Abstract
Macrophages have diverse functions in the pathogenesis, resolution, and repair of inflammatory processes. Elegant studies have elucidated the metabolomic and transcriptomic profiles of activated macrophages. However, the versatility of macrophage responses in inflammation is likely due, at least in part, to their ability to rearrange their repertoire of bioactive lipids, including fatty acids and oxylipins. This review will describe the fatty acids and oxylipins generated by macrophages and their role in type 1 and type 2 immune responses. We will highlight lipidomic studies that have shaped the current understanding of the role of lipids in macrophage polarization.
1. Introduction
Macrophages exist in virtually every tissue. Ontogenetically, macrophages deriving from the primitive yolk sac, home to various tissues, including the brain and fetal liver [1]. The second wave of proliferation originates from fetal liver seeding remaining tissues, including bone marrow [2]. This process defines the first line of heterogeneity. An additional diversification of macrophages happens in the particular niche in which they develop and it is due to the stimulation by environmental cues and pathogens. Thus, tissue macrophages acquire peculiar characteristics, including lipid composition [3,4].
Another level of complexity in the study of macrophages is due to the activation in vitro of bone-marrow-derived (BM)-macrophages, or human monocyte-derived macrophages, which resemble in vivo counterparts, mirroring T helper (Th)1 (type 1) and Th2 cell (type 2) activation. Classically activated macrophages or M1 are generated by exposure to type 1 stimuli, including LPS and IFNγ, and produce IL-12, IL-1β, TNF-α, and the chemokines CXCL10, CXCL9, and CXCL11. M2 macrophages are activated by type 2 cytokines, particularly IL-4, the prototypical stimulus for alternative activated macrophages. M2 macrophages express CD206, arginase-1 (Arg-1), and produce IL-13, IL-33, and the chemokines CCL22 and CCL21 [5]. Although it is helpful to classify macrophages into different groups, it is likely that in vivo differentially activated macrophages coexist, continuously changing depending on the microenvironment in which they develop.
Macrophages are a rich source of bioactive lipids, including fatty acids (FAs) and their oxygenated metabolites oxylipins. FAs are characterized by a carbon backbone of various lengths. They are classified based on their degree of saturation in saturated fatty acids (SFAs, no double bonds), mono- or polyunsaturated (MUFAs or PUFAs, one or more double bonds, respectively). FAs may act directly at cognate receptors in an autocrine or paracrine fashion. They can bind specific G-protein-coupled receptors (GPCRs), including free fatty acid receptors (FFARs)1-4 and GPR84, expressed on macrophages and target cells [6,7,8], thus serving as signaling molecules [9,10].
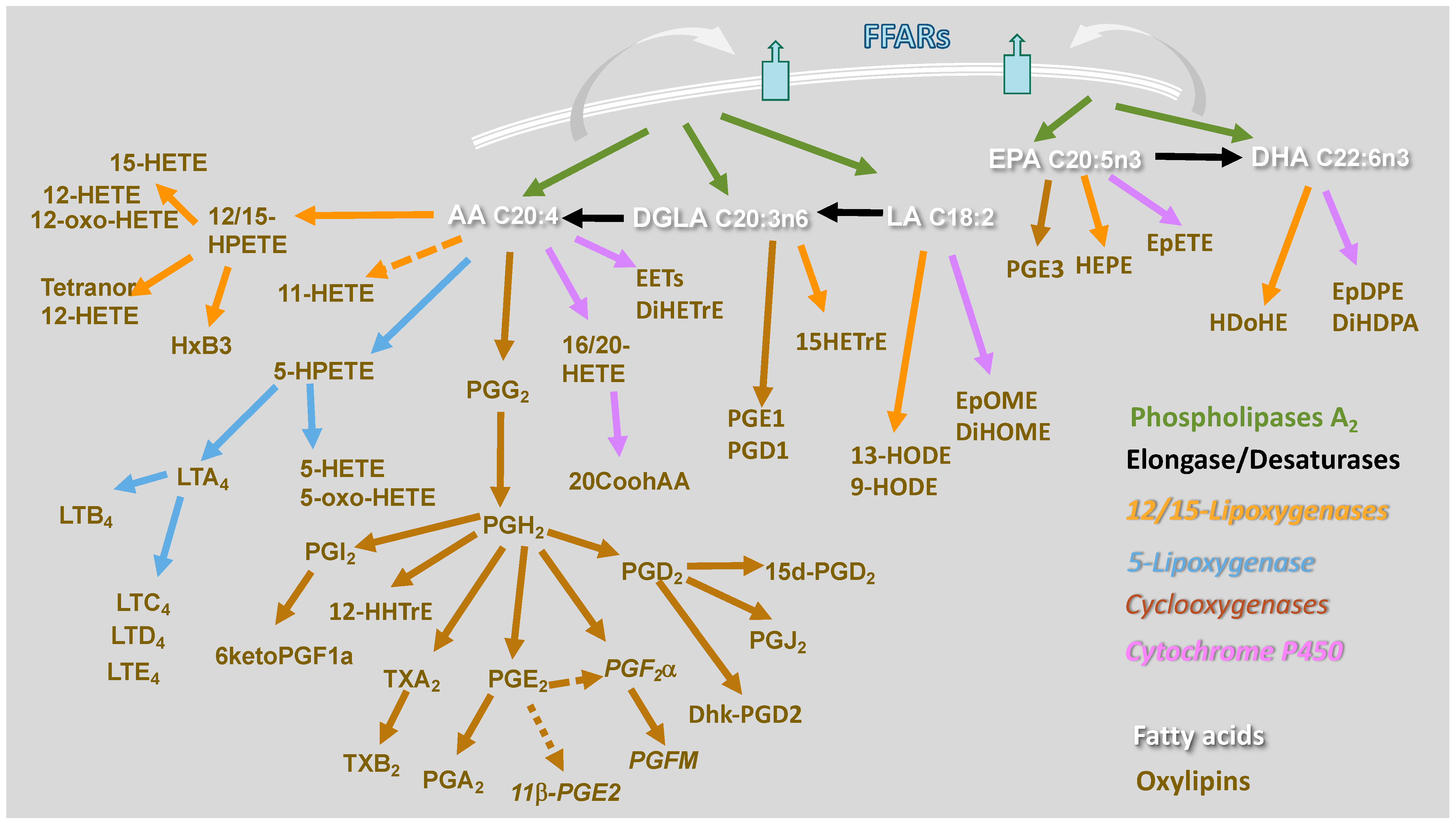
Macrophages incorporate and utilize FAs through several pathways. Linoleic acid (LA, C18:0 N6) and alpha-linolenic acid (ALA, C18:3 N3) are essential fatty acids that mammalian cells cannot synthesize. Therefore, they are provided to macrophages either through lipoproteins or as non-esterified fatty acids bound to albumin. Then, they are transported intracellularly via CD36 or fatty acid transporters (FATs), respectively, and shuttled intracellularly to various organelles via fatty acid-binding protein (FABP)-4 and 5 [11,12]. Phagocytosis and efferocytosis also contribute to enriching the FA repertoire of macrophages. MUFAs and PUFAs are synthesized in the cell from LA and ALA by the action of fatty acid elongase (ELOVL) and desaturase (FADS) enzymes [13]. Through acetyl-CoA synthases (ACSs), which also serve as FA transporters [14], FAs may enter the cell and be esterified into ceramide, phospholipids, and triglyceride, therefore performing many functions [15]. FAs incorporated into neutral lipids such as triglycerides are a source of stored energy, or FAs can be incorporated into structural lipids such as membrane phospholipids, sphingolipids, and plasmalogens. Additionally, FAs present in the second position of membrane phospholipids are hydrolyzed by phospholipase A2 (PLA2) enzymes [16,17]. PUFAs liberated from membrane phospholipids by PLA2 are metabolized into oxylipins through three major enzymatic pathways, cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450), and other nonenzymatic pathways. Each pathway comprises multiple enzymes that produce several bioactive lipid mediators (Figure 1).
