Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation
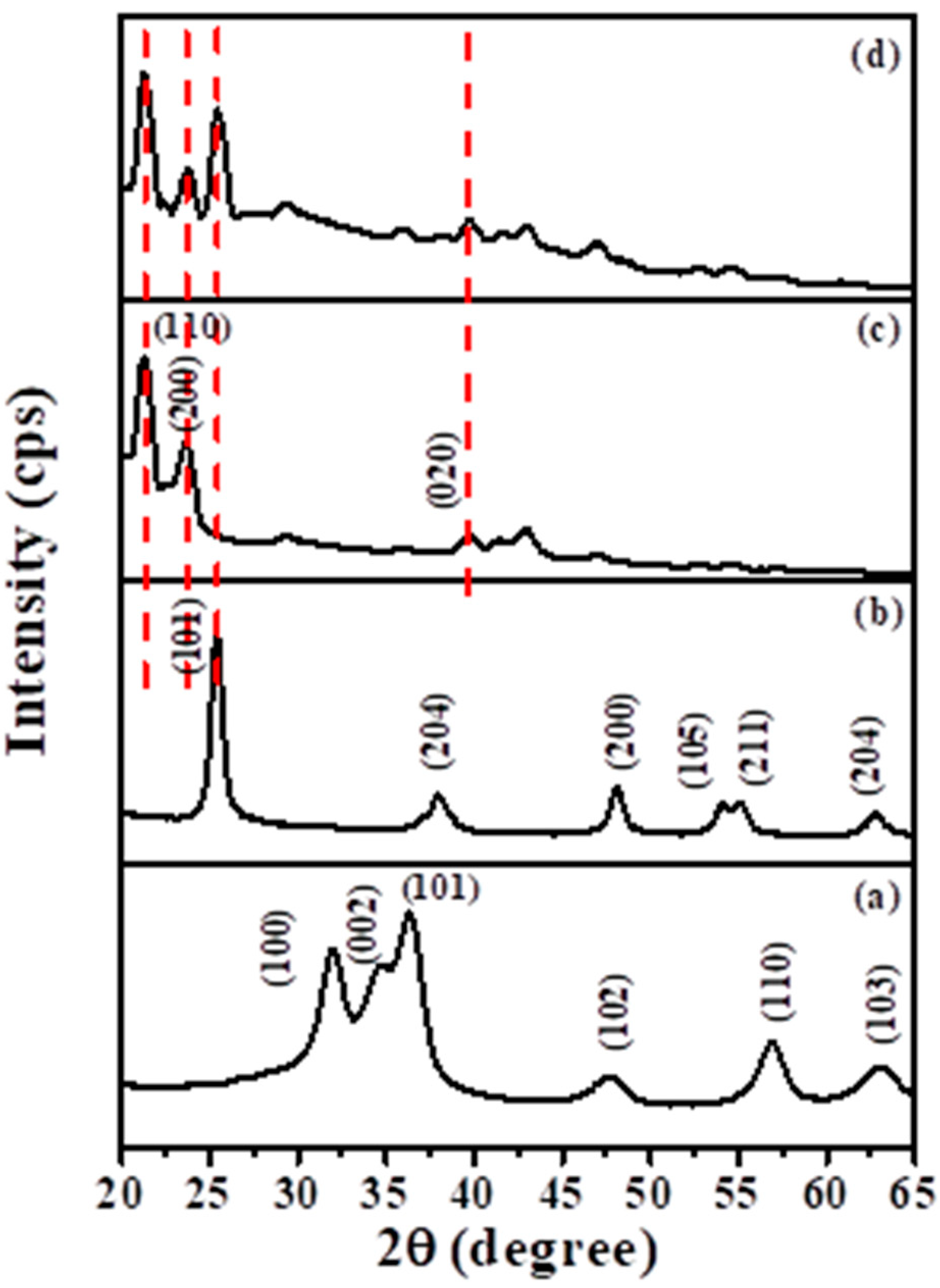
