Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements
Abstract
1. Introduction
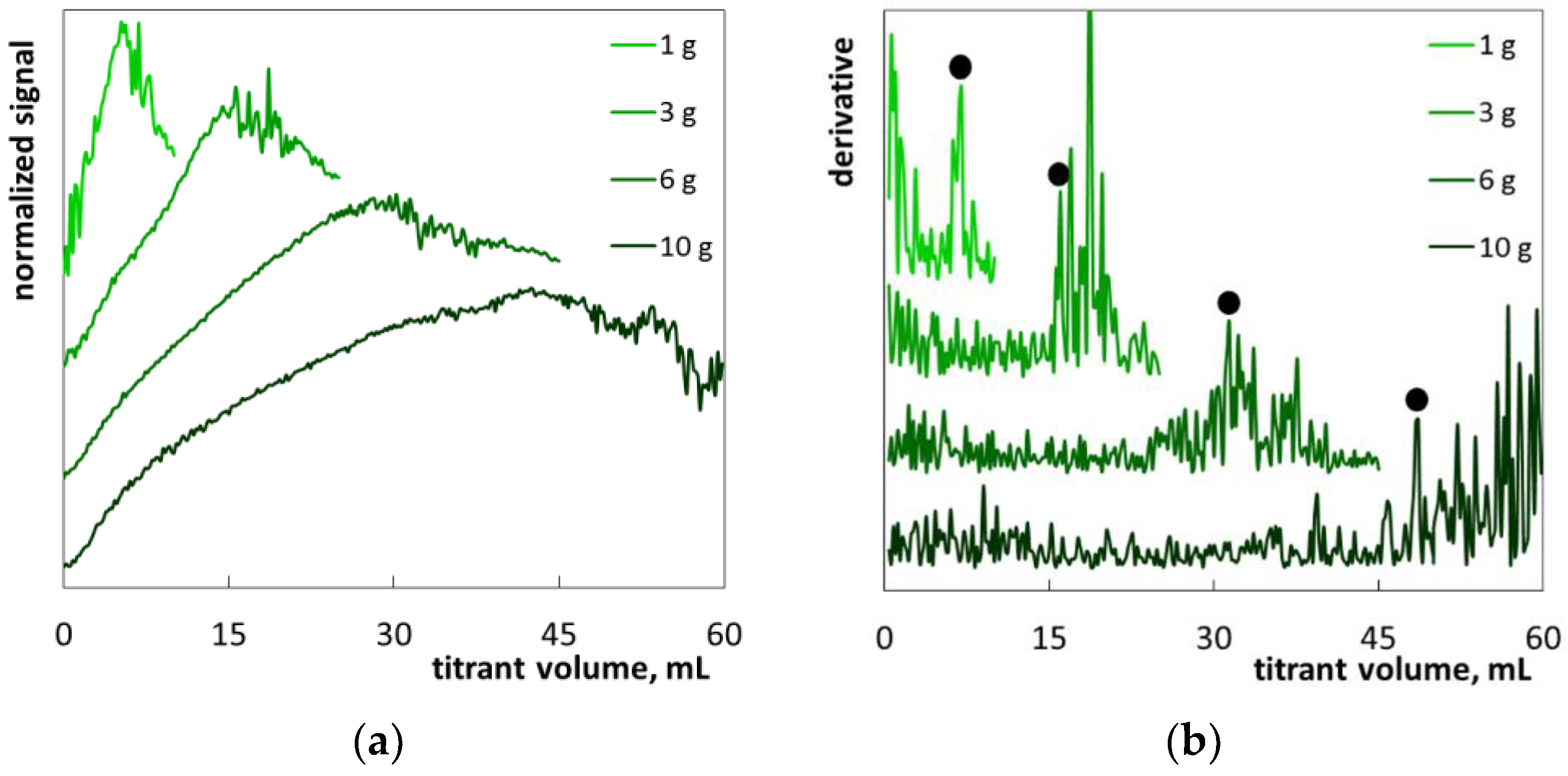
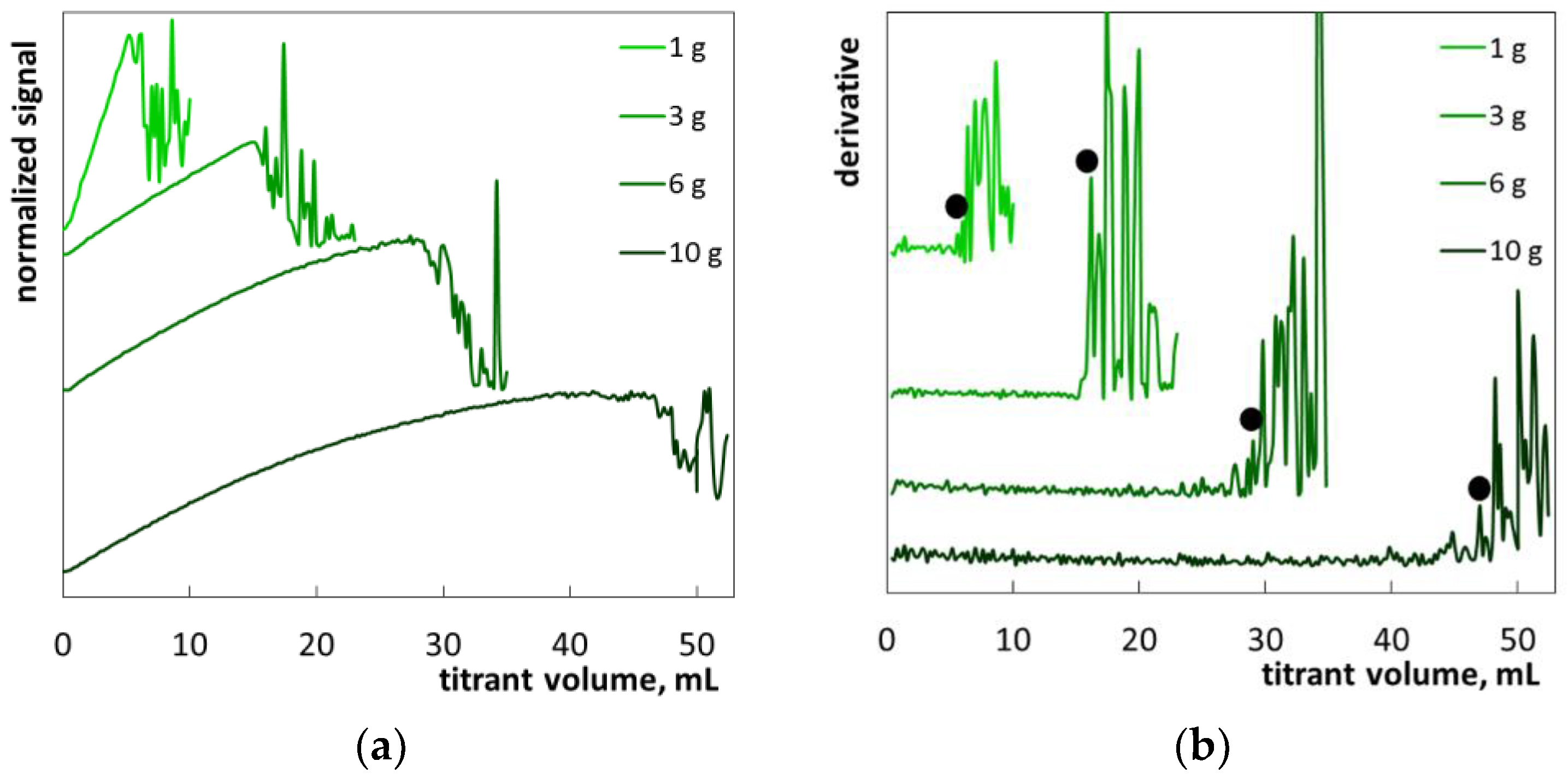
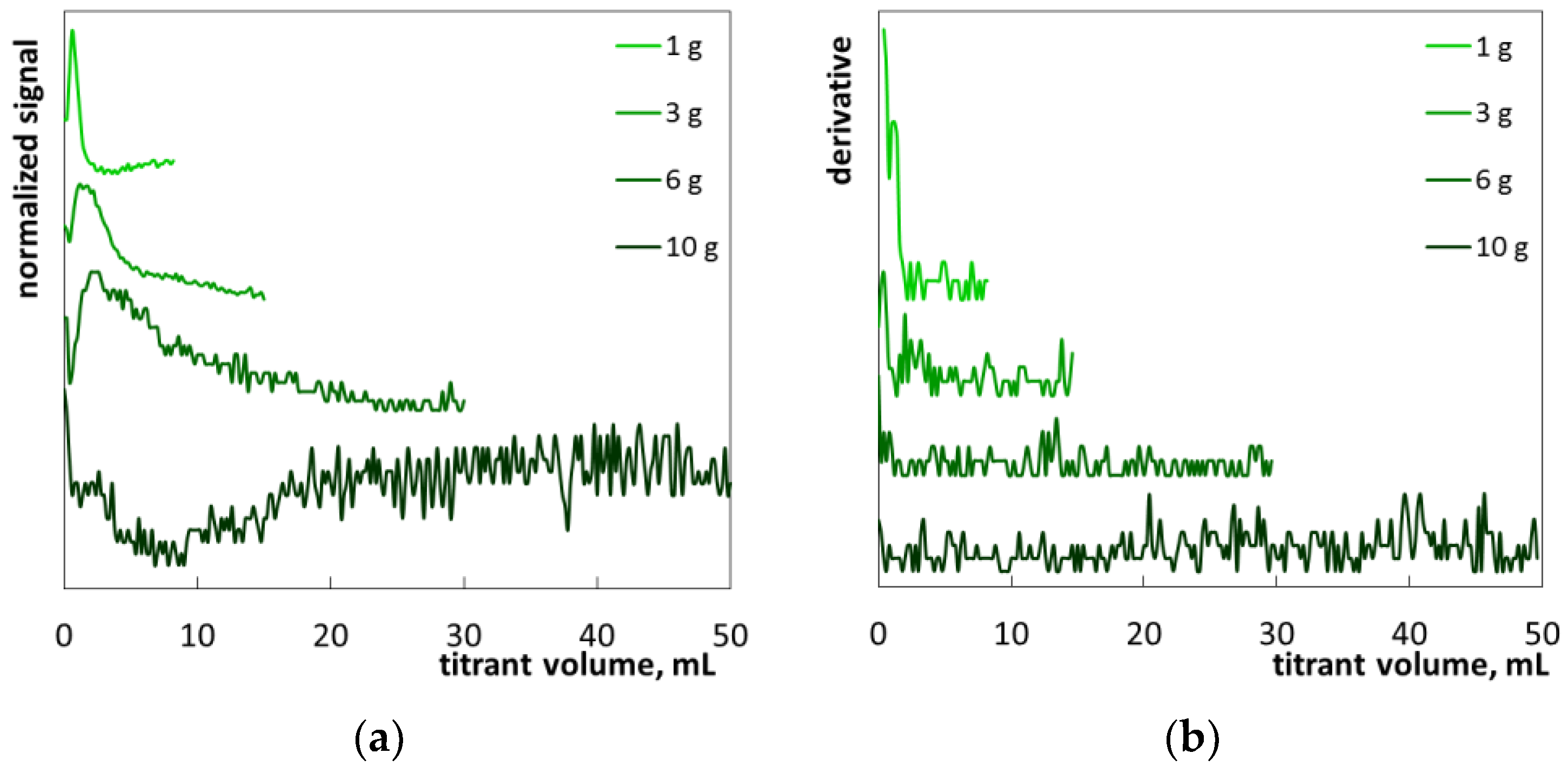
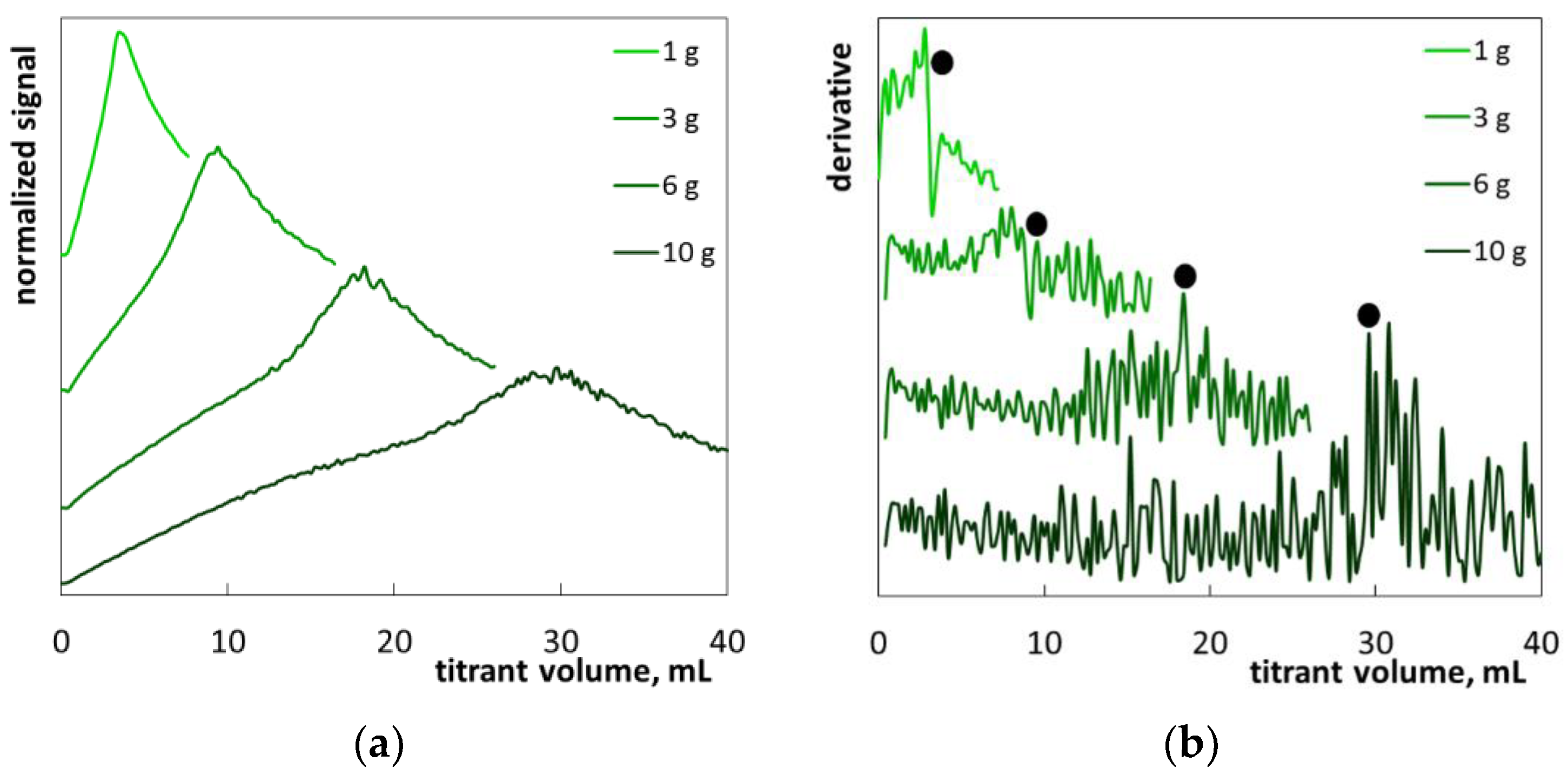
2. Results and Discussion
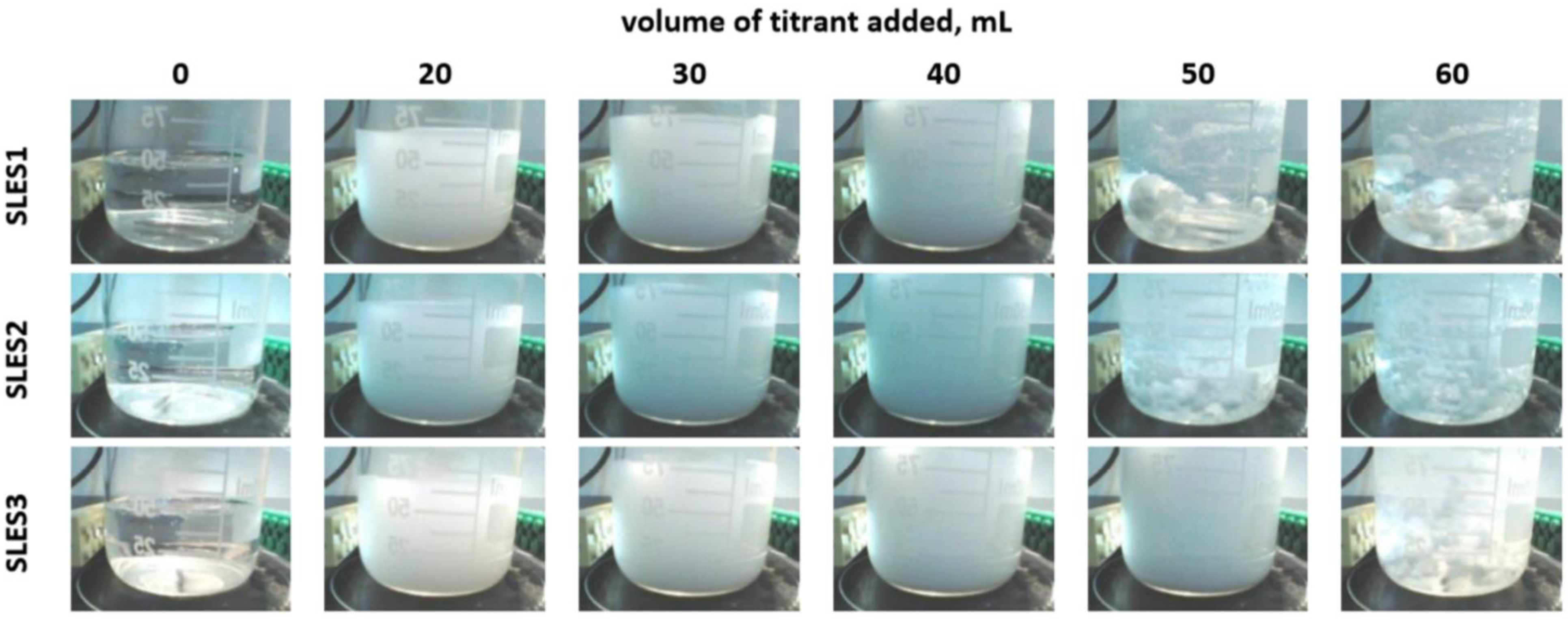
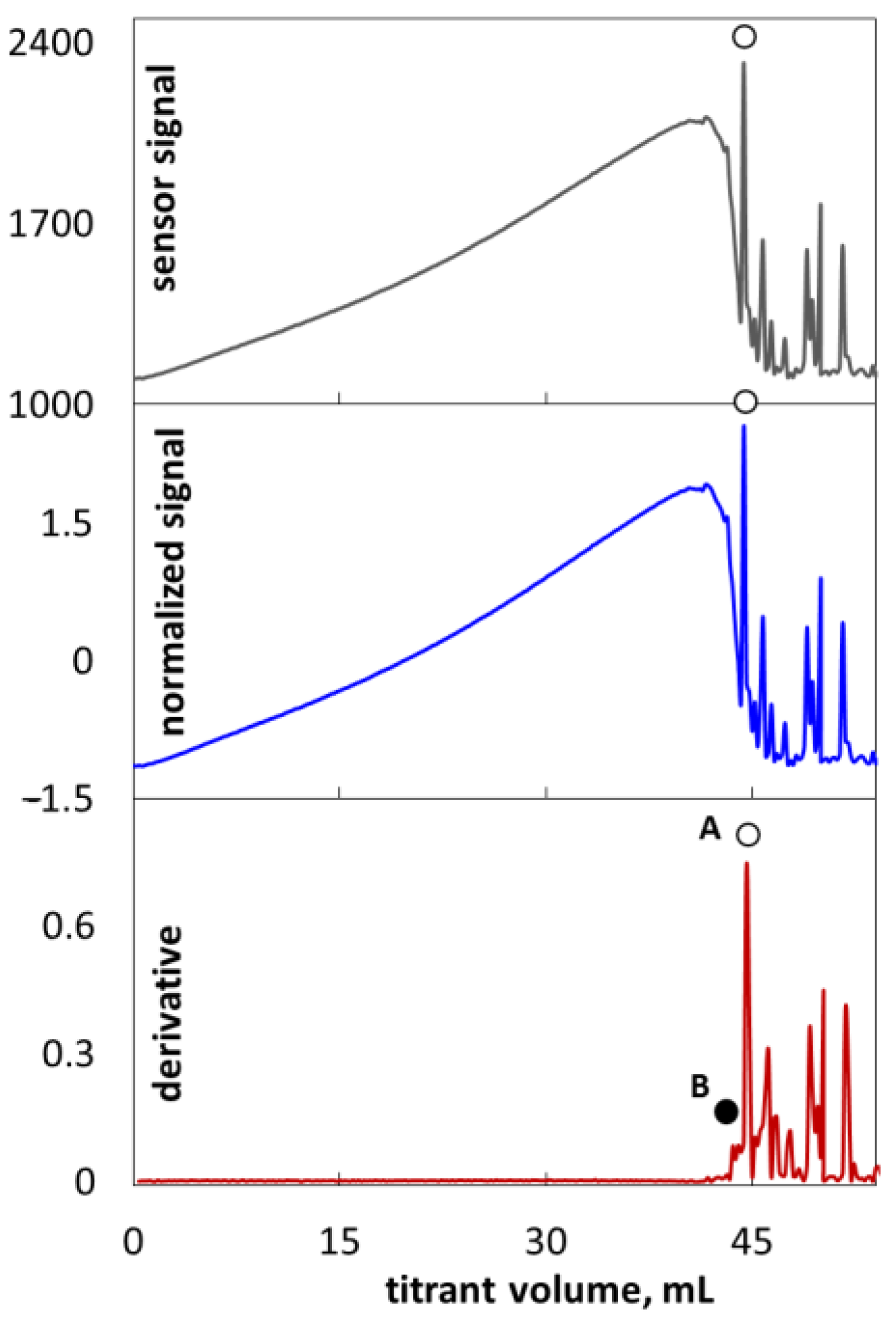
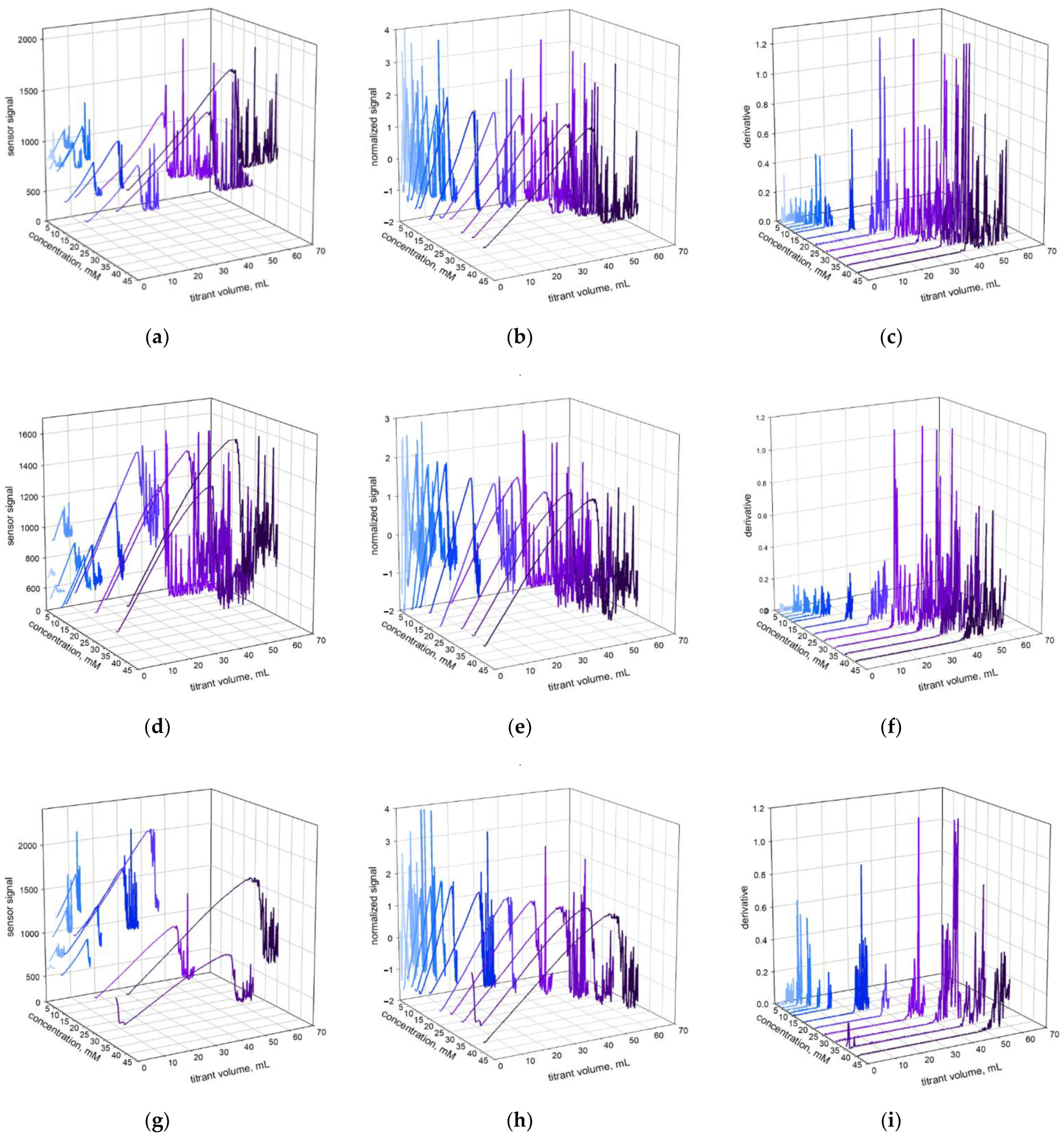
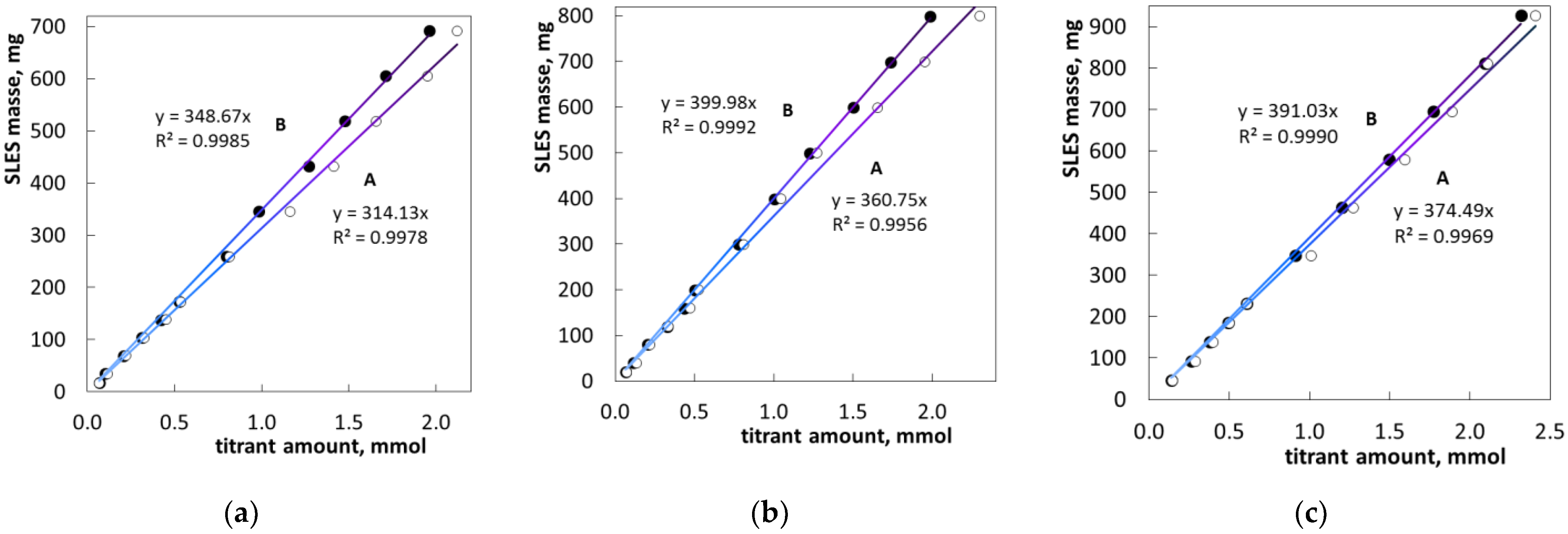
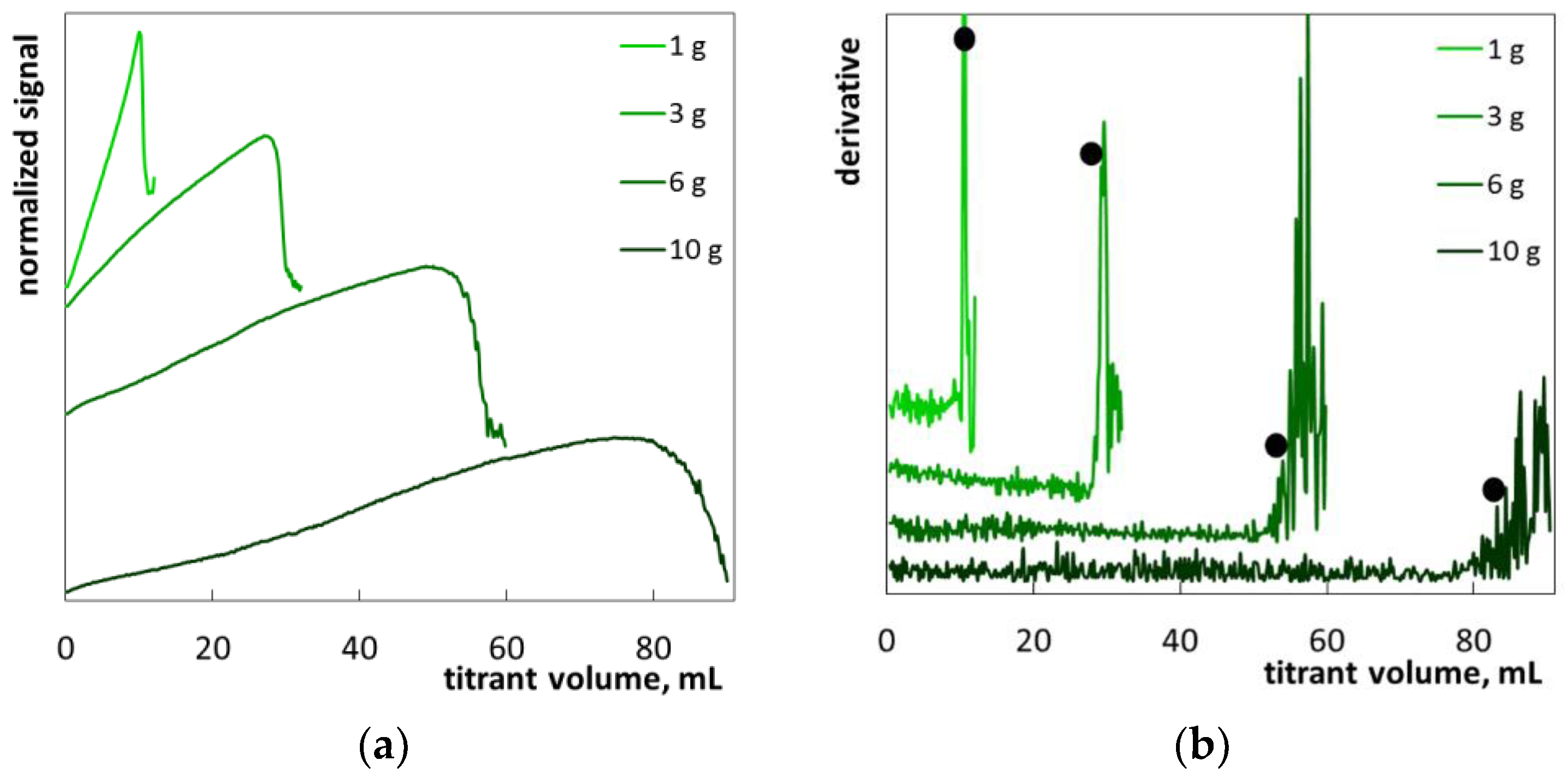
2.1. Colloid Titration Method
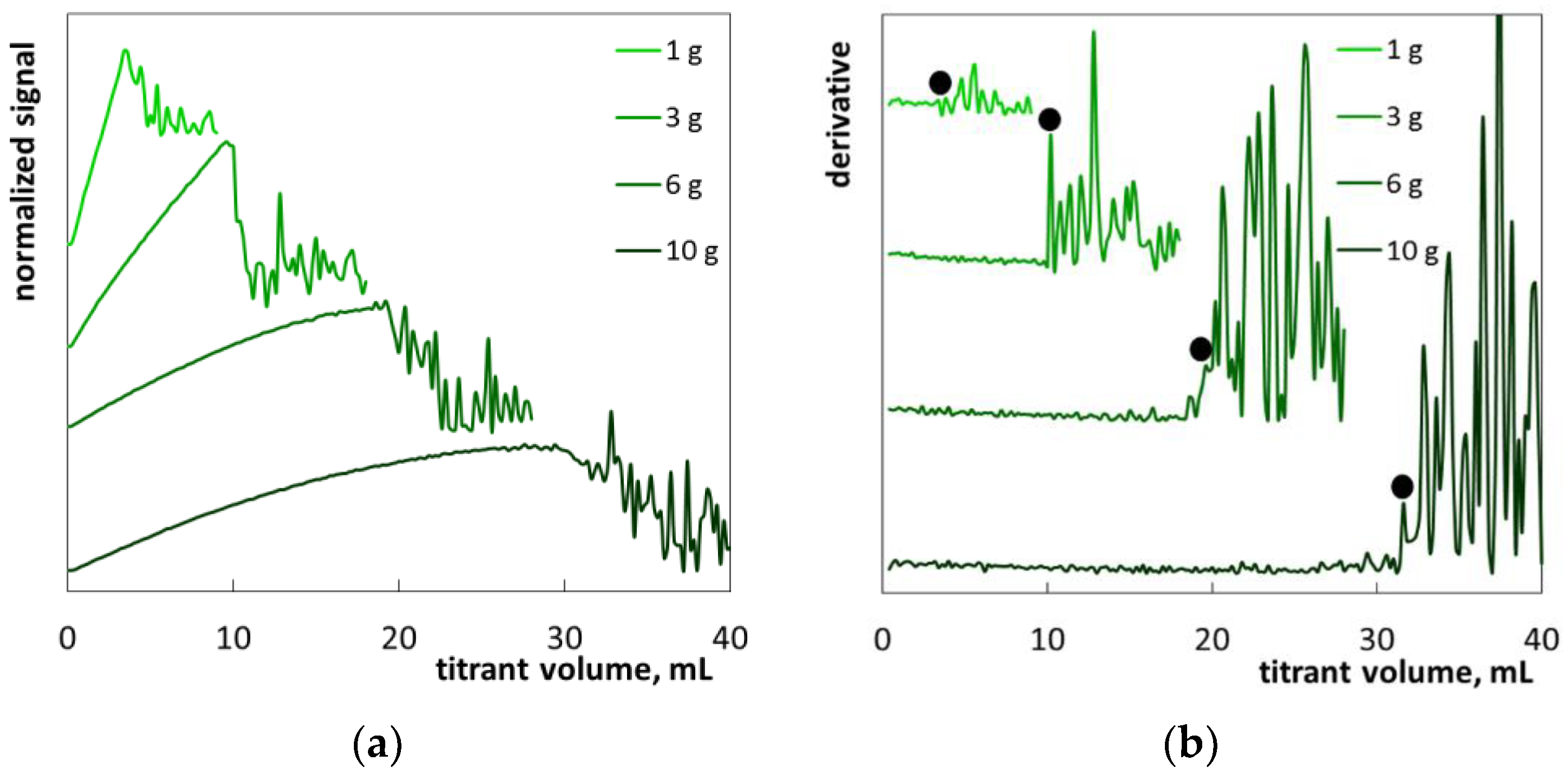
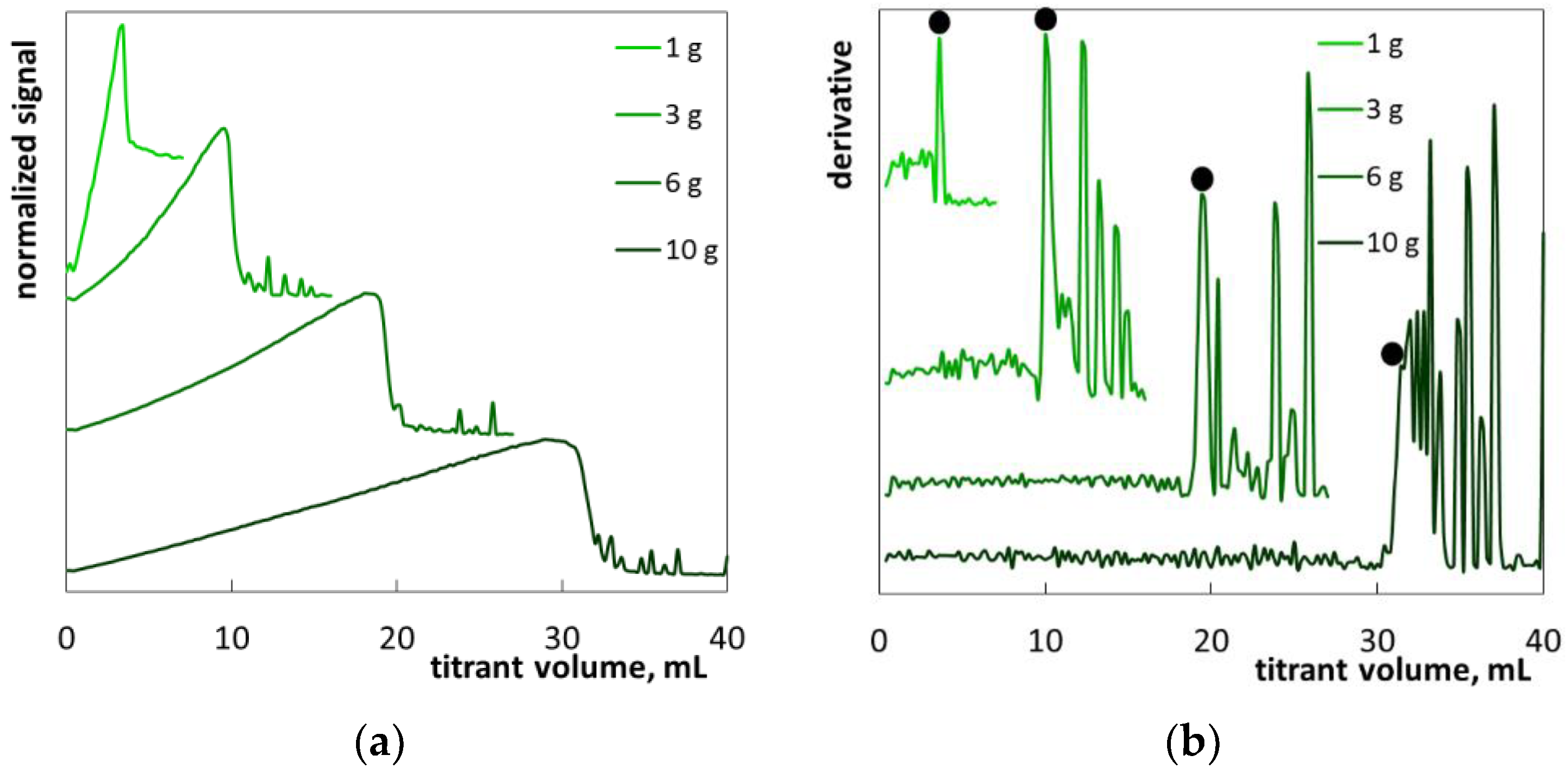
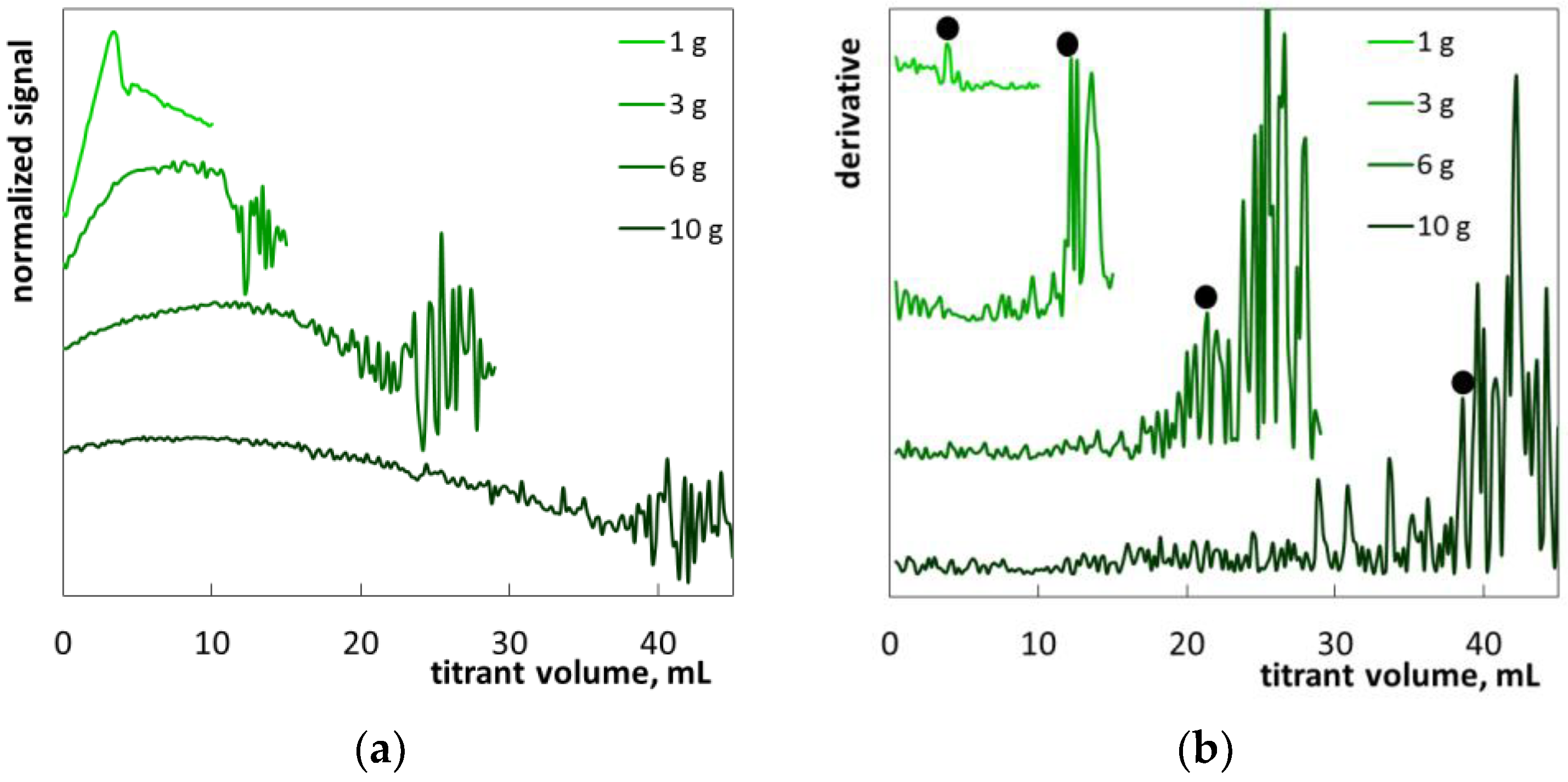
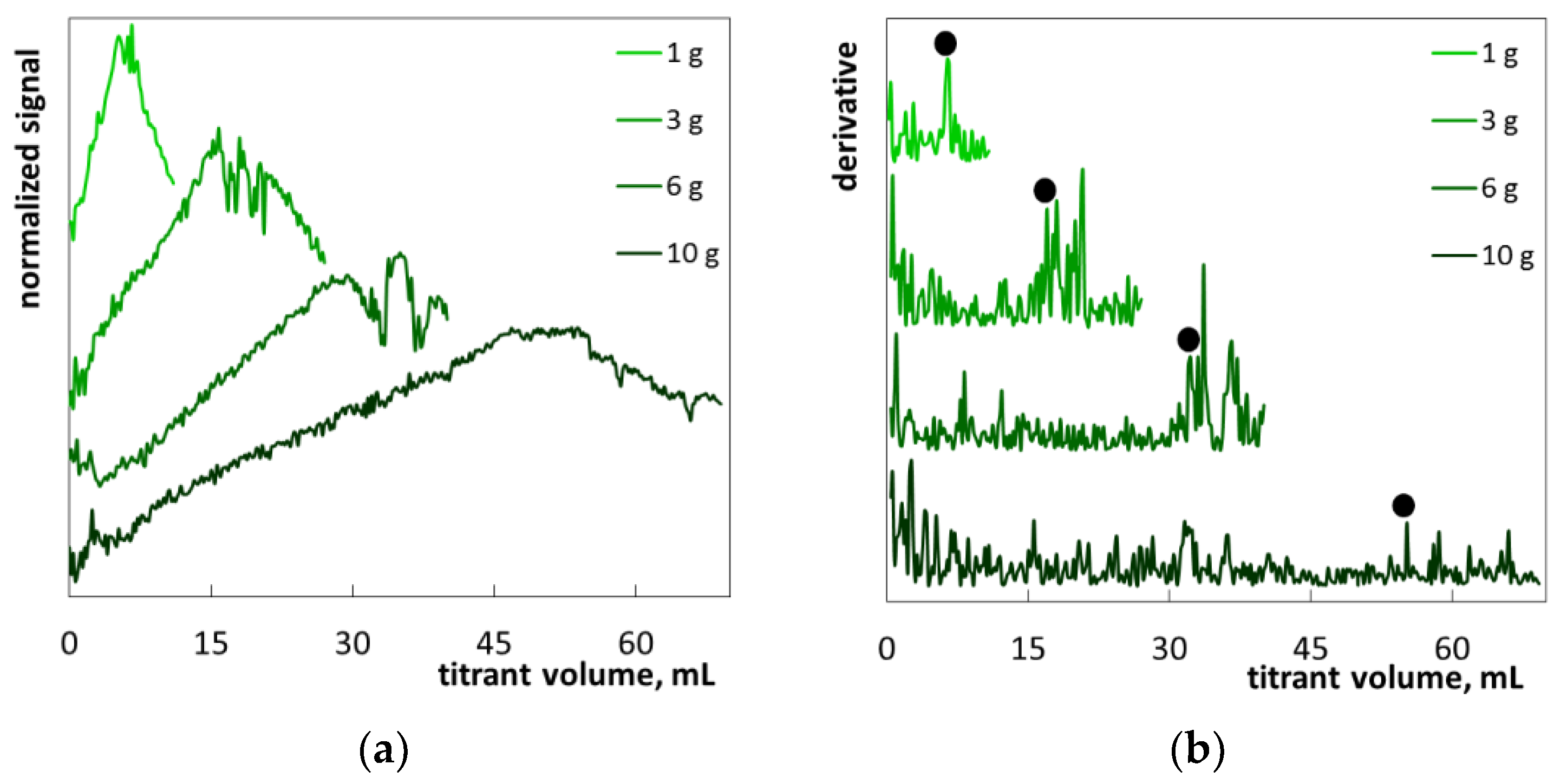
2.2. The Determination of SLES in Commercial Products
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Couteau, C.; Diarra, H.; Schmitt, Z.; Coiffard, L. Study of the composition of 140 shampoos: Similarities and differences de-pending on the sales channel used. Eur. J. Dermatol. 2019, 29, 141–159. [Google Scholar] [CrossRef]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2017, 40, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Ż.; Ruman, M.; Namieśnik, J. Similar concentration of surfactants in rural and urban areas. Environ. Chem. Lett. 2015, 13, 97–104. [Google Scholar] [CrossRef]
- Ramcharan, T.; Bissessur, A. Analysis of Linear Alkylbenzene Sulfonate in Laundry Wastewater by HPLC–UV and UV–Vis Spectrophotometry. J. Surfactants Deterg. 2016, 19, 209–218. [Google Scholar] [CrossRef]
- Shyichuk, A.; Ziółkowska, D. Determination of Anionic Surfactants by Means of Photometric Titration with Methylene Blue Dye. J. Surfactants Deterg. 2016, 19, 425–429. [Google Scholar] [CrossRef]
