Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins
Abstract
:1. Introduction
2. Fatty Acids
2.1. Medium-Chain and Uneven Saturated Fatty Acids
2.2. Long-Chain Saturated Fatty Acids
2.3. Monounsaturated Fatty Acids
2.4. Polyunsaturated Fatty Acids
2.4.1. Linoleic Acid
2.4.2. Gamma-Linolenic Acid and Dihomo-Gamma-Linolenic Acid
2.4.3. Eicosatrienoic Acid
2.4.4. Arachidonic Acid
2.4.5. Adrenic Acid
2.4.6. Docosapentaenoic Acid
2.5. Omega-3 PUFAs
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Ginhoux, F. Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; LaVine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeksema, M.A.; Glass, C.K. Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis 2019, 281, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, X.; Simonavicius, N.; Tian, H.; Ling, L. Medium-chain Fatty Acids as Ligands for Orphan G Protein-coupled Receptor GPR64. J. Biol. Chem. 2006, 281, 34457–34464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberger, T.; Schaller, H.; Hellebrand, S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J. Mol. Biol. 2001, 307, 799–813. [Google Scholar] [CrossRef]
- Suzuki, M.; Takaishi, S.; Nagasaki, M.; Onozawa, Y.; Iino, I.; Maeda, H.; Komai, T.; Oda, T. Medium-chain Fatty Acid-sensing Receptor, GPR84, Is a Proinflammatory Receptor. J. Biol. Chem. 2013, 288, 10684–10691. [Google Scholar] [CrossRef] [Green Version]
- Vieira, W.A.; Gijsen, H.S.-V.; Ferris, W.F. Free fatty acid G-protein coupled receptor signaling in M1 skewed white adipose tissue macrophages. Cell. Mol. Life Sci. 2016, 73, 3665–3676. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Feingold, K.R.; Kazemi, M.R.; Magra, A.L.; McDonald, C.M.; Chui, L.G.; Shigenaga, J.K.; Patzek, S.M.; Chan, Z.W.; Londos, C.; Grunfeld, C. ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 2010, 209, 81–88. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid–binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef]
- Watkins, P.A.; Maiguel, D.; Jia, Z.; Pevsner, J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 2007, 48, 2736–2750. [Google Scholar] [CrossRef] [Green Version]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Fatty acid transport across the cell membrane: Regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 149–154. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Omega-3 versus Omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A2s. J. Lipid Res. 2021, 62, 100113. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Deems, R.A.; Harkewicz, R.; Quehenberger, O.; Brown, H.A.; Milne, S.B.; Myers, D.S.; Glass, C.K.; Hardiman, G.; Reichart, D.; et al. A Mouse Macrophage Lipidome. J. Biol. Chem. 2010, 285, 39976–39985. [Google Scholar] [CrossRef] [Green Version]