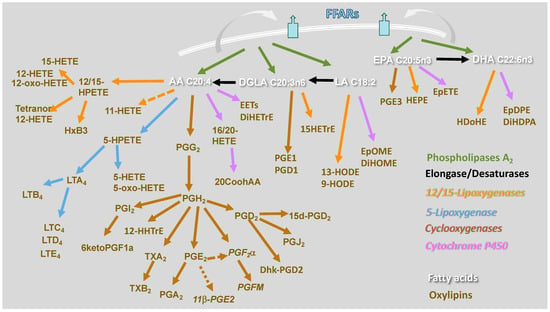
Figure 1.
Enzymatic pathways and oxylipins derived from the omega-6 FAs, arachidonic acid (AA), di-homo-gamma-linolenic acid (DGLA), and linoleic acid (LA), and the omega-3 FAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Enzymatic pathways are color coded. Phospholipases A2 are depicted in green, elongases/desaturases in black, 12/15-lipoxygenase in orange, 5-lipoxygenase in blue, cyclooxygenases in brown, cytochrome P450 in purple. 20-carboxyAA (20CooHAA), dihydroxy-eicosatrienoic acid (DiHETrE), dihydroxy-docosa-pentaenoic acid (DiHDPA), 13,14-dihydro-15-keto prostaglandin D2 (Dhk-PGD2), dihydroxy-octadecenoic acid (DiHOME), epoxy-docosapentaenoic acid (EpDPE), epoxy-eicosatrienoic acid (EET), epoxy-octadecenoic acid (EpOME), hydroxy-eicosatrienoic acid (HETrE), hydroxy-docosahexaenoic acid (HDoHE), hydroxy-eicosatetraenoic acid (HETE), 5-oxo-eicosatetraenoic acid (5-oxo-HETE), hydroperoxy-eicosatetraenoic acid (HPETE), hydroxy-eicosapentaenoic acid (HEPE), hepoxilin B3 (HXB3), 12-hydroxy-heptadecatrenoic acid (12-HHTrE), hydroxy-octadecadienoic acid (HODE), leukotriene A4 (LTA4), leukotriene B4 (LTB4), leukotriene C4 (LTC4), leukotriene D4 (LTD4), leukotriene E4 (LTE4), prostaglandin H2 (PGH2), prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostaglandin F metabolite (PGFM), and thromboxane B2 (TxB2).
The first comprehensive mass spectrometry lipid analysis of macrophages was conducted on RAW264.7 cells using an M1-like stimulation with Kdo2-lipid A (KLA), a toll-like receptor 4 (TLR4) agonist [18]. The report correlated the changes in lipids induced by KLA to gene expression. Remarkably, the generation of FAs and oxylipins correlated with the changes in the expression of PLA2s and other enzymes involved in the production of oxylipins. KLA induced the increased expression of several enzymes, including group V PLA2 (Pla2g5), prostaglandin-endoperoxide synthase 2 (Ptgs2), also known as cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase 2 (Pges2), which correlated with the increase in prostaglandin (PG)E2, PGF2, PGD2, and its metabolites PGJ2, 15-deoxy-12,14-PGD2, and 15-deoxy-12,14-PGJ2. Interestingly, unsaturated fatty acids were reduced, especially at 24 h stimulation, likely due to conversion to oxylipins. Indeed, KLA, similarly to the effects of other TLRs [19], increased PGD2 > PGE2 > PGF2α, but not cysteinyl leukotrienes (CysLTs). However, a shorter stimulation of less than 60 min was sufficient to induce Ca++ flux and leukotriene (LT)C4 release [19]. These effects were likely dependent on subcellular lipid distribution [20] or specific transcriptomic and metabolomic programs induced in M1-polarized macrophages, for instance, the regulation of the TCA pathway, which is suppressed in M1 and activated in M2 macrophages [21].
Thus, FAs serve multiple purposes in the cell: they contribute to the membrane structure; they are a source of energy; and they are second messengers and signaling molecules in immune responses, directly or indirectly through the generation of oxylipins. This review will summarize FAs and derived oxylipins produced by macrophages as revealed by comprehensive lipidomic studies and we will correlate them with the functions of M1 and M2 macrophages in type 1 and type 2 immune responses.
2. Fatty Acids
FAs have different activities based on the degree of saturation (SFAs, MUFAs, and PUFAs) and the length of the fatty acid chain, medium-chain FAs having 12–15 carbons and long-chain FAs containing 16 to 26 carbons. Unsaturated FAs are further classified based on the position of the final double bond at n-9, n-6, or n-3 from the methyl end, which define, respectively, omega-9, omega-6, or omega-3 FAs (Table 1).

Table 1.
Fatty acids produced by activated macrophages and their role in inflammation.
