Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff
Abstract
1. Introduction
2. Results and Discussion
2.1. Techno-Functional Properties of Pomace Powder (PP)
2.1.1. Moisture Content and Microbiological Status of the Pomace Powders
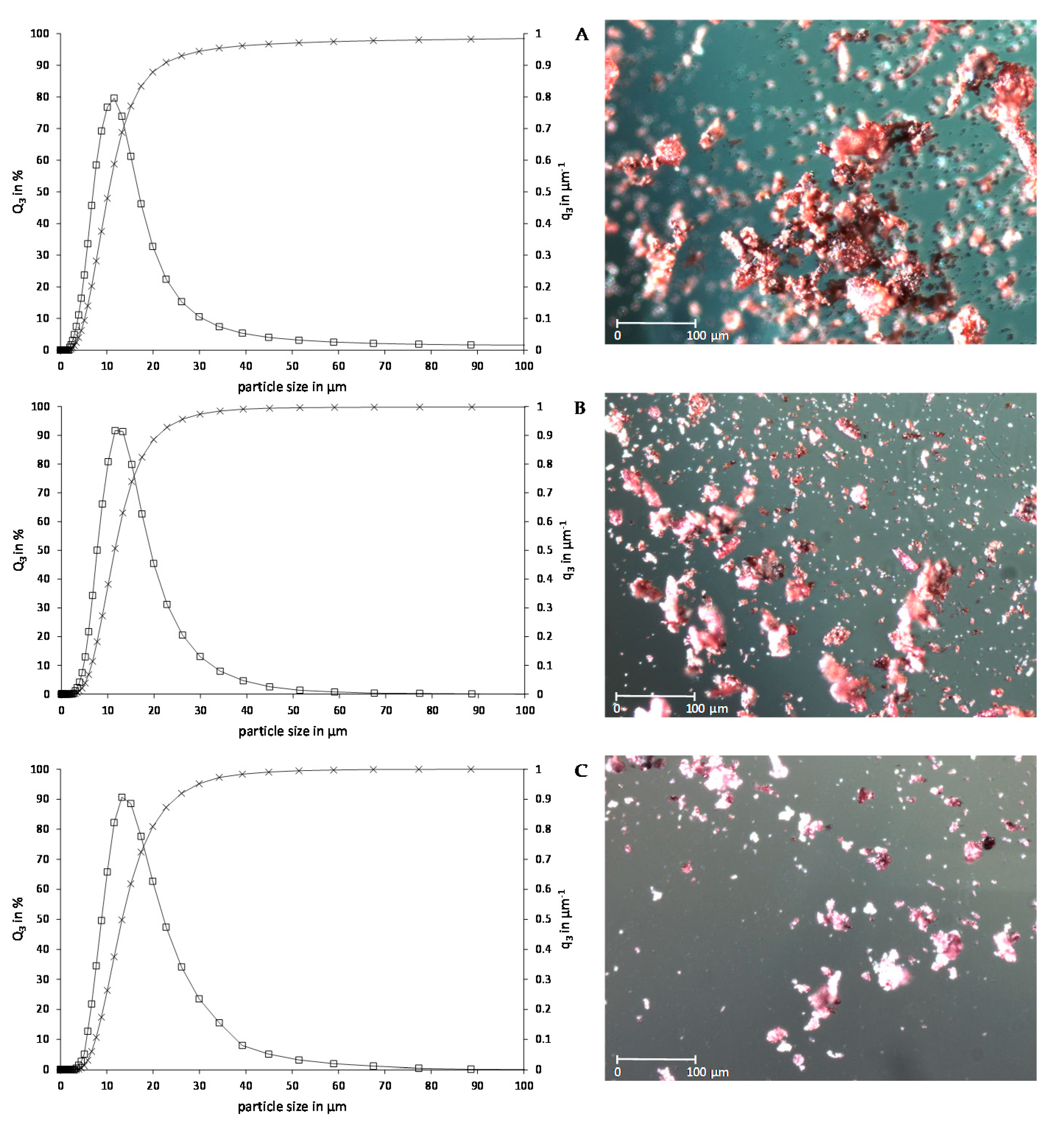
2.1.2. Particle Size Distribution and Morphology
2.1.3. Hygroscopicity
2.1.4. Bulk Density
2.1.5. Water-Binding and Oil Absorption Capacity
2.1.6. Cold-Water Solubility
2.1.7. Sedimentation Velocity
2.1.8. Swelling Capacity
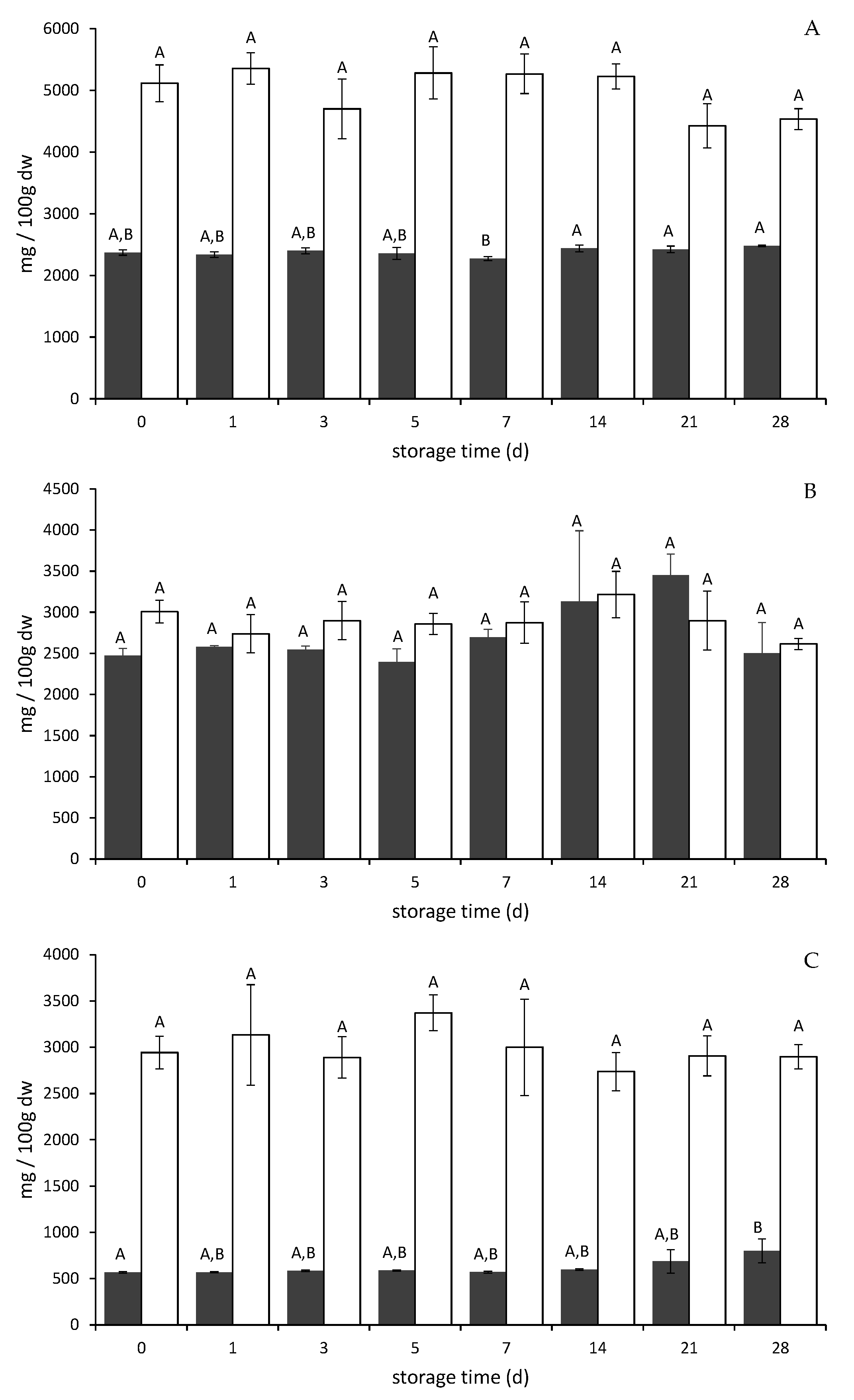
2.2. Anthocyanin and Total Phenolic Contents in Pomace Powder (PP)
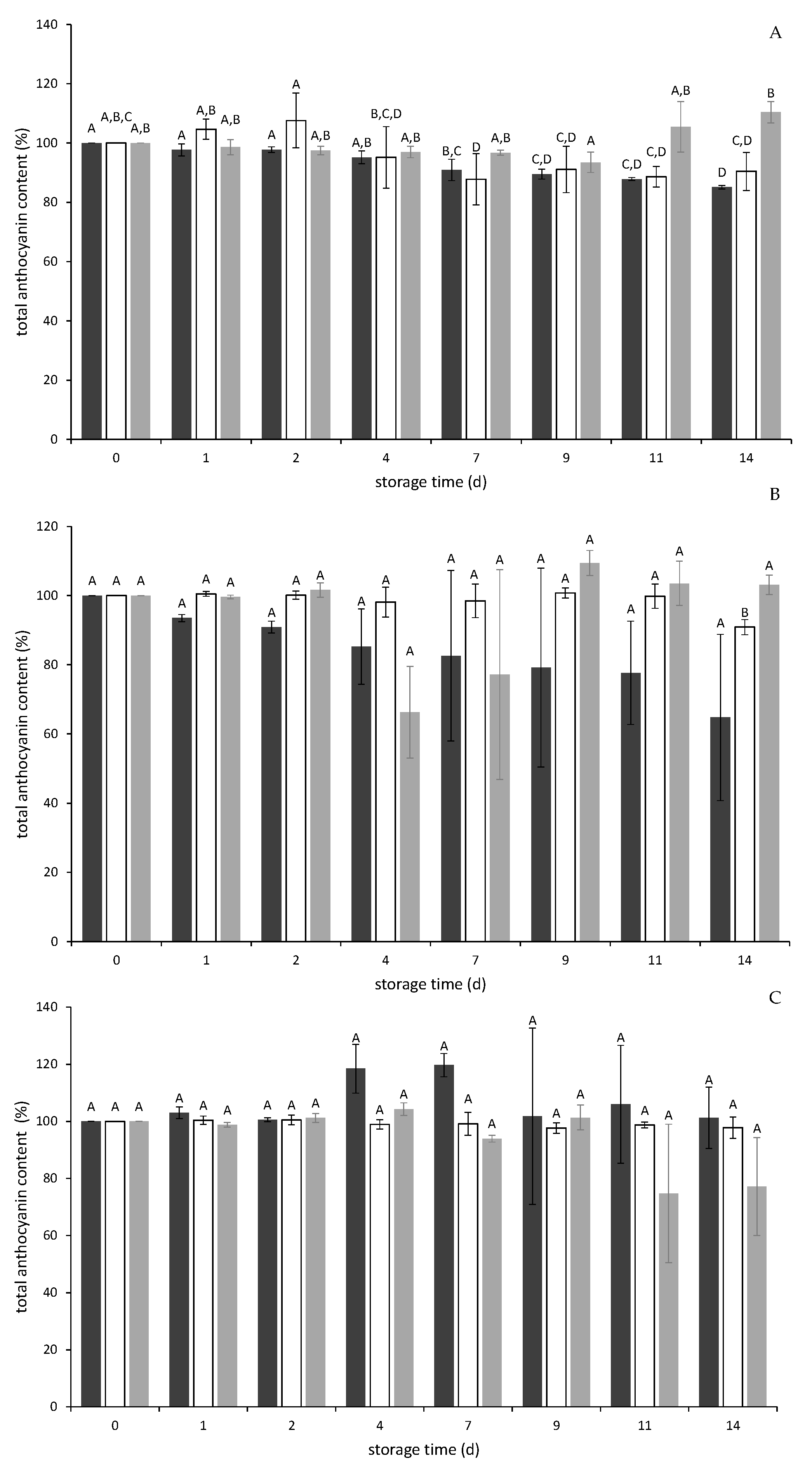
2.2.1. Storage Stability of Anthocyanins and Phenolic Compounds in PP
2.2.2. Color Parameters of the three PP
2.3. Anthocyanin and Total Phenolic Content in a Yogurt Model Application over Storage and the Effect on Color Parameters
2.3.1. Storage Stability of Anthocyanins during a Yogurt Model Application
2.3.2. Storage Stability of Phenolic Compounds during a Yogurt Model Application
2.3.3. Color Parameters of the Yogurt Model Applications
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals and Standards
3.1.2. Berry Pomace as a Raw Material for the Production of Pomace Powder (PP)
3.2. Methods
3.2.1. Storage of Pomace Powder (PP)
3.2.2. Storage of Colored Yogurt
3.2.3. Extraction and Quantification of Anthocyanins in Stored PP and Yogurt