2.1.1. X-ray Diffraction (XRD) Analysis
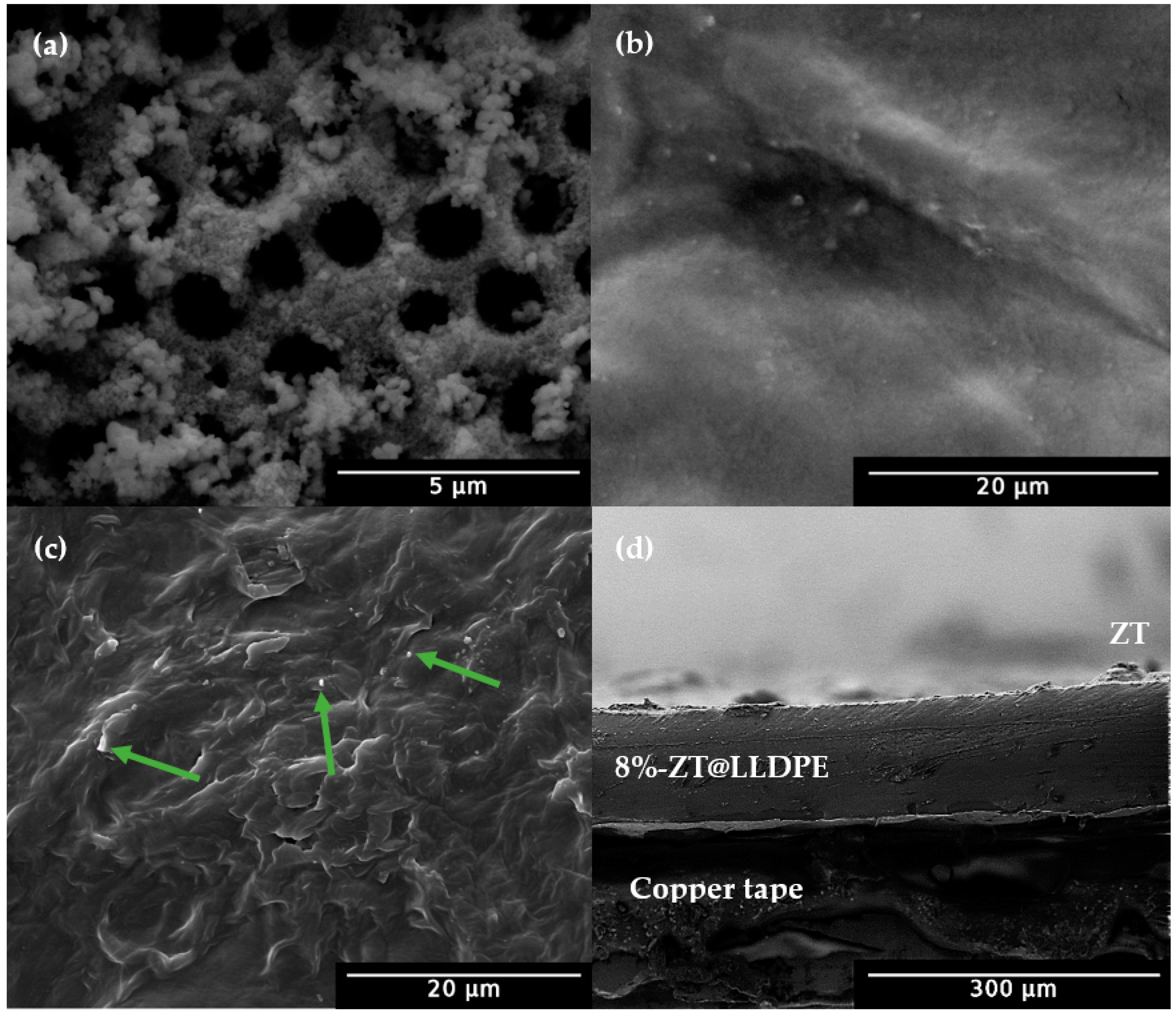
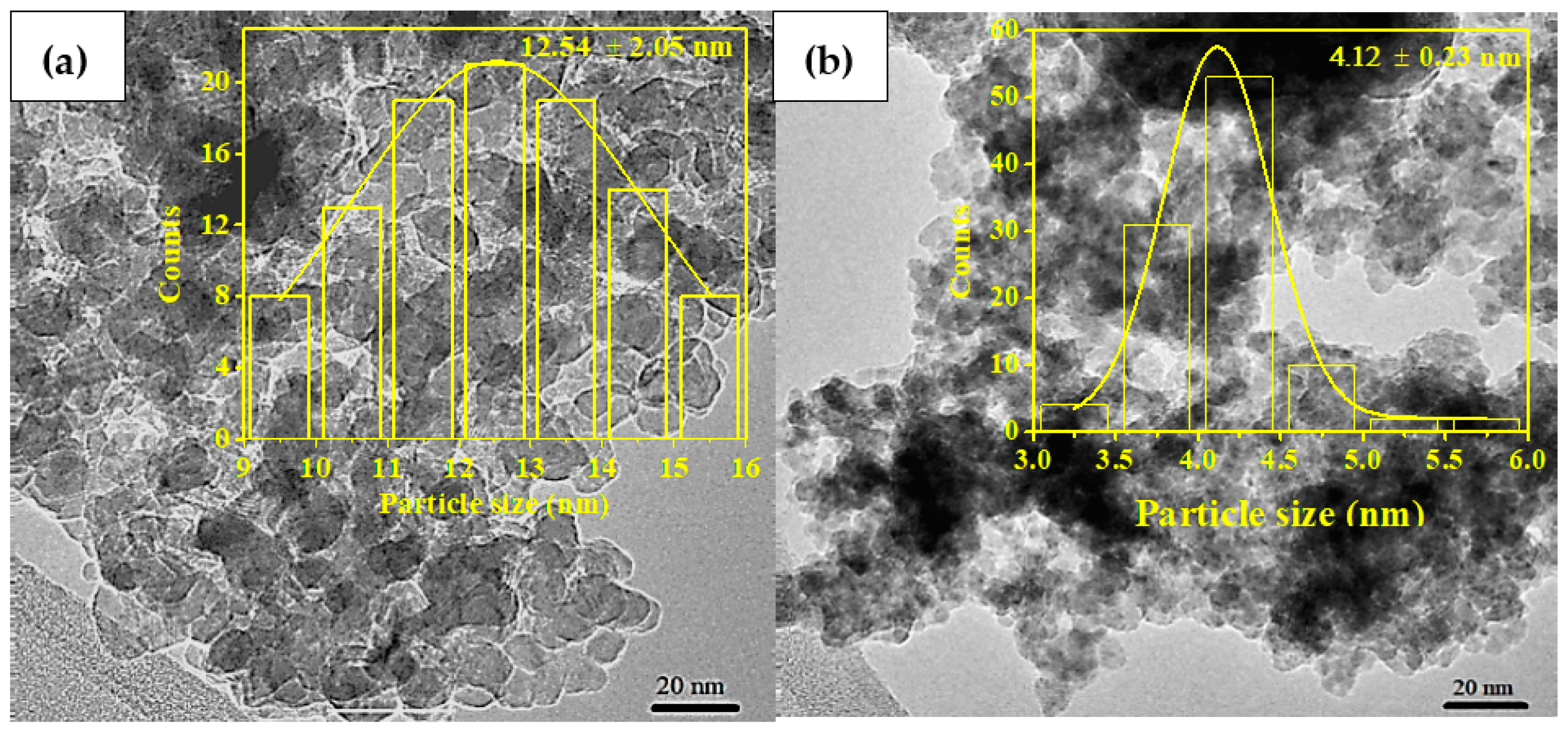