- Buczynski, M.W.; Stephens, D.L.; Bowers-Gentry, R.C.; Grkovich, A.; Deems, R.A.; A Dennis, E. TLR-4 and Sustained Calcium Agonists Synergistically Produce Eicosanoids Independent of Protein Synthesis in RAW264.7 Cells. J. Biol. Chem. 2007, 282, 22834–22847. [Google Scholar] [CrossRef] [Green Version]
- Andreyev, A.Y.; Fahy, E.; Guan, Z.; Kelly, S.; Li, X.; McDonald, J.G.; Milne, S.; Myers, D.; Park, H.; Ryan, A.; et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010, 51, 2785–2797. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Bentley, J.K.; Han, M.; Jaipalli, S.; Hinde, J.L.; Lei, J.; Ishikawa, T.; Goldsmith, A.M.; Rajput, C.; Hershenson, M.B. Myristoylated rhinovirus VP4 protein activates TLR2-dependent proinflammatory gene expression. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L57–L70. [Google Scholar] [CrossRef]
- Rowe, D.C.; McGettrick, A.F.; Latz, E.; Monks, B.G.; Gay, N.J.; Yamamoto, M.; Akira, S.; O’Neill, L.; Fitzgerald, K.; Golenbock, D.T. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc. Natl. Acad. Sci. USA 2006, 103, 6299–6304. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated Fatty Acids, but Not Unsaturated Fatty Acids, Induce the Expression of Cyclooxygenase-2 Mediated through Toll-like Receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Ye, J.; Gao, Z.; Youn, H.S.; Lee, W.H.; Zhao, L.; Sizemore, N.; Hwang, D.H. Reciprocal Modulation of Toll-like Receptor-4 Signaling Pathways Involving MyD88 and Phosphatidylinositol 3-Kinase/AKT by Saturated and Polyunsaturated Fatty Acids. J. Biol. Chem. 2003, 278, 37041–37051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, A.W.B.; Kim, H.; Huy, T.X.N.; Vu, S.H.; Nguyen, T.T.; Kang, C.K.; Min, W.; Lee, H.J.; Lee, J.H.; Kim, S. Immune-metabolic receptor GPR84 surrogate and endogenous agonists, 6-OAU and lauric acid, alter Brucella abortus 544 infection in both in vitro and in vivo systems. Microb. Pathog. 2021, 158, 105079. [Google Scholar] [CrossRef]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.T.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Yoo, W.; Gjuka, D.; Stevenson, H.; Song, X.; Shen, H.; Yoo, S.Y.; Wang, J.; Fallon, M.; Ioannou, G.N.; Harrison, S.A.; et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS ONE 2017, 12, e0189965. [Google Scholar] [CrossRef] [Green Version]
- Okuno, T.; Gijon, M.A.; Zarini, S.; Martin, S.A.; Barkley, R.M.; Johnson, C.A.; Ohba, M.; Yokomizo, T.; Murphy, R.C. Altered eicosanoid production and phospholipid remodeling during cell culture. J. Lipid Res. 2018, 59, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiyama, J.; Taguchi, R.; Akasaka, Y.; Shibata, S.; Ito, M.; Nagasawa, M.; Murakami, K. Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 2011, 52, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, E.R.; Kulkarni, M.; Gabriel, J.; Cichon, M.J.; Riedl, K.; Belury, M.A.; Lake, J.E.; Richardson, B.; Cameron, C.; Cameron, M.; et al. Altered Lipidome Composition Is Related to Markers of Monocyte and Immune Activation in Antiretroviral Therapy Treated Human Immunodeficiency Virus (HIV) Infection and in Uninfected Persons. Front. Immunol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef] [PubMed]