3.2.4. Determination of Total Phenolic Content by the Folin-Ciocalteu Assay
3.2.5. Determination of Techno-Functional Properties of PP
Particle Size
Hygroscopicity
Color Parameters (CIELab Color Metrics)
Microbiological Status
Water-Binding and Oil Absorption Capacity
Solubility
Bulk Density
Dry Matter
Sedimentation Velocity
Swelling Capacity
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Gras, C.C. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Color; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publ; CRC Press: Amsterdam, The Netherlands, 2016; pp. 385–428. [Google Scholar]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Stich, E. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Color; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publ; CRC Press: Amsterdam, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Kendrick, A. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Color; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publ; CRC Press: Amsterdam, The Netherlands, 2016; pp. 163–178. [Google Scholar]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours-selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Lakshmi, C.G. Food coloring: The natural way. Res. J. Chem. Sci. 2014, 4, 87–96. [Google Scholar]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. Lwt Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Schieber, A. Side streams of plant food processing as a source of valuable compounds: Selected examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Gras, C.C.; Bogner, H.; Carle, R.; Schweiggert, R.M. Effect of genuine non-anthocyanin phenolics and chlorogenic acid on color and stability of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) anthocyanins. Food Res. Int. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Gras, C.C.; Nemetz, N.; Carle, R.; Schweiggert, R.M. Anthocyanins from purple sweet potato (Ipomoea batatas (L.) Lam.) and their color modulation by the addition of phenolic acids and food-grade phenolic plant extracts. Food Chem. 2017, 235, 265–274. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- May, N.; Guenther, E. Shared benefit by material flow cost accounting in the food supply chain—The case of berry pomace as upcycled by-product of a black currant juice production. J. Clean. Prod. 2020, 245, 118946. [Google Scholar] [CrossRef]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H.; Hernando, I. Fiber from fruit pomace: A review of applications in cereal-based products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Singh, A.; Gu, Y.; Castellarin, S.D.; Kitts, D.D.; Pratap-Singh, A. Development and characterization of the edible packaging films incorporated with blueberry pomace. Foods 2020, 9, 1599. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J. Agric. Food Chem. 2010, 58, 4037–4042. [Google Scholar] [CrossRef]
- Weber, F.; Larsen, L.R. Influence of fruit juice processing on anthocyanin stability. Food Res. Int. 2017, 100, 354–365. [Google Scholar] [CrossRef]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding value to fruit processing waste: Innovative ways to incorporate fibers from berry pomace in baked and extruded cereal-based foods-A SUSFOOD project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food Bioprocess Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crop. Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Perez-Gregorio, M.R.; Simal-Gandara, J. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Beer, A.; Struck, S.; Rohm, H. Pre-hydrated berry pomace in wheat bread: An approach considering requisite water in fiber enrichment. Foods 2020, 9, 1600. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.; Shere, D.M. Comparative evaluation of physico-chemical and functional properties of apple, carrot and beetroot pomace powders. Int. J. Food Ferment. Technol. 2017, 7, 317–323. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- German Society for Hygiene and Microbiology. Mikrobiologische Richt- und Warnwerte zur Beurteilung von Lebensmitteln. Available online: https://www.dghm-richt-warnwerte.de/de (accessed on 21 April 2020).
