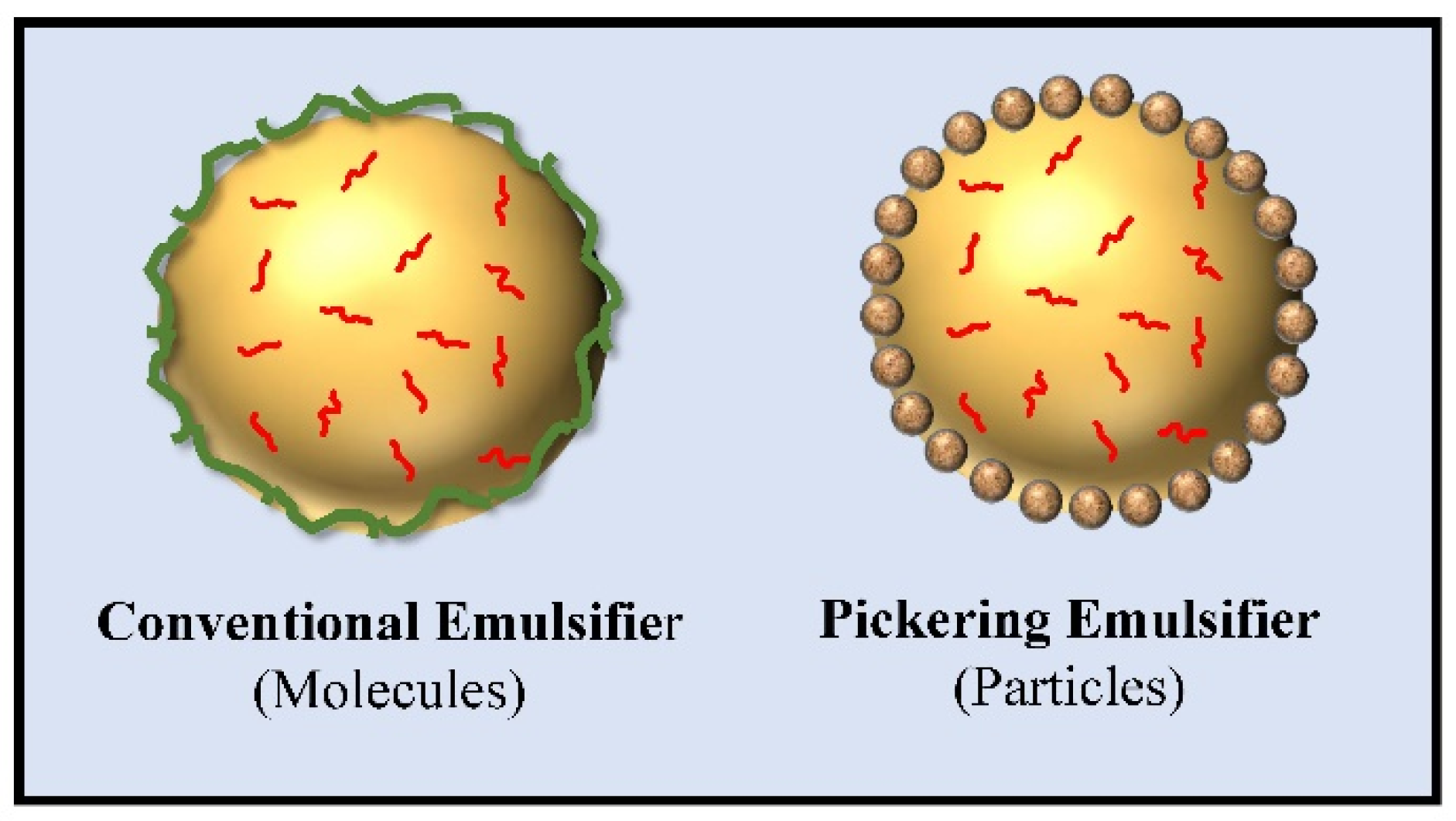
3.1. Emulsifying
3.2. Gelling
3.3. Structure Forming
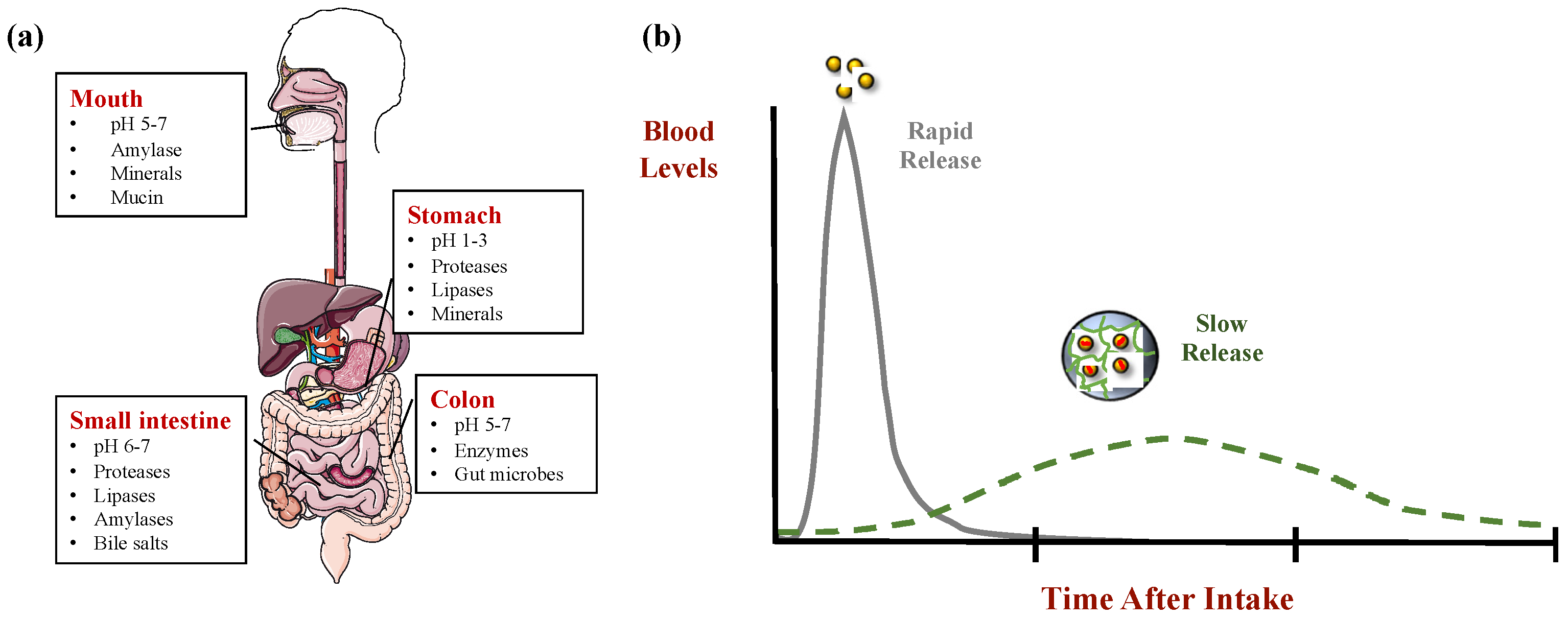
4. Delivery System Types
4.1. Biopolymer Nanoparticles
4.2. Microgels
4.3. Nanoemulsions
4.4. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
4.5. Nanoliposomes
5. Applications
5.1. Oil-Soluble Vitamins
5.2. Omega-3 Oils
5.3. Nutraceuticals
5.3.1. Carotenoids
5.3.2. Curcuminoids
5.3.3. Polyphenols
6. Potential Toxicity
7. Conclusions and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- GFI. State of the Industry Report: Plant-Based Meat, Eggs, and Dairy; Good Food Institute: Washington, DC, USA, 2021; pp. 1–85. [Google Scholar]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Baur, A.C.; Brandsch, C.; Konig, B.; Hirche, F.; Stangl, G.I. Plant Oils as Potential Sources of Vitamin D. Front. Nutr. 2016, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Kadhum, A.A.; Shamma, M.N. Edible lipids modification processes: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 48–58. [Google Scholar] [CrossRef]
- Yilmaz, E.; Keskin Uslu, E.; Öz, C. Oleogels of Some Plant Waxes: Characterization and Comparison with Sunflower Wax Oleogel. J. Am. Oil Chem. Soc. 2021, 98, 643–655. [Google Scholar] [CrossRef]
- Sandoval, J.M.; Carelli, A.; Palla, C.; Baumler, E. Preparation and characterization of oleogel emulsions: A comparative study between the use of recovered and commercial sunflower waxes as structuring agent. J. Food Sci. 2020, 85, 2866–2878. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Oleogel-based emulsions: Concepts, structuring agents, and applications in food. J. Food Sci. 2021, 86, 2785–2801. [Google Scholar] [CrossRef]
- Gunstone, F.D. Phospholipid Technology and Applications; The Oily Press: Dundee, UK, 2008. [Google Scholar]
- Toro-Uribe, S.; Ibanez, E.; Decker, E.A.; McClements, D.J.; Zhang, R.; Lopez-Giraldo, L.J.; Herrero, M. Design, Fabrication, Characterization, and In Vitro Digestion of Alkaloid-, Catechin-, and Cocoa Extract-Loaded Liposomes. J. Agric. Food Chem. 2018, 66, 12051–12065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja Saponin Characteristics and Functional Properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef]
- Matsumiya, K.; Murray, B.S. Soybean protein isolate gel particles as foaming and emulsifying agents. Food Hydrocoll. 2016, 60, 206–215. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Popović, L.; Petrović, L.; Milinković, J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016, 60, 271–278. [Google Scholar] [CrossRef]
- Han, M.M.; Yi, Y.; Wang, H.X.; Huang, F. Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities. Molecules 2017, 22, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, D.M.; Babiker, E.E.; Eltinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Sarkar, A.; Kamaruddin, H.; Bentley, A.; Wang, S. Emulsion stabilization by tomato seed protein isolate: Influence of pH, ionic strength and thermal treatment. Food Hydrocoll. 2016, 57, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Deng, X.; Liu, T.; Yang, B.; Zhao, M.; Zhao, Q. Influence of NaCl on the oil/water interfacial and emulsifying properties of walnut protein-xanthan gum. Food Hydrocoll. 2017, 72, 73–80. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Tdrens Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Funami, T. In vivo and rheological approaches for characterizing food oral processing and usefulness of polysaccharides as texture modifiers—A review. Food Hydrocoll. 2017, 68, 2–14. [Google Scholar] [CrossRef]