- L’Homme, L.; Esser, N.; Riva, L.; Scheen, A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J. Lipid Res. 2013, 54, 2998–3008. [Google Scholar] [CrossRef] [Green Version]
- Tam, T.H.; Chan, K.L.; Boroumand, P.; Liu, Z.; Brozinick, J.T.; Bui, H.H.; Roth, K.; Wakefield, C.B.; Penuela, S.; Bilan, P.J.; et al. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J. Biol. Chem. 2020, 295, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Riera-Borrull, M.; Cuevas, V.D.; Alonso, B.; Vega, M.A.; Joven, J.; Izquierdo, E.; Corbí, Á.L. Palmitate Conditions Macrophages for Enhanced Responses toward Inflammatory Stimuli via JNK Activation. J. Immunol. 2017, 199, 3858–3869. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The Orphan G Protein-coupled Receptor GPR40 Is Activated by Medium and Long Chain Fatty Acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Mancini, A.D.; Bertrand, G.; Vivot, K.; Carpentier, É.; Tremblay, C.; Ghislain, J.; Bouvier, M.; Poitout, V. β-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J. Biol. Chem. 2015, 290, 21131–21140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, Y.; Wang, F.; Wang, Z.; Lu, Y.; Xu, Y.; Wang, K.; Shen, H.; Yang, P.; Li, S.; et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci. Rep. 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosquera-Restrepo, S.F.; Caro, A.C.; Peláez-Jaramillo, C.A.; Rojas, M. Mononuclear phagocyte accumulates a stearic acid derivative during differentiation into macrophages. Effects of stearic acid on macrophage differentiation and Mycobacterium tuberculosis control. Cell. Immunol. 2016, 303, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic Acid Accumulation in Macrophages Induces Toll-Like Receptor 4/2-Independent Inflammation Leading to Endoplasmic Reticulum Stress–Mediated Apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; Koyama, Y.; Sato-Tomita, A.; Matsuzaka, T.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 744–756. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Misialek, J.R.; Jing, M.; Tsai, M.Y.; Eckfeldt, J.H.; Steffen, L.M.; Knopman, D.; Wruck, L.; Gottesman, R.; Mosley, T.H.; et al. Plasma phospholipid very-long-chain SFAs in midlife and 20-year cognitive change in the Atherosclerosis Risk in Communities (ARIC): A cohort study. Am. J. Clin. Nutr. 2020, 111, 1252–1258. [Google Scholar] [CrossRef]
