1. Introduction
Insects have been eaten for over 5 million years, when our ancestors—the first hominids—consumed insects as protein supplements [
1]. Some insect species are disruptive to humans and animals, for example, crop and grain storage pests [
2,
3]. However, we have far more benefits from the presence of insects in all the globe. Pollinating insects play a large role in nature, and eating insects can be helpful in fighting hunger in the world. At least 2 billion people in almost 80% of countries eat insects in various forms [
4]. In many countries, insects are consumed because of their taste and nutritional value (they can be a source of nutritious protein, fats, and other nutrients) [
5,
6]. To date, more than 1900 species of insects have been described as food for humans [
7,
8].
In the near future, food production methods and the criteria for its choice by consumers probably will change [
9], because people are increasingly aware of the need to reduce consumption and live a “zero waste” life. Production of one kilo of beef requires around ten kilograms of feed [
10]. The same ten kilograms can also be a source of food for nine kilograms of insects [
11]. Global meat requirements have been increasing intensively in recent years [
12] and the production of “insect meat” would be a good approach for a solution to this problem. At the same time, the meat industry is one of the largest sources of pollution [
13] and zoonoses such as “mad cow disease”, bird flu, or swine flu [
14]. We can treat insects as an environmentally friendly animal protein in our food [
15,
16,
17]. Insect protein is one of the four main trends (insects, vertical agriculture, aquaponics, and laboratory-grown meat) that can affect future world nutrition trends [
18,
19,
20]. Insect meat is rich in amino acids, fats, sugars, and has a high concentration of some vitamins (e.g., B and K) [
21,
22].
Most Europeans do not consider insects as food, it is a food taboo [
23,
24,
25]. Until 31 December 2017, the legal status of insects as food was unclear. Some experts believe that insects are food for general consumption, the same as farm animals. Others claimed that insects are so-called “novel food” and it is necessary to obtain the appropriate authorization [
26,
27]. Others thought insects were not food. In countries such as the United Kingdom, the Netherlands, or France, serving insects as a food was allowed and in Poland it was not. From January 1, 2018, insects and their parts can be placed on the market as “novel food” if they obtain the approval of the European Commission [
28]. Novel food is defined as food that has not been consumed to any significant degree in the EU before 15 May 1997 (when the first novel food legislation entered into force) [
29]. This can be a newly developed, innovative food or food produced using new technologies and production processes, as well as food traditionally eaten outside of the EU. For example, one of the Finnish food companies has introduced bread with the addition of insects (ground cricket powder) [
30]. Insect foods can also be found in stores in Belgium, Great Britain, Denmark, and the Netherlands [
31,
32]. In addition, processed animal protein derived from insects may be used for feeding aquaculture animals and fur animals [
33,
34].
Insects are most often consumed whole (blanched, chilled, dried, fried) or ground (powdered or paste) [
35,
36,
37]. Protein and fat isolates from insects are also used [
38,
39]. Known examples are protein bars with insect protein, paste with flies’ eggs, cricket flour, caterpillar burgers, larvae dumplings, crunchy fried locusts, or tempura grasshopper. The taste of insects is very diverse but it is not unusual and we can compare it to dishes known to us. It depends on the insect species and the stage of development of the insect and the method of preparation. For example, roasted grasshoppers taste like salted and oiled sardines, butter-fried locusts taste like shrimp, ant larvae have a watermelon flavor, and adult ants taste like lemon, while termites seem to taste like hazelnuts [
40]. In recent years, interest in researching the biological activity of chemical compounds obtained from insects has increased. For example, peptides derived from insect proteins have anti-fungal, anti-bacterial, anti-oxidant, anti-diabetic, and antihypertensive (angiotensin-converting inhibitors (ACE)) [
41,
42,
43].
The Maillard reactions (non-enzymatic browning reactions) were first described by Louis-Camille Maillard in 1912 [
44]. It is a group of chemical reactions between amino acids and reducing sugars, usually occurring at elevated temperature, during heat treatment of food products [
45]. Then, hundreds of different flavor and aroma compounds are formed in subsequent reactions. Treatment with elevated temperature causes many changes in the chemical composition and affects the nutritional value of food and its taste and smell [
46]. This process creates compounds considered to be carcinogenic or mutagenic [
47,
48], as well as antioxidant substances with potential positive effects on the human body [
49]. Some of the Maillard reaction products formed during the thermal processing of food have been known recently thanks to the development of modern separation and identification techniques [
50]. Determining the chemical structures and biological properties of compounds allows improving technological processes in terms of food safety and functionality.