- Grundy, M.M.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Yang, X.; Gao, S.; Yao, J.; McClements, D.J. Influence of Hydrocolloids (Dietary Fibers) on Lipid Digestion of Protein-Stabilized Emulsions: Comparison of Neutral, Anionic, and Cationic Polysaccharides. J. Food Sci. 2016, 81, C1636–C1645. [Google Scholar] [CrossRef]
- Qi, X.; Al-Ghazzewi, F.H.; Tester, R.F. Dietary Fiber, Gastric Emptying, and Carbohydrate Digestion: A Mini-Review. Starch Stärke 2018, 70, 1700346. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Caston, M.J.; Catasta, G.; Toti, E.; Cambrodon, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S194–S218. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.X.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [Green Version]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease: How does the evidence stack up? Trends Food Sci. Technol. 2017, 69, 192–202. [Google Scholar] [CrossRef]
- Song, T.; Xiong, Z.; Shi, T.; Yuan, L.; Gao, R. Effect of glutamic acid on the preparation and characterization of Pickering emulsions stabilized by zein. Food Chem. 2022, 366, 130598. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.-Q.; Chen, X.-W.; Deng, Z.-Y.; Yang, X.-Q. Whole cereal protein-based Pickering emulsions prepared by zein-gliadin complex particles. J. Cereal Sci. 2019, 87, 46–51. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy protein nanoparticle aggregates as pickering stabilizers for oil-in-water emulsions. J. Agric. Food Chem. 2013, 61, 8888–8898. [Google Scholar] [CrossRef] [PubMed]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Porfiri, M.C.; Vaccaro, J.; Stortz, C.A.; Navarro, D.A.; Wagner, J.R.; Cabezas, D.M. Insoluble soybean polysaccharides: Obtaining and evaluation of their O/W emulsifying properties. Food Hydrocoll. 2017, 73, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Mechanisms involved in the formation and structure of soya protein cold-set gels: A molecular and supramolecular investigation. Food Hydrocoll. 2008, 22, 550–559. [Google Scholar] [CrossRef]
- Wang, X.; Luo, K.; Liu, S.; Zeng, M.; Adhikari, B.; He, Z.; Chen, J. Textural and Rheological Properties of Soy Protein Isolate Tofu-Type Emulsion Gels: Influence of Soybean Variety and Coagulant Type. Food Biophys. 2018, 13, 324–332. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; de Jong, G.A. Characterization of heat-set gels from RuBisCO in comparison to those from other proteins. J. Agric. Food Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.M.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of gels made with different commercial pea protein isolate: Rheological, structural and functional properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Simsek, S.; Chen, B.; Rao, J. Alginate-based double-network hydrogel improves the viability of encapsulated probiotics during simulated sequential gastrointestinal digestion: Effect of biopolymer type and concentrations. Int. J. Biol. Macromol. 2020, 165, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta, M.; Gil-Izquierdo, A.; Garcia-Viguera, C.; Dominguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Teng, Z.; Wang, Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Zhang, L.; Dai, L.; Yang, S.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Influence of calcium ions on the stability, microstructure and in vitro digestion fate of zein-propylene glycol alginate-tea saponin ternary complex particles for the delivery of resveratrol. Food Hydrocoll. 2020, 106, 105886. [Google Scholar] [CrossRef]