- Liu, M.; Zuo, L.-S.-Y.; Sun, T.-Y.; Wu, Y.-Y.; Liu, Y.-P.; Zeng, F.-F.; Chen, Y.-M. Circulating Very-Long-Chain Saturated Fatty Acids Were Inversely Associated with Cardiovascular Health: A Prospective Cohort Study and Meta-Analysis. Nutrients 2020, 12, 2709. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Astudillo, A.; Meana, C.; Bermúdez, M.; Pérez-Encabo, A.; Balboa, M.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Pillon, N.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Théret, M.; Chazaud, B.; Klip, A. Palmitoleate Reverses High Fat-induced Proinflammatory Macrophage Polarization via AMP-activated Protein Kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.O.; Teixeira, A.A.; Biondo, L.A.; Silveira, L.S.; Breda, C.N.D.S.; Braga, T.T.; Camara, N.O.; Belchior, T.; Festuccia, W.; Diniz, T.A.; et al. Palmitoleic acid reduces high fat diet-induced liver inflammation by promoting PPAR-γ-independent M2a polarization of myeloid cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158776. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, Y.M.; Lee, K.H.; Jeong, S.-W.; Kwon, O.-J. Oleate protects macrophages from palmitate-induced apoptosis through the downregulation of CD36 expression. Biochem. Biophys. Res. Commun. 2017, 488, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Pardo, V.; González-Rodríguez, Á.; Guijas, C.; Balsinde, J.; Valverde, Á.M. Opposite Cross-Talk by Oleate and Palmitate on Insulin Signaling in Hepatocytes through Macrophage Activation. J. Biol. Chem. 2015, 290, 11663–11677. [Google Scholar] [CrossRef] [Green Version]
- Camell, C.; Smith, C.W. Dietary Oleic Acid Increases M2 Macrophages in the Mesenteric Adipose Tissue. PLoS ONE 2013, 8, e75147. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Weidinger, C.; Schmidt, F.; Keye, J.; Friedrich, M.; Yerinde, C.; Willimsky, G.; Qin, Z.; Siegmund, B.; Glauben, R. Oleate but not stearate induces the regulatory phenotype of myeloid suppressor cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hunsche, C.; Hernandez, O.; Gheorghe, A.; Díaz, L.E.; Marcos, A.; De La Fuente, M. Immune dysfunction and increased oxidative stress state in diet-induced obese mice are reverted by nutritional supplementation with monounsaturated and n-3 polyunsaturated fatty acids. Eur. J. Nutr. 2017, 57, 1123–1135. [Google Scholar] [CrossRef]
- Freigang, S.; Ampenberger, F.; Weiss, A.; Kanneganti, T.-D.; Iwakura, Y.; Hersberger, M.; Kopf, M. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 2013, 14, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas-García, M.; Bressan, J.; Martínez, J.; Milagro, F. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: Effects of fatty acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Samuchiwal, S.; Quehenberger, O.; Boyce, J.A.; Balestrieri, B. Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms. Mucosal Immunol. 2018, 11, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Koganesawa, M.; Yamaguchi, M.; Samuchiwal, S.K.; Balestrieri, B. Lipid Profile of Activated Macrophages and Contribution of Group V Phospholipase A2. Biomolecules 2020, 11, 25. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; De Maeyer, R.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.; Bishop-Bailey, D. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef] [Green Version]