2.1. Medium-Chain and Uneven Saturated Fatty Acids
Medium-chain (MC)SFAs include lauric acid (LU, C12:0), myristic acid (MA, C14:0), and pentadecanoic (PdA, C15:0). One of the least abundant FAs in macrophages is MA, which has a prominent function in post-translational modification of proteins carrying the amino acids methionine-glycine (MG) at the N-terminus. The N-myristoleic enzymes 1 and 2 are expressed in macrophages, where targeted proteins are directed to lipid reach subcellular structures such as Golgi and caveolae. Myristoylation increases type 1 immune responses [22,23] (Table 2). Lauric acid (LU, C12:0) composes 45% coconut oil. LU has proinflammatory functions in type 1 inflammation [24,25,26,27,28] (Table 2). However, the function of MA and LU in M2 macrophages and type 2 immune responses are less known.

Table 2.
Functions of indicated FAs in type 1 inflammation.
Pentadecanoic acid (PdA, C15:0) and heptadecanoic acid or margaric acid (MaA, C17:0) are uneven SFAs found in dairy products [29]. Recent reports suggest a protective effect of PdA in type 1 inflammation [29,30] (Table 2).
2.2. Long-Chain Saturated Fatty Acids
Long-chain (LC)SFAs are essential in macrophage development, polarization and, type 1 inflammation [21,31]. Particularly, PA (C16:0) and stearic acid (SA, C18:0) increase during macrophage differentiation [32]. Furthermore, LCSFAs can induce macrophage activation and cytokine release in M1 conditions. Indeed, levels of LCSFAs are increased in the serum of patients with type 2 diabetes, cardiovascular diseases, and obesity, pathologies in which macrophages have a prominent pathogenetic role. These effects have been linked to palmitate-induced endoplasmic reticulum (ER) stress, which, in macrophages, is dependent on the presence of fatty acid-binding protein 4 (FABP4) and can be prevented by unsaturated FAs [32,33]. An increase in LCSFAs has also been reported in HIV patients, likely contributing to HIV pathogenesis due to SA and PA activation of human monocytes [34].
PA is introduced into the cells from the diet. Once in the cell, PA is metabolized into lysophosphatidylcholine, diacylglycerol, and ceramide. PA exerts its proinflammatory functions in macrophages by activating TLR2 and TLR4 through direct and indirect mechanisms. In THP-1 monocyte-macrophages, PA increased the secretion of IL-1β, TNF-α, and IL-8 by activating the nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 (NLRP3) inflammasome, and through ceramide production. At the same time, LA and OA reduced IL-1β secretion not by reducing its synthesis but by reducing its processing by the NLRP3 inflammasome [35,36]. In BM-macrophages, PA upregulated the channel forming molecule pannexin-1 allowing the release of nucleotides attracting neutrophils [37] and increased LPS-induced production of IL-6, TNF-α, and IL1-β [38]. PA binds the FFAR1 or GPR40 [39,40]. Indeed, in HEK293, PA acted as a partial β-arrestin agonist and a Gq agonist [41]. However, FFAR-1 is the receptor for other medium- to long-chain FAs expressed in macrophages, neutrophils, and muscular cells [39], and it is also highly expressed in beta cells, where it regulates insulin secretion [40]. Therefore, the functions of PA in vivo may reflect the effects of PA on multiple cells.
Macrophage development involves the rearrangement of the lipids composing the plasma membrane [21,31,42]. SA has been reported to accumulate in the supernatant of human monocyte-derived macrophages [43]. Its effects included increased maturation of macrophages and shifting macrophages toward a classical M1 phenotype. Furthermore, mouse BM-macrophages cultured with SA acquired a more mature phenotype based on increased CD11c expression, a feature not shared with LA and OA [35]. Mouse peritoneal macrophages activated by SA increase the production of proinflammatory cytokines, including IL-1α, TNF-α, and ER stress, leading to apoptosis [44]. Furthermore, like PA, SA activated NLRP3 by creating intracellular crystals [45].
Arachidic acid (ArA, C20:0), and other very-long-chain SFAs (VLCSFAs) docosanoic acid (C22:0), tricosanoic acid (C23:0), tetracosanoic acid (C24:0), and hexacosanoic acid (C26:0) are derived from elongation of SA or PA or from the diet. Several studies have suggested a potential inverse correlation between these circulating VLCSFAs and the risk of cardiovascular disease [46,47,48]. However, ArA may target TLR4 to induce a proinflammatory response and ER stress, particularly in macrophages, contributing to obesity [49]. Therefore, further studies are needed to understand the mechanisms underlying these associations and the specific role of each VLCSFA in macrophage activation and type 2 inflammation.
2.3. Monounsaturated Fatty Acids
Palmitoleic acid (PoA, C16:1) is synthesized from PA and modulates type 1 inflammation. Indeed, PoA counteracted the effect of PA on the J774A.1 mouse macrophage cell line by reducing the secretion of TNF-α and the expression of COX-2 and TLR2. Furthermore, resident peritoneal macrophages from Swiss male mice released PoA following stimulation with zymosan, suggesting that PoA or its metabolites [50] could exert an anti-inflammatory function in the contest of type 1 inflammation [51]. Others reported inhibitory functions of PoA, including prevention of PA-induced NF-kB activation; reduced expression of type 1 inflammatory molecules, including IL-6, IL-12β, and Nos2 in BM-macrophages fed a high-fat diet [52]; and the increased expression of molecules associated with type 2 inflammation, including Mannose receptor C-type 1 (Mrc1), IL-10, and Transforming growth factor beta-1 (Tgf-β1). Furthermore, administration of PoA in vivo increased M2 polarization of liver macrophages and reduced insulin resistance in mice fed a high-fat diet [53].
Oleic acid (OA, C18:1) is a dietary lipid that may exert opposing proinflammatory or anti-inflammatory functions depending on whether additional stimuli or environmental factors are involved. In vitro, OA reduced inflammasome activation in mouse peritoneal macrophages primed by LPS and stimulated by SFAs [45]. In RAW264.7 macrophages, 500 μM PA increased apoptosis and reactive oxygen species production, which were reduced by the addition of OA [54]. PA, but not OA, increased TNF-α, IL-6, IL-1β, and MCP-1 in RAW264.7 cells [55], while OA increased the expression of Arg-1, CD206, and KLRT4 [56]. In vivo, OA reduced proinflammatory effects of SA by developing regulatory myeloid suppressive cells [57], characterized by accumulation of lipid droplets and production of nitric oxide (NO). Furthermore, OA reduced high-fat-diet–induced oxidative stress [58] and IL-1α production coupled with vascular inflammation and atherosclerosis [59]. These anti-inflammatory functions could be exerted by activation of microRNA let-7b, at least in the THP-1 monocytic cell line [60]. In a model of Alternaria alternata-induced pulmonary inflammation, the release of OA and LA from macrophages increased the activation of innate lymphoid cells type 2 (ILC2s), innate cells central to the development of type 2 inflammation, likely by binding to FFAR1 [61]. These data suggest opposing functions of OA in type 1 and type 2 inflammation.