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef]
- Mokhtar, S.M.; Swailam, H.M.; Embaby, H.E.-S. Physicochemical properties, nutritional value and techno-functional properties of goldenberry (Physalis peruviana) waste powder concise title: Composition of goldenberry juice waste. Food Chem. 2018, 248, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Tapia, A.; Pérez-Chabela, M.d.L.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Valorization of pomace powder obtained from native mexican apple (Malus domestica var. rayada): Chemical, techno-functional and antioxidant properties. Plant Foods Hum. Nutr. 2015, 70, 310–316. [Google Scholar] [CrossRef]
- Gurak, P.D.; Bona GS de Tessaro, I.C.; Marczak, L.D.F. Jaboticaba pomace powder obtained as a co-product of juice extraction: A comparative study of powder obtained from peel and whole fruit. Food Res. Int. 2014, 62, 786–792. [Google Scholar] [CrossRef]
- Sahni, P.; Shere, D.M. Utilization of fruit and vegetable pomace as functional ingredient in bakery products: A review. Asian J. Dairy Food Res. 2018, 37, 202–211. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Effects of fruit juice concentrate production on individual anthocyanin species in elderberry. Int. J. Food Sci. Technol. 2016, 51, 641–648. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. Lwt Food Sci. Technol. 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Larsen, L.R.; Buerschaper, J.; Schieber, A.; Weber, F. Interactions of anthocyanins with pectin and pectin fragments in model solutions. J. Agric. Food Chem. 2019, 67, 9344–9353. [Google Scholar] [CrossRef] [PubMed]
- Heffels, P.; Weber, F.; Schieber, A. Influence of accelerated solvent extraction and ultrasound-assisted extraction on the anthocyanin profile of different Vaccinium species in the context of statistical models for authentication. J. Agric. Food Chem. 2015, 63, 7532–7538. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Garcia-Herrera, P.; Pérez-Rodríguez, M.-L.; Aguilera-Delgado, T.; Labari-Reyes, M.-J.; Olmedilla-Alonso, B.; Camara, M.; de Pascual-Teresa, S. Anthocyanin profile of red fruits and black carrot juices, purees and concentrates by HPLC-DAD-ESI/MS-QTOF. Int. J. Food Sci. Technol. 2016, 51, 2290–2300. [Google Scholar] [CrossRef]
- Tanner, H.; Brunner, H.R. Getränkeanalytik, 2nd ed.; Heller Chemie und Verwaltungsgesellschaft: Schwäbisch Hall, Germany, 1987. [Google Scholar]
- Ritter, G. Die Bedeutung der phenolischen Saft- und Weininhaltsstoffe während der Verarbeitung von Äpfeln, Speierling und weissen Trauben. Doctoral Thesis, University of Giessen, Gießen, Germany, 1994. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substances and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–177. [Google Scholar]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
| Chokeberry | Bilberry | Elderberry | ||
|---|---|---|---|---|
| Dry matter content (%) | 94.70 ± 0.14 B | 94.76 ± 0.15 B | 96.23 ± 0.36 A | |
| Microbiological status (CFU/g) | PC | <1 × 103 | <1 × 103 | 4.6 × 104 |
| YGC | <1 × 103 | <1 × 103 | <1 × 103 |
| d10 (µm) | d50 (µm) | d90 (µm) | |
|---|---|---|---|
| Chokeberry | 5.21 ± 0.04 C | 10.36 ± 0.06 C | 21.93 ± 0.74 B |
| Bilberry | 6.50 ± 0.46 B | 11.53 ± 0.83 B | 20.93 ± 2.67 B |