- Lezana, P.; García-Mayoral, M.; Lamothe, B.; Pena-Abaurrea, M. Comprehensive ethoxymer characterization of complex alcohol ethoxy sulphate products by mixed-mode high-performance liquid chromatography coupled to charged aerosol detection. J. Chromatogr. A 2021, 1639, 461927. [Google Scholar] [CrossRef]
- Moldovan, Z.; Avram, V.; Marincas, O.; Petrov, P.; Ternes, T. The determination of the linear alkylbenzene sulfonate isomers in water samples by gas-chromatography/mass spectrometry. J. Chromatogr. A 2011, 1218, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kurrey, R.; Mahilang, M.; Deb, M.K.; Shrivas, K. Analytical approach on surface active agents in the environment and challenges. Trends Environ. Anal. Chem. 2019, 21, 00061. [Google Scholar] [CrossRef]
- Jozanović, M.; Sakač, N.; Karnaš, M.; Medvidović-Kosanović, M. Potentiometric Sensors for the Determination of Anionic Surfactants—A Review. Crit. Rev. Anal. Chem. 2021, 51, 115–137. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the end point potential break values: A case of potentiometric titration of lipophilic anions with cetylpyridinium chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- Abd-Rabboh, H. Batch and Flow-Injection Analysis of Lauryl Sulfate in Industrial Products and Wastes Using Membrane Sensors Based on Methyltrioctylammonium Chloride. Int. J. Electrochem. Sci. 2020, 15, 3704–3714. [Google Scholar] [CrossRef]