The role of other MUFAs (heptadecenoic acid, gadoleic acid, docosenoic acid, and tetracosenoic acid) in macrophage-mediated inflammation remains poorly understood.
2.4. Polyunsaturated Fatty Acids
PUFAs liberated from membrane phospholipids by PLA2 enzymes can be metabolized into oxylipins through three major enzymatic pathways (Figure 1). To understand the pathways involved in the activation macrophages, here, we compare FAs and oxylipins liberated by wild-type mouse BM-macrophages unstimulated (M0) or stimulated with LPS + IFNγ (M1) or IL-4 (M2) [61,62]. The hierarchical clustering analysis shows that, compared to M1 or M2 macrophages, unstimulated BM-macrophages (M0) prefer to generate SFAs (Figure 2). Instead, M1 macrophages produce mainly COX- and 15-LOX oxylipins derived from omega-6 FAs arachidonic acid (AA) and LA and omega-3 DHA, suggesting a broad and potent activation. M2 macrophages mainly generated AA-derived COX products. However, given the heterogeneity of macrophages, it is likely that activation of macrophages, particularly in vivo, involves additional pathways and FAs.

Figure 2.
Hierarchical clustering of FAs and oxylipins generated in BM-macrophages activated with IL-4 (M2), LPS + IFNγ (M1), or unstimulated controls (M0). BM-macrophages were cultured as previously described [61,62]. FAs and oxylipins were analyzed in cell pellets and supernatants, respectively, and previously shown as pmol/mg of protein (FAs) or pmol/mL (oxylipins) [61,62]. Data are from 3 independent experiments; shown are mean intensities of lipids that reached p < 0.05 by Anova.
2.4.1. Linoleic Acid
LA is an essential FA from which are generated oxylipins and PUFAs. In a model of zymosan-induced peritoneal inflammation, dihydroxy-eicosatrienoic acids (DHETs) and dihydroxy-octadecenoic acid (DiHOME) derived, respectively, from AA and LA by the action of CYP450 epoxygenases limited recruitment of proinflammatory monocytes CX3CR1low, CCR2high, and CCL2high [63], supporting the notion of a protective role of CYP450 derived epoxy-eicosatrienoic acids (EETs). However, 9-hydroxy-octadecadienoic acid (HODE) and 13-HODE, the LOX products of LA (Figure 1), increased the expression of the chemokine receptor CX3CR1 and reduced CCR2, favoring the adhesion of macrophages to coronary artery smooth muscle cells [64,65]. Additionally, 13-HODE, but not LA, stimulation of RAW 264.7 macrophages induced the expression of ATP-binding cassette transporter A1 (ABCA1) and increased cholesterol efflux in the presence of apolipoprotein A I [66]. Consistently OA and LA reduced cholesterol efflux in the presence of apolipoprotein A I by increasing ABCA1 degradation [67]. These studies may explain the protective function of LA-derived CYP450 products and the proinflammatory function of LA in atherosclerosis.
In type 2 inflammation, IL-4 upregulated 15-LOX in human monocyte-derived macrophages and 12/15-LOX in thioglycollate-elicited peritoneal macrophages. Indeed 15-HETE and 12/15-HETE were major products of macrophages activated by IL-4 in the presence of AA, while 13-HODE was the primary oxidative product of LA [68]. In a model of allergen-induced pulmonary inflammation, LA produced by macrophages induced activation of ILC2 expressing FFAR1 [61]. Another study supporting a proinflammatory role for LA in type 2 inflammation showed that IL-13 induced the release of 13-HODE from RAW 264.7 macrophages, thus suggesting that, at least in an ovalbumin (OVA) model of airway inflammation, macrophages contribute to epithelial injury [69]. Furthermore, LA was found to significantly increase in the serum obtained from patients with asthma COPD overlap [70].
2.4.2. Gamma-Linolenic Acid and Dihomo-Gamma-Linolenic Acid
An elongation product of LA is Gamma-linolenic acid (GLA, C18-3 N6). Despite being an omega-6 FA, GLA reduced the inflammatory response in LPS-activated RAW 256.7 macrophages by reducing phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and c-Jun N-terminal kinase (JNK)-1 [71]. Another elongation product of LA is eicosadienoic acid (EDA, C20:2), which is rapidly metabolized to di-homo-gamma-linolenic acid (DGLA, C20:3n6) and AA (C20:4). Indeed, stimulation of RAW 264.7 macrophages with EDA resulted in increased AA incorporation into membrane phospholipids and increased PGE2 production [72]. On the contrary, the addition of DGLA to human monocyte-derived macrophages resulted in reduced chemokine production and macrophage migration [73]. Macrophages activated with LPS and DGLA produce PGE1 and PGD1 [74], molecules that could compete with the series 2 of lipids derived from AA. Therefore, the beneficial effects of DGLA in type 1 inflammation could be direct or indirect.
2.4.3. Eicosatrienoic Acid
Eicosatrienoic acid (ETA, C20:3 N9) is an omega-9 fatty acid that is usually scarce unless there is a drastic reduction in the availability of AA. Indeed, following the reduction in AA, through the Lands cycle, the membrane was replenished with ETA N9 [31]. Although the function of ETA N9 in macrophage-driven inflammation needs to be further investigated, its presence indicates a deficiency in essential fatty acids [75].
2.4.4. Arachidonic Acid
AA is the primary FA of the cell plasma membrane. The importance of AA in type 1 inflammation driven by macrophages has been recently validated using mice lacking the long-chain acyl-CoA synthetase 4 (ACSL4), which preferentially incorporated AA into membrane phospholipids [76]. In a model of zymosan-induced peritoneal inflammation, the absence of ACSL4, specifically in macrophages, reduced neutrophil recruitment and LTB4 and PGE2 generation. Recently, the lipid profile of human monocyte-derived macrophages obtained by negative selection, cultivated in M-CSF for 7 days and polarized with LPS + IFNγ, or IL-4, revealed a significant increase in TxB2 in LPS + IFNγ activation compared to controls and IL-4 [77]. These results align with data obtained in mouse BM-macrophages (Figure 2) since TxB2 was increased in M1 compared to M2 and M0. However, as described above about LA, EETs derived from AA by CYP450 epoxygenases (Figure 1) reduced the expression of M1 molecules (iNOS, MCP-1, IL-6) and increased the expression of M2 molecules (Arg-1, CD206) in human monocytic cell lines [78]. Consistently, we found that 20-carboxyAA (20CooHAA), an eicosanoid produced from AA by cytochrome P450 (CYP) omega-oxidases, was increased in IL-4 activated mouse BM-macrophages and human monocyte-derived macrophages in a Pla2g5-dependent fashion [62], confirming the importance of this pathway in IL-4 activation.