2.1.2. Field Emission Scanning Electron Microscopy (FESEM) Analysis
2.1.3. Field Emission Scanning Electron Microscope (FESEM) Analysis
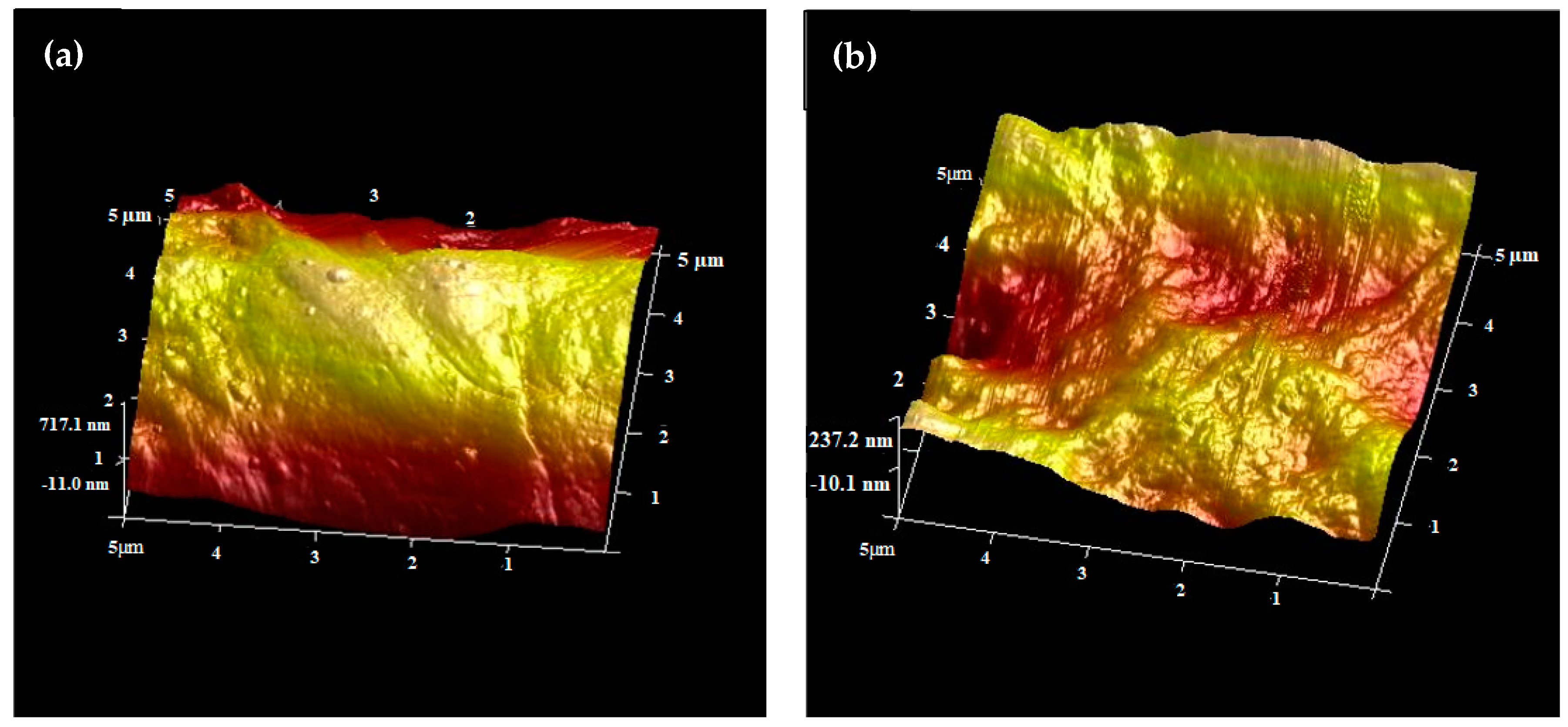
2.1.4. Atomic Force Microscope (AFM) Analysis