Therefore, the aim of the present research was to investigate the roasting of two insect species: Tenebrio molitor and Zophobas morio larvae at different temperatures (160, 180, and 200 °C) and to detect the Maillard compounds that were formed in the process. Furthermore, it was planned to see how these compounds would affect the odor profile of the roasted insects.
3. Discussion
Roasting refers to the dry thermal treatment of food in an oven and is usually applied to meat. The tested larvae
Tenebrio molitor and
Zophobas morio lost on average about 50% of their water when baked at three different temperatures (160, 180, and 200 °C) (
Table 1). During roasting, juices (moisture) are lost and heat-labile (e.g., some vitamins are easily destroyed by heat). The study shows that the three temperatures used do not show appreciable variations in water loss. Roasting can improve the palatability and appearance of food by enhancing and preserving natural flavors. It can also improve food safety by destroying pathogenic microorganisms. It aims to increase the absorption of nutrients, allows the consumption of certain products, and gives flavor. Unfortunately, despite the overall improvement in the digestibility of, e.g., protein or carbohydrates, many ingredients are lost during thermal processing, so it is not recommended in every situation and with every product. The cooking loss is a combination of liquid and soluble substances lost from the meat during cooking. Overall, it can affect the nutritional value of food positively or negatively.
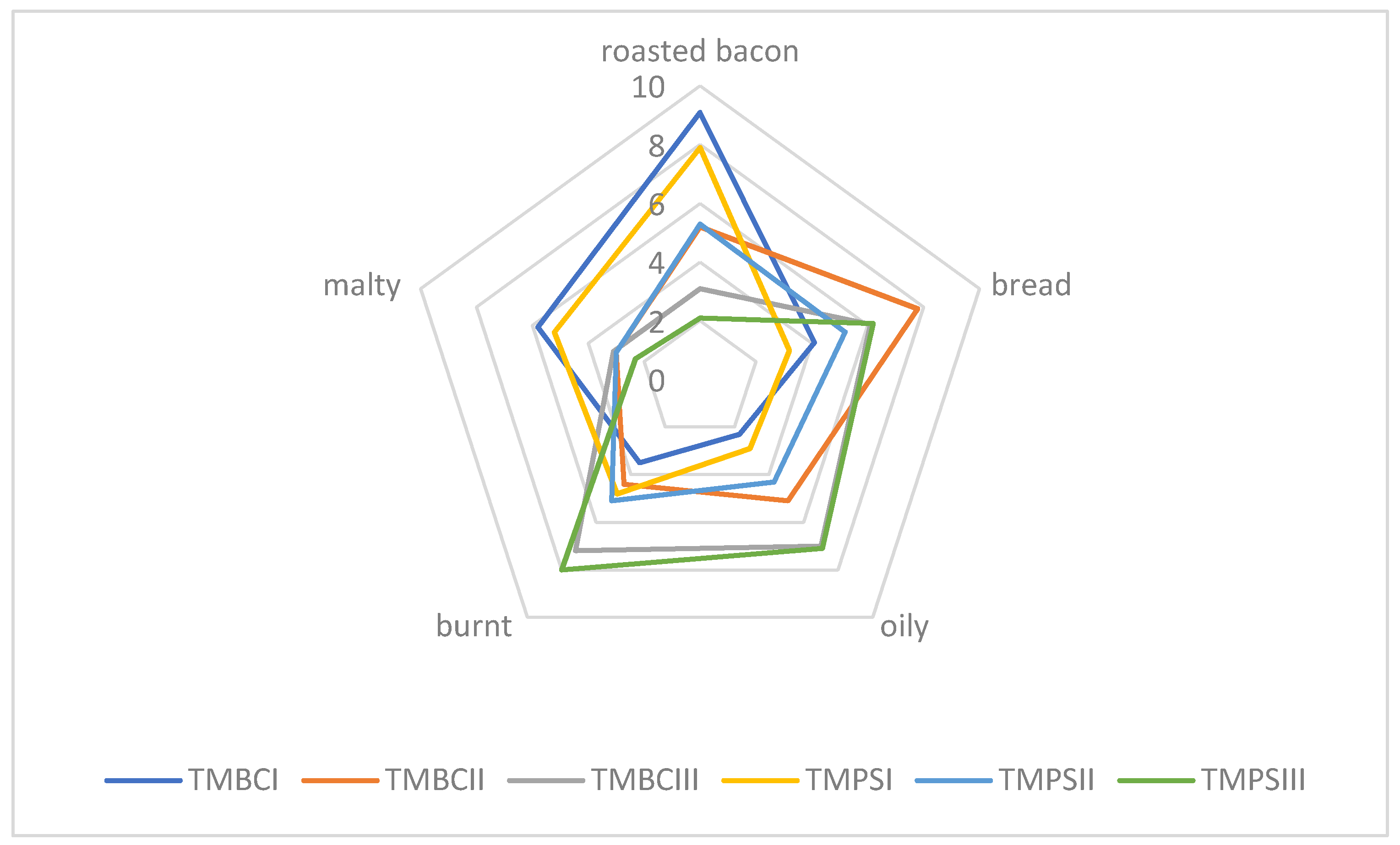
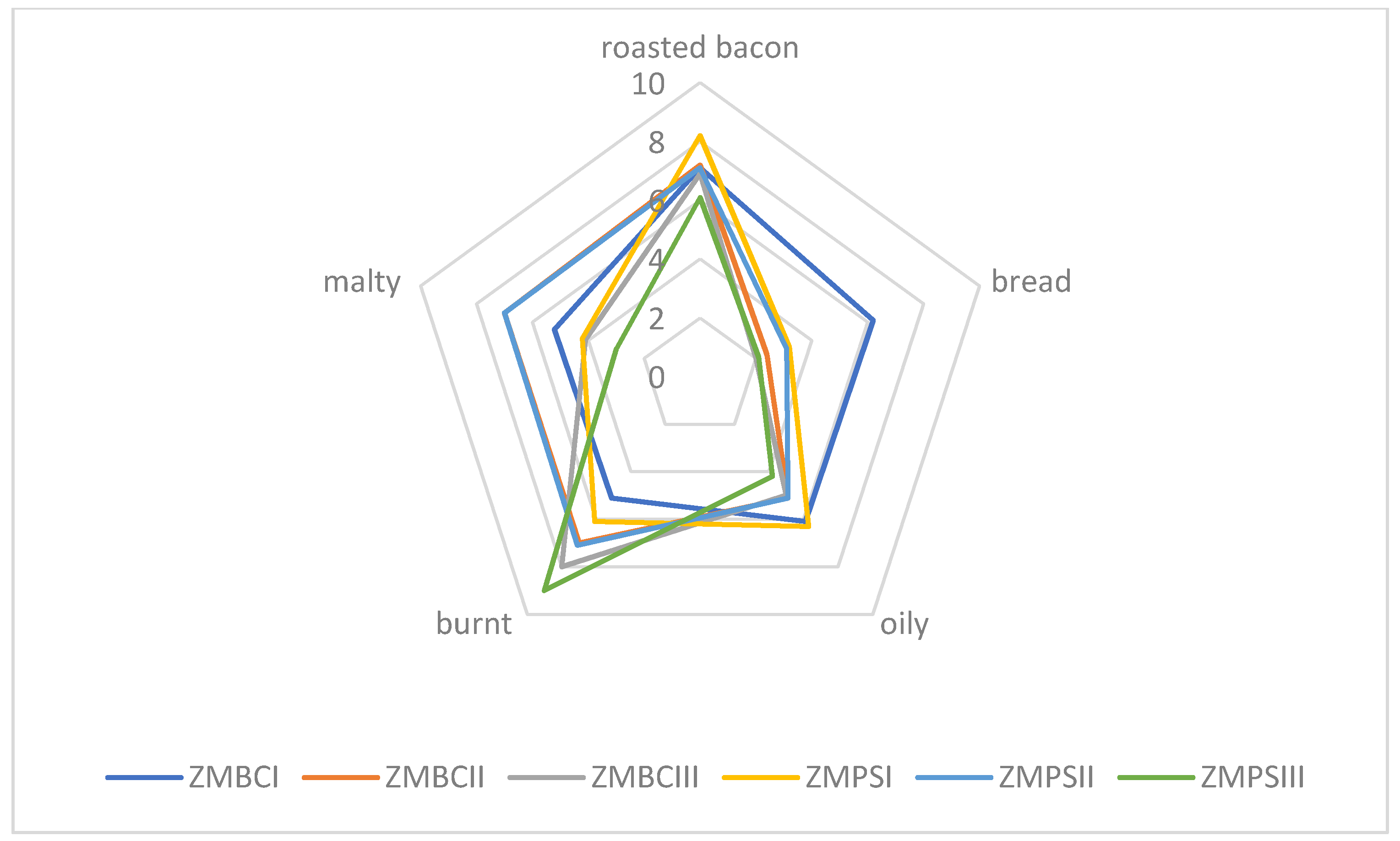
