The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture
Abstract
1. Introduction
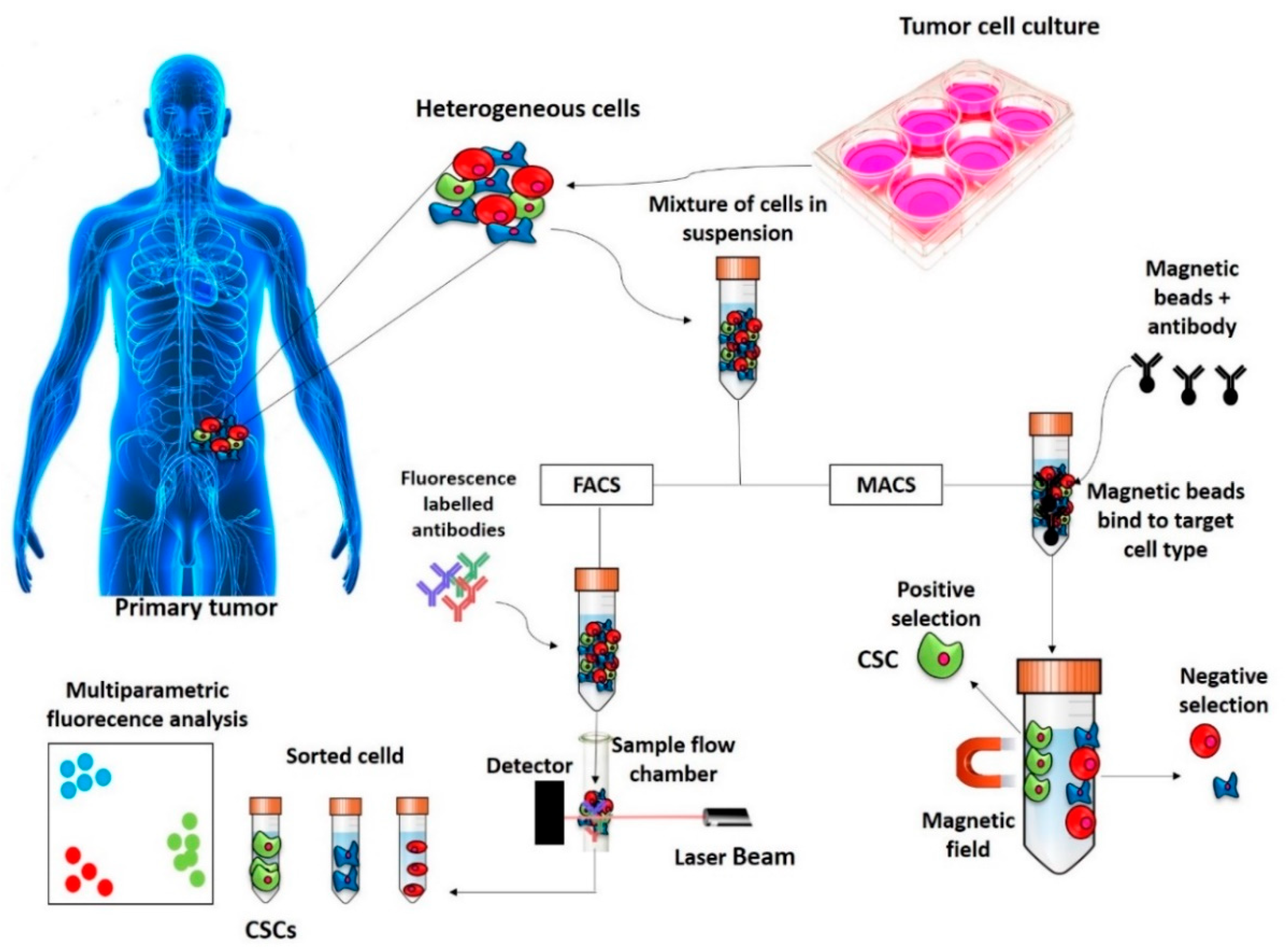
2. Methods of Identification, Isolation and Enrichment of CSCs
2.1. Surface Markers Screening Assays
2.1.1. FACS
2.1.2. MACS
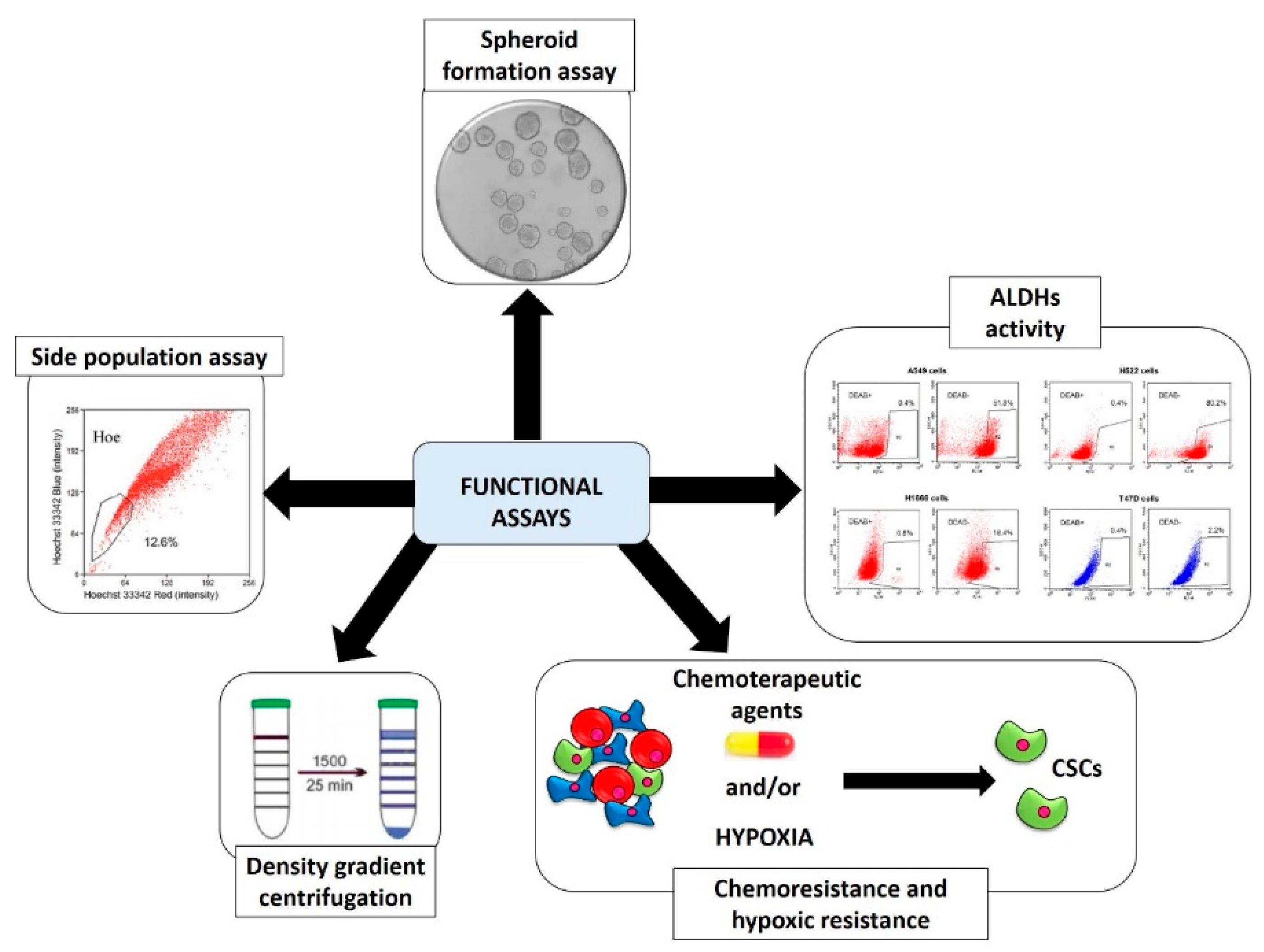
2.2. Functional Screening Assays
2.2.1. Spheroid Formation Assay
2.2.2. Aldehyde Dehydrogenases (ALDHs) Activity
2.2.3. Side Population (SP) Assay
2.2.4. Chemoresistance and Hypoxic Resistance
2.2.5. Physical CSCs Properties (Density Gradient Centrifugation)
2.2.6. Other Functional Isolation Methods
3. Advantages and Disadvantages of a Three-Dimensional Culture in the Study of CSCs
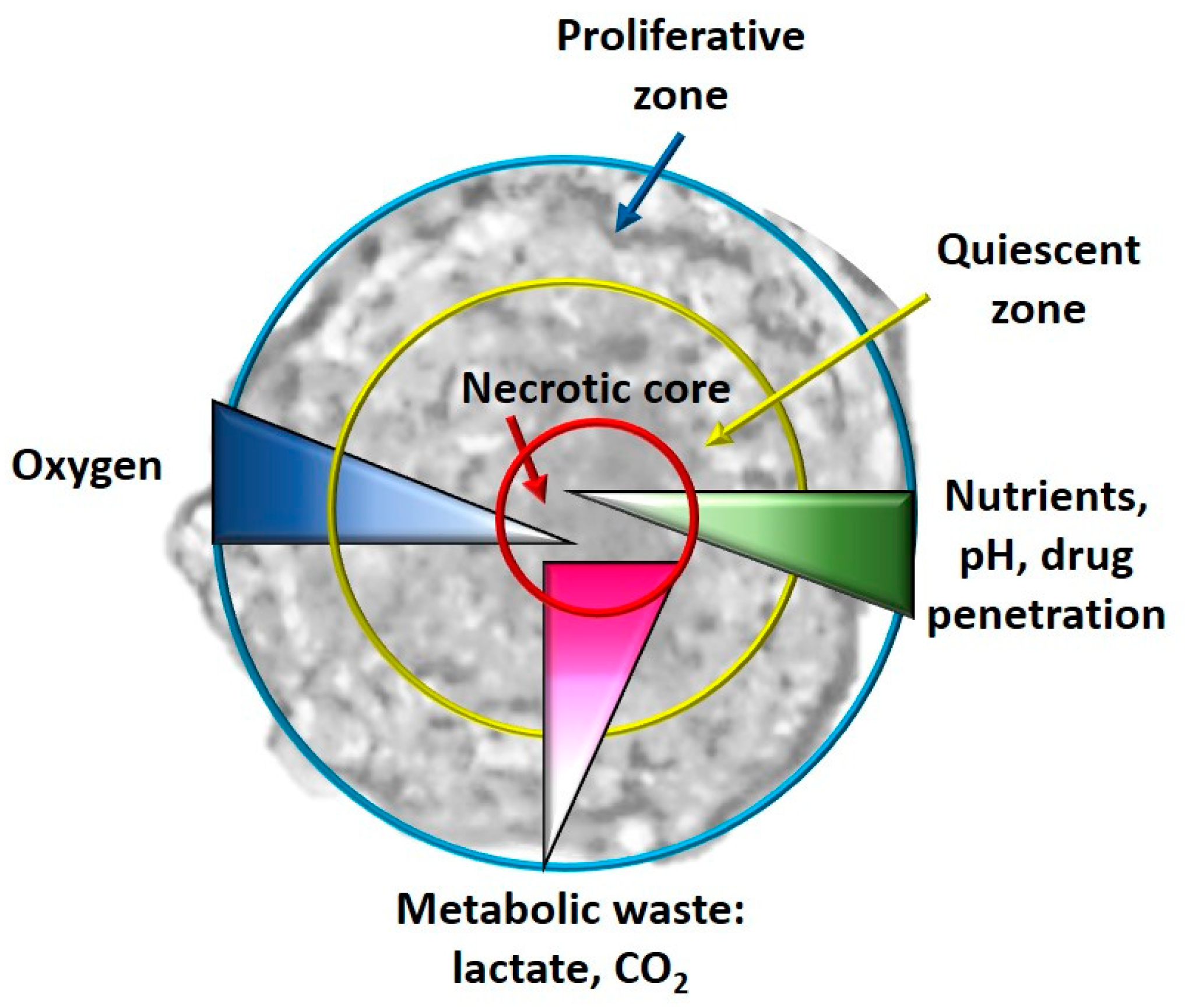
- Hypoxia and metabolism in 3D culture models: In general, in the tumor masses a condition of hypoxia is present in vivo, especially for the cells located in the core of the tumor bulk; this condition is impossible to reproduce in a monolayer culture system. When the cells, especially tumor cells, are grown with 3D culture methods, the formed spheroids