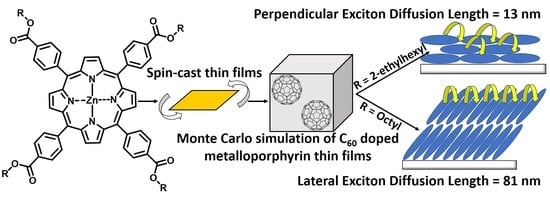
Self-Assembly-Directed Exciton Diffusion in Solution-Processable Metalloporphyrin Thin Films
Abstract
1. Introduction
2. Results
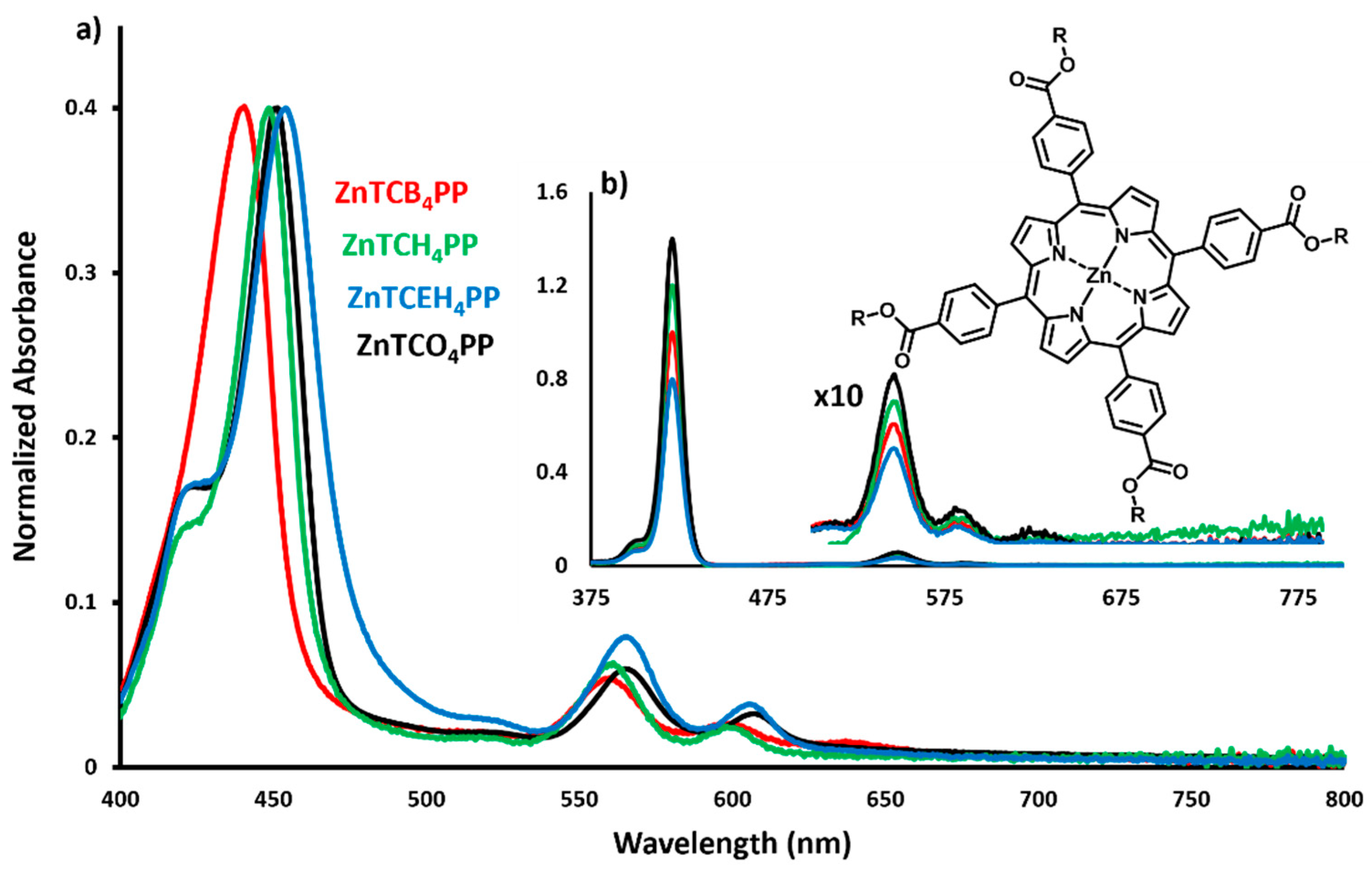
2.1. Zn Porphyrin Thin-Film and Solution UV-Vis Spectra
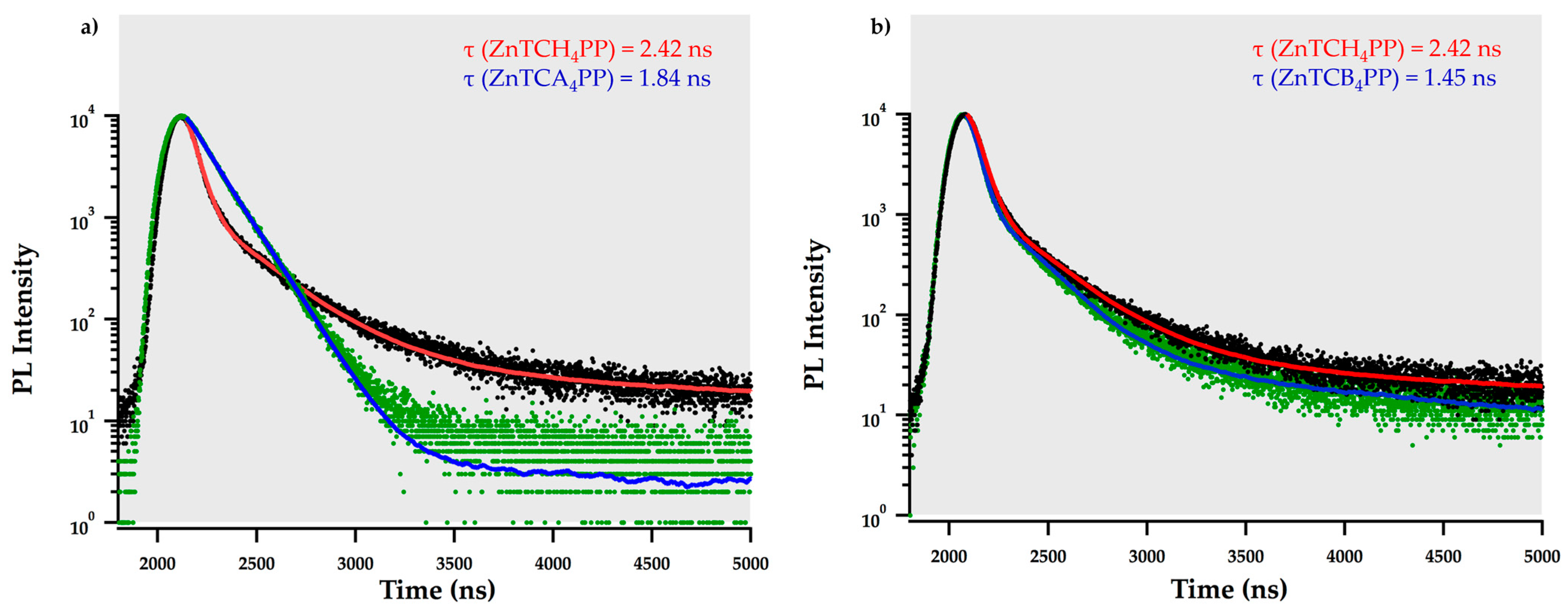
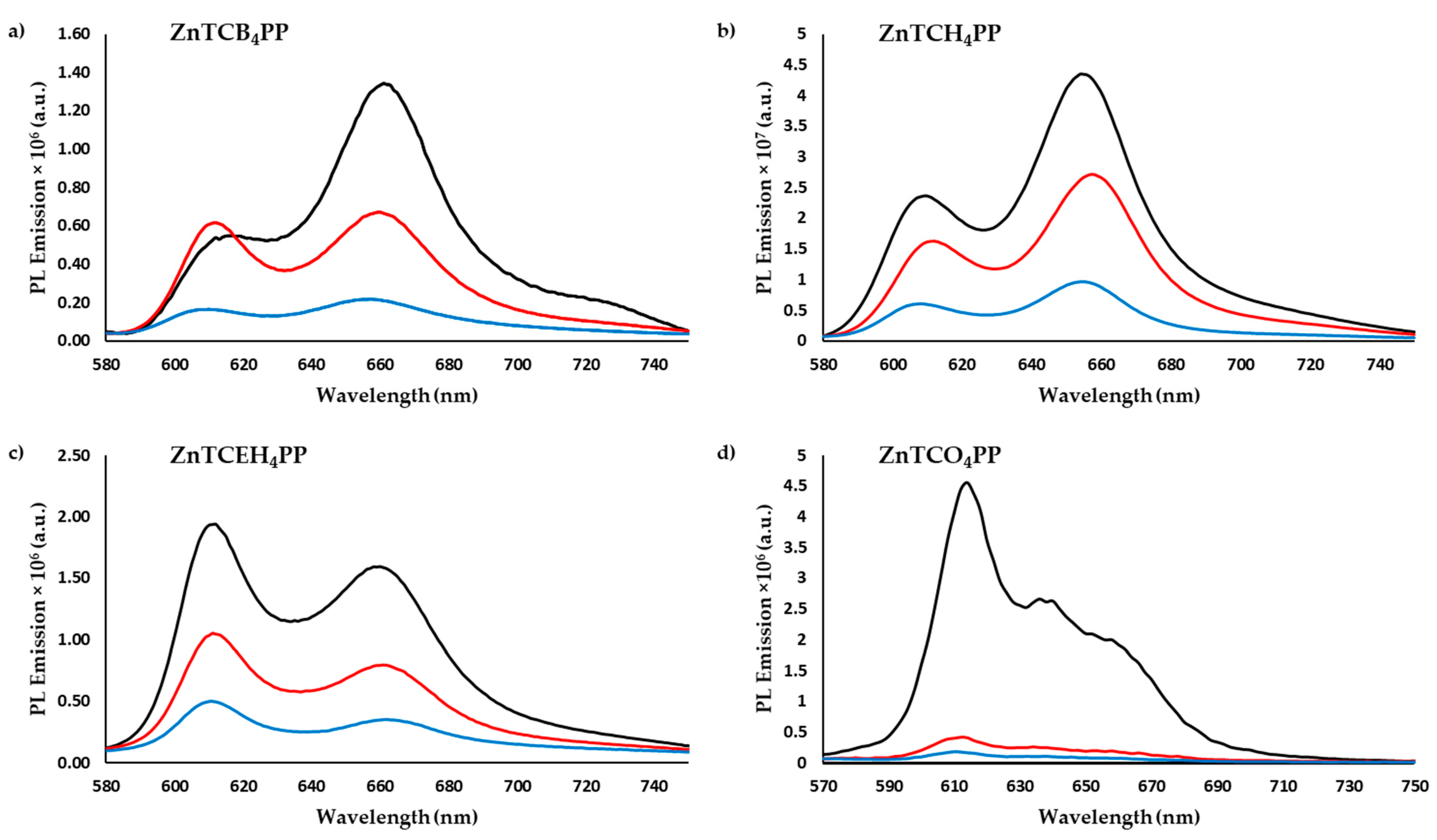
2.2. Exciton Diffusion Coefficient (D) and Diffusion Lengths (LD)
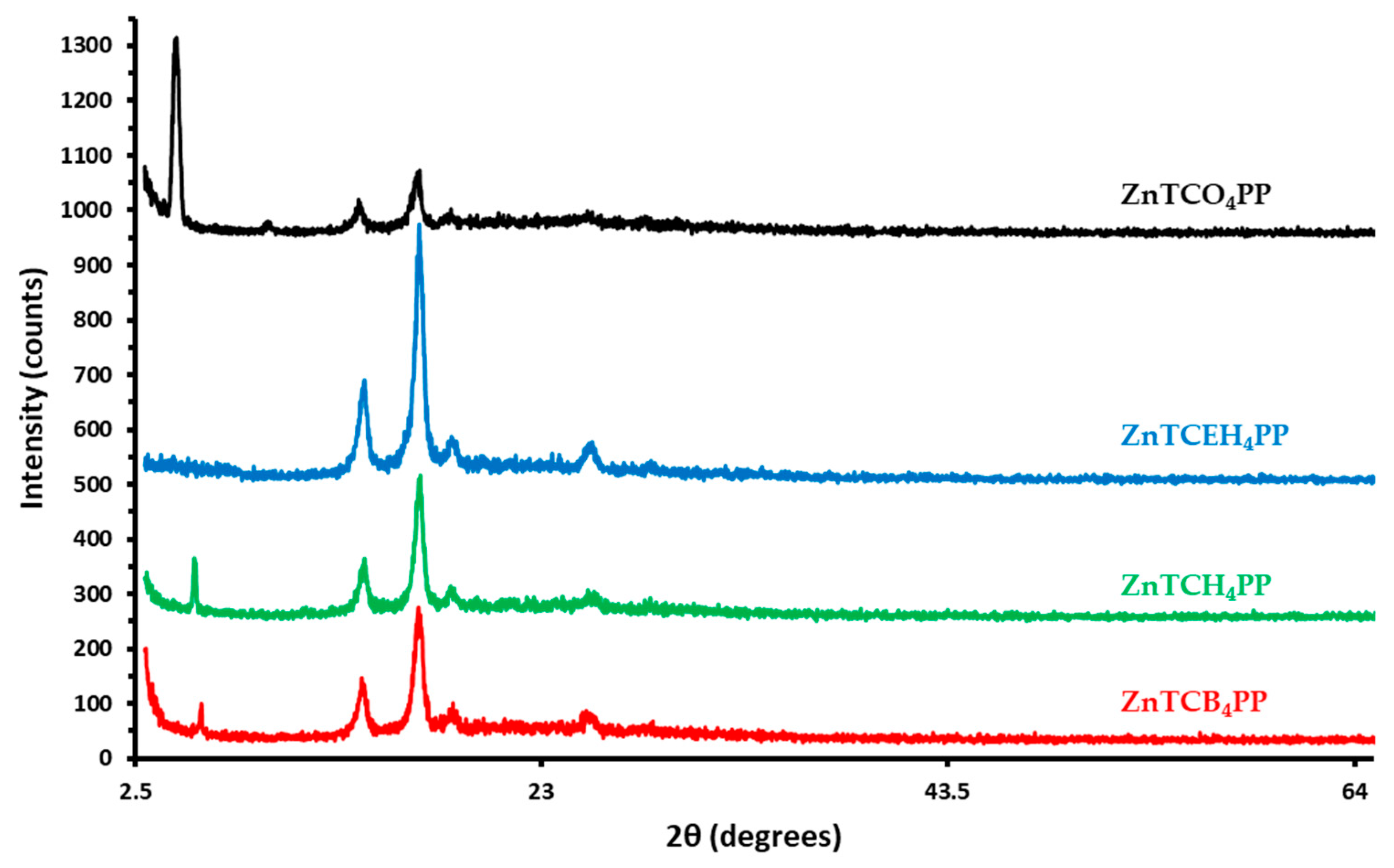
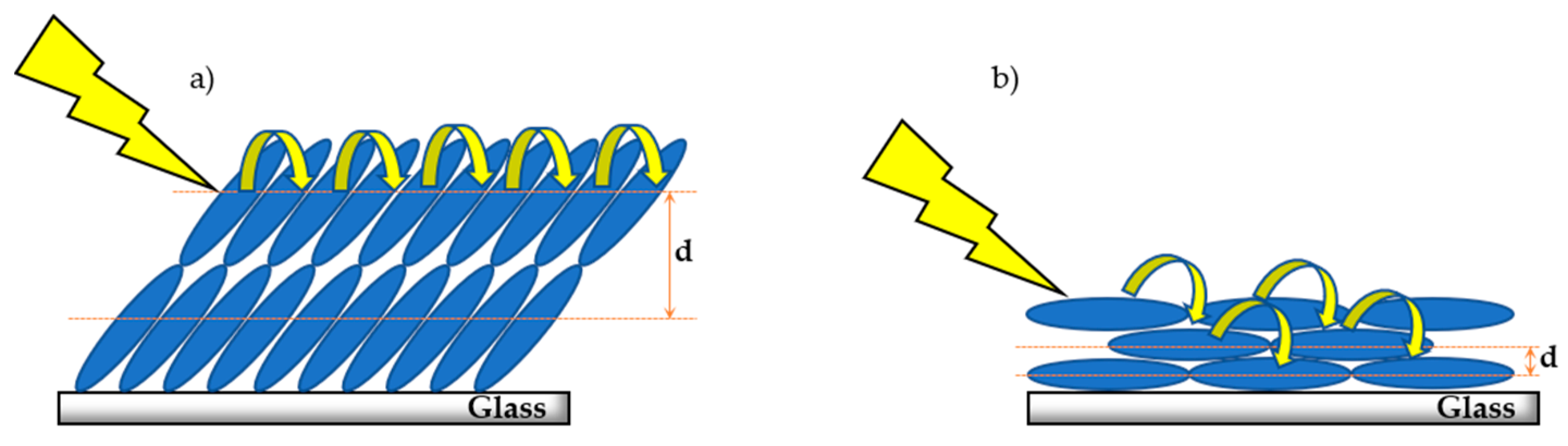
2.3. Porphyrin Thin-Film XRD Patterns
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Preparation of Porphyrin Derivatives
4.3. Zn-Porphyrin Thin-Film Preparation and Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rabinowitch, E. Photosynthesis and energy transfer. J. Phys. Chem. 1957, 61, 870–878. [Google Scholar] [CrossRef]
- Engel, G.S.; Calhoun, T.R.; Read, E.L.; Ahn, T.-K.; Mančal, T.; Cheng, Y.-C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef]
- Bennett, D.I.; Fleming, G.R.; Amarnath, K. Energy-dependent quenching adjusts the excitation diffusion length to regulate photosynthetic light harvesting. Proc. Natl. Acad. Sci. USA 2018, 115, E9523–E9531. [Google Scholar] [CrossRef]
- Fujita, T.; Brookes, J.C.; Saikin, S.K.; Aspuru-Guzik, A. Memory-assisted exciton diffusion in the chlorosome light-harvesting antenna of green sulfur bacteria. J. Phys. Chem. Lett. 2012, 3, 2357–2361. [Google Scholar] [CrossRef][Green Version]
- Jin, L.; Lv, S.; Miao, Y.; Liu, D.; Song, F. Recent development of porous porphyrin-based nanomaterials for photocatalysis. ChemCatChem 2021, 13, 140–152. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ogumi, K.; Jeon, I.; Wang, H.; Nakagawa, T. Recent progress in porphyrin-and phthalocyanine-containing perovskite solar cells. RSC Adv. 2020, 10, 32678–32689. [Google Scholar] [CrossRef]