Sensory quality is one of the most important features in food, including meat products or insects, too. There are many factors affecting food quality, like, for example, feedstuff type and its composition or heat treatment. Feed components influence the nutritional and physio-chemical properties of meat and its sensory characteristics, which in turn are reflected in the quality of meat products. We assume that for insects, it will be similar. Due to the size and delicate nature of insects as food, they should be baked carefully so that they do not turn bitter and black (burnt and unpalatable). Sensory evaluation defined as “the systematic study of human reaction to physicochemical properties” enables obtaining information about the sensitivity of the human sense of taste and smell [
64,
65]. In sensory analysis, the respective groups were divided by the effect of feeding and temperature in the group of insect species. Standard deviation and Duncan’s test (
p < 0.05) were applied. The corresponding
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 can be found in the
Supplementary Materials S15–S20. Statistical tests show that there were in most cases significant statistical differences in all groups. The applied baking temperature had a greater effect on the smell of the insects. Mealworm larvae roasted at 160 °C (TMPSI, TMBCI) were characterized by the aroma of baked bacon, those roasted at 180 °C (TMPSII, TMBCII) showed a bread smell, and those roasted at 200 °C (TMPSIII, TMBCIII) had a burnt smell (
Figure 1). In the case of superworm larvae, samples roasted at the lowest of the temperatures tested (ZMPCI, ZMBCI) were also characterized by an intense smell of roasted bacon (