simulate the tumor mass. It has been observed that real oxygen gradients, nutrients and metabolites are generated in spheroids miming those observed in vivo, even if, for example, cellular heterogeneity is not created in single-cell spheroids, but it is, however, a system that is the closest to the real one. In fact, in the spheroids, as happens in vivo, it has been observed that in their cores, there are cells that live in conditions of hypoxia and have gene alterations compared to all the other tumor mass [92]. Furthermore, necrotic cells are present in the core of the spheroid, as happens in vivo, and quiescent cells are present immediately outside the necrotic core. As regards the hypoxia-CSCs relationship, this is a fundamental parameter as hypoxia is an essential condition for the formation and expansion of this cellular niche [93]. In some studies, it has been observed that these hypoxic conditions activate some pathways closely related to CSCs, such as Wnt, Hedgehog (Hh) and Notch [94]. Cells grown in 2D, on the other hand, have uniform contact with oxygen and therefore it is impossible to observe an oxygen gradient [86].
- Angiogenesis in 3D CSC models: Angiogenesis is the process which, in tumor bulk, supplies oxygen and nutrients to cancer cells, allowing their growth, invasion and metastasis. CSCs are closely linked to the phenomenon of angiogenesis [95]. From the point of view of the expression of some genes involved in angiogenesis, 3D multicellular models with endothelial cells are, in particular, much closer to the in vivo conditions than a normal 2D culture, where it is not possible to recreate the angiogenic process [96].
- EMT in 3D culture models: Another difference in the gene expression patterns between 2D and 3D cultures is found in the EMT process, in which CSCs play a fundamental role. In 2D models the shape of cells is flattened, while in 3D cultures the cells take on shapes more similar to what they really are, forming aggregates and cell–cell interactions that are fundamental characteristics for studying this process, interactions that are absolutely not recreated in a 2D cellular model [97].