- Panda, M.K.; Ladomenou, K.; Coutsolelos, A.G. Porphyrins in bio-inspired transformations: Light-harvesting to solar cell. Coord. Chem. Rev. 2012, 256, 2601–2627. [Google Scholar] [CrossRef]
- Soudi, N.; Nanayakkara, S.; Jahed, N.M.; Naahidi, S. Rise of nature-inspired solar photovoltaic energy convertors. Sol. Energy 2020, 208, 31–45. [Google Scholar] [CrossRef]
- Walter, M.G.; Rudine, A.B.; Wamser, C.C. Porphyrins and phthalocyanines in solar photovoltaic cells. J. Porphyr. Phthalocyanines 2010, 14, 759–792. [Google Scholar] [CrossRef]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Mahmood, A.; Hu, J.-Y.; Xiao, B.; Tang, A.; Wang, X.; Zhou, E. Recent progress in porphyrin-based materials for organic solar cells. J. Mater. Chem. A 2018, 6, 16769–16797. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, J.H.; Jang, W.D. Applications of porphyrins in emerging energy conversion technologies. Coord. Chem. Rev. 2020, 407, 213157. [Google Scholar] [CrossRef]
- Lin, G.; Ding, H.; Chen, R.; Peng, Z.; Wang, B.; Wang, C. 3D Porphyrin-Based Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Goswami, S.; Young, R.M.; Schlesinger, I.; Mian, M.R.; Enciso, A.E.; Zhang, X.; Hornick, J.E.; Farha, O.K.; Wasielewski, M.R.; et al. Photon Upconversion in a Glowing Metal–Organic Framework. J. Am. Chem. Soc. 2021, 143, 5053–5059. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhu, Y.F.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem.-Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; Rosa, L.P.; de Jesus, I.M.; de Oliveira Santos, G.P.; Inada, N.M.; Blanco, K.C.; Araújo, T.S.D.; Bagnato, V.S. Total mouth photodynamic therapy mediated by red LED and porphyrin in individuals with AIDS. Lasers Med. Sci. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, M.; Magna, G.; Emam-djomeh, Z.; Yarmand, M.S.; Paolesse, R.; Di Natale, C. Porphyrin-Functionalized Zinc Oxide Nanostructures for Sensor Applications. Sensors 2018, 18, 2279. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Xu, J.; Liang, L.; Mai, C.-L.; Ren, L.; Zhou, Q.; Yu, Y.; Zhang, B.; Gao, P. Structural, photophysical, electrochemical and spintronic study of first-row metal Tetrakis (meso-triphenylamine)-porphyrin complexes: A combined experimental and theoretical study. Dye Pigments 2021, 193, 109469. [Google Scholar] [CrossRef]
- Kaushal, M.; Ortiz, A.L.; Kassel, J.A.; Hall, N.; Lee, T.D.; Singh, G.; Walter, M.G. Enhancing exciton diffusion in porphyrin thin films using peripheral carboalkoxy groups to influence molecular assembly. J. Mater. Chem. C 2016, 4, 5602–5609. [Google Scholar] [CrossRef]
- Ghosh, T.; Gerbig, L.; Lambov, M.; Dechant, M.; Lehmann, M. Liquid crystals from shape-persistent porphyrin stars with intrinsic free space. J. Mater. Chem. C 2020, 8, 5562–5571. [Google Scholar] [CrossRef]
- Martin, C.R.; Park, K.C.; Corkill, R.E.; Kittikhunnatham, P.; Leith, G.A.; Mathur, A.; Abiodun, S.L.; Greytak, A.B.; Shustova, N.B. Photoresponsive frameworks: Energy transfer in the spotlight. Faraday Discuss. 2021, 231, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Tamiaki, H. Supramolecular chlorophyll aggregates inspired from specific light-harvesting antenna “chlorosome”: Static nanostructure, dynamic construction process, and versatile application. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100385. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.L.; Collier, G.S.; Marin, D.M.; Kassel, J.A.; Ivins, R.J.; Grubich, N.G.; Walter, M.G. The effects of heavy atoms on the exciton diffusion properties in photoactive thin films of tetrakis(4-carbomethoxyphenyl)porphyrins. J. Mater. Chem. C 2015, 3, 1243–1249. [Google Scholar] [CrossRef]
- Sajjad, M.T.; Ruseckas, A.; Samuel, I.D. Enhancing Exciton Diffusion Length Provides New Opportunities for Organic Photovoltaics. Matter 2020, 3, 341–354. [Google Scholar] [CrossRef]
- Otsuki, J. Supramolecular approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 2018, 6, 6710–6753. [Google Scholar] [CrossRef]
- Huijser, A.; Savenije, T.J.; Kotlewski, A.; Picken, S.J.; Siebbeles, L.D. Efficient Light-Harvesting Layers of Homeotropically Aligned Porphyrin Derivatives. Adv. Mater. 2006, 18, 2234–2239. [Google Scholar] [CrossRef]
- Walter, M.G.; Wamser, C.C.; Ruwitch, J.; Zhao, Y.; Braden, D.; Stevens, M.; Denman, A.; Pi, R.; Rudine, A.; Pessiki, P.J. Syntheses and optoelectronic properties of amino/carboxyphenylporphyrins for potential use in dye-sensitized TiO2 solar cells. J. Porphyr. Phthalocyanines 2007, 11, 601–612. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, C. Excited State Energy Transfer in Metal-Organic Frameworks. Adv. Mater. 2021, 33, 2005819. [Google Scholar] [CrossRef]
- Feron, K.; Belcher, W.J.; Fell, C.J.; Dastoor, P.C. Organic Solar Cells: Understanding the Role of Forster Resonance Energy Transfer. Int. J. Mol. Sci. 2012, 13, 17019–17047. [Google Scholar] [CrossRef] [PubMed]
