Application of CO2-Switchable Oleic-Acid-Based Surfactant for Reducing Viscosity of Heavy Oil
Abstract
:1. Introduction
2. Results and Discussion
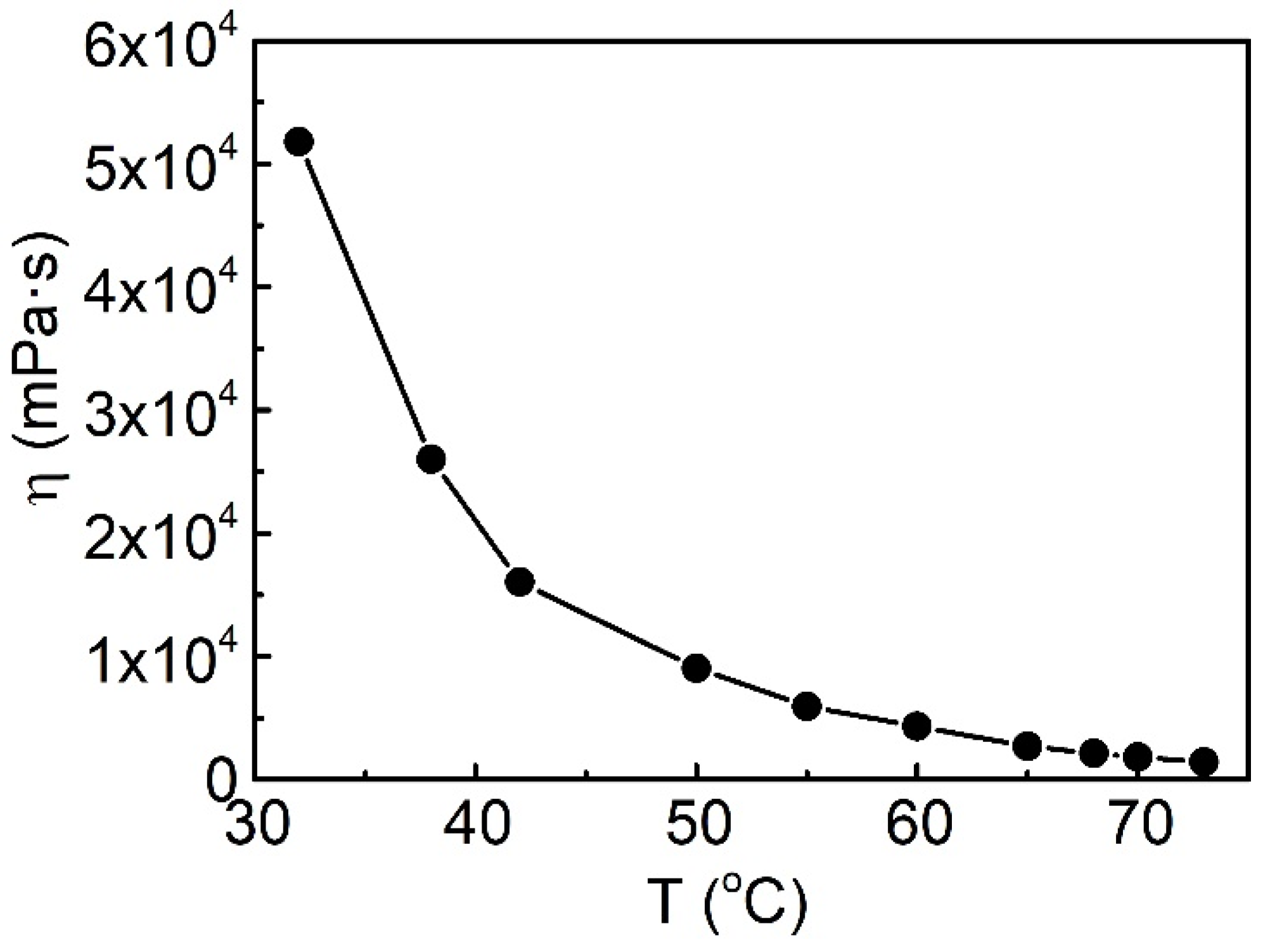
2.1. Properties of Heavy Oil
2.2. Properties of Mixed OA/Cyclen as a CO2-Switchable Surfactant
2.2.1. Surface Tension
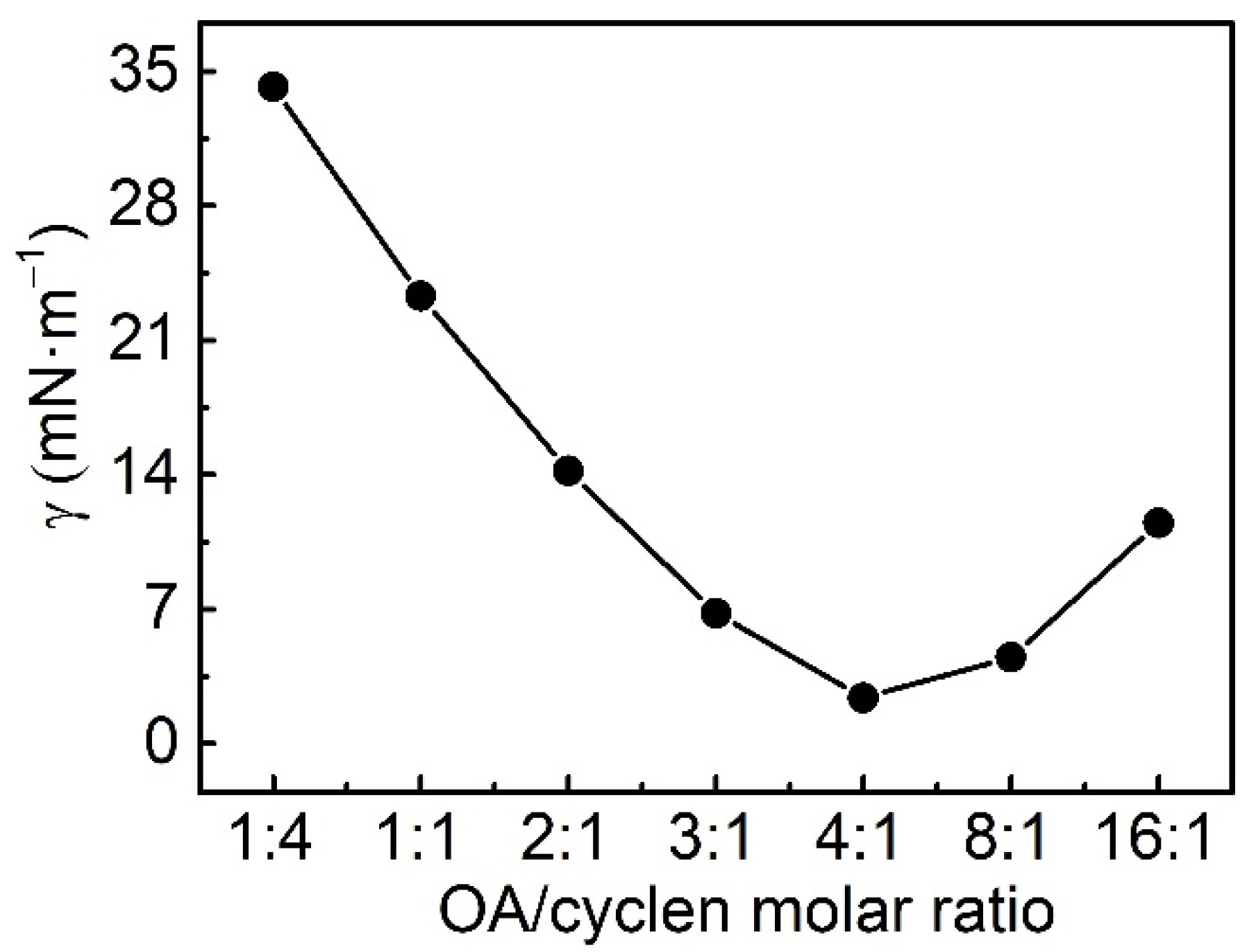
2.2.2. Interfacial Tension
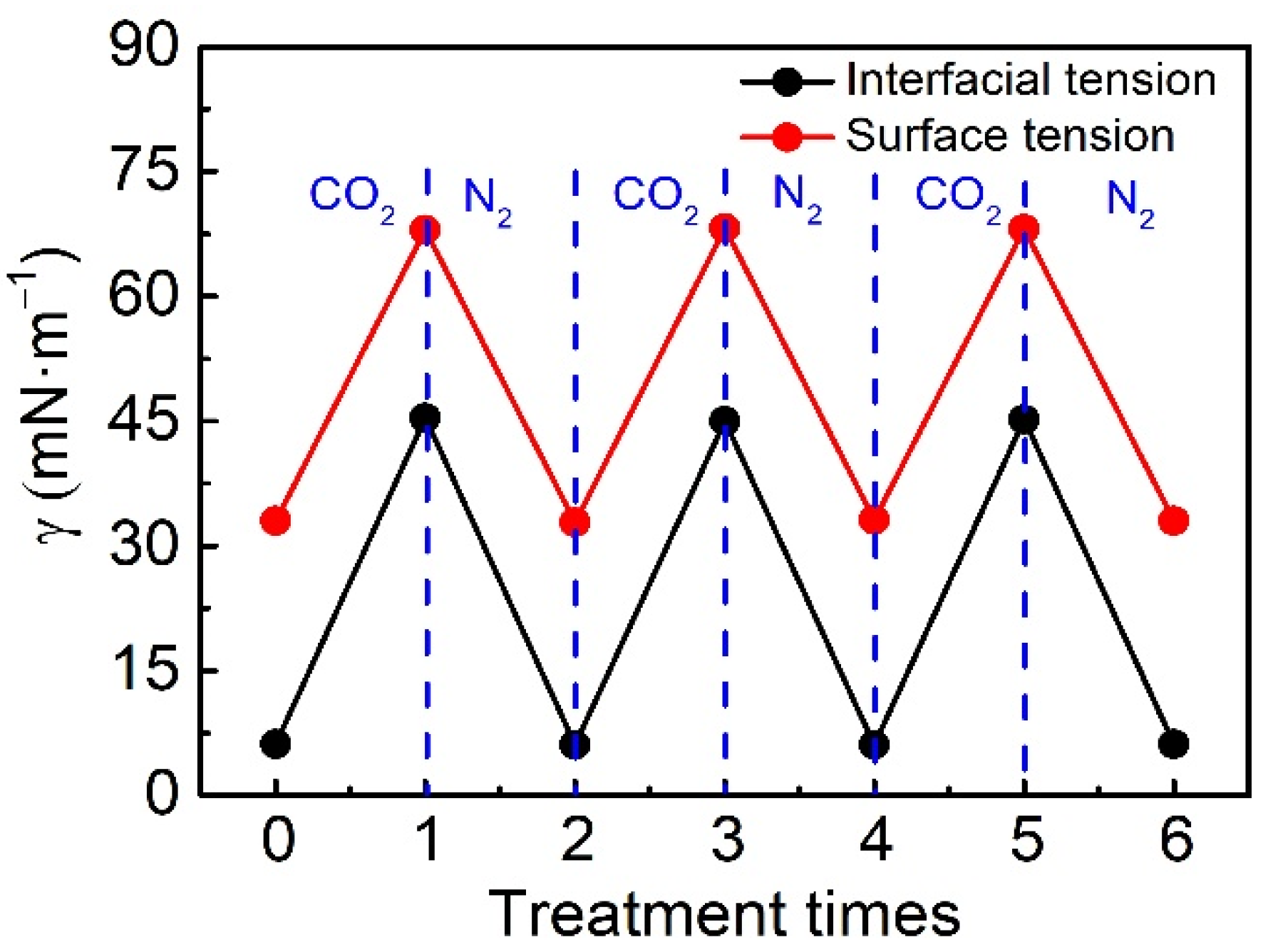
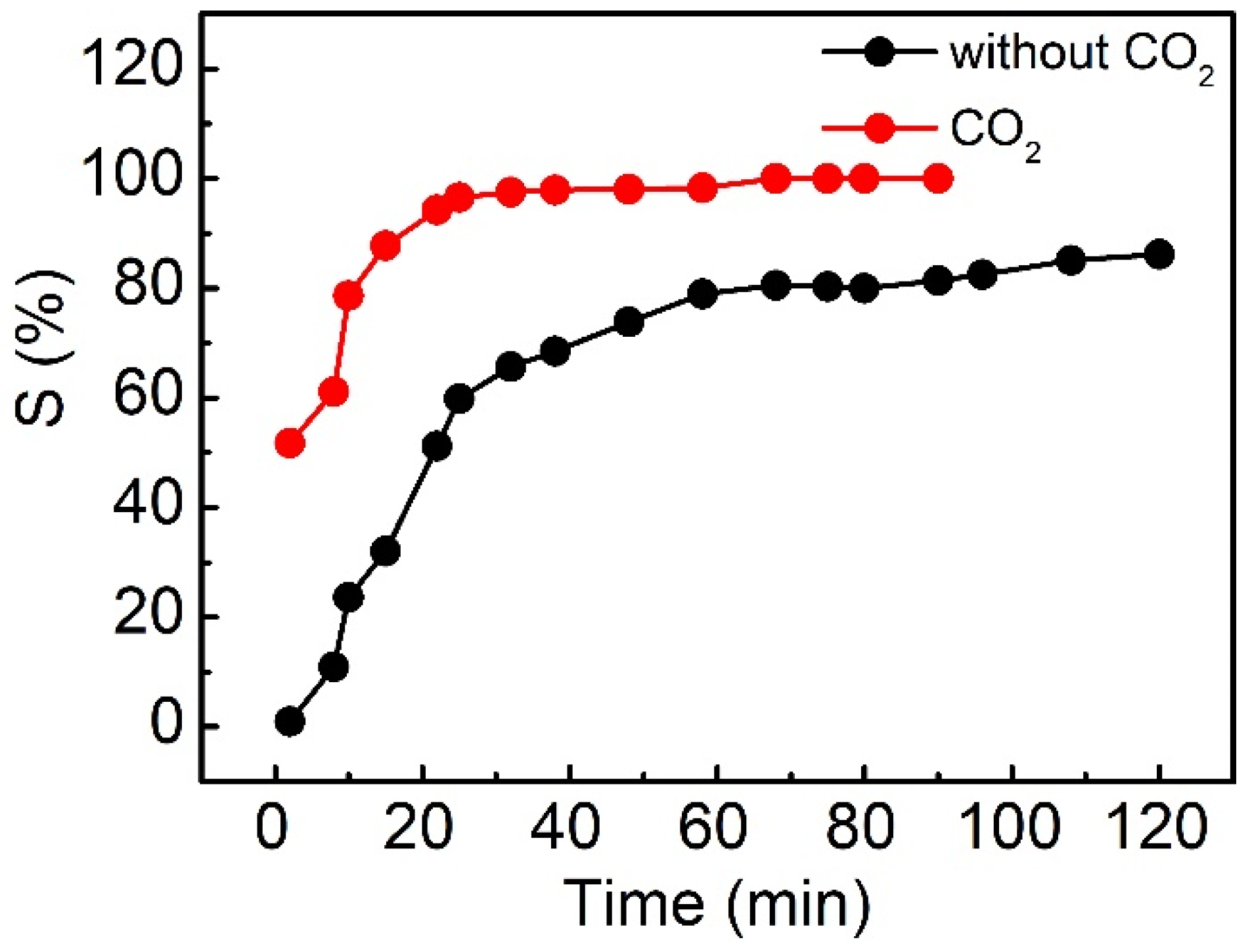
2.2.3. CO2 Switching Behavior of OA/Cyclen Aqueous Solution
2.3. Heavy Oil Viscosity Reduction
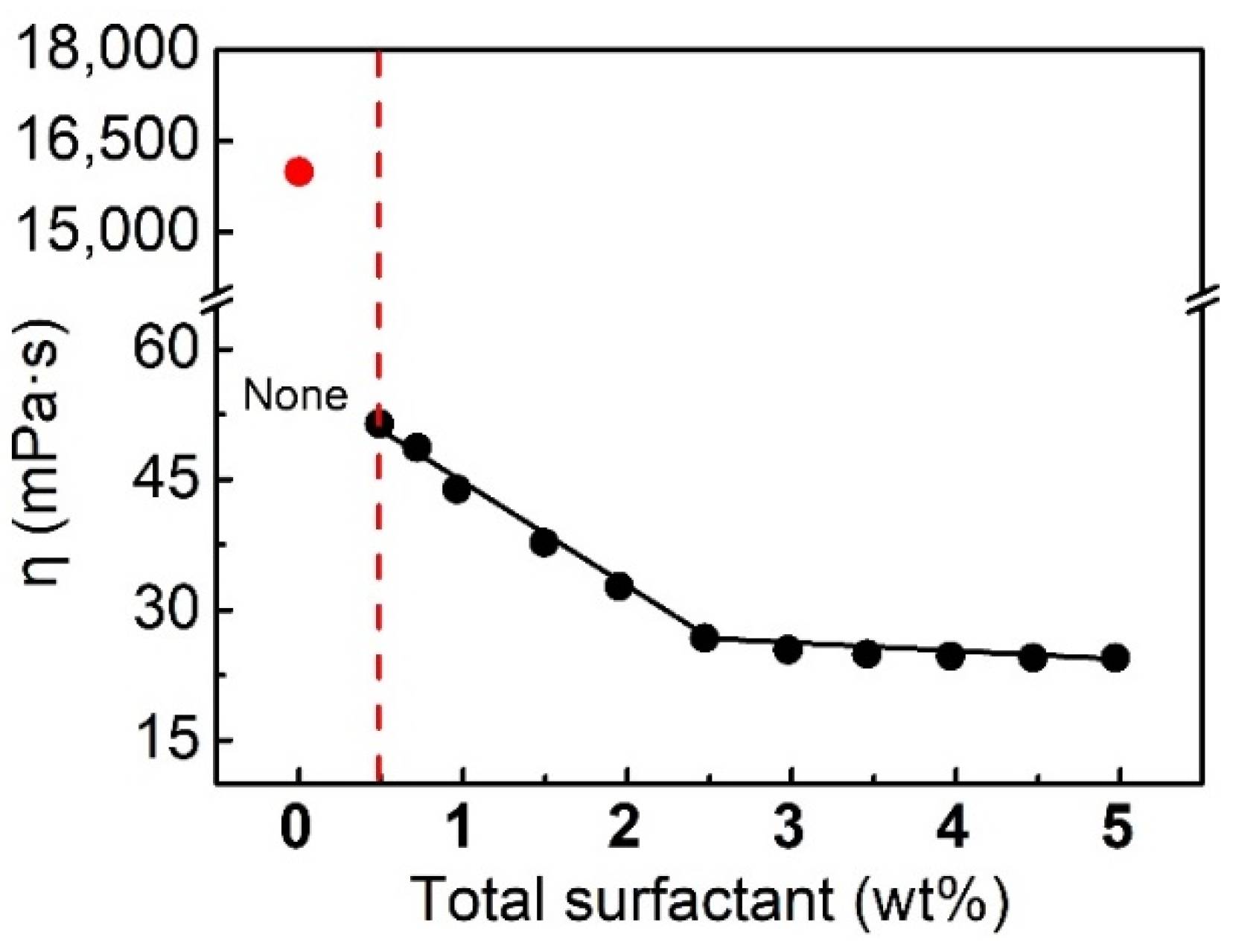
2.3.1. Effect of Concentration
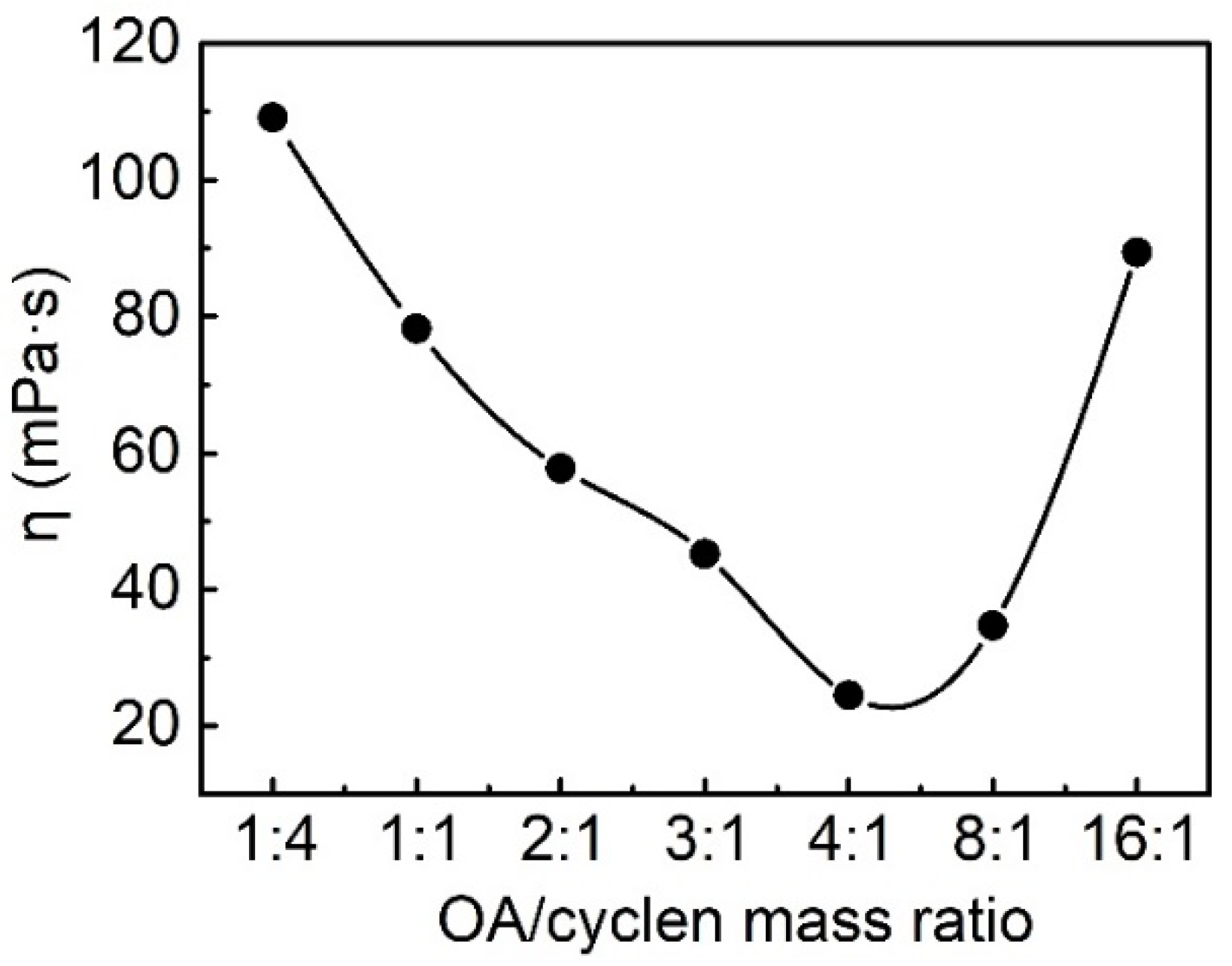
2.3.2. Effect of Molar Ratio
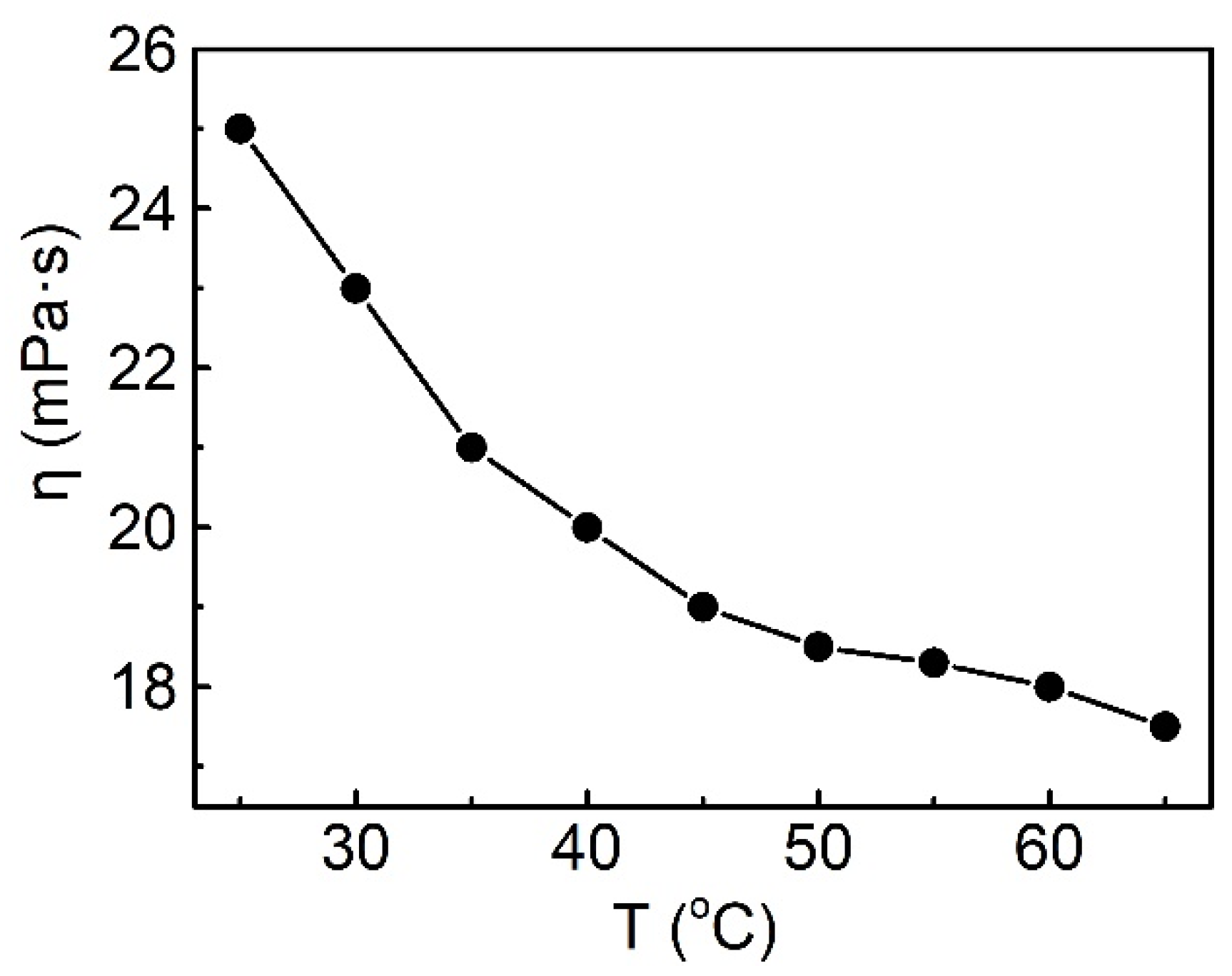
2.3.3. Effect of Temperature
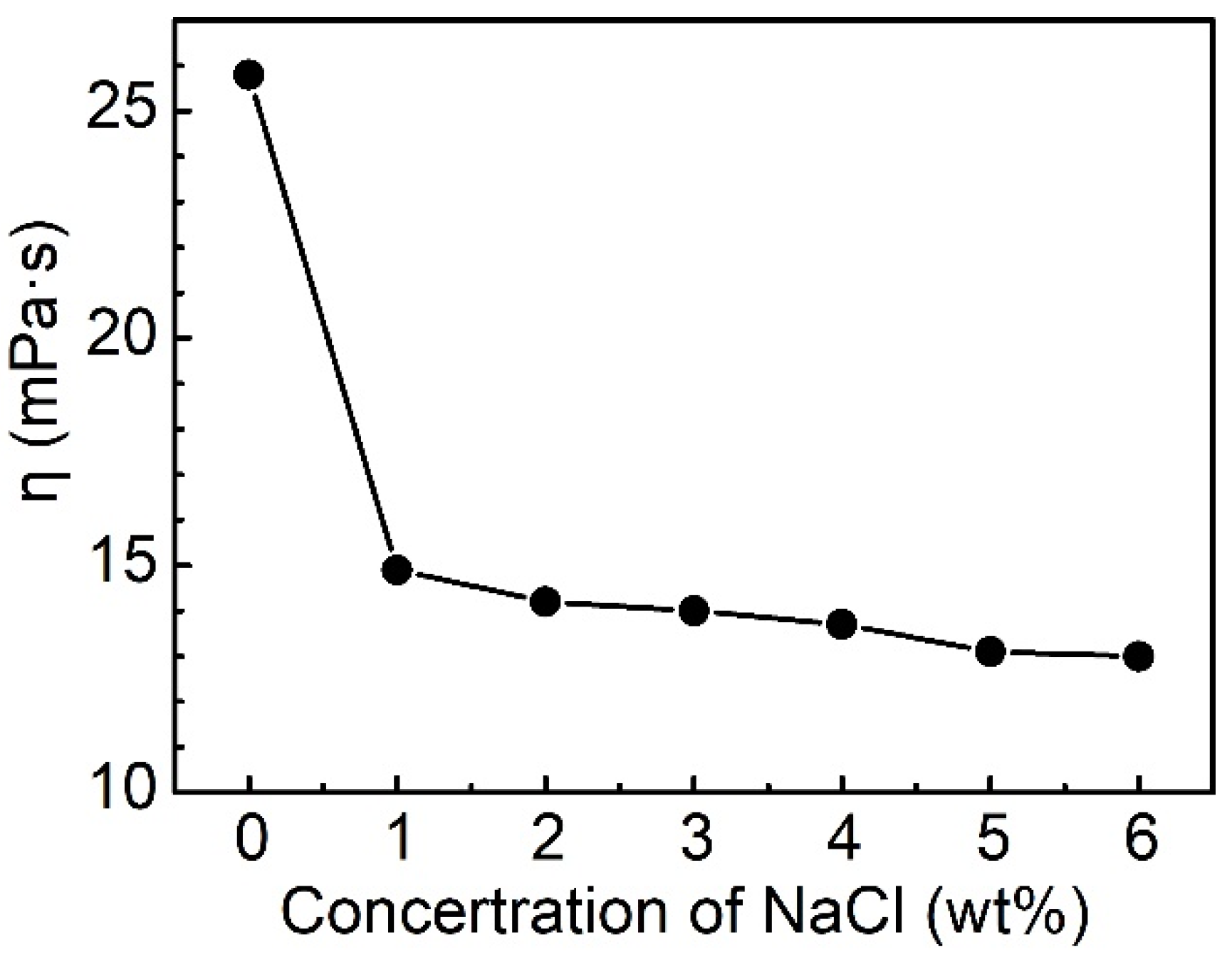
2.3.4. Effect of Salinity
2.4. CO2 Demulsification of Viscosity Reduction System
3. Experimental Procedures
3.1. Materials