- Barlic, J.; Zhang, Y.; Foley, J.F.; Murphy, P.M. Oxidized Lipid-Driven Chemokine Receptor Switch, CCR2 to CX3CR1, Mediates Adhesion of Human Macrophages to Coronary Artery Smooth Muscle Cells Through a Peroxisome Proliferator-Activated Receptor γ–Dependent Pathway. Circulation 2006, 114, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Barlic, J.; Zhang, Y.; Murphy, P.M. Atherogenic Lipids Induce Adhesion of Human Coronary Artery Smooth Muscle Cells to Macrophages by Up-regulating Chemokine CX3CL1 on Smooth Muscle Cells in a TNFα-NFκB-dependent Manner. J. Biol. Chem. 2007, 282, 19167–19176. [Google Scholar] [CrossRef] [Green Version]
- Kämmerer, I.; Ringseis, R.; Biemann, R.; Wen, G.; Eder, K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis. 2011, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Kanter, J.E.; Tang, C.; Oram, J.F.; Bornfeldt, K.E. Acyl-CoA synthetase 1 is required for oleate and linoleate mediated inhibition of cholesterol efflux through ATP-binding cassette transporter A1 in macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.; Conrad, D.; Glass, C.K. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, srep01540. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Choudhury, P.; Kaushik, S.R.; Arya, R.; Nanda, R.; Bhattacharyya, P.; Roychowdhury, S.; Banerjee, R.; Chaudhury, K. Metabolomic fingerprinting and systemic inflammatory profiling of asthma COPD overlap (ACO). Respir. Res. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Sun, H.-L.; Lii, C.-K.; Chen, H.-W.; Chen, P.-Y.; Liu, K.-L. Gamma-Linolenic Acid Inhibits Inflammatory Responses by Regulating NF-κB and AP-1 Activation in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Huang, W.-C.; Li, C.-W.; Chuang, L.-T. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol. Cell. Biochem. 2011, 358, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.; Williams, J.O.; Ferekidis, N.; Ismail, A.; Chan, Y.-H.; Michael, D.R.; Guschina, I.A.; Tyrrell, V.J.; O’Donnell, V.B.; Harwood, J.L.; et al. Dihomo-γ-linolenic acid inhibits several key cellular processes associated with atherosclerosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Kawashima, H.; Tanaka, T.; Shiraishi-Tateishi, A.; Kiso, Y. Uptake of dihomo-γ-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ichi, I.; Kono, N.; Arita, Y.; Haga, S.; Arisawa, K.; Yamano, M.; Nagase, M.; Fujiwara, Y.; Arai, H. Identification of genes and pathways involved in the synthesis of Mead acid (20:3n−9), an indicator of essential fatty acid deficiency. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 204–213. [Google Scholar] [CrossRef]