- Mikhnenko, O.V.; Azimi, H.; Scharber, M.; Morana, M.; Blom, P.W.M.; Loi, M.A. Exciton diffusion length in narrow bandgap polymers. Energy Environ. Sci. 2012, 5, 6960–6965. [Google Scholar] [CrossRef]
- de Sousa, L.E.; Bueno, F.T.; Ribeiro, L.; Ribeiro Junior, L.A.; da Silva Filho, D.A.; de Oliveira Neto, P.H. Role of Exciton Density in Organic Materials: Diffusion Length, Lifetime, and Quantum Efficiency. Chem. Mater. 2019, 31, 6818–6823. [Google Scholar] [CrossRef]
- Raul, B.A.; Luponosov, Y.N.; Yang, W.; Surin, N.M.; Douhéret, O.; Min, J.; Jansen, T.L.; Ponomarenko, S.A.; Pshenichnikov, M.S. Excited state dynamics and exciton diffusion in triphenylamine/dicyanovinyl push–pull small molecule for organic optoelectronics. Sci. Rep. 2020, 10, 21198. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Bangsund, J.S.; Barriocanal, J.G.; Holmes, R.J. Impact of molecular structure on singlet and triplet exciton diffusion in phenanthroline derivatives. J. Mater. Chem. C 2020, 8, 6118–6123. [Google Scholar] [CrossRef]
- Mikhnenko, O.V.; Kuik, M.; Lin, J.; van der Kaap, N.; Nguyen, T.Q.; Blom, P.W. Trap-limited exciton diffusion in organic semiconductors. Adv. Mater. 2014, 26, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Mikhnenko, O.V.; Lin, J.; Shu, Y.; Anthony, J.E.; Blom, P.W.; Nguyen, T.-Q.; Loi, M.A. Effect of thermal annealing on exciton diffusion in a diketopyrrolopyrrole derivative. Phys. Chem. Chem. Phys. 2012, 14, 14196–14201. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D.M. Fullerenes—How 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef]