- Vleugels, L.F.W.; Pollet, J.; Tuinier, R. Polycation–Sodium Lauryl Ether Sulfate-Type Surfactant Complexes: Influence of Ethylene Oxide Length. J. Phys. Chem. B 2015, 119, 6338–6347. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Determination of Sodium Dodecyl Sulfate via Turbidimetric Titration with Poly(Diallyldimethylammonium Chloride). J. Surfactants Deterg. 2020, 23, 913–920. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Baghdadli, N.; Ortega, F.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of poly(diallyldimethylammonium chloride)—sodium methyl-cocoyl-taurate complexes onto solid surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 505, 150–157. [Google Scholar] [CrossRef]
- Chatterjee, S.; Prajapati, R.; Bhattacharya, A.; Mukherjee, T.K. Microscopic Evidence of “Necklace and Bead”-Like Morphology of Polymer–Surfactant Complexes: A Comparative Study on Poly(vinylpyrrolidone)–Sodium Dodecyl Sulfate and Poly(diallyldimethylammonium chloride)–Sodium Dodecyl Sulfate Systems. Langmuir 2014, 30, 9859–9865. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.; Varga, Z.; Kardos, A.; Mészáros, R. Impact of local inhomogeneities on the complexation between poly(diallyldimethylammoniumchloride) and sodium dodecyl sulfate. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 574, 21–28. [Google Scholar] [CrossRef]
- Plazzotta, B.; Fegyver, E.; Mészáros, R.; Pedersen, J.S. Anisometric Polyelectrolyte/Mixed Surfactant Nanoassemblies Formed by the Association of Poly(diallyldimethylammonium chloride) with Sodium Dodecyl Sulfate and Dodecyl Maltoside. Langmuir 2015, 31, 7242–7250. [Google Scholar] [CrossRef]
- Del Sorbo, G.R.; Cristiglio, V.; Clemens, D.; Hoffmann, I.; Schneck, E. Influence of the Surfactant Tail Length on the Viscosity of Oppositely Charged Polyelectrolyte/Surfactant Complexes. Macromolecules 2021, 54, 2529–2540. [Google Scholar] [CrossRef]