- Chemoresistance in 3D culture models: CSCs play a fundamental role in chemoresistance, which is one of the main causes of therapeutic failures in cancer treatments. 3D culture models could better simulate the in vivo situation for studying drug penetration, response and resistance. Indeed, it has been observed that the 3D cultures themselves and even more those that allow the enrichment of CSCs possess greater resistance to drugs related to the type of architecture of the spheroid [98]. In 2D cultures, for example, the size of the surface (surface/volume ratio) is very large and this allows an easy absorption of the treatment, which is not the case of a 3D culture or even in vivo [99].
- Among the disadvantages of 3D cultures, there are the costs and times that are higher than 2D culture systems, poor reproducibility, standardization, automation and comparison with literature data. Furthermore, adapting the protocols used in 2D to the conditions of 3D is not easy and there is low standardization, especially for cytotoxicity tests, but also for other analyses, such as Western blot. Moreover, greater ability and expertise in sample handling are also required [100].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cianciosi, D.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Reboredo-Rodriguez, P.; Zhang, J.; Quiles, J.L.; Nabavi, S.F.; Battino, M.; et al. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol. Res. 2018, 135, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.N. Cancer stem cells: Understanding tumor hierarchy and heterogeneity. Medicine 2016, 95, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.; Maroufi, N.F.; Tazehkand, A.P.; Akbarzadeh, M.; Bastani, S.; Safdari, R.; Farzane, A.; Fattahi, A.; Nejabati, H.R.; Nouri, M.; et al. Current approaches in identification and isolation of cancer stem cells. J. Cell. Physiol. 2019, 234, 14759–14772. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Herheliuk, T.; Perepelytsina, O.; Ugnivenko, A.; Ostapchenko, L.; Sydorenko, M. Investigation of multicellular tumor spheroids enriched for a cancer stem cell phenotype. Stem Cell Investig. 2019, 6, 21. [Google Scholar] [CrossRef]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Glumac, P.M.; Lebeau, A.M. The role of CD133 in cancer: a concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Li, Z. CD133: a stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef]
- Mak, A.B.; Nixon, A.M.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.-C.; Mazitschek, R.; Neel, B.G.; Stagljar, I.; et al. Regulation of CD133 by HDAC6 Promotes β-Catenin Signaling to Suppress Cancer Cell Differentiation. Cell Rep. 2012, 2, 951–963. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, G.; Yang, Y.; Jiang, Z.; Cai, J.; Hu, H. Inhibition of CD133 Overcomes Cisplatin Resistance Through Inhibiting PI3K/AKT/mTOR Signaling Pathway and Autophagy in CD133-Positive Gastric Cancer Cells. Technol. Cancer Res. Treat. 2019, 18, 1533033819864311. [Google Scholar] [CrossRef]
- Wang, H.; Gong, P.; Li, J.; Fu, Y.; Zhou, Z.; Liu, L. Role of CD133 in human embryonic stem cell proliferation and teratoma formation. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Handgretinger, R.; Kuçi, S. CD133-Positive Hematopoietic Stem Cells: From Biology to Medicine. Adv. Exp. Med. Biol. 2013, 777, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kong, W.; Falk, A.; Hu, J.; Zhou, L.; Pollard, S.; Smith, A. CD133 (Prominin) Negative Human Neural Stem Cells Are Clonogenic and Tripotent. PLoS ONE 2009, 4, e5498. [Google Scholar] [CrossRef]
- Griend, D.J.V.; Karthaus, W.L.; Dalrymple, S.; Meeker, A.; DeMarzo, A.M.; Isaacs, J.T. The Role of CD133 in Normal Human Prostate Stem Cells and Malignant Cancer-Initiating Cells. Cancer Res. 2008, 68, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell. Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef]
- Hough, M.R.; Rosten, P.M.; Sexton, T.L.; Kay, R.; Humphries, R. Mapping of CD24 and Homologous Sequences to Multiple Chromosomal Loci. Genomics 1994, 22, 154–161. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Naujok, O.; Lenzen, S. A Critical Re-Evaluation of CD24-Positivity of Human Embryonic Stem Cells Differentiated into Pancreatic Progenitors. Stem Cell Rev. Rep. 2012, 8, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Gracz, A.D.; Fuller, M.K.; Wang, F.; Li, L.; Stelzner, M.; Dunn, J.C.; Martin, M.G.; Magness, S.T. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 2013, 31, 2024–2030. [Google Scholar] [CrossRef]