- Zeeb, B.; Mi-Yeon, L.; Gibis, M.; Weiss, J. Growth phenomena in biopolymer complexes composed of heated WPI and pectin. Food Hydrocoll. 2018, 74, 53–61. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Food-grade nanoparticles for encapsulation, protection and delivery of curcumin: Comparison of lipid, protein, and phospholipid nanoparticles under simulated gastrointestinal conditions. RSC Adv. 2016, 6, 3126–3136. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid. Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Liang, L.; Zhang, Z.; Deng, Z.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocoll. 2017, 63, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Soukoulis, C.; Cambier, S.; Hoffmann, L.; Bohn, T. Chemical stability and bioaccessibility of β-carotene encapsulated in sodium alginate o/w emulsions: Impact of Ca2+ mediated gelation. Food Hydrocoll. 2016, 57, 301–310. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of delivery system type on curcumin stability: Comparison of curcumin degradation in aqueous solutions, emulsions, and hydrogel beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Chen, F.; Deng, Z.; Zhang, Z.; Zhang, R.; Xu, Q.; Fan, G.; Luo, T.; McClements, D.J. Controlling lipid digestion profiles using mixtures of different types of microgel: Alginate beads and carrageenan beads. J. Food Eng. 2018, 238, 156–163. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.M.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Du, Y.M.; Xiao, H.; McClements, D.J. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocoll. 2011, 25, 122–130. [Google Scholar] [CrossRef]
- van Leusden, P.; den Hartog, G.J.M.; Bast, A.; Postema, M.; van der Linden, E.; Sagis, L.M.C. Lipase diffusion in oil-filled, alginate micro- and macrobeads. Food Hydrocoll. 2018, 85, 242–247. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, R.J.; Chen, L.; Tong, Q.Y.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ansari, V.A.; Ahmad, U.; Akhtar, J.; Jahan, A. Nanoemulsion: An advanced vehicle for efficient drug delivery. Drug Res. 2017, 67, 617–631. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: Lentil, pea, and faba bean proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gan, J.; Li, Y.; Nirasawa, S.; Cheng, Y. Conformation and emulsifying properties of deamidated wheat gluten-maltodextrin/citrus pectin conjugates and their abilities to stabilize β-carotene emulsions. Food Hydrocoll. 2019, 87, 129–141. [Google Scholar] [CrossRef]
- Shrestha, S.; Sadiq, M.B.; Anal, A.K. Culled banana resistant starch-soy protein isolate conjugate based emulsion enriched with astaxanthin to enhance its stability. Int. J. Biol. Macromol. 2018, 120, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- O’Sullivan, J.J.; Kurukji, D.; Norton, I.T.; Spyropoulos, F. Investigation of the fabrication and subsequent emulsifying capacity of potato protein isolate/κ-carrageenan electrostatic complexes. Food Hydrocoll. 2017, 71, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and Technofunctional Properties of Protein-Polysaccharide Complexes: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant. Food Hydrocoll. 2020, 101, 105447. [Google Scholar] [CrossRef]