Figure 2). Similar results were obtained for larvae roasted at 180 °C (ZMPSII, ZMBCII), and the malty aroma was also recognized. Samples roasted at the highest temperature (ZMPSIII, ZMBCIII) were found to be burnt. Based on our own experience, we can conclude that depending on the species, we should bake insects at a temperature of 160 to 180 °C. Those temperatures are appropriate and they contribute in the panel test to major pleasantness. From the experiments carried out, the optimum temperature for baking the selected insect larvae is 160 °C (
Figure 1 and
Figure 2).
Among the many aromatic compounds found in food, only those that are present in a concentration greater than their sensory detection threshold are significant. The odor detection threshold and odor activity value (OAV) are important for the importance of a volatile compound to the aroma of a food product. Because most essential odorants have low odor detection thresholds, the odor of these compounds can be detected when they are at low concentrations. To estimate odor potency, the OAV is used. This is the ratio of the concentration of a volatile compound to its odor detection threshold. The relevant odor descriptors of the compounds determined by GC-MS (SPME) in roasted samples of the insect larvae studied can be divided into general groups: fruity-floral, roast, bread-like, and burnt. The results obtained in the sensory analysis are supported by the chemical analysis of the aroma compounds and in respect with their OAV values (%OAV). For insects roasted at the lowest temperature (TMPSI, TMBCI, MPSI, ZMBCI), the highest OAV was found for isobutylpyrazine (
34) and 2,5-dimethylpyrazine (
7) and was more than 90% and about 20%, respectively (
Table 5 and
Table 6). Their presence determined mainly the aroma of the roast. As the roasting temperature of the insects was increased, a burning smell developed. The isobutylpyrazine (
34) content (%OAV) decreased (roast aroma) and the 2,5-dimethylpyrazine (
7) content increased (burnt aroma).