2.4.5. Adrenic Acid
Although Adrenic acid (AdA, C22:4) is a FA derived by elongation of AA, AdA likely has specific functions in macrophages. Mouse peritoneal macrophages released AdA by the calcium-independent group VIA PLA2 following zymosan stimulation [79]. However, in inflammatory conditions, it is plausible that the liberation of AA from phospholipids is followed by its conversion into AdA [80] and subsequent re-acylation. Therefore, it has been hypothesized that AdA may function in the resolution phase of inflammation [81].
2.4.6. Docosapentaenoic Acid
Docosapentaenoic acid (DPA, C22:5 n6) is formed from AA. Interestingly, incubation of RAW 264.7 cells with the 15-LOX derived oxidation product 17-HDPA reduced M1 markers iNOs and TNF-α mRNA, and increased scavenger receptor mRNA, an M2 marker [82].
2.5. Omega-3 PUFAs
ALA (C18:3 N3) and other omega-3 PUFAs have been reported to increase certain features of M2 macrophage activation. BM-macrophages activated with IL-4 and ALA showed an increase in Arg-1 mRNA and CD206 expression, likely through the activation of GPR40 (FFAR1) and PPARγ [83]. In the THP-1 human monocytic cell line activated with LPS and IFNγ, ALA reduced the production of inflammatory cytokines and increased the release of oxylipins derived from ALA and LA [84].
Docosahexaenoic acid (DHA, C22:6 N3) in vivo has antidiabetic functions and downregulates type 1 inflammation likely by acting through GPR120 [85] and, at least in part, through cytosolic PLA2 activation and PGE2 generation [86]. BM-macrophages isolated from mice lacking ELOVL2, the enzyme catalyzing the conversion of PUFA C22 to C24 and activated with LPS + IFNγ or IL-4, resulted in increased expression of M1 molecules (iNOS, IL-6, IL-12, CD86) and reduced expression of M2 molecules (CD206, CCL22, CCL17), but not Arg-1, respectively. The addition of DHA to macrophage cultures could restore these defects [87]. Furthermore, the anti-inflammatory effect of DHA on RAW 264.7 cells included reduced expression of the histone deacetylases, particularly HDAC3, 4, and 9 [88].
Interestingly, in BM-macrophages, DHA and eicosapentaenoic acid (EPA, 20:5N3) inhibited inflammasome activation and LPS-induced IL-1β secretion, likely signaling through both FFAR1 and FFAR4 [89]. EPA reduced cholesterol efflux in human THP-1 macrophages [90], which may help explain the protective effects of EPA in chronic inflammation, namely atherosclerosis [91]. Indeed, administration of a combination of DHA and EPA reduced atherosclerosis lesions in mice fed a western diet, and incubation of RAW 264.7 macrophages with EPA and DHA reduced MCP-1 (at low dose) and TNF-α (at high dose) [92]. Similarly, in another study, incubation of RAW 264.7 macrophages with EPA reduced NO and PGE2 production induced by LPS. These effects were partially reproduced (only reduction of NO) also with Eicosatrienoic acid N3 (ETA, C20:3 N3) [93]. Reduced NO production and nuclear factor κB (NFκB) activation were also obtained by the incubation of LPS-stimulated RAW 264.7 macrophages with Stearidonic acid (SDA 18:4 N3) [94]. It is likely that the incorporation in the macrophage plasma membrane of omega-3 FAs, including EPA, DHA, and Docosapentaenoic acid (DPA, C22:5 N3), shifts the balance of TLR-4 induced macrophage activation from AA-derived eicosanoids to omega-3-derived oxylipins [95].
3. Concluding Remarks
Since the first comprehensive lipid analysis of mouse macrophages [18], the availability of lipid mass spectrometry has drastically expanded the studies on lipid functions related to the many faces of macrophage activation in health and disease. Furthermore, the identification of bioactive lipids produced by macrophages in various conditions has improved our understanding of the role of lipids in the pathogenesis of many diseases.
Studies of macrophage development highlight the relevance of FAs in the biology of macrophages. Intriguingly, the differentiation of human monocytes into macrophages was associated with increased FA synthesis and desaturation, with PA being the most abundant FA in differentiated macrophages [96]. Furthermore, the same authors reported an increase in glycerophospholipids (PLs) during macrophage differentiation, particularly phosphatidylcholine (PC), as expected since the volume of macrophages is larger than monocytes. Inhibition of FA synthesis reduced not only PLs content but also the expression of CD11b, CD36, and Mrc1, markers of macrophage differentiation, and phagocytosis. These effects are likely due to both MUFAs and PUFAs, since OA, LA, DHA, and EPA increased CD36 expression in THP-1 monocyte-macrophages [97].
Type 1 inflammation requires that macrophages kill pathogens and produce cytokine and chemokines necessary to mount an inflammatory response. SFAs and oxylipins strongly contribute to type 1 inflammation, as supported by many studies (Table 1) and recent lipidomic analysis (Figure 2). IL-4 activation of macrophages is one of the many M2 “alternatives” to M1 macrophages. However, M2 macrophages seem to produce fewer FAs and oxylipins than M1 macrophages, although studies are still scarce (Table 1). Therefore, M2 macrophages could offer an alternative to type one inflammation by replacing M1 macrophages.
The combination of lipidomic, metabolomic, and transcriptomic will likely offer in the future a more comprehensive evaluation of the pathways engaged by FAs and oxylipins in type 1 and type 2 inflammation, thus allowing the identification of new therapeutic targets.