- Yang, J.; Mao, L.; Yang, W.; Sun, C.; Dai, L.; Gao, Y. Evaluation of non-covalent ternary aggregates of lactoferrin, high methylated pectin, EGCG in stabilizing beta-carotene emulsions. Food Chem. 2018, 240, 1063–1071. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Zhang, X.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Comparative study on lipid digestion and carotenoid bioaccessibility of emulsions, nanoemulsions and vegetable-based in situ emulsions. Food Hydrocoll. 2019, 87, 119–128. [Google Scholar] [CrossRef]
- Scheuble, N.; Schaffner, J.; Schumacher, M.; Windhab, E.J.; Liu, D.; Parker, H.; Steingoetter, A.; Fischer, P. Tailoring Emulsions for Controlled Lipid Release: Establishing in vitro-in Vivo Correlation for Digestion of Lipids. ACS Appl. Mater. Interfaces 2018, 10, 17571–17581. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Xu, H.N.; Liu, Y.; Zhang, L. Salting-out and salting-in: Competitive effects of salt on the aggregation behavior of soy protein particles and their emulsifying properties. Soft Matter 2015, 11, 5926–5932. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Carrillo, C.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancing nutraceutical bioavailability from raw and cooked vegetables using excipient emulsions: Influence of lipid type on carotenoid bioaccessibility from carrots. J. Agric. Food Chem. 2015, 63, 10508–10517. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified alpha-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Marquis, M.; Anton, M.; Marze, S. Studying the real-time interplay between triglyceride digestion and lipophilic micronutrient bioaccessibility using droplet microfluidics. 2 application to various oils and (pro)vitamins. Food Chem. 2019, 275, 661–667. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef]
- Gao, S.; McClements, D.J. Formation and stability of solid lipid nanoparticles fabricated using phase inversion temperature method. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing beta-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Guo, Q.; Bellissimo, N.; Rousseau, D. The Physical State of Emulsified Edible Oil Modulates Its in Vitro Digestion. J. Agric. Food Chem. 2017, 65, 9120–9127. [Google Scholar] [CrossRef]
- Jannin, V.; Dellera, E.; Chevrier, S.; Chavant, Y.; Voutsinas, C.; Bonferoni, C.; Demarne, F. In vitro lipolysis tests on lipid nanoparticles: Comparison between lipase/co-lipase and pancreatic extract. Drug Dev. Ind. Pharm. 2015, 41, 1582–1588. [Google Scholar] [CrossRef]
- Chen, F.; Fan, G.Q.; Zhang, Z.; Zhang, R.; Deng, Z.Y.; McClements, D.J. Encapsulation of omega-3 fatty acids in nanoemulsions and microgels: Impact of delivery system type and protein addition on gastrointestinal fate. Food Res. Int. 2017, 100, 387–395. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Soler, C.; Lopez-Rubio, A. Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll. 2016, 61, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Zhou, H.; Zhang, Z.; McClements, D.J. Bioaccessibility of oil-soluble vitamins (A, D, E) in plant-based emulsions: Impact of oil droplet size. Food Func. 2021, 12, 3883–3897. [Google Scholar] [CrossRef]
- Sun, M.; Li, X.; McClements, D.J.; Xiao, M.; Chen, H.; Zhou, Q.; Xu, S.; Chen, Y.; Deng, Q. Reducing off-flavors in plant-based omega-3 oil emulsions using interfacial engineering: Coating algae oil droplets with pea protein/flaxseed gum. Food Hydrocol. 2022, 122, 107069. [Google Scholar] [CrossRef]
- Li, R.; Tan, Y.; Dai, T.; Zhang, R.; Fu, G.; Wan, Y.; Liu, C.; McClements, D.J. Bioaccessibility and stability of β-carotene encapsulated in plant-based emulsions: Impact of emulsifier type and tannic acid. Food Funct. 2019, 10, 7239–7252. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Muriel Mundo, J.; McClements, D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): Emulsifier type. Food Res. Int. 2020, 137, 109739. [Google Scholar] [CrossRef]