3.2. Determination of Viscosity–Temperature Relation of Heavy Oil
3.3. Preparation of OA/cyclen Mixture
3.4. Viscosity Reduction Experiments
3.5. Surface Tension Analysis
3.6. Interfacial Tension Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, Z.; Lu, H.S.; Huang, Z.Y. A CO2-switchable polymer surfactant copolymerized with DMAEMA and am as a heavy oil emulsifier. J. Dispersion Sci. Technol. 2015, 37, 1200–1207. [Google Scholar] [CrossRef]
- Storm, D.A.; Sheu, E.Y. Characterization of colloidal asphaltenic particles in heavy oil. Fuel 1995, 74, 1140–1145. [Google Scholar] [CrossRef]
- Burg, P.; Selves, J.L.; Colin, J.P. Prediction of kinematic viscosity of crude oil from chromatographic data. Fuel 1997, 76, 1005–1011. [Google Scholar] [CrossRef]
- Wyk, V.D.; Ja, A. Viscosity of binary mixtures. Nature 1936, 138, 845–846. [Google Scholar]
- Jamaluddin, A.; Nazarko, T.W. Controlling sand production during downhole emulsification. J. Can. Pet. Technol. 1995, 34, 22–28. [Google Scholar] [CrossRef]
- Zhao, F.J.; Liu, Y.J.; Lu, N.; Xu, T.X.; Zhu, G.M.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- He, F.A.; Zhang, L.M. Organo-modified ZnAl layered double hydroxide as new catalyst support for the ethylene polymerization. J. Colloid Interface Sci. 2007, 315, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Engels, T.; Foerster, T.; Rybinski, W.V. The influence of coemulsifier type on the stability of oil-in-water emulsions. Colloids Surf. A 1995, 99, 141–149. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Y.; Yang, F.; Xu, Z.; Liu, Q. Advanced switchable molecules and materials for oil recovery and oily waste cleanup. Adv. Sci. 2021, 8, 2004082. [Google Scholar] [CrossRef]
- Morse, A.J.; Armes, S.P.; Thompson, K.L.; Dupin, D.; Fielding, L.A.; Mills, P.; Swart, R. Novel pickering emulsifiers based on pH-responsive poly(2-(diethylamino)ethyl methacrylate) latexes. Langmuir 2013, 29, 5466–5475. [Google Scholar] [CrossRef] [Green Version]