- Hubbe, M.; Altevogt, P. Heat-stable antigen/CD24 on mouse T lymphocytes: Evidence for a costimulatory function. Eur. J. Immunol. 1994, 24, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Pruszak, J.; Ludwig, W.; Blak, A.; Alavian, K.; Isacson, O. CD15, CD24 and CD29 Define a Surface Biomarker Code for Neural Lineage Differentiation of Stem Cells. Stem Cells 2009, 27, 2928–2940. [Google Scholar] [CrossRef]
- Teye, K.; Numata, S.; Ishii, N.; Krol, R.P.; Tsuchisaka, A.; Hamada, T.; Koga, H.; Karashima, T.; Ohata, C.; Tsuruta, D.; et al. Isolation of All CD44 Transcripts in Human Epidermis and Regulation of Their Expression by Various Agents. PLoS ONE 2016, 11, e0160952. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 1–23. [Google Scholar] [CrossRef]
- Cao, H.; Heazlewood, S.Y.; Williams, B.; Cardozo, D.; Nigro, J.; Oteiza, A.; Nilsson, S.K. The role of CD44 in fetal and adult hematopoietic stem cell regulation. Haematologica 2015, 101, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef]
- Luong, M.X.; Tam, J.; Lin, Q.; Hagendoorn, J.; Moore, K.J.; Padera, T.P.; Seed, B.; Fukumura, D.; Kucherlapati, R.; Jain, R.K. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. J. Cell. Physiol. 2009, 219, 430–437. [Google Scholar] [CrossRef]
- Kennel, S.J.; Lankford, T.K.; Foote, L.J.; Shinpock, S.G.; Stringer, C. CD44 expression on murine tissues. J. Cell Sci. 1993, 104, 373–382. [Google Scholar] [CrossRef]
- Lachance, C.; Arbour, N.; Cashman, N.R.; Talbot, P.J. Involvement of Aminopeptidase N (CD13) in Infection of Human Neural Cells by Human Coronavirus 229E. J. Virol. 1998, 72, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Endres, J.; Behbahani-Nejad, N.; Phillips, K.; Ruth, J.H.; Friday, S.C.; Edhayan, G.; Lanigan, T.; Urquhart, A.; Chung, K.C.; et al. Expression and function of aminopeptidase N/CD13 produced by fibroblast-like synoviocytes in rheumatoid arthritis: Role of CD13 in chemotaxis of cytokine-activated T cells independent of enzymatic ac-tivity. Arthritis Rheumatol. 2015, 67, 74–85. [Google Scholar] [CrossRef]
- Sun, J.-H.; Luo, Q.; Liu, L.-L.; Song, G.-B. Liver cancer stem cell markers: Progression and therapeutic implications. World J. Gastroenterol. 2016, 22, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Subramani, J.; Ghosh, M.; Denninger, J.K.; Takeda, K.; Fong, G.-H.; Carlson, M.E.; Shapiro, L.H. CD13 promotes mesenchymal stem cell-mediated regeneration of ischemic muscle. Front. Physiol. 2014, 4, 402. [Google Scholar] [CrossRef]
- Verma, A.; Kapoor, R.; Mittal, R.D. Genetic Variation in CD166 Gene and Its Association with Bladder Cancer Risk in North Indian Population. Indian J. Clin. Biochem. 2016, 32, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Davies, P.S.; Levin, T.G.; Gallagher, A.C.; Keene, D.R.; Sengupta, S.K.; Wieghard, N.; El Rassi, E.; Wong, M.H. Cell Adhesion Molecule CD166/ALCAM Functions Within the Crypt to Orchestrate Murine Intestinal Stem Cell Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Ashry, R.; Tran, S.D. Head and neck cancer management and cancer stem cells implication. Saudi Dent. J. 2019, 31, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.G.; Powell, A.E.; Davies, P.S.; Silk, A.D.; Dismuke, A.D.; Anderson, E.C.; Swain, J.R.; Wong, M.H. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology. 2010, 139, 2072–2082. [Google Scholar] [CrossRef]
- Brinkhof, B.; Zhang, B.; Cui, Z.; Ye, H.; Wang, H. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene X 2020, 5, 100031. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tatsumi, K.; Kosaka, K.; Sato, Y.; Higuchi, T.; Yoshioka, S.; Maeda, M.; Ueda, M.; Fujii, S. Human Blastocysts and Endometrial Epithelial Cells Express Activated Leukocyte Cell Adhesion Molecule (ALCAM/CD166). J. Clin. Endocrinol. Metab. 2003, 88, 3437–3443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases. Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Imrich, S.; Hachmeister, M.; Gires, O. EpCAM and its potential role in tumor-initiating cells. Cell Adhes. Migr. 2012, 6, 30–38. [Google Scholar] [CrossRef]