Author Contributions
Conceptualization, B.B.; writing—original draft preparation, B.B.; formal analysis, D.F.D. and D.D.C.; writing—review and editing, B.B., D.F.D. and D.D.C.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Heart, Lung, and Blood Institute, Grant Number 113071.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Alpha-linolenic acid (ALA), arachidonic acid (AA), arginase-1 (Arg-1), cyclooxygenase-2 (COX-2), cytochrome P450 (CYP450), dihydroxy-eicosatrienoic acids (DHETs), dihydroxy-octadecenoic acid (DiHOME), linoleic acid (LA), 20-carboxyAA (20CooHAA), cysteinyl leukotrienes (CysLTs), fatty acids (FAs), desaturase (FAD), elongase (ELOVL), free fatty acid receptor (FFAR), group V phospholipase A2 (Pla2g5), hydroxy-octadecadienoic acid (HODE), innate lymphoid cells type 2 (ILC2s), lipoxygenase (LOX), Mannose receptor C-type 1 (Mrc1), monounsaturated fatty acids (MUFAs), nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 (NLRP3), polyunsaturated (PUFAs), saturated fatty acids (SFAs), Transforming growth factor beta-1 (Tgf-β1).
References
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Ginhoux, F. Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; LaVine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeksema, M.A.; Glass, C.K. Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis 2019, 281, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, X.; Simonavicius, N.; Tian, H.; Ling, L. Medium-chain Fatty Acids as Ligands for Orphan G Protein-coupled Receptor GPR64. J. Biol. Chem. 2006, 281, 34457–34464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberger, T.; Schaller, H.; Hellebrand, S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J. Mol. Biol. 2001, 307, 799–813. [Google Scholar] [CrossRef]
- Suzuki, M.; Takaishi, S.; Nagasaki, M.; Onozawa, Y.; Iino, I.; Maeda, H.; Komai, T.; Oda, T. Medium-chain Fatty Acid-sensing Receptor, GPR84, Is a Proinflammatory Receptor. J. Biol. Chem. 2013, 288, 10684–10691. [Google Scholar] [CrossRef] [Green Version]
- Vieira, W.A.; Gijsen, H.S.-V.; Ferris, W.F. Free fatty acid G-protein coupled receptor signaling in M1 skewed white adipose tissue macrophages. Cell. Mol. Life Sci. 2016, 73, 3665–3676. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Feingold, K.R.; Kazemi, M.R.; Magra, A.L.; McDonald, C.M.; Chui, L.G.; Shigenaga, J.K.; Patzek, S.M.; Chan, Z.W.; Londos, C.; Grunfeld, C. ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 2010, 209, 81–88. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid–binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef]
- Watkins, P.A.; Maiguel, D.; Jia, Z.; Pevsner, J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 2007, 48, 2736–2750. [Google Scholar] [CrossRef] [Green Version]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Fatty acid transport across the cell membrane: Regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 149–154. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Omega-3 versus Omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A2s. J. Lipid Res. 2021, 62, 100113. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Deems, R.A.; Harkewicz, R.; Quehenberger, O.; Brown, H.A.; Milne, S.B.; Myers, D.S.; Glass, C.K.; Hardiman, G.; Reichart, D.; et al. A Mouse Macrophage Lipidome. J. Biol. Chem. 2010, 285, 39976–39985. [Google Scholar] [CrossRef] [Green Version]
- Buczynski, M.W.; Stephens, D.L.; Bowers-Gentry, R.C.; Grkovich, A.; Deems, R.A.; A Dennis, E. TLR-4 and Sustained Calcium Agonists Synergistically Produce Eicosanoids Independent of Protein Synthesis in RAW264.7 Cells. J. Biol. Chem. 2007, 282, 22834–22847. [Google Scholar] [CrossRef] [Green Version]
- Andreyev, A.Y.; Fahy, E.; Guan, Z.; Kelly, S.; Li, X.; McDonald, J.G.; Milne, S.; Myers, D.; Park, H.; Ryan, A.; et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010, 51, 2785–2797. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Bentley, J.K.; Han, M.; Jaipalli, S.; Hinde, J.L.; Lei, J.; Ishikawa, T.; Goldsmith, A.M.; Rajput, C.; Hershenson, M.B. Myristoylated rhinovirus VP4 protein activates TLR2-dependent proinflammatory gene expression. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L57–L70. [Google Scholar] [CrossRef]
- Rowe, D.C.; McGettrick, A.F.; Latz, E.; Monks, B.G.; Gay, N.J.; Yamamoto, M.; Akira, S.; O’Neill, L.; Fitzgerald, K.; Golenbock, D.T. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc. Natl. Acad. Sci. USA 2006, 103, 6299–6304. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated Fatty Acids, but Not Unsaturated Fatty Acids, Induce the Expression of Cyclooxygenase-2 Mediated through Toll-like Receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Ye, J.; Gao, Z.; Youn, H.S.; Lee, W.H.; Zhao, L.; Sizemore, N.; Hwang, D.H. Reciprocal Modulation of Toll-like Receptor-4 Signaling Pathways Involving MyD88 and Phosphatidylinositol 3-Kinase/AKT by Saturated and Polyunsaturated Fatty Acids. J. Biol. Chem. 2003, 278, 37041–37051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, A.W.B.; Kim, H.; Huy, T.X.N.; Vu, S.H.; Nguyen, T.T.; Kang, C.K.; Min, W.; Lee, H.J.; Lee, J.H.; Kim, S. Immune-metabolic receptor GPR84 surrogate and endogenous agonists, 6-OAU and lauric acid, alter Brucella abortus 544 infection in both in vitro and in vivo systems. Microb. Pathog. 2021, 158, 105079. [Google Scholar] [CrossRef]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.T.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Yoo, W.; Gjuka, D.; Stevenson, H.; Song, X.; Shen, H.; Yoo, S.Y.; Wang, J.; Fallon, M.; Ioannou, G.N.; Harrison, S.A.; et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS ONE 2017, 12, e0189965. [Google Scholar] [CrossRef] [Green Version]
- Okuno, T.; Gijon, M.A.; Zarini, S.; Martin, S.A.; Barkley, R.M.; Johnson, C.A.; Ohba, M.; Yokomizo, T.; Murphy, R.C. Altered eicosanoid production and phospholipid remodeling during cell culture. J. Lipid Res. 2018, 59, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiyama, J.; Taguchi, R.; Akasaka, Y.; Shibata, S.; Ito, M.; Nagasawa, M.; Murakami, K. Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 2011, 52, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, E.R.; Kulkarni, M.; Gabriel, J.; Cichon, M.J.; Riedl, K.; Belury, M.A.; Lake, J.E.; Richardson, B.; Cameron, C.; Cameron, M.; et al. Altered Lipidome Composition Is Related to Markers of Monocyte and Immune Activation in Antiretroviral Therapy Treated Human Immunodeficiency Virus (HIV) Infection and in Uninfected Persons. Front. Immunol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef] [PubMed]