- Long, Y.; Hedley, G.J.; Ruseckas, A.; Chowdhury, M.; Roland, T.; Serrano, L.A.; Cooke, G.; Samuel, I.D.W. Effect of Annealing on Exciton Diffusion in a High Performance Small Molecule Organic Photovoltaic Material. ACS Appl. Mater. Interfaces 2017, 9, 14945–14952. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.T.; Ward, A.J.; Kästner, C.; Ruseckas, A.; Hoppe, H.; Samuel, I.D.W. Controlling Exciton Diffusion and Fullerene Distribution in Photovoltaic Blends by Side Chain Modification. J. Phys. Chem. Lett. 2015, 6, 3054–3060. [Google Scholar] [CrossRef]
- Siegmund, B.; Sajjad, M.T.; Widmer, J.; Ray, D.; Koerner, C.; Riede, M.; Leo, K.; Samuel, I.D.; Vandewal, K. Exciton diffusion length and charge extraction yield in organic bilayer solar cells. Adv. Mater. 2017, 29, 1604424. [Google Scholar] [CrossRef] [PubMed]
- Mikhnenko, O.V.; Blom, P.W.M.; Nguyen, T.-Q. Exciton diffusion in organic semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Rotas, G.; Thomas, M.B.; Canton-Vitoria, R.; D’Souza, F.; Tagmatarchis, N. Preparation, Photophysical and Electrochemical Evaluation of an Azaborondipyrromethene/Zinc Porphyrin/Graphene Supramolecular Nanoensemble. Chem.–Eur. J. 2020, 26, 6652–6661. [Google Scholar] [CrossRef]
- Das, S.K.; Song, B.; Mahler, A.; Nesterov, V.N.; Wilson, A.K.; Ito, O.; D’Souza, F. Electron Transfer Studies of High Potential Zinc Porphyrin–Fullerene Supramolecular Dyads. J. Phys. Chem. C 2014, 118, 3994–4006. [Google Scholar] [CrossRef]
- Huijser, A.; Savenije, T.J.; Meskers, S.C.; Vermeulen, M.J.; Siebbeles, L.D. The mechanism of long-range exciton diffusion in a nematically organized porphyrin layer. J. Am. Chem. Soc. 2008, 130, 12496–12500. [Google Scholar] [CrossRef] [PubMed]