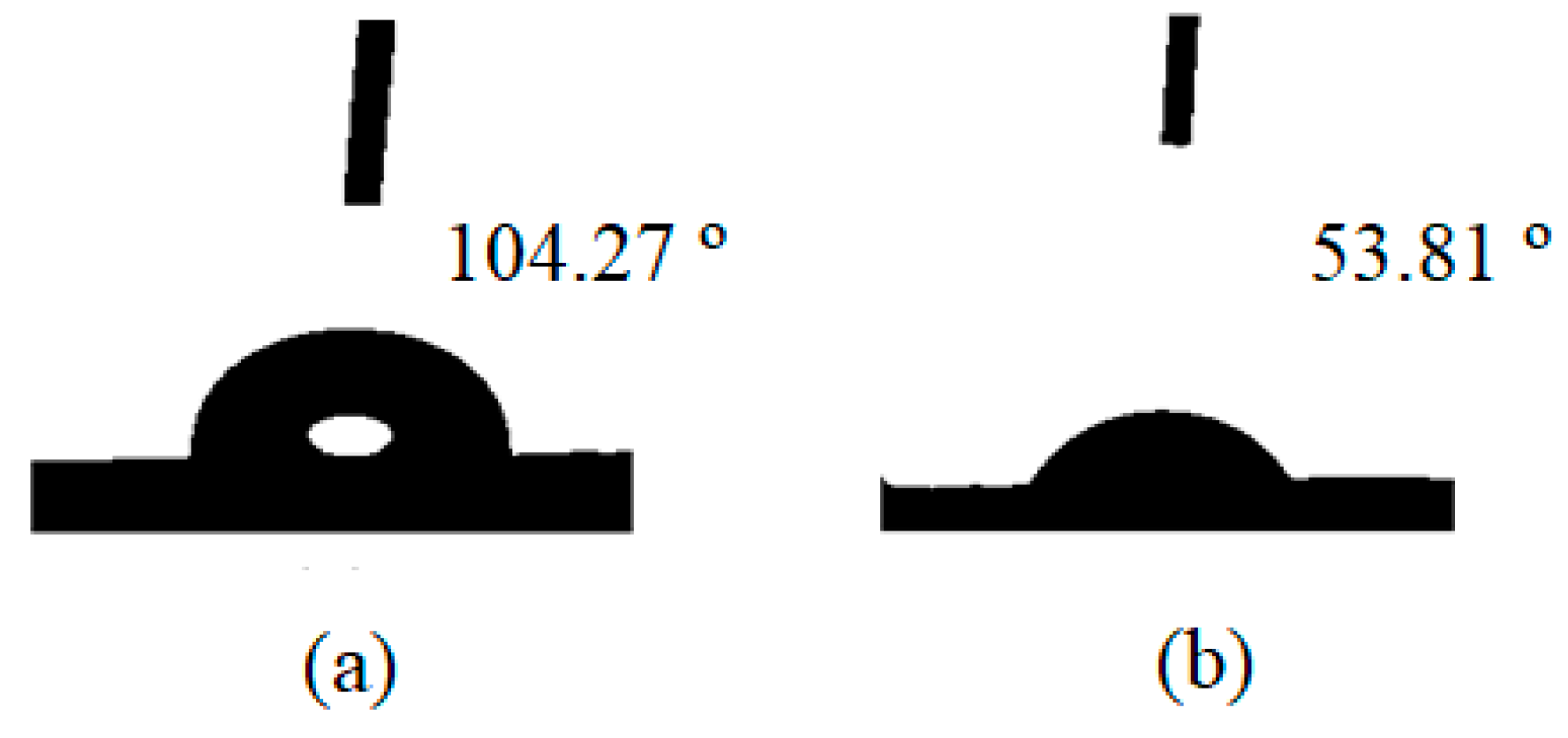
2.1.5. Contact Angle Results
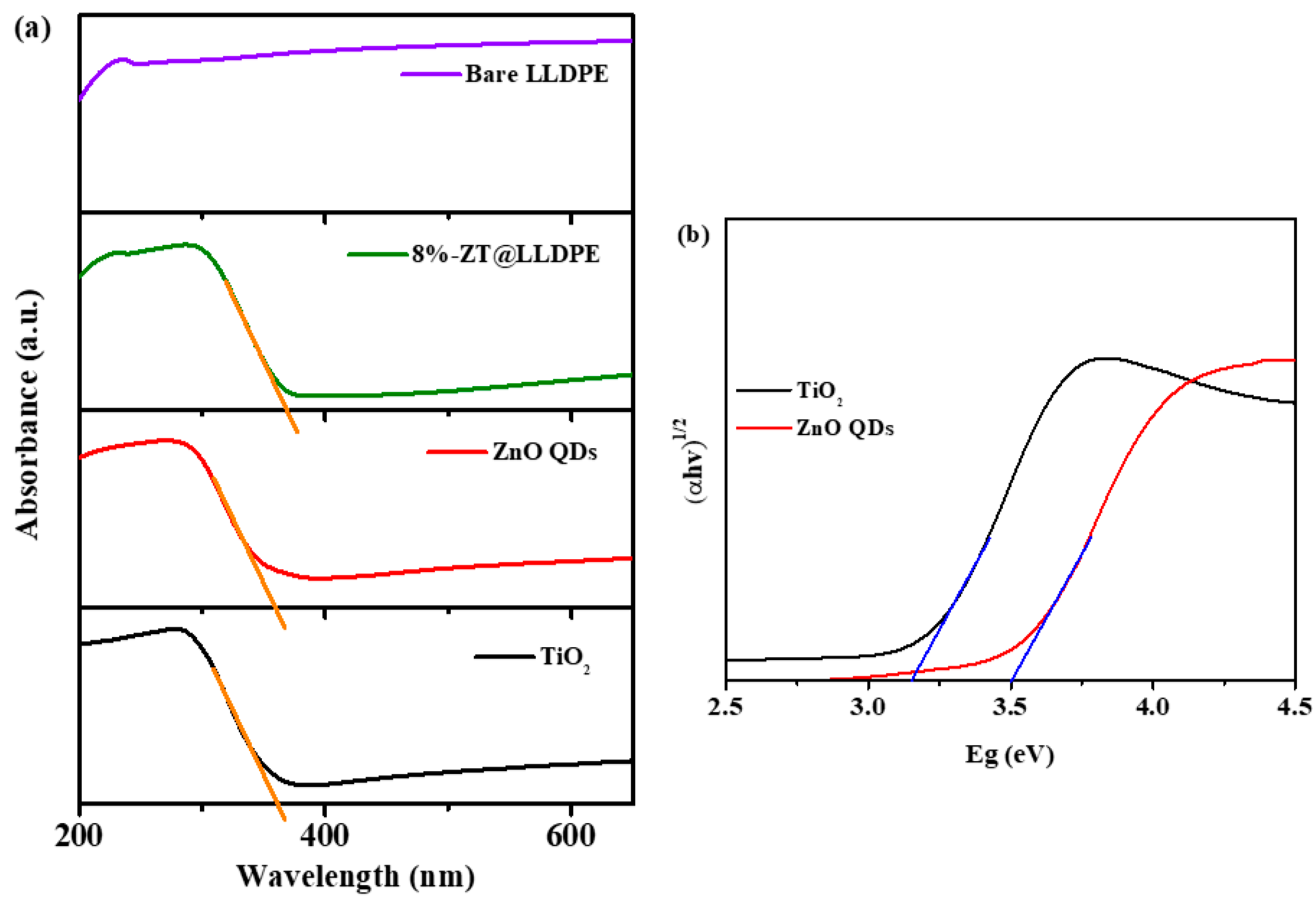
2.1.6. UV–Visible