- Reeves, A.R.; Sansbury, B.E.; Pan, M.; Han, X.; Spite, M.; Greenberg, A.S. Myeloid-Specific Deficiency of Long-Chain Acyl CoA Synthetase 4 Reduces Inflammation by Remodeling Phospholipids and Reducing Production of Arachidonic Acid–Derived Proinflammatory Lipid Mediators. J. Immunol. 2021, 207, 2744–2753. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Sutton, J.A.; Rogers, L.M.; Milne, G.L.; McLean, J.A.; Aronoff, D.M. Lipid profiling of polarized human monocyte-derived macrophages. Prostaglandins Other Lipid Mediat. 2016, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wu, L.; He, Z.; Zhang, S.; Chen, C.; Xu, X.; Wang, P.; Gruzdev, A.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic Acids Regulate Macrophage Polarization and Prevent LPS-Induced Cardiac Dysfunction. J. Cell. Physiol. 2015, 230, 2108–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monge, P.; Garrido, A.; Rubio, J.M.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. The Contribution of Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A2s to Adrenic Acid Mobilization in Murine Macrophages. Biomolecules 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Guijas, C.; Astudillo, A.M.; Gil-De-Gómez, L.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipid sources for adrenic acid mobilization in RAW 264.7 macrophages. Comparison with arachidonic acid. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, H.; Jónasdóttir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; Gonzalez-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, I.; et al. Anti-Inflammatory and Proresolving Effects of the Omega-6 Polyunsaturated Fatty Acid Adrenic Acid. J. Immunol. 2020, 205, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Gomolka, B.; Dierkes, C.; Huang, N.R.; Schroeder, M.; Purschke, M.; Manstein, D.; Dangi, B.; Weylandt, K.H. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm. Res. 2012, 61, 967–976. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Yasuoka, Y.; Goto, T.; Kitamura, N.; Park, S.; Kishino, S.; Kimura, I.; Kasubuchi, M.; Takahashi, H.; Li, Y.; et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. 2018, 32, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauls, S.; Rodway, L.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, L.-Y.; Sokolowska, M.; Eberlein, M.; Alsaaty, S.; Anton, A.M.; Logun, C.; Qi, H.-Y.; Shelhamer, J.H. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A2via GPR120 receptor to produce prostaglandin E2and plays an anti-inflammatory role in macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef]