Pyrazines occur naturally in heat-treated food products and are produced on a mass scale by extraction from natural products, chemical synthesis, and biocatalysis (enzymatic, microbiological) [
66]. They are used as flavor- and aroma-enhancing compounds. In the case of alkyl pyrazines, the increasing demand can no longer be supplied economically from natural sources. The natural occurrence of the main seven alkyl pyrazines in foods in Europe in tons per year was 2157 tons per year (in 2004). In contrast, the annual volume of use of the same pyrazine derivatives used as flavoring agents in Europe was 2157 kg per year, which represents 0.85% of naturally occurring compounds [
67]. Therefore, new, preferably natural, sources of pyrazines are being sought. Roasted insects can find use as food additives that are a source of natural pyrazines. In January 2021, specialists from the European Food Safety Authority (EFSA) gave a positive opinion on insect-based food products [
68]. The project has not yet been approved by the European Commission, but specialist voices indicate that such approval is likely to be given. The novelty of using insects in food has aroused great interest among the public (potential consumers). Various insect-derived foods could be applied as a source of protein, lipids, vitamins, macro- and microelements, or volatile compounds for the diet.
4. Materials and Methods
4.1. Insect Breeding
Mealworms (TM) are the larval form of the mealworm beetle,
Tenebrio molitor, a species of darkling beetle. Darkling beetle is the common name of the large family of beetles Tenebrionidae. In nature, it lives where it is dark, warm, humid, and there are lots of decaying organic matter, i.e., under decaying pieces of bark of deciduous trees. Moreover,
Tenebrio molitor is a pest of grain, flour, and food stores [
69]. The life cycle of
Tenebrio molitor is of variable length, from 280 to 630 days. Larvae hatch after 10–12 days (at 18–20 °C) and become mature after a variable number of stages (8 to 20), typically after 3–4 months, but the larva stage can last up to 18 months. Mealworms have short life cycles, and are easy to breed [
69].
Superworms (ZM), Morio worms, or Zophobas,
Zophobas morio (Fabricius, 1776) (Coleoptera: Tenebrionidae) are a globally recognized feed for reptiles. In the wild, superworms larvae occur in dead (diseased) trees, where they feed on this wood. It is a popular food insect due to its ease of breeding and nutrition. Recent research efforts indicate that this insect could also be used as a partial replacement of fishmeal for farmed tilapia [
70]. The life cycle of
Z. morio is like other beetles, as it has an egg, larva, pupa, and adult stage. Larvae are similar to mealworm larvae, although they are much larger and more fat.
Insects for described research were purchased at a local terrarium store (Wrocław, Poland). The feeding medium for the insect larvae consisted of 100% blue corn flour (BC) or potato starch (PS). Rearing of larvae was carried out in plastic containers (in triplicate for each of the food variants) at 26 °C for 10 days. For 500 g of insect biomass, 500 g of blue corn flour (BC) and potato starch (PS) were added, respectively. After 5 days, another 200 g of feed was added.
At the end of the ten-day growth period, the larvae were separated from the feeding media by manual sieving and immediately preserved by freezing at −28 °C. The larvae were weighed about 1 g into separate glass screw-cap containers and stored at −28 °C until analysis. The mean larval weight for mealworms was 0.13 g and for superworms was 0.51 g. The larvae were baked whole and were crushed before SPME analysis.
4.2. Insect Samples
On the day of the analysis of the profile of fragrances (SPME), insects were thawed to room temperature and then baked according to the three following variants: I. 160 °C for 20 min; II. 180 °C for 15 min; III. 200 °C for 10 min (
Table 7). After the baking process, the dishes were sealed and the samples were prepared for SPME analysis (in sub-replicate, three times).
4.3. Water Loss (WL)
Non-enzymatic browning reactions are not only chemically complicated. Physical phenomena also have an impact on Maillard’s reaction. One of the factors conditioning the reaction of non-enzymatic browning during heat treatment food is water activity (aw). In the tested materials, water loss during baking was expressed as g/100 g and was calculated by weighing the insect samples before (WB) and after roasting (WA), as follows: WL =100 × (WB − WA) / WB. The determination of each variant was carried out in triplicate.