- Ryuzaki, S.; Hasegawa, T.; Onoe, J. X-ray diffraction and infrared multiple-angle incidence resolution spectroscopic studies on the crystal structure and molecular orientation of zinc-porphyrin thin films on a SiO2/Si substrate. J. Appl. Phys. 2009, 105, 113529. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Yu, J.; Shen, Q.; Luo, G.; Chen, X.; Xue, P.; Wang, Z.; Hu, J. Assembled Exciton Dynamics in Porphyrin Metal–Organic Framework Nanofilms. Nano Lett. 2021, 21, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, M.; Douvas, A.M.; Georgiadou, D.G.; Constantoudis, V.; Davazoglou, D.; Kennou, S.; Palilis, L.C.; Daphnomili, D.; Coutsolelos, A.G.; Argitis, P. Large work function shift of organic semiconductors inducing enhanced interfacial electron transfer in organic optoelectronics enabled by porphyrin aggregated nanostructures. Nano Res. 2014, 7, 679–693. [Google Scholar] [CrossRef]
- Kumaran, N.; Veneman, P.A.; Minch, B.A.; Mudalige, A.; Pemberton, J.E.; O’Brien, D.F.; Armstrong, N.R. Self-Organized Thin Films of Hydrogen-Bonded Phthalocyanines: Characterization of Structure and Electrical Properties on Nanometer Length Scales. Chem. Mater. 2010, 22, 2491–2501. [Google Scholar] [CrossRef]
- Ðorđević, L.; Demitri, N.; Bonifazi, D. Solvent-dependent moulding of porphyrin-based nanostructures: Solid state, solution and on surface self-assembly. Supramol. Chem. 2016, 28, 753–761. [Google Scholar] [CrossRef]
- Huijser, A.; Savenije, T.J.; Kroeze, J.E.; Siebbeles, L.D.A. Exciton Diffusion and Interfacial Charge Separation in meso-Tetraphenylporphyrin/TiO2 Bilayers: Effect of Ethyl Substituents. J. Phys. Chem. B 2005, 109, 20166–20173. [Google Scholar] [CrossRef]