- Schumers, J.M.; Fustin, C.A.; Gohy, J.F. Light-responsive block copolymers. Macromol. Rapid Commun. 2010, 31, 1588–1607. [Google Scholar] [CrossRef]
- Shibata, Y.; Hyde, A.; Asakuma, Y.; Phan, C. Thermal response of a non-ionic surfactant layer at the water/oil interface during microwave heating. Colloids Surf. A 2018, 556, 127–133. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ke, Y.C.; Hu, X.; Peng, F.F.; Yu, C.C.; Xing, L. Synthesis and switchable behavior of a CO2 responsive polymeric surfactant acting as emulsifier. J. Dispersion Sci. Technol. 2019, 42, 407–415. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, J.; Kowal, E.; Cunningham, M.F.; Jessop, P. 2-(diethyl)aminoethyl methacrylate as a CO2-switchable comonomer for the preparation of readily coagulated and redispersed polymer latexes. ACS Macro Lett. 2012, 1, 1103–1107. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Wang, W.J.; Li, B.G.; Zhu, S. Preparation of CO2/N2-triggered reversibly coagulatable and redispersible polyacrylate latexes by emulsion polymerization using a polymeric surfactant. Macromol. Rapid Commun. 2012, 33, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Lin, S.J.; Feng, Y.J.; Theato, P. CO2-responsive polymer materials. Polym. Chem. 2017, 8, 12–23. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Secience 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Manica, R.; Liu, Q.; Xu, Z. Enhancing oil–solid and oil–water separation in heavy oil recovery by CO2-responsive surfactants. AIChE J. 2020, 67, e17033. [Google Scholar]
- Lu, Y.; Sun, D.; Ralston, J.; Liu, Q.; Xu, Z. CO2-responsive surfactants with tunable switching ph. J. Colloid Interface Sci. 2019, 557, 185–195. [Google Scholar] [CrossRef]