- Liu, X.; Bi, J.; Xiao, H.; McClements, D.J. Enhancement of Nutraceutical Bioavailability using Excipient Nanoemulsions: Role of Lipid Digestion Products on Bioaccessibility of Carotenoids and Phenolics from Mangoes. J. Food Sci. 2016, 81, N754–N761. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Kadappan, A.S.; Guo, C.; Gumus, C.E.; Bessey, A.; Wood, R.J.; McClements, D.J.; Liu, Z. The efficacy of nanoemulsion-based delivery to improve vitamin D absorption: Comparison of in vitro and in vivo studies. Mol. Nutr. Food Res. 2018, 62, 1700836. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, B.; McClements, D.J. Encapsulation of lipophilic polyphenols in plant-based nanoemulsions: Impact of carrier oil on lipid digestion and curcumin, resveratrol and quercetin bioaccessibility. Food Funct. 2021, 12, 3420–3432. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Fletcher, R.H. Vitamins for chronic disease prevention in adults: Scientific review. JAMA 2002, 287, 3116–3126. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Fontan, L.; Moench-Pfanner, R.; Laillou, A.; Berger, J.; Renaud, C.; Avallone, S. Influence of light exposure and oxidative status on the stability of vitamins A and D(3) during the storage of fortified soybean oil. Food Chem. 2015, 184, 90–98. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Improving the bioavailability of oil-soluble vitamins by optimizing food matrix effects: A review. Food Chem. 2021, 348, 129148. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J.; Decker, E. Interfacial Antioxidants: A Review of Natural and Synthetic Emulsifiers and Coemulsifiers That Can Inhibit Lipid Oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Rahiminezhad, Z.; Hashemi Gahruie, H.; Esteghlal, S.; Mesbahi, G.R.; Golmakani, M.-T.; Hosseini, S.M.H. Oxidative stability of linseed oil nano-emulsions filled in calcium alginate hydrogels. Lwt 2020, 127, 109392. [Google Scholar] [CrossRef]
- Hu, M.; Xie, F.; Zhang, S.; Qi, B.; Li, Y. Effect of nanoemulsion particle size on the bioavailability and bioactivity of perilla oil in rats. J. Food Sci. 2021, 86, 206–214. [Google Scholar] [CrossRef]
- Inapurapu, S.P.; Ibrahim, A.; Kona, S.R.; Pawar, S.C.; Bodiga, S.; Bodiga, V.L. Development and characterization of ω-3 fatty acid nanoemulsions with improved physicochemical stability and bioaccessibility. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125515. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Chacon-Ordonez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of carotenoids from plant and animal foods. J. Sci. Food Agric. 2019, 99, 3220–3239. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Liu, J.; Xiao, H.; McClements, D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): Oil droplet size. Food Funct. 2020, 11, 9936–9946. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancement of carotenoid bioaccessibility from carrots using excipient emulsions: Influence of particle size of digestible lipid droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional Curcumin Mediate Multitherapeutic Effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhou, H.; Mundo, J.L.M.; McClements, D.J. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J. Food Eng. 2019, 254, 1–9. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of β-carotene emulsions. Food Hydrocoll. 2016, 61, 578–588. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Npj Sci. Food 2017, 1, 1–13. [Google Scholar] [CrossRef]