- Siebbeles, L.D.A.; Huijser, A.; Savenije, T.J. Effects of molecular organization on exciton diffusion in thin films of bioinspired light-harvesting molecules. J. Mater. Chem. 2009, 19, 6067–6072. [Google Scholar] [CrossRef]
| Material | τS1 (ns) | Q0.06% | D × 10−4 (cm2/s) | LD (nm) | Q0.2% | LD (nm) |
|---|---|---|---|---|---|---|
| ZnTCB4PP | 1.52 ± 0.07 | 0.37 | 1.0 ± 0.3 | 9.42 ± 1.2 | 0.77 | 8.93 ± 0.4 |
| ZnTCH4PP | 2.30 ± 0.11 | 0.37 | 0.90 ± 0.2 | 11.16 ± 1.2 | 0.78 | 9.31 ± 0.4 |
| ZnTCEH4PP | 1.88 ± 0.04 | 0.46 | 1.68 ± 0.2 | 13.74 ± 0.7 | 0.73 | 7.38 ± 0.1 |
| ZnTCO4PP | 2.02 ± 0.05 | 0.89 | 50.20 ± 4.1 | 77.98 ± 3.0 | 0.95 | 28.16 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibu, A.; Middleton, C.; Kwiatkowski, C.O.; Kaushal, M.; Gillen, J.H.; Walter, M.G. Self-Assembly-Directed Exciton Diffusion in Solution-Processable Metalloporphyrin Thin Films. Molecules 2022, 27, 35. https://doi.org/10.3390/molecules27010035
Shibu A, Middleton C, Kwiatkowski CO, Kaushal M, Gillen JH, Walter MG. Self-Assembly-Directed Exciton Diffusion in Solution-Processable Metalloporphyrin Thin Films. Molecules. 2022; 27(1):35. https://doi.org/10.3390/molecules27010035
Chicago/Turabian StyleShibu, Abhishek, Camilla Middleton, Carly O. Kwiatkowski, Meesha Kaushal, Jonathan H. Gillen, and Michael G. Walter. 2022. "Self-Assembly-Directed Exciton Diffusion in Solution-Processable Metalloporphyrin Thin Films" Molecules 27, no. 1: 35. https://doi.org/10.3390/molecules27010035
APA StyleShibu, A., Middleton, C., Kwiatkowski, C. O., Kaushal, M., Gillen, J. H., & Walter, M. G. (2022). Self-Assembly-Directed Exciton Diffusion in Solution-Processable Metalloporphyrin Thin Films. Molecules, 27(1), 35. https://doi.org/10.3390/molecules27010035