- Lu, H.; Guan, X.; Dai, S.; Huang, Z. Application of CO2-triggered switchable surfactants to form emulsion with xinjiang heavy oil. J. Dispersion Sci. Technol. 2014, 35, 655–662. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Quan, H.; Su, X. Soybean-oil-based CO2-switchable surfactants with multiple heads. Molecules 2021, 26, 4342. [Google Scholar] [CrossRef]
- Hoseini-Moghadam, S.M.A.; Ghiasimehr, B.; Torkaman, M.; Mirmarghabi, P. The role of temperature and porous media morphology on the performance of anionic and cationic surfactants for enhanced heavy oil recovery. J. Mol. Liq. 2021, 339, 116051. [Google Scholar] [CrossRef]
- Khan, M.R. Rheological properties of heavy oils and heavy oil emulsions. Energy Sources 1996, 18, 385–391. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.; Wang, Y.; Mao, H.; Zhang, Z. Interaction between star-like block copolymer and sodium oleate in aqueous solutions. Eur. Polym. J. 2008, 44, 3246–3255. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Li, D.; Yin, T.; Zhang, P.; Dong, Z.; Lin, M.; Zhang, J. New method based on CO2-switchable wormlike micelles for controlling CO2 breakthrough in a tight fractured oil reservoir. Energy Fuels 2019, 33, 4806–4815. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef] [PubMed]

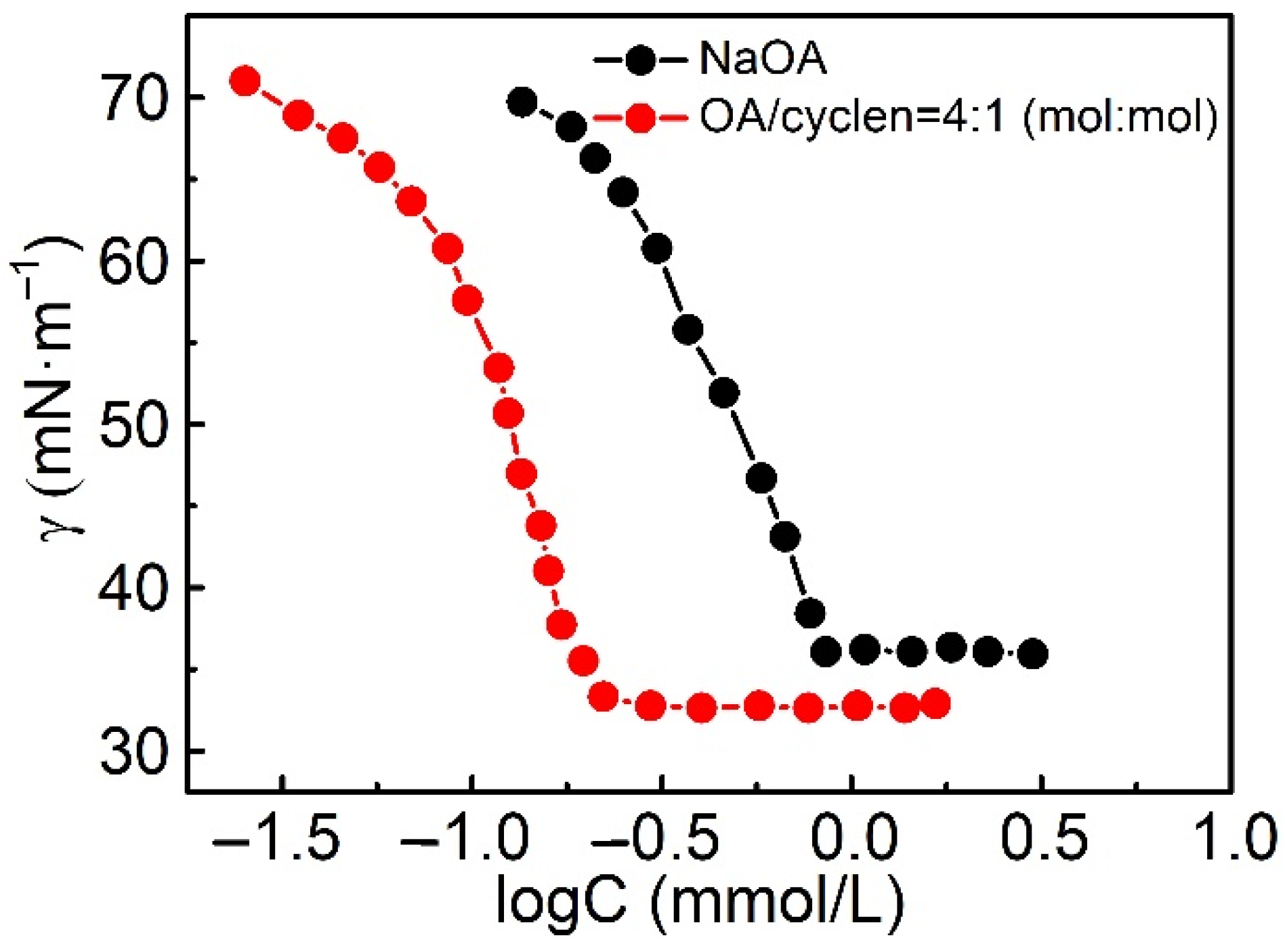
| Molecular Structure of Surfactant | Type | CMC (mM) | γcmc (mN·m−1) | Source of Data |
|---|---|---|---|---|
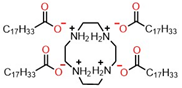 | Pseudo-tetrameric | 0.30 | 32.75 | This work |
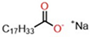 | Monomeric | 0.86 | 36.07 | This work |
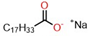 | Monomeric | 1.20 | 25.70 | Ref. [27] |
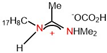 | Monomeric | 9.46 | 45.05 | Ref. [23] |
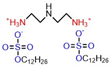 | Dimeric | 0.67 | 30.25 | Ref. [28] |