- Luengo, G.S.; Guzman, E.; Fernández-Peña, L.; Leonforte, F.; Ortega, F.; Rubio, R.G. Interaction of Polyelectrolytes and Sur-factants on Hair Surfaces. Deposits and their Characterization. In Surface Science and Adhesion in Cosmetics; Mittal, K.L., Bui, H.S., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Benhur, A.M.; Diaz, J.; Amin, S. Impact of polyelectrolyte-surfactant interactions on the rheology and wet lubrication performance of conditioning shampoo. Int. J. Cosmet. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Yang, J. Hair Care Cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–615. [Google Scholar] [CrossRef]
- Zhan, S.; Li, D.; Liang, S.; Chen, X.; Li, X. A Novel Flexible Room Temperature Ethanol Gas Sensor Based on SnO2 Doped Poly-Diallyldimethylammonium Chloride. Sensors 2013, 13, 4378–4389. [Google Scholar] [CrossRef]
- Tyagi, C.; Sharma, A. Optimization of structural and dielectric properties of CdSe loaded poly(diallyl dimethyl ammonium chloride) polymer in a desired frequency and temperature window. J. Appl. Phys. 2016, 119, 014108. [Google Scholar] [CrossRef]
- Nizri, G.; Lagerge, S.; Kamyshny, A.; Major, D.T.; Magdassi, S. Polymer–surfactant interactions: Binding mechanism of sodium dodecyl sulfate to poly(diallyldimethylammonium chloride). J. Colloid Interface Sci. 2008, 320, 74–81. [Google Scholar] [CrossRef]
- Ábrahám, Á.; Mezei, A.; Mészáros, R. The effect of salt on the association between linear cationic polyelectrolytes and sodium dodecyl sulfate. Soft Matter 2009, 5, 3718–3726. [Google Scholar] [CrossRef]