- L’Homme, L.; Esser, N.; Riva, L.; Scheen, A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J. Lipid Res. 2013, 54, 2998–3008. [Google Scholar] [CrossRef] [Green Version]
- Tam, T.H.; Chan, K.L.; Boroumand, P.; Liu, Z.; Brozinick, J.T.; Bui, H.H.; Roth, K.; Wakefield, C.B.; Penuela, S.; Bilan, P.J.; et al. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J. Biol. Chem. 2020, 295, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Riera-Borrull, M.; Cuevas, V.D.; Alonso, B.; Vega, M.A.; Joven, J.; Izquierdo, E.; Corbí, Á.L. Palmitate Conditions Macrophages for Enhanced Responses toward Inflammatory Stimuli via JNK Activation. J. Immunol. 2017, 199, 3858–3869. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The Orphan G Protein-coupled Receptor GPR40 Is Activated by Medium and Long Chain Fatty Acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Mancini, A.D.; Bertrand, G.; Vivot, K.; Carpentier, É.; Tremblay, C.; Ghislain, J.; Bouvier, M.; Poitout, V. β-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J. Biol. Chem. 2015, 290, 21131–21140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, Y.; Wang, F.; Wang, Z.; Lu, Y.; Xu, Y.; Wang, K.; Shen, H.; Yang, P.; Li, S.; et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci. Rep. 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosquera-Restrepo, S.F.; Caro, A.C.; Peláez-Jaramillo, C.A.; Rojas, M. Mononuclear phagocyte accumulates a stearic acid derivative during differentiation into macrophages. Effects of stearic acid on macrophage differentiation and Mycobacterium tuberculosis control. Cell. Immunol. 2016, 303, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic Acid Accumulation in Macrophages Induces Toll-Like Receptor 4/2-Independent Inflammation Leading to Endoplasmic Reticulum Stress–Mediated Apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; Koyama, Y.; Sato-Tomita, A.; Matsuzaka, T.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 744–756. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Misialek, J.R.; Jing, M.; Tsai, M.Y.; Eckfeldt, J.H.; Steffen, L.M.; Knopman, D.; Wruck, L.; Gottesman, R.; Mosley, T.H.; et al. Plasma phospholipid very-long-chain SFAs in midlife and 20-year cognitive change in the Atherosclerosis Risk in Communities (ARIC): A cohort study. Am. J. Clin. Nutr. 2020, 111, 1252–1258. [Google Scholar] [CrossRef]
- Liu, M.; Zuo, L.-S.-Y.; Sun, T.-Y.; Wu, Y.-Y.; Liu, Y.-P.; Zeng, F.-F.; Chen, Y.-M. Circulating Very-Long-Chain Saturated Fatty Acids Were Inversely Associated with Cardiovascular Health: A Prospective Cohort Study and Meta-Analysis. Nutrients 2020, 12, 2709. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Astudillo, A.; Meana, C.; Bermúdez, M.; Pérez-Encabo, A.; Balboa, M.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Pillon, N.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Théret, M.; Chazaud, B.; Klip, A. Palmitoleate Reverses High Fat-induced Proinflammatory Macrophage Polarization via AMP-activated Protein Kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.O.; Teixeira, A.A.; Biondo, L.A.; Silveira, L.S.; Breda, C.N.D.S.; Braga, T.T.; Camara, N.O.; Belchior, T.; Festuccia, W.; Diniz, T.A.; et al. Palmitoleic acid reduces high fat diet-induced liver inflammation by promoting PPAR-γ-independent M2a polarization of myeloid cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158776. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, Y.M.; Lee, K.H.; Jeong, S.-W.; Kwon, O.-J. Oleate protects macrophages from palmitate-induced apoptosis through the downregulation of CD36 expression. Biochem. Biophys. Res. Commun. 2017, 488, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Pardo, V.; González-Rodríguez, Á.; Guijas, C.; Balsinde, J.; Valverde, Á.M. Opposite Cross-Talk by Oleate and Palmitate on Insulin Signaling in Hepatocytes through Macrophage Activation. J. Biol. Chem. 2015, 290, 11663–11677. [Google Scholar] [CrossRef] [Green Version]
- Camell, C.; Smith, C.W. Dietary Oleic Acid Increases M2 Macrophages in the Mesenteric Adipose Tissue. PLoS ONE 2013, 8, e75147. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Weidinger, C.; Schmidt, F.; Keye, J.; Friedrich, M.; Yerinde, C.; Willimsky, G.; Qin, Z.; Siegmund, B.; Glauben, R. Oleate but not stearate induces the regulatory phenotype of myeloid suppressor cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hunsche, C.; Hernandez, O.; Gheorghe, A.; Díaz, L.E.; Marcos, A.; De La Fuente, M. Immune dysfunction and increased oxidative stress state in diet-induced obese mice are reverted by nutritional supplementation with monounsaturated and n-3 polyunsaturated fatty acids. Eur. J. Nutr. 2017, 57, 1123–1135. [Google Scholar] [CrossRef]
- Freigang, S.; Ampenberger, F.; Weiss, A.; Kanneganti, T.-D.; Iwakura, Y.; Hersberger, M.; Kopf, M. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 2013, 14, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas-García, M.; Bressan, J.; Martínez, J.; Milagro, F. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: Effects of fatty acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Samuchiwal, S.; Quehenberger, O.; Boyce, J.A.; Balestrieri, B. Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms. Mucosal Immunol. 2018, 11, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Koganesawa, M.; Yamaguchi, M.; Samuchiwal, S.K.; Balestrieri, B. Lipid Profile of Activated Macrophages and Contribution of Group V Phospholipase A2. Biomolecules 2020, 11, 25. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; De Maeyer, R.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.; Bishop-Bailey, D. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef] [Green Version]
- Barlic, J.; Zhang, Y.; Foley, J.F.; Murphy, P.M. Oxidized Lipid-Driven Chemokine Receptor Switch, CCR2 to CX3CR1, Mediates Adhesion of Human Macrophages to Coronary Artery Smooth Muscle Cells Through a Peroxisome Proliferator-Activated Receptor γ–Dependent Pathway. Circulation 2006, 114, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Barlic, J.; Zhang, Y.; Murphy, P.M. Atherogenic Lipids Induce Adhesion of Human Coronary Artery Smooth Muscle Cells to Macrophages by Up-regulating Chemokine CX3CL1 on Smooth Muscle Cells in a TNFα-NFκB-dependent Manner. J. Biol. Chem. 2007, 282, 19167–19176. [Google Scholar] [CrossRef] [Green Version]