- Talamonti, E.; Pauter, A.M.; Asadi, A.; Fischer, A.; Chiurchiù, V.; Jacobsson, A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: Implications for DHA supplementation during inflammation. Cell. Mol. Life Sci. 2017, 74, 2815–2826. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.X.; Bae, M.; Lee, Y.; Park, Y.-K.; Lee, J.-Y. Transcriptional and posttranscriptional repression of histone deacetylases by docosahexaenoic acid in macrophages. J. Nutr. Biochem. 2018, 57, 162–169. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Fournier, N.; Sayet, G.; Vedie, B.; Nowak, M.; Allaoui, F.; Solgadi, A.; Caudron, E.; Chaminade, P.; Benoist, J.-F.; Paul, J.-L. Eicosapentaenoic acid membrane incorporation impairs cholesterol efflux from cholesterol-loaded human macrophages by reducing the cholesteryl ester mobilization from lipid droplets. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1079–1091. [Google Scholar] [CrossRef]
- Dakroub, H.; Nowak, M.; Benoist, J.-F.; Noël, B.; Vedie, B.; Paul, J.-L.; Fournier, N. Eicosapentaenoic acid membrane incorporation stimulates ABCA1-mediated cholesterol efflux from human THP-1 macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2021, 1866, 159016. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 2016, 254, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chuang, L.-T.; Chen, S.-N. Incorporation of Eicosatrienoic Acid Exerts Mild Anti-inflammatory Properties in Murine RAW264.7 Cells. Inflammation 2014, 38, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Jeon, H.; Kim, I.-H.; Jeong, H.S.; Lee, J. Anti-Inflammatory Effects of Stearidonic Acid Mediated by Suppression of NF-κB and MAP-Kinase Pathways in Macrophages. Lipids 2017, 52, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.; Dennis, E.A. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 8517–8522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, J.; Liebisch, G.; Englmaier, M.; Grandl, M.; Robenek, H.; Schmitz, G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 7817–7822. [Google Scholar] [CrossRef] [Green Version]
- Vallvé, J.-C.; Uliaque, K.; Girona, J.; Cabré, A.; Ribalta, J.; Heras, M.; Masana, L. Unsaturated fatty acids and their oxidation products stimulate CD36 gene expression in human macrophages. Atherosclerosis 2002, 164, 45–56. [Google Scholar] [CrossRef]
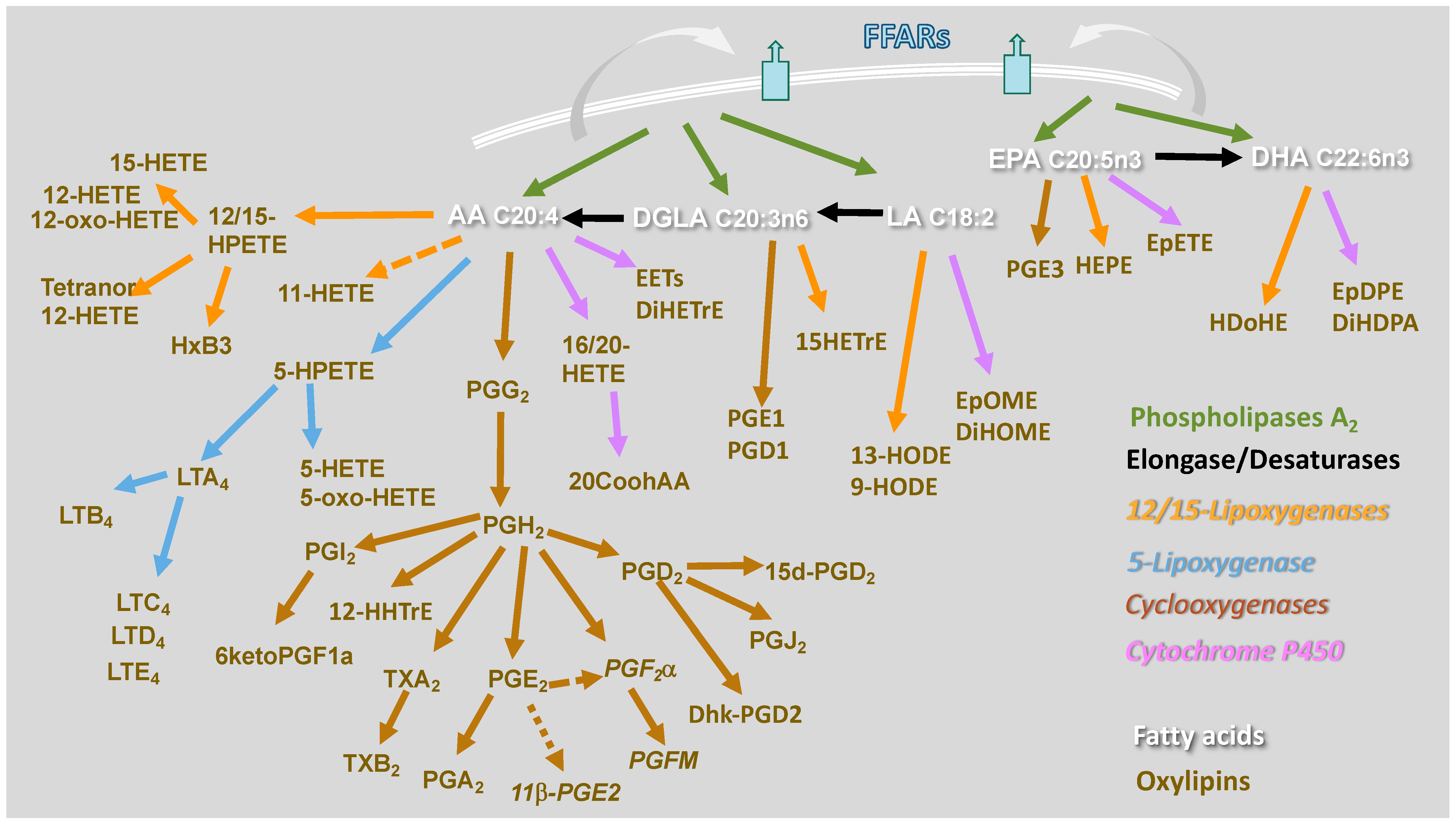
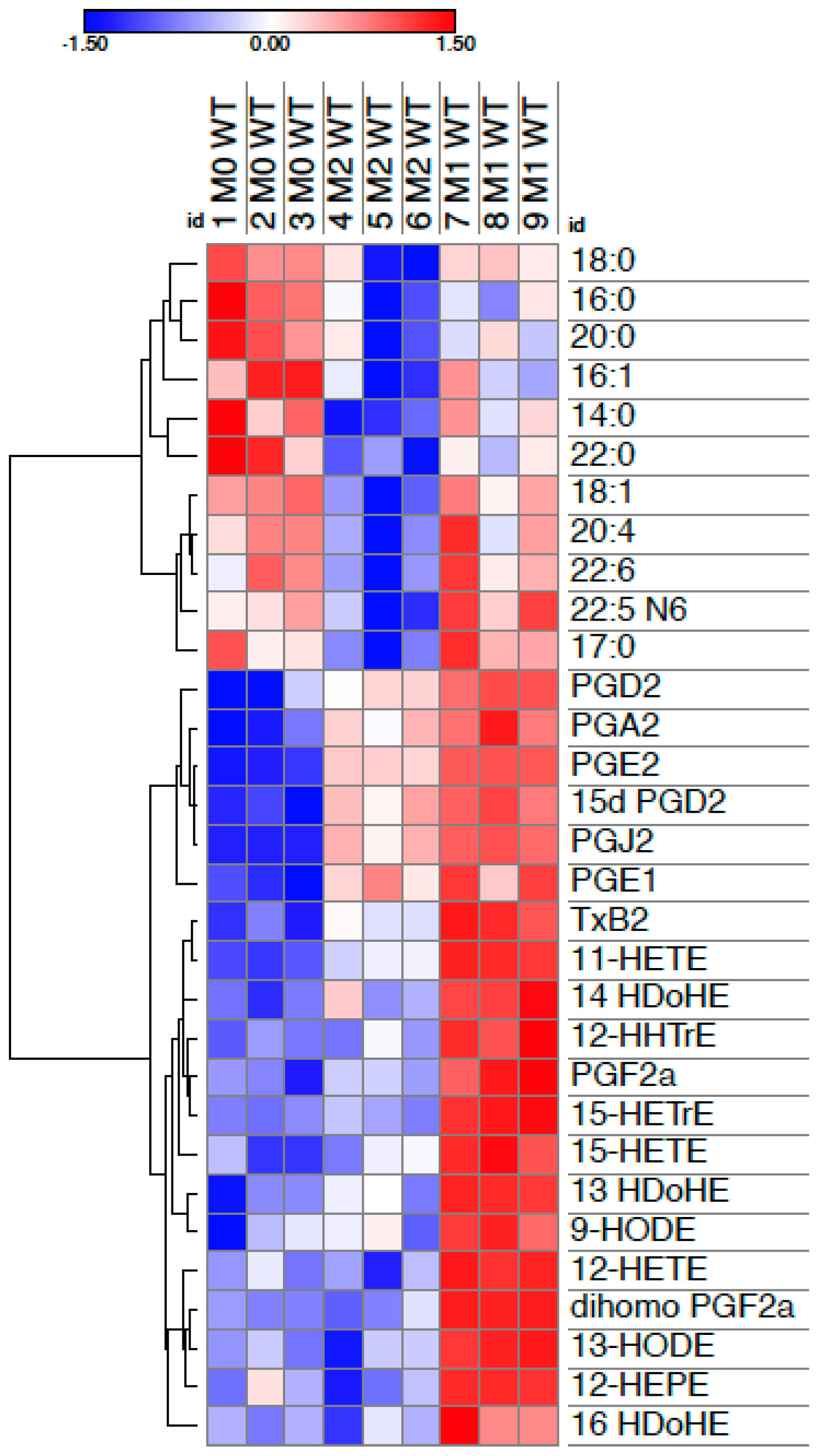
| Symbol | Name | Abbreviation | Effect on Inflammation | |
|---|---|---|---|---|
| Type 1 | Type 2 | |||
| Medium-Chain Saturated Fatty Acids | ||||
| 12:0 | Lauric acid | LU | ↑↓ | ? |
| 14:0 | Myristic acid | MA | ↑ | ? |
| Uneven Saturated Fatty Acids | ||||
| 15:0 | Pentadecanoic acid | PdA | ↓ | ? |
| 17:0 | Heptadecanoic or Margaric acid | MaA | ? | ? |
| Long-Chain Saturated Fatty Acids | ||||
| 16:0 | Palmitic acid | PA | ↑ | ? |
| 18:0 | Stearic acid | SA | ↑ | ? |
| 20:0 | Arachidic acid | ArA | ↑ | ? |
| 22:0 | Docosanoic acid | ? | ? | |
| 23:0 | Tricosanoic acid | ? | ? | |
| 24:0 | Tetracosanoic acid | ? | ? | |
| 26:0 | Hexacosanoic acid | ? | ? | |
| Monounsaturated Fatty Acids | ||||