- Dollé, L.; Theise, N.D.; Schmelzer, E.; Boulter, L.; Gires, O.; Van Grunsven, L.A. EpCAM and the biology of hepatic stem/progenitor cells. Am. J. Physiol. Liver Physiol. 2015, 308, G233–G250. [Google Scholar] [CrossRef]
- Greve, B.; Kelsch, R.; Spaniol, K.; Eich, H.T.; Götte, M. Flow cytometry in cancer stem cell analysis and separation. Cytom. Part A 2012, 81, 284–293. [Google Scholar] [CrossRef]
- Tzur, A.; Moore, J.K.; Jorgensen, P.; Shapiro, H.M.; Kirschner, M.W. Optimizing Optical Flow Cytometry for Cell Volume-Based Sorting and Analysis. PLoS ONE 2011, 6, e16053. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Pohnert, G. Rapid Estimation of Astaxanthin and the Carotenoid-to-Chlorophyll Ratio in the Green Microalga Chromochloris zofingiensis Using Flow Cytometry. Mar. Drugs 2017, 15, 231. [Google Scholar] [CrossRef]
- Baca, O.G.; Crissman, H.A. Correlation of DNA, RNA, and protein content by flow cytometry in normal and Coxiella bur-netii-infected L929 cells. Infect. Immun. 1987, 55, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Dingley, S.; Chapman, K.A.; Falk, M.J. Fluorescence-Activated Cell Sorting Analysis of Mitochondrial Content, Membrane Potential, and Matrix Oxidant Burden in Human Lymphoblastoid Cell Lines. Methods Mol. Biol. 2011, 837, 231–239. [Google Scholar] [CrossRef]
- Menon, V.; Thomas, R.; Ghale, A.R.; Reinhard, C.; Pruszak, J. Flow Cytometry Protocols for Surface and Intracellular Antigen Analyses of Neural Cell Types. J. Vis. Exp. 2014, 94, 52241. [Google Scholar] [CrossRef]
- Gorry, M.; Yoneyama, T.; Vujanovic, L.; Moss, M.L.; Garlin, M.A.; A Miller, M.; Herman, J.; Stabile, L.P.; Vujanovic, N.L. Development of flow cytometry assays for measuring cell-membrane enzyme activity on individual cells. J. Cancer 2020, 11, 702–715. [Google Scholar] [CrossRef]
- Suni, M.A.; Maino, V.C. Flow cytometric analysis of cell signaling proteins. Methods Mol. Biol. 2011, 717, 155–169. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, srep19103. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey poly-phenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 126881. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Chang, Y.-C.; Nieh, S.; Liu, C.-L.; Yang, C.-Y.; Lin, Y.-S. Nonadhesive culture system as a model of rapid sphere for-mation with cancer stem cell properties. PLoS ONE 2012, 7, e31864. [Google Scholar] [CrossRef]
- Maliszewska-Olejniczak, K.; Brodaczewska, K.K.; Bielecka, Z.F.; Solarek, W.; Kornakiewicz, A.; Szczylik, C.; Porta, C.; Czarnecka, A.M. Development of extracellular matrix supported 3D culture of renal cancer cells and renal cancer stem cells. Cytotechnology 2019, 71, 149–163. [Google Scholar] [CrossRef]
- Leng, Z.; Yang, Z.; Li, L.; Zhong, X.; Zhou, H.; Li, Y.; Yang, G.; Zhang, G.; Xiong, Y.; Zhou, T.; et al. A reliable method for the sorting and identification of ALDH high cancer stem cells by flow cytometry. Exp. Ther. Med. 2017, 14, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef]
- Clark, D.W.; Palle, K. Aldehyde dehydrogenases in cancer stem cells: Potential as therapeutic targets. Ann. Transl. Med. 2016, 4, 518. [Google Scholar] [CrossRef]
- Shimoda, M.; Ota, M.; Okada, Y. Isolation of Cancer Stem Cells by Side Population Method. Methods Mol. Biol. 2017, 1692, 49–59. [Google Scholar] [CrossRef]
- Candeil, L.; Gourdier, I.; Peyron, D.; Vezzio, N.; Copois, V.; Bibeau, F.; Orsetti, B.; Scheffer, G.L.; Ychou, M.; Khan, Q.A.; et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotec-an-treated metastases. Int. J. Cancer 2004, 109, 848–854. [Google Scholar] [CrossRef]
- Francipane, M.G.; Bulanin, D.; Lagasse, E. Establishment and Characterization of 5-Fluorouracil-Resistant Human Colorectal Cancer Stem-Like Cells: Tumor Dynamics under Selection Pressure. Int. J. Mol. Sci. 2019, 20, 1817. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lin, Q.; Glazer, P.M.; Yun, Z. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res. 2018, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Wang, X.; You, N.; Tao, K.-S.; Wang, T.; Tang, L.-J.; Dou, K.-F. Efficient Enrichment of Hepatic Cancer Stem-Like Cells from a Primary Rat HCC Model via a Density Gradient Centrifugation-Centered Method. PLoS ONE 2012, 7, e35720. [Google Scholar] [CrossRef]