4.4. SPME/GC-MS Conditions
For HS-SPME analysis (30 min exposure to a 2 cm DVB/CAR/PDMS fiber (Supelco, Bellefonte, PA, USA)), about 0.5 g of roasted sample was put in to headspace vials and kept in a laboratory water bath at 50 °C. Next, 0.1 µg of equilibrium mixture of 3-ethyl-2,5-dimethylpyrazine and 2-ethyl-3,5-dimethylpyrazines (Sigma Aldrich, Saint Louis, MO, USA) as an internal standard was added. Calibration function was constructed for 3-ethyl-2,5-dimethylpyrazine and 2-ethyl-3,5-dimethylpyrazines ranging from 0.001 to 1 microgram (in vial suspended and intensively shaken in water before use), with excellent linearity, with an R2 value 0.993. We observed two signals with equal ratio. Semi-quantification of compounds was based on calculation of the area of unknown signals and comparison with the regression equation for the internal standard.
Analyte desorption (220 °C for 3 min) was performed on Shimadzu apparatus (Shimadzu, Kyoto, Japan) equipped with a Zebron ZB-5 MSI (30 m × 0.25 mm × 0.25 µm) column (Phenomenex, Shim-Pol, Warsaw, Poland). Fiber composition was chosen due to previous optimizations [
71]. The potential OAV was calculated by dividing the concentration of the compounds in the sample by the sensory thresholds obtained from the literature. The concentration of the compounds was established by a standard calibration curve. The Kovats retention index values were calculated for each according to Adams [
72], with a comparison of the obtained data with the values presented in NIST17 (NIST/EPA/NIH Mass Spectral Library) database peaks by comparing their retention characteristics with those of the two closest eluting aliphatic hydrocarbons from the retention index standard, analyzed under identical conditions. Presumptive identification can often be made by comparing the Kovats retention index value with a value previously published in literature references. Identification of the compounds was done by comparison: I: spectrum presented in NIST17 (NIST/EPA/NIH Mass Spectral Library); II: calculated retention index values with database NIST17; III: retention times of unknown compounds with available standards (1, 3, 5, 8, 11, 14, 15, 16, 18, 20, 23, 30, 32, 39, 40, 43, 44, 45, 47, 48).
4.5. Sensory Evaluation
In this study, for sensory evaluation, we chose descriptive sensory analysis. A nine-hedonic scale (
Table 8) was used to investigate the degree of preference of the roasted larvae (160, 180, and 200 °C by 20, 15, or 10 min, respectively) of
Tenebrio molitor (TM) and
Zophobas morio (ZM) fed with potato starch (PS) or blue corn flour (BC) (sample codes in
Table 7). Samples were roasted and 250 g of each sample were used. After thermal treatment, the insects were put in glass containers and stored in a fridge (−24 °C) until sensory and GC-MS analysis. Nine panelists were chosen from the teaching staff, graduate students, and master degree students of The Faculty of Biotechnology and Food Science, Wrocław University of Environmental and Life Sciences. The age distribution of the panelists was between 23 and 48. Among them were four men and five women. The panelists were trained for three one-hour sessions. To assist the panelists in establishing a framework for each attribute, reference smells were used during training to establish minimum and maximum intensities for each attribute. The samples were evaluated for sensory quality of roasted bacon, bread, oily, burnt, and malty aroma, and consistency using a varying scale from 9—which means like extremely, to 1—which means dislike extremely (
Table 8). Descriptors for the evaluation of roasted insects were designated from the literature data about roasted food and in preliminary tests. A set of reference solutions in water (0.01–0.1%; concentrations well above the threshold, but assessed as not very intense) was prepared based on the odor descriptor set, which consisted of coffee (no. 12), bread crust (no. 85), bread (no. 86), beef found (no. 178), pork found (no. 179 (Sosa Ingredients, S.L., Spain)), malty aroma (soya milk, Mona Naturprodukte GmbH, Austria) and caramel (burned sugar). The sensory tests were done in a specially designed laboratory, which met relevant standards. The three treatments were evaluated in one session. The data were recorded on paper. The samples were coded and randomized. The insect samples were served on small plates. After each sample, the panelists drank water to restore their original tasting conditions.
4.6. Statistical Analysis
The data from quantitative volatile constituents were subjected to the analysis of variance using Duncan’s test (p < 0.05), all using the STATISTICA 13.3 software for Windows (StatSoft, Krakow, Poland).