| Delivery System Type | Size | Charge | Composition | Loading Properties | Stability | Digestion Properties | References |
|---|---|---|---|---|---|---|---|
| Nanoparticle, nanoliposome, nanoemulsion | Nanoparticle and nanoliposome: 99 nm; nanoemulsion: 168 nm | Nanoparticle: 20 mV; nanoliposome: −5.2 mV; nanoemulsion: −6.5 mV | Curcumin; zein nanoparticle; phospholipid nanoliposome; Tween 20 stabilized corn oil nanoemulsion | Loading capacity: nanoparticle: 12%; nanoliposome: 3%; nanoemulsion 0.4% | Chemical stability of curcumin in GIT: nanoemulsion ≈ nanoparticle > nanoliposome | Curcumin bioaccessibility: nanoemulsion (92%) > nanoliposome (74%) > nanoparticle (52%) | [51] |
| Nanoparticle | 86–200 nm | −27 to −11 mV as increasing calcium level | Vitamin D; zein nanoparticles with different levels of carboxymethyl chitosan and calcium | Loading capacity decreased from 3.9 to 1.7%; encapsulation efficiency increased from 52% to 88% as increasing chitosan or calcium level. | Photochemical stability increased from ~70% to 80% with the chitosan coating, compared to 30% of free vitamin D. | Vitamin D release in the GIT decreased with the chitosan coating (~55%) compared to ~100% for zein nanoparticles. | [47] |
| Nanoparticle | 267–728 nm as increasing calcium level | −23 to −14 mV as increasing calcium level | Resveratrol; zein-propylene glycol alginate-tea saponin complex at different calcium level | Encapsulation efficiency increased with tea saponin (58% to 77%), irrespective of calcium level. Loading capacity was the same for all treatments. | Tea saponin increased photo and thermal stability of resveratrol; calcium increased the environmental stability (thermal, pH, ionic) of the particle. | Calcium reduced the release of resveratrol in the stomach but promoted the release in the small intestinal phase. | [49] |
| Solid lipid nanoparticle (SLN), nanoemulsion | Corn oil: 0.5 μm; cocoa butter: 0.9 μm | Corn oil: −29 mV; cocoa butter: −42 mV | β-Carotene; cocoa butter, cocoa oil, corn oil, Tween 80 | - | β-Carotene stability decreased in cocoa oil with a compact crystalized lipid core and in small oil droplet size. | Triglycerides (TAG) disappearance: 90% for both; bioaccessibility: 64% for corn oil, 82% for cocona butter. | [87] |
| Nanostructured lipid carrier (NLC) | 94 nm | −24 mV | Astaxanthin; oil phase: glyceryl behenate, oleic acid, lecithin, α-tocopherol; aqueous phase: Tween 80 and EDTA | - | Carbonation and thermal pasteurization increased particle size and astaxanthin loss. | - | [85] |
| SLN, NLC | <200 nm | −40 to −20 mV | Curcumin; oil phase: glyceryl behenate (SLN) or mixed with oleic acid oil (NLC); aqueous phase: casein, Tween 80, pectin | Tween 80, oil blending, and pectin coating improved loading capacity and encapsulation efficiency (~35% to 66%). | The size of SLN was more stable than NLC during storage. | SLN showed less curcumin release than NLC. | [86] |
| Microgel | Alginate: 626 μm; carrageenan: 849 μm; mixture: 763 μm | Alginate: −24 mV; carrageenan: −16 mV; mixture: −21 mV | Corn oil droplets stabilized by Tween 80; gelling structure: alginate, carrageenan, and mixture | - | Alginate beads were intact, while carrageenan beads were deformed and dissociated in the GIT. | The free fatty acid (FFA) release rate decreased as carrageenan (~80%), mixture, alginate (~50%). | [57] |
| Emulsion, microgel bead | Emulsion: 0.27 μm; alginate bead: ~400 μm; chitosan bead: ~250 μm | Emulsion: −20 mV; alginate beads: −17 mV; chitosan beads: −23 mV at pH 7 | Curcumin; emulsion: corn oil droplets stabilized by Tween 80; microgel beads: alginate vs. chitosan | High encapsulation of the lipid droplets in both alginate and chitosan beads. | At pH 7, curcumin degradation under 55 °C increased as: chitosan < emulsion < solution < alginate; at pH 3, the order was: emulsion < solution < chitosan < alginate. | - | [56] |
| Nanoemulsion vs. microgel | Nanoemulsion: 0.2 μm; microgel: ~500 μm | Nanoemulsion: −74 mV; microgel: −46 mV | Flaxseed oil nanoemulsion stabilized by quillaja saponin; alginate microgel bead; casein as an antioxidant | - | Particle size was the same under 55 °C. Microgel inhibited lipid oxidation (hydroperoxides and TBARS). | FFA release: ~100% for emulsion, ~50% for microgel | [54,91] |
| Microgel | Chitosan microgel was much smaller than gelatin microgel | - | (-)-Epigallocatechin gallate (EGCG); microgel beads: gelatin vs chitosan | Encapsulation efficiency: 95% for gelatin; 82% for chitosan | The initial release of the EGCG was faster in the chitosan microgel, but the final release was the same. | Release of EGCG reduced as: Gelatin > chitosan, which was significantly less than free EGCG. | [92] |
| Emulsion | 0.15, 1.6, 11 μm | −68 to −57 mV | Vitamin A, D, E; soy oil emulsion stabilized by quillaja saponin | - | Oil droplet size remained unchanged during GIT. Vitamin stability in GIT was the same for different oil droplet sizes. | FFA release decreased from 125% to 99% as increasing oil droplet size. Bioaccessibility: vitamin A: 87%, 68%, 39%; vitamin D: 76%, 76%, 44%; vitamin E: 77%, 40%, 21% as increasing oil droplet size. | [93] |
| Emulsion | Pea protein: 36 μm; complex with flaxseed gum: 20 to 54 μm | Pea protein: 33 mV; complex with flaxseed gum: −3 mV | Algae oil emulsion stabilized by pea protein with or without flaxseed gum | - | The emulsion was stable in pure protein or high flaxseed gum level but unstable at low flaxseed gum level (0.01%). Flaxseed gum reduces lipid oxidation and the release of undesirable volatiles. | - | [94] |
| Emulsion | 0.3–0.4 μm | −25 to −20 mV | Fish oil droplets stabilized by lentil, pea, and faba bean proteins | - | All emulsions were unstable in stomach or under different pH, ionic strength, temperature. Lentil protein emulsion was most stable. All plant proteins inhibited lipid oxidation. | FFA reached 100% for all samples. | [66,95,96] |
| Emulsion | Quillaja saponin: 0.13 μm; gum arabic: 0.33 μm | Quillaja saponin: −63 mV; gum arabic: −32 mV | β-Carotene; flaxseed oil droplets stabilized by either quillaja saponin or gum arabic, tannic acid as an antioxidant | - | Emulsions were relatively stable at 55 °C and in GIT. | Quillaja saponin slightly reduced lipid digestion to 90%. The β-carotene bioaccessibility was the same (45%) for all emulsions. | [95] |
| Emulsion | Tween 20, quillaja saponin and casein: 0.17 μm; lysolecithin: 0.33 μm; gum Arabic: 0.48 μm | Casein, lysolecithin and gum Arabic: −42 to −33 mV; quillaja saponin: −72 mV; Tween 20: −18 mV | β-Carotene, corn oil droplets stabilized by Tween 20, quillaja saponin, casein, gum arabic, or lysolecithin | - | Casein and lysolecithin stabilized emulsions were unstable in GIT. | FFA release: Tween 20 and quillaja saponin (>100%) > gum arabic (99%) > soy lysolecithin and casein (93%); β-carotene bioaccessibility: Tween 20 (62%) > quillaja saponin (56%) > casein (55%) > gum arabic (51%) > lysolecithin (25%). | [96] |