| 16:1 | Palmitoleic acid | PoA | ↓ | ↑? |
| 17:1 | Heptadecenoic acid | ? | ? | |
| 18:1 | Oleic acid | OA | ↓ | ↑ |
| 20:1 | Gadoleic acid | ? | ? | |
| 22:1 | Docosenoic acid | ? | ? | |
| 24:1 | Tetracosenoic acid | ? | ? | |
| Polyunsaturated Fatty Acids Omega-6 and Omega-9 | ||||
| 18:2 | Linoleic acid | LA | ↑↓ | ↑ |
| 18:3 N6 | Gamma-linolenic acid | GLA | ↓ | ? |
| 20:2 | Eicosadienoic acid | EDA | ↑ | ? |
| 20:3 N6 | Dihomo-gamma-linolenic acid | DGLA | ↓ | ? |
| 20:3 N9 | Eicosatrienoic acid or mead acid | ETA N9 | ? | ? |
| 20:4 | Arachidonic acid | AA | ↑ | ↑ |
| 22:4 | Adrenic acid | AdA | ↑ | ? |
| 22:5 N6 | Docosapentaenoic acid | DPA N6 | ↓ | ↑ |
| Polyunsaturated Fatty Acids Omega-3 | ||||
| 18:3 N3 | Alpha-linolenic acid | ALA | ↓ | ↑ |
| 18:4 N3 | Stearidonic acid | SDA | ↓ | ↑? |
| 20:3 N3 | Eicosatrienoic acid | ETA N3 | ↓ | ? |
| 20:5 N3 | Eicosapentaenoic acid | EPA | ↓ | ? |
| 22:5 N3 | Docosapentaenoic acid | DPA N3 | ↓ | ? |
| 22:6 N3 | Docosahexaenoic acid | DHA | ↓ | ? |
| Fatty Acid | Findings | Selected References |
|---|---|---|
| MA | Myristoylation of viral protein-4 (VP4) increased TLR2 aggregation with MyD88 in mouse BM-macrophages and chemokine production in human alveolar macrophages | [22] |
| In human embryonic kidney (HEK)293 cells, myristoylation of TRIF-related adaptor molecule (TRAM), followed by its translocation to the plasma membrane, was essential for TLR4 signaling and LPS activation | [23] | |
| LU | LU increased TLR signaling and COX-2 expression in RAW 264.7 macrophages | [24,25] |
| LU increased killing of Brucella abortus in vitro and in vivo likely through GPR84 | [26] | |
| Improved insulin resistance and reduced inflammation in THP-1 macrophages and in vivo | [27,28] | |
| PdA | In a model of nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet, administration of PdA reduced ceroid-laden macrophages | [30] |
| PdA reduced reactive oxygen species in human hepatic cell line and production of type 1 proinflammatory cytokines and chemokines in peripheral blood mononuclear cells | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrieri, B.; Di Costanzo, D.; Dwyer, D.F. Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins. Molecules 2022, 27, 152. https://doi.org/10.3390/molecules27010152
Balestrieri B, Di Costanzo D, Dwyer DF. Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins. Molecules. 2022; 27(1):152. https://doi.org/10.3390/molecules27010152
Chicago/Turabian StyleBalestrieri, Barbara, David Di Costanzo, and Daniel F. Dwyer. 2022. "Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins" Molecules 27, no. 1: 152. https://doi.org/10.3390/molecules27010152
APA StyleBalestrieri, B., Di Costanzo, D., & Dwyer, D. F. (2022). Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins. Molecules, 27(1), 152. https://doi.org/10.3390/molecules27010152