- Fegyver, E.; Mészáros, R. The impact of nonionic surfactant additives on the nonequilibrium association between oppositely charged polyelectrolytes and ionic surfactants. Soft Matter 2014, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Fegyver, E.; Mészáros, R. Fine-Tuning the Nonequilibrium Behavior of Oppositely Charged Macromolecule/Surfactant Mixtures via the Addition of Nonionic Amphiphiles. Langmuir 2014, 30, 15114–15126. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Determination of Sodium Dodecyl Sulfate by Means of Photometric Titration with o-Toluidine Blue Dye. J. Surfactants Deterg. 2018, 21, 751–756. [Google Scholar] [CrossRef]


| Sample Code | Brand Name | Values from the Manufacturer’s Leaflet | Measured Dry Mass, (% m/m) | ||
|---|---|---|---|---|---|
| Ethoxy Group Content | Molecular Weight, (g/mol) | Active Substance Content, (% m/m) | |||
| SLES1 | SULFOROKAnol L170/1 | 1–2.5 | approx. 340 | 68–72 | 71.2 |
| SLES2 | SULFOROKAnol L270/1 | 1–2.5 | approx. 384 | 68–72 | 72.8 |
| SLES3 | SULFOROKAnol L370 | >2.5 | approx. 435 | 68–72 | 74.6 |
| Sample | Method | Average 1, mM | Variance 1 | SD 1, mM | RSD 1, % |
|---|---|---|---|---|---|
| SLES1 | A | 18.73 | 1.01 | 1.00 | 5.36 |
| SLES1 | B | 20.09 | 0.03 | 0.17 | 0.85 |
| SLES2 | A | 19.17 | 0.29 | 0.53 | 2.79 |
| SLES2 | B | 20.24 | 0.03 | 0.18 | 0.88 |
| SLES3 | A | 20.23 | 0.14 | 0.38 | 1.86 |
| SLES3 | B | 20.38 | 0.02 | 0.12 | 0.60 |
| Sample | SLES Determined in mmol/g at Different Sample Masses | Average Content, mmol/g | Average Content, % m/m 1 | |||
|---|---|---|---|---|---|---|
| 1 g | 3 g | 6 g | 10 g | |||
| S1 laundry liquid | (0.170) | 0.152 | 0.148 | 0.141 | 0.147 | 5.5 |
| S2 hair shampoo | (0.161) | 0.149 | 0.145 | 0.141 | 0.145 | 5.5 |