Diffuse Reflectance Analysis
2.2. Photocatalytic Activity of the LLDPE Hybrid Films
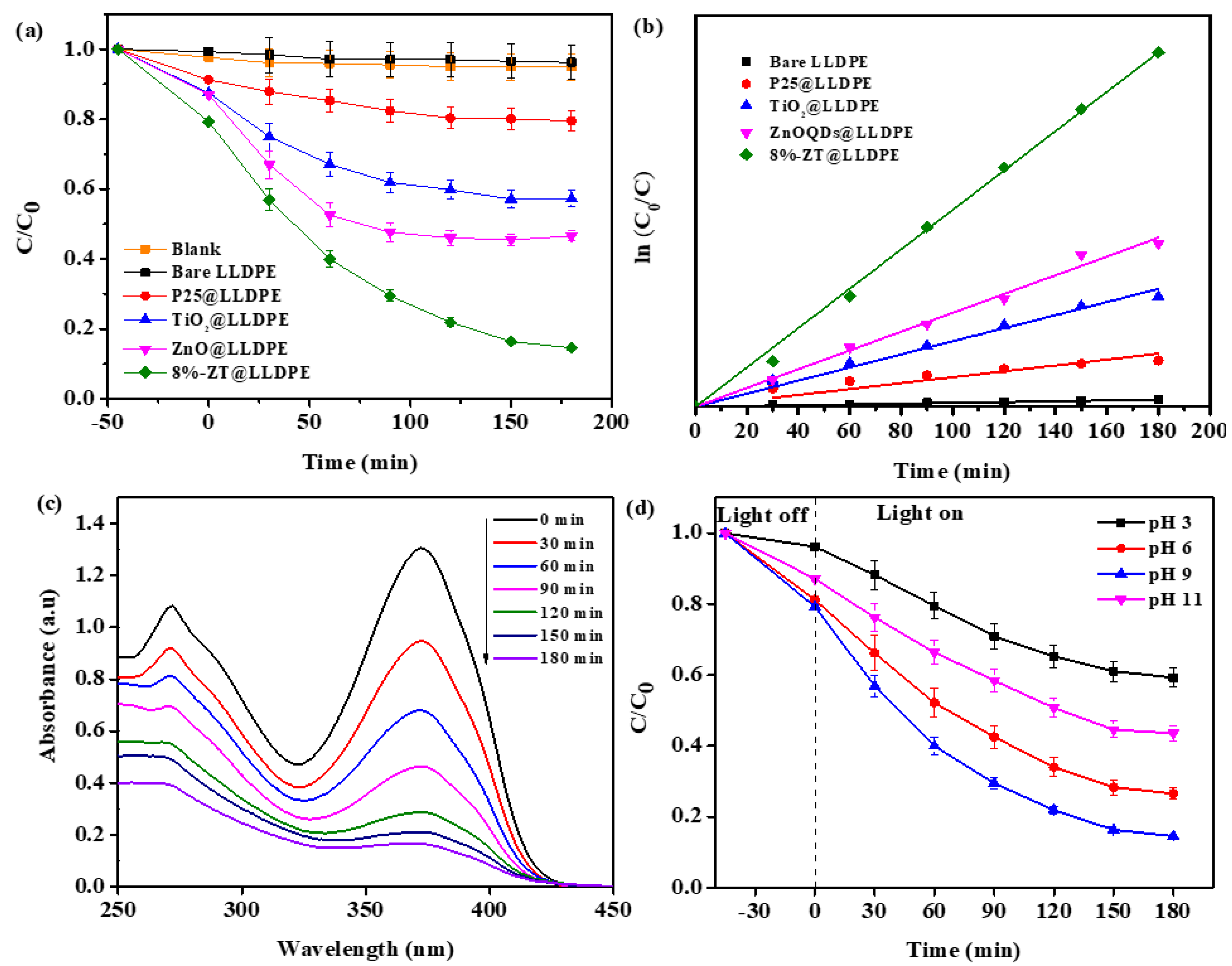