| Excipient emulsion | 0.2, 0.5, 10 μm | −72 to −42 mV as increasing oil droplet size | Carotenoids from carrots; whey protein stabilized corn oil emulsion | - | The emulsion was unstable in the stomach phase. | FFA release: over 100% for 0.2 and 0.5 μm, >90% for 10 μm and bulk oil; Bioaccessibility: α-carotene and β-carotene decreased as increasing oil droplet size (32% to 7%). | [80] |
| Excipient emulsion | 0.7–0.9 μm | - | Carotenoids from carrot or tomato purees, sucrose ester, stabilized emulsion, olive (OO), soybean (SO), or linseed (LO) oils | - | All emulsions were unstable in the stomach phase. | TAG disappearance: 43–44% for OO, 42–45% for SO, 34–36% for LO. Bioaccessibility: β-carotene from carrots: 13% for OO, 8–10% for SO and LO; cis-lycopene from tomato 27% for OO, 15% for SO and LO. | [79] |
| Excipient emulsion | <0.2 μm | LCT: −23 mV; MCT: −14 mV | Carotenoids and phenolics from mangoes; Tween 20 stabilized emulsion: corn oil (LCT) vs. MCT | - | All emulsions were stable in GIT. | FFA release: MCT (>100%) > LCT (80%). Phenolics bioaccessibility: 80%, 100%, 80%; carotenoids bioaccessibility: 35%, 60%, 80%, for buffer solution, MCT, LCT emulsions respectively. | [97] |
| Emulsion | <0.2 μm | −45 mV | Curcumin loaded by pH driven method, heat-driven method, or conventional oil loading method; corn oil emulsion stabilized by quillaja saponin | Encapsulation efficiency: pH driven (93%) > heat-driven (76%) > conventional oil loading (56%) | Emulsions were stable in GIT. | FFA release (~80%), curcumin stability (76–92%) and bioaccessibility (74–79%) were similar. | [98] |
| Nanoemulsion | ~0.2 μm | −46 to −40 mV | Vitamin E acetate, quillaja saponin stabilized emulsions: corn oil (LCT) or MCT | - | - | FFA release: MCT (> 100%) > LCT (~80%); vitamin E bioaccessibility: LCT (39%) >MCT (17%); vitamin E conversion: LCT (29%) > MCT (17%) | [81] |
| Emulsion and nanoemulsion | 0.2, 20 μm | −60 to −50 mV | Vitamin D; corn oil emulsion stabilized by quillaja saponin | - | - | FFA release: 100% to 69%; vitamin D bioaccessibility: 1.8 μg/mL, 0.5 μg/mL; bioavailability: 22 ng/mL, 18 ng/mL as increasing oil droplet size | [99] |
| Emulsion | <0.2 μm | −70 to −60 mV | Curcumin, resveratrol, and quercetin; quillaja saponin stabilized emulsion: coconut, sunflower, and flaxseed oil | Encapsulation efficiency: 70–90%, higher in long-chain TAGs | Long-chain TAG promoted higher gastrointestinal stability of polyphenol. | Long chain TAG and resveratrol retarded lipid digestion. Resveratrol: 86%, 80%, 77%; curcumin: 52%, 53%, 59%; quercetin: 48%, 75%, 69%, for coconut oil, sunflower oil and flaxseed oil respectively. | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; McClements, D.J. Plant-Based Colloidal Delivery Systems for Bioactives. Molecules 2021, 26, 6895. https://doi.org/10.3390/molecules26226895
Tan Y, McClements DJ. Plant-Based Colloidal Delivery Systems for Bioactives. Molecules. 2021; 26(22):6895. https://doi.org/10.3390/molecules26226895
Chicago/Turabian StyleTan, Yunbing, and David Julian McClements. 2021. "Plant-Based Colloidal Delivery Systems for Bioactives" Molecules 26, no. 22: 6895. https://doi.org/10.3390/molecules26226895
APA StyleTan, Y., & McClements, D. J. (2021). Plant-Based Colloidal Delivery Systems for Bioactives. Molecules, 26(22), 6895. https://doi.org/10.3390/molecules26226895