| Elderberry | 7.57 ± 0.41 A | 13.28 ± 0.66 A | 24.69 ± 1.54 A |
| Chokeberry | Bilberry | Elderberry | |
| Moisture absorption after 24 h (%) | 4.09 ± 0.13 A | 3.31 ± 0.12 B | 3.63 ± 0.29 A,B |
| Bulk density (g/mL) | 0.73 ± 0.03 A | 0.63 ± 0.00 C | 0.45 ± 0.01 B |
| Water-binding capacity (g water/g dw) | 2.43 ± 0.10 A | 3.10 ± 0.07 A | 2.38 ± 0.76 A |
| Oil absorption capacity (g canola oil/g dw) | 1.74 ± 0.18 B | 1.79 ± 0.09 B | 2.24 ± 0.10 A |
| Oil absorption capacity (g sunflower oil/g dw) | 1.76 ± 0.08 B | 1.85 ± 0.05 B | 2.13 ± 0.03 A |
| Cold-water solubility (%) | 10.98 ± 0.03 B | 10.98 ± 0.17 B | 16.42 ± 0.26 A |
| Sedimentation velocity (mL/min) | 0.02 ± 0.00 A | 0.02 ± 0.00 A | 0.02 ± 0.00 B |
| Swelling capacity (mL/g dw) | 5.08 ± 0.10 B | 5.16 ± 0.10 B | 10.16 ± 0.18 A |
| Chokeberry | Bilberry | Elderberry | ||
|---|---|---|---|---|
| C* | day 0 | 17.44 ± 0.02 | 14.40 ± 0.02 | 9.99 ± 0.02 |
| day 28 | 14.72 ± 0.01 a | 14.21 ± 0.02 a | 8.93 ± 0.04 a | |
| h° | day 0 | 15.03 ± 0.09 | 13.71 ± 0.06 | 14.26 ± 0.01 |
| day 28 | 13.97 ± 0.05 a | 12.82 ± 0.06 | 11.55 ± 0.26 a | |
| ∆E b | 3.55 ± 0.03 A | 0.38 ± 0.05 C | 2.32 ± 0.19 B | |
| Total Phenolic Content (mg GAE/100 g fw Yogurt) | ||
|---|---|---|
| Day 0 | Day 14 | |
| Chokeberry PP | 71.27 ± 2.85 | 73.45 ± 2.49 |
| PSP reference chokeberry | 23.62 ± 4.66 | 29.68 ± 7.13 |
| BC reference chokeberry | 17.24 ± 4.66 | 29.75 ± 7.13 |
| Bilberry PP | 71.97 ± 11.43 | 65.14 ± 9.09 |
| PSP reference bilberry | 25.09 ± 3.33 | 17.32 ± 0.71 |
| BC reference bilberry | 21.54 ± 2.50 | 11.21 ± 0.80 |
| Elderberry PP | 65.38 ± 8.47 | 85.75 ± 4.15 a |
| PSP reference elderberry | 18.91 ± 3.09 | 37.17 ± 12.41 |
| BC reference elderberry | 10.92 ± 4.54 | 17.27 ± 5.71 |
| ∆E | h° | C* | |||
|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | ||
| Chokeberry PP | 1.87 | 1.79 | 0.46 | 19.74 | 19.68 |
| PSP reference chokeberry | 3.72 | 12.92 | 10.37 | 19.61 | 17.39 |
| BC reference chokeberry | 1.96 | 1.75 | 0.70 | 20.29 | 19.11 |
| Bilberry PP | 2.17 | 10.42 | 9.98 | 16.78 | 16.30 |
| PSP reference bilberry | 2.94 | 10.05 | 7.62 | 16.27 | 14.83 |
| BC reference bilberry | 2.61 | 0.75 | 2.97 | 16.04 | 14.90 |
| Elderberry PP | 4.89 | 3.59 | 4.2 | 15.16 | 15.61 |
| PSP reference elderberry | 5.72 | 9.20 | 4.20 | 15.50 | 13.17 |
| BC reference elderberry | 2.94 | 2.79 | 5.35 | 14.33 | 13.30 |
| Chokeberry | Bilberry | Elderberry | |
|---|---|---|---|
| Purple sweet potato anthocyanins (%) | 0.43 | 0.33 | 0.27 |
| Black carrot concentrate (%) | 0.32 | 0.18 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemetz, N.J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. https://doi.org/10.3390/molecules26092689
Nemetz NJ, Schieber A, Weber F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules. 2021; 26(9):2689. https://doi.org/10.3390/molecules26092689
Chicago/Turabian StyleNemetz, Nicole Jasmin, Andreas Schieber, and Fabian Weber. 2021. "Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff" Molecules 26, no. 9: 2689. https://doi.org/10.3390/molecules26092689
APA StyleNemetz, N. J., Schieber, A., & Weber, F. (2021). Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules, 26(9), 2689. https://doi.org/10.3390/molecules26092689