2.2.1. Effect of Various Photocatalysts Immobilised on LLDPE Film
2.2.2. Effect of the Initial pH of the Solution
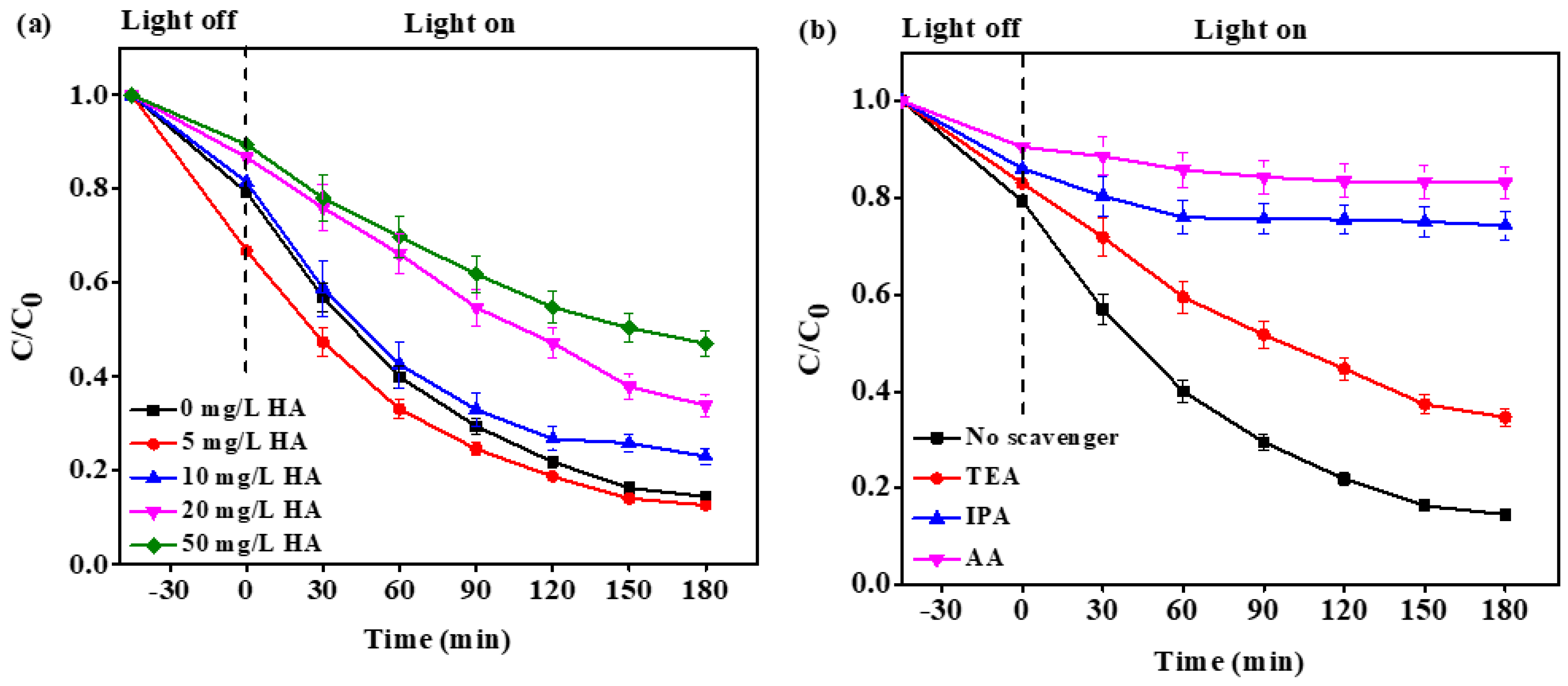
2.2.3. Effect of Natural Organic Matter (NOM)
2.2.4. Scavenging Test