| S3 hair shampoo | 0.170 | 0.182 | 0.160 | 0.173 | 0.171 | 6.4 |
| S4 hair shampoo | (0.286) | 0.254 | 0.242 | b/r | 0.248 | 9.3 |
| S5 hair shampoo | (0.286) | 0.239 | 0.225 | b/r | 0.232 | 8.7 |
| S6 baby shampoo | 0.251 | 0.239 | 0.216 | b/r | 0.235 | 8.9 |
| S7 baby shampoo | ~0 | ~0 | ~0 | ~0 | 0 | 0 |
| S8 intimate hygiene gel | (0.170) | 0.143 | 0.139 | 0.132 | 0.138 | 5.2 |
| S9 shower oil | 0.474 | 0.442 | b/r | b/r | 0.458 | 17.2 |
| Sample Code | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|
| Type of Product | Laundry Liquid | Hair Shampoo | Hair Shampoo | Hair Shampoo | Hair Shampoo | Baby Shampoo | Baby Shampoo | Intimate Hygiene Gel | Shower Oil |
| aqua | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Sodium Laureth Sulfate | ● | ● | ● | ● | ● | ● | ● | ||
| MIPA-Laureth Sulfate | ● | ||||||||
| Sodium C10-13 Alkyl Benzenosulfonate | ● | ● | |||||||
| Laureth-7 Citrate | ● | ||||||||
| Hydrogenated Palm Glycerides Citrate | ● | ● | |||||||
| Cocamidopropyl Hydroxysultaine | ● | ||||||||
| Cocamidopropyl Betaine | ● | ● | ● | ● | ● | ● | |||
| Lauramidopropyl Betaine | ● | ||||||||
| Betaine | ● | ||||||||
| Cocamide MEA | ● | ||||||||
| Cocamide DEA | ● | ● | |||||||
| Laureth 2 | ● | ||||||||
| Laureth 3 | ● | ||||||||
| Laureth-4 | ● | ● | |||||||
| Ceteareth-18 | ● | ||||||||
| Trideceth-10 | ● | ||||||||
| PEG-3 Distearate | ● | ● | |||||||
| PEG-40 Hydrogenated Castor Oil | ● | ||||||||
| PEG-75 Lanolin | ● | ||||||||
| PEG-14M | ● | ||||||||
| PEG/PPG-120/10 Trimethylolpropane Trioleate | ● | ||||||||
| Lauryl Glucoside | ● | ● | |||||||
| Coco-Glucoside | ● | ● | |||||||