- Mauri, F.A.; Pinato, D.J.; Trivedi, P.; Sharma, R.; Shiner, R.J. Isogeneic comparison of primary and metastatic lung cancer identifies CX3CR1 as a molecular determinant of site-specific metastatic diffusion. Oncol. Rep. 2012, 28, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.-J.; Qiu, W.; Xu, S.-L.; Wang, B.; Ye, X.-Z.; Ping, Y.-F.; Zhang, X.; Bian, X.-W.; Yu, S.-C. Strategies for Isolating and Enriching Cancer Stem Cells: Well Begun Is Half Done. Stem Cells Dev. 2013, 22, 2221–2239. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lorenzo, I.; Dorado, J.; Lonardo, E.; Alcala, S.; Serrano, A.G.; Clausell-Tormos, J.; Cioffi, M.; Megias, D.; Zagorac, S.; Balic, A. Intracellular autofluorescence: A biomarker for epithelial cancer stem cells. Nat. Methods 2014, 11, 1161–1169. [Google Scholar] [CrossRef]
- Tang, B.; Raviv, A.; Esposito, D.; Flanders, K.C.; Daniel, C.; Nghiem, B.T.; Garfield, S.; Lim, L.; Mannan, P.; Robles, A.I.; et al. A flexible reporter system for direct observation and isolation of cancer stem cells. Stem Cell Reports 2015, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nat. Cell Biol. 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Wang, D.-G.; Guo, F.-F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer 2014, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, M.; Liu, J.; Mehta, G.U.; Patel, S.J.; Roychoudhuri, R.; Crompton, J.G.; Klebanoff, C.A.; Ji, Y.; Li, P.; Yu, Z.; et al. Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell Metab. 2016, 23, 63–76. [Google Scholar] [CrossRef] [PubMed]
- A Siclari, V.; Qin, L. Targeting the osteosarcoma cancer stem cell. J. Orthop. Surg. Res. 2010, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Akrap, N.; Andersson, D.; Bom, E.; Gregersson, P.; Ståhlberg, A.; Landberg, G. Identification of Distinct Breast Cancer Stem Cell Populations Based on Single-Cell Analyses of Functionally Enriched Stem and Progenitor Pools. Stem Cell Rep. 2016, 6, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Tseng, H.-C.; Arasteh, A.; Saadat, S.; Christensen, R.E.; Cacalano, N.A. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr. Drug Deliv. 2012, 9, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nat. Cell Biol. 2017, 545, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, M.; Liu, J.; Zhang, S.; Noordam, L.; Verstegen, M.M.A.; Wang, L.; Ma, B.; Li, S.; Wang, W.; et al. LGR5 marks targetable tumor-initiating cells in mouse liver cancer. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Dong, D.-L.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Jin, G.-Z.; Xuan, Y. 3D culture technologies of cancer stem cells: Promising ex vivo tumor models. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef]
- Li, Y.; Kilian, K.A. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv. Heal. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Souza, A.G.; Silva, I.B.B.; Campos-Fernandez, E.; Barcelos, L.S.; Souza, J.B.; Marangoni, K.; Goulart, L.R.; Alonso-Goulart, V. Comparative Assay of 2D and 3D Cell Culture Models: Proliferation, Gene Expression and Anticancer Drug Response. Curr. Pharm. Des. 2018, 24, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug dis-covery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Eke, I.; Hehlgans, S.; Zong, Y.; Cordes, N. Comprehensive analysis of signal transduction in three-dimensional ECM-based tumor cell cultures. J. Biol. Methods 2015, 2, e31. [Google Scholar] [CrossRef]
- Uematsu, N.; Zhao, Y.; Kiyomi, A.; Yuan, B.; Onda, K.; Kanaka, S.; Sugiyama, K.; Sugiura, M.; Takagi, N.; Hayakawa, A.; et al. Chemo-sensitivity of Two-dimensional Monolayer and Three-dimensional Spheroid of Breast Cancer MCF-7 Cells to Daunorubicin, Docetaxel, and Arsenic Disulfide. Anticancer Res. 2018, 38, 2101–2108. [Google Scholar] [CrossRef]
- Kim, H.-S.; Sinha, N.; Sharma, A.R.; Ryu, B.-Y.; Kim, L.-S.; Hong, M.-G.; Kang, H.J.; Lee, S.-S.; Nam, J.-S. Abstract 1003: The inhibitory role of quercetin-induced Dickkopf-1 on the growth of 4T1 breast cancer cell line. Cell. Mol. Biol. 2011, 71, 1003. [Google Scholar] [CrossRef]
- Permlid, A.M.; Roci, P.; Fredlund, E.; Fält, F.; Önell, E.; Johansson, F.; Oredsson, S. Unique animal friendly 3D culturing of human cancer and normal cells. Toxicol. Vitr. 2019, 60, 51–60. [Google Scholar] [CrossRef]