- Kämmerer, I.; Ringseis, R.; Biemann, R.; Wen, G.; Eder, K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis. 2011, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Kanter, J.E.; Tang, C.; Oram, J.F.; Bornfeldt, K.E. Acyl-CoA synthetase 1 is required for oleate and linoleate mediated inhibition of cholesterol efflux through ATP-binding cassette transporter A1 in macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.; Conrad, D.; Glass, C.K. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, srep01540. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Choudhury, P.; Kaushik, S.R.; Arya, R.; Nanda, R.; Bhattacharyya, P.; Roychowdhury, S.; Banerjee, R.; Chaudhury, K. Metabolomic fingerprinting and systemic inflammatory profiling of asthma COPD overlap (ACO). Respir. Res. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Sun, H.-L.; Lii, C.-K.; Chen, H.-W.; Chen, P.-Y.; Liu, K.-L. Gamma-Linolenic Acid Inhibits Inflammatory Responses by Regulating NF-κB and AP-1 Activation in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Huang, W.-C.; Li, C.-W.; Chuang, L.-T. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol. Cell. Biochem. 2011, 358, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.; Williams, J.O.; Ferekidis, N.; Ismail, A.; Chan, Y.-H.; Michael, D.R.; Guschina, I.A.; Tyrrell, V.J.; O’Donnell, V.B.; Harwood, J.L.; et al. Dihomo-γ-linolenic acid inhibits several key cellular processes associated with atherosclerosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Kawashima, H.; Tanaka, T.; Shiraishi-Tateishi, A.; Kiso, Y. Uptake of dihomo-γ-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ichi, I.; Kono, N.; Arita, Y.; Haga, S.; Arisawa, K.; Yamano, M.; Nagase, M.; Fujiwara, Y.; Arai, H. Identification of genes and pathways involved in the synthesis of Mead acid (20:3n−9), an indicator of essential fatty acid deficiency. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 204–213. [Google Scholar] [CrossRef]
- Reeves, A.R.; Sansbury, B.E.; Pan, M.; Han, X.; Spite, M.; Greenberg, A.S. Myeloid-Specific Deficiency of Long-Chain Acyl CoA Synthetase 4 Reduces Inflammation by Remodeling Phospholipids and Reducing Production of Arachidonic Acid–Derived Proinflammatory Lipid Mediators. J. Immunol. 2021, 207, 2744–2753. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Sutton, J.A.; Rogers, L.M.; Milne, G.L.; McLean, J.A.; Aronoff, D.M. Lipid profiling of polarized human monocyte-derived macrophages. Prostaglandins Other Lipid Mediat. 2016, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wu, L.; He, Z.; Zhang, S.; Chen, C.; Xu, X.; Wang, P.; Gruzdev, A.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic Acids Regulate Macrophage Polarization and Prevent LPS-Induced Cardiac Dysfunction. J. Cell. Physiol. 2015, 230, 2108–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monge, P.; Garrido, A.; Rubio, J.M.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. The Contribution of Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A2s to Adrenic Acid Mobilization in Murine Macrophages. Biomolecules 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Guijas, C.; Astudillo, A.M.; Gil-De-Gómez, L.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipid sources for adrenic acid mobilization in RAW 264.7 macrophages. Comparison with arachidonic acid. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, H.; Jónasdóttir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; Gonzalez-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, I.; et al. Anti-Inflammatory and Proresolving Effects of the Omega-6 Polyunsaturated Fatty Acid Adrenic Acid. J. Immunol. 2020, 205, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Gomolka, B.; Dierkes, C.; Huang, N.R.; Schroeder, M.; Purschke, M.; Manstein, D.; Dangi, B.; Weylandt, K.H. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm. Res. 2012, 61, 967–976. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Yasuoka, Y.; Goto, T.; Kitamura, N.; Park, S.; Kishino, S.; Kimura, I.; Kasubuchi, M.; Takahashi, H.; Li, Y.; et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. 2018, 32, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauls, S.; Rodway, L.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, L.-Y.; Sokolowska, M.; Eberlein, M.; Alsaaty, S.; Anton, A.M.; Logun, C.; Qi, H.-Y.; Shelhamer, J.H. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A2via GPR120 receptor to produce prostaglandin E2and plays an anti-inflammatory role in macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef]
- Talamonti, E.; Pauter, A.M.; Asadi, A.; Fischer, A.; Chiurchiù, V.; Jacobsson, A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: Implications for DHA supplementation during inflammation. Cell. Mol. Life Sci. 2017, 74, 2815–2826. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.X.; Bae, M.; Lee, Y.; Park, Y.-K.; Lee, J.-Y. Transcriptional and posttranscriptional repression of histone deacetylases by docosahexaenoic acid in macrophages. J. Nutr. Biochem. 2018, 57, 162–169. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Fournier, N.; Sayet, G.; Vedie, B.; Nowak, M.; Allaoui, F.; Solgadi, A.; Caudron, E.; Chaminade, P.; Benoist, J.-F.; Paul, J.-L. Eicosapentaenoic acid membrane incorporation impairs cholesterol efflux from cholesterol-loaded human macrophages by reducing the cholesteryl ester mobilization from lipid droplets. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1079–1091. [Google Scholar] [CrossRef]
- Dakroub, H.; Nowak, M.; Benoist, J.-F.; Noël, B.; Vedie, B.; Paul, J.-L.; Fournier, N. Eicosapentaenoic acid membrane incorporation stimulates ABCA1-mediated cholesterol efflux from human THP-1 macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2021, 1866, 159016. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 2016, 254, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chuang, L.-T.; Chen, S.-N. Incorporation of Eicosatrienoic Acid Exerts Mild Anti-inflammatory Properties in Murine RAW264.7 Cells. Inflammation 2014, 38, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Jeon, H.; Kim, I.-H.; Jeong, H.S.; Lee, J. Anti-Inflammatory Effects of Stearidonic Acid Mediated by Suppression of NF-κB and MAP-Kinase Pathways in Macrophages. Lipids 2017, 52, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.; Dennis, E.A. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 8517–8522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, J.; Liebisch, G.; Englmaier, M.; Grandl, M.; Robenek, H.; Schmitz, G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 7817–7822. [Google Scholar] [CrossRef] [Green Version]
- Vallvé, J.-C.; Uliaque, K.; Girona, J.; Cabré, A.; Ribalta, J.; Heras, M.; Masana, L. Unsaturated fatty acids and their oxidation products stimulate CD36 gene expression in human macrophages. Atherosclerosis 2002, 164, 45–56. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).