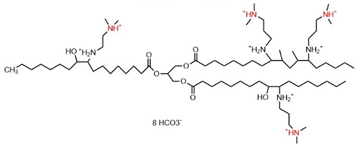 | Oligomeric | 0.20 | 35.80 | Ref. [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; He, S.; Tang, L.; Yang, S.; Ma, T.; Su, X. Application of CO2-Switchable Oleic-Acid-Based Surfactant for Reducing Viscosity of Heavy Oil. Molecules 2021, 26, 6273. https://doi.org/10.3390/molecules26206273
Liu L, He S, Tang L, Yang S, Ma T, Su X. Application of CO2-Switchable Oleic-Acid-Based Surfactant for Reducing Viscosity of Heavy Oil. Molecules. 2021; 26(20):6273. https://doi.org/10.3390/molecules26206273
Chicago/Turabian StyleLiu, Lulu, Shuai He, Lu Tang, Shu Yang, Tao Ma, and Xin Su. 2021. "Application of CO2-Switchable Oleic-Acid-Based Surfactant for Reducing Viscosity of Heavy Oil" Molecules 26, no. 20: 6273. https://doi.org/10.3390/molecules26206273
APA StyleLiu, L., He, S., Tang, L., Yang, S., Ma, T., & Su, X. (2021). Application of CO2-Switchable Oleic-Acid-Based Surfactant for Reducing Viscosity of Heavy Oil. Molecules, 26(20), 6273. https://doi.org/10.3390/molecules26206273