- Delnero, P.; Lane, M.; Verbridge, S.S.; Kwee, B.; Kermani, P.; Hempstead, B.; Stroock, A.; Fischbach, C. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 2015, 55, 110–118. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Hiyama, A.; Skubutyte, R.; Markova, D.; Anderson, D.G.; Yadla, S.; Sakai, D.; Mochida, J.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011, 63, 1355–1364. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- E Timmins, N.; Dietmair, S.; Nielsen, L.K. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 2004, 7, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chi, B.H.; Yoo, J.J.; Ju, Y.M.; Whang, Y.M.; Chang, I.H. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS ONE 2019, 14, e0223689. [Google Scholar] [CrossRef] [PubMed]
- Ward Rashidi, M.R.; Mehta, P.; Bregenzer, M.; Raghavan, S.; Fleck, E.M.; Horst, E.N.; Harissa, Z.; Ravikumar, V.; Brady, S.; Bild, A.; et al. Engineered 3D Model of Cancer Stem Cell Enrichment and Chemoresistance. Neoplasia 2019, 21, 822–836. [Google Scholar] [CrossRef]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.-L.; Baird, A.-M.; Vaz, G.; Urquhart, A.J.; Senge, H.; Richard, D.J.; O’Byrne, K.J.; Davies, A.M. Drug Discovery Ap-proaches Utilizing Three-Dimensional Cell Culture. Assay Drug Dev. Technol. 2016, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
| Methods | Advantages | Disadvantages |
|---|---|---|
| Surface markers with FACS 1 | -multiparametric analysis | -large number of cells is needed |
| Surface markers with MACS 2 | -easy method -use of few cells | -single-parametric analysis |
| Spheroid formation assay | -use of a 3D cellular model | -moderate cellular heterogeneity |
| ALDHs 3 activity | -consolidated method | -many nontumor stem cells have also high activity of ALDHs |
| SP assay | -high specificity | -lack of real standardization of the method |
| Chemoresistance and hypoxic resistance | -simplicity of the method | -lack of protocol standardization -moderate cellular heterogeneity |
| Physical CSCs properties | -simplicity and speed of the experiment | -low homogeneity |
| CSCs Marker | Expression in ESCs | Expression in ASCs | Expression in Normal Tissues/Cells | Expression in CSCs |
|---|---|---|---|---|
| CD133 | yes | hematopoietic, neural, prostatic | rare | pancreas, colon, liver, prostate, lung, brain |
| CD24 | yes | intestinal | rare, neural cells, lymphocytes | kidney, bladder, breast, ovary |
| CD44 | no | hematopoietic, adipose, mesenchymal | yes, lymphatic and epithelial tissue | colon, prostate, stomach, ovary, breast |
| CD13 | no | mesenchymal | rare | liver |
| CD166 | weak | intestinal, adipose | epithelial cells | colon, lung, head and neck |
| EpCAM | yes | intestinal | rare | breast, colon, liver |
| Advantages and Disadvantages | 2D Culture | 3D Culture |
|---|---|---|
| Chemical gradient formation | − | + |
| Physiological architecture | − | + |
| 3D cell migration/interaction | − | + |
| Drug resistance | − | + |
| In vivo-like gene expression | −/+ | + |
| Protocol standardization | + | − |
| Reproducibility | + | +/− |
| Comparison in scientific literature | + | +/− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciosi, D.; Ansary, J.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M.; et al. The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture. Molecules 2021, 26, 2615. https://doi.org/10.3390/molecules26092615
Cianciosi D, Ansary J, Forbes-Hernandez TY, Regolo L, Quinzi D, Gracia Villar S, Garcia Villena E, Tutusaus Pifarre K, Alvarez-Suarez JM, Battino M, et al. The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture. Molecules. 2021; 26(9):2615. https://doi.org/10.3390/molecules26092615
Chicago/Turabian StyleCianciosi, Danila, Johura Ansary, Tamara Y. Forbes-Hernandez, Lucia Regolo, Denise Quinzi, Santos Gracia Villar, Eduardo Garcia Villena, Kilian Tutusaus Pifarre, José M. Alvarez-Suarez, Maurizio Battino, and et al. 2021. "The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture" Molecules 26, no. 9: 2615. https://doi.org/10.3390/molecules26092615
APA StyleCianciosi, D., Ansary, J., Forbes-Hernandez, T. Y., Regolo, L., Quinzi, D., Gracia Villar, S., Garcia Villena, E., Tutusaus Pifarre, K., Alvarez-Suarez, J. M., Battino, M., & Giampieri, F. (2021). The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture. Molecules, 26(9), 2615. https://doi.org/10.3390/molecules26092615