| Decyl Glucoside | ● | ||||||||
| Glyceryl Oleate | ● | ● | |||||||
| Glycol Distearate | ● | ● | |||||||
| Laurdimonium Hydroxypropyl Hydrolyzed Wheat Protein | ● | ||||||||
| Laurdimonium Hydroxypropyl Hydrolyzed Wheat Starch | ● | ||||||||
| Hydrolyzed Silk | ● | ● | |||||||
| Hydrolyzed Milk Protein | ● | ||||||||
| Hydrogenated Starch Hydrolysate | ● | ||||||||
| Glycerol | ● | ● | ● | ||||||
| Propylene Glycol | ● | ● | ● | ||||||
| Panthenol | ● | ● | ● | ||||||
| Allantoin | ● | ||||||||
| Urea | ● | ||||||||
| Biotin | ● | ||||||||
| Polyquaternium-10 | ● | ● | ● | ||||||
| Guar Hydroxypropyltrimonium Chloride | ● | ||||||||
| Lecithin | ● | ||||||||
| Sodium Chloride | ● | ● | ● | ● | ● | ||||
| Tetrasodium EDTA | ● | ||||||||
| Disodium Phosphate | ● | ||||||||
| Citric acid | ● | ● | ● | ● | ● | ● | ● | ● | |
| Lactic Acid | ● | ● | |||||||
| Sodium Hydroxide | ● | ● | |||||||
| Aloe Barbadensis Leaf Juice | ● | ||||||||
| Gossypium Herbaceum Seed Extract | ● | ||||||||
| Prunus Amygdalus Dulcis Seed Extract | ● | ||||||||
| Macadamia Ternifolia Seed Oil | ● | ||||||||
| Glicine Soja Oil | ● | ||||||||
| Ricinus Communis Seed Oil | ● | ||||||||
| Dimethiconol | ● | ||||||||
| Lanolin Alcohol | ● | ||||||||
| Cetearyl Alcohol | ● | ||||||||
| Tocopherol | ● | ● | |||||||
| BHT | ● | ||||||||
| Propyl Gallate | ● | ||||||||
| Ascorbyl Palmitate | ● | ||||||||
| Parfum | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Alpha-Isomethyl Ionone | ● | ||||||||
| Geraniol | ● | ● | ● | ● | |||||
| Hexyl Cinnamal | ● | ● | |||||||
| Butylphenyl Methylpropional | ● | ||||||||
| Limonene | ● | ● | ● | ● | |||||
| Linalool | ● | ● | ● | ● | |||||
| Citronellol | ● | ● | |||||||
| Coumarin | ● | ||||||||
| Benzyl Alcohol | ● | ● | ● | ||||||
| Benzyl Salicylate | ● | ● | |||||||
| Caprylic/Capric Triglyceride | ● | ||||||||
| CI 19140 | ● | ||||||||
| CI 42090 | ● | ||||||||
| CI 75810 | ● | ||||||||
| Benzophenone-4 | ● | ||||||||
| Diethylhexyl Syringylidenemalonate | ● | ||||||||
| Zinc Pyrithione | ● | ||||||||
| Sodium Pyrithione | ● | ||||||||
| Potassium Sorbate | ● | ● | |||||||
| Phenoxyethanol | ● | ● | |||||||
| Methylparaben | ● | ||||||||
| Propylparaben | ● | ||||||||
| Methylchloroisothiazolinone | ● | ● | |||||||
| Methylisothiazolinone | ● | ● | |||||||
| Benzisothiazolinone | ● | ||||||||
| DMDM Hydantoin | ● | ● | |||||||
| Sodium Benzoate | ● | ● | ● | ||||||
| Benzoic Acid | ● | ||||||||
| p-Anisic Acid | ● |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkowska, D.; Syrotynska, I.; Shyichuk, A.; Lamkiewicz, J. Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements. Molecules 2021, 26, 2716. https://doi.org/10.3390/molecules26092716
Ziółkowska D, Syrotynska I, Shyichuk A, Lamkiewicz J. Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements. Molecules. 2021; 26(9):2716. https://doi.org/10.3390/molecules26092716
Chicago/Turabian StyleZiółkowska, Dorota, Iryna Syrotynska, Alexander Shyichuk, and Jan Lamkiewicz. 2021. "Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements" Molecules 26, no. 9: 2716. https://doi.org/10.3390/molecules26092716
APA StyleZiółkowska, D., Syrotynska, I., Shyichuk, A., & Lamkiewicz, J. (2021). Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements. Molecules, 26(9), 2716. https://doi.org/10.3390/molecules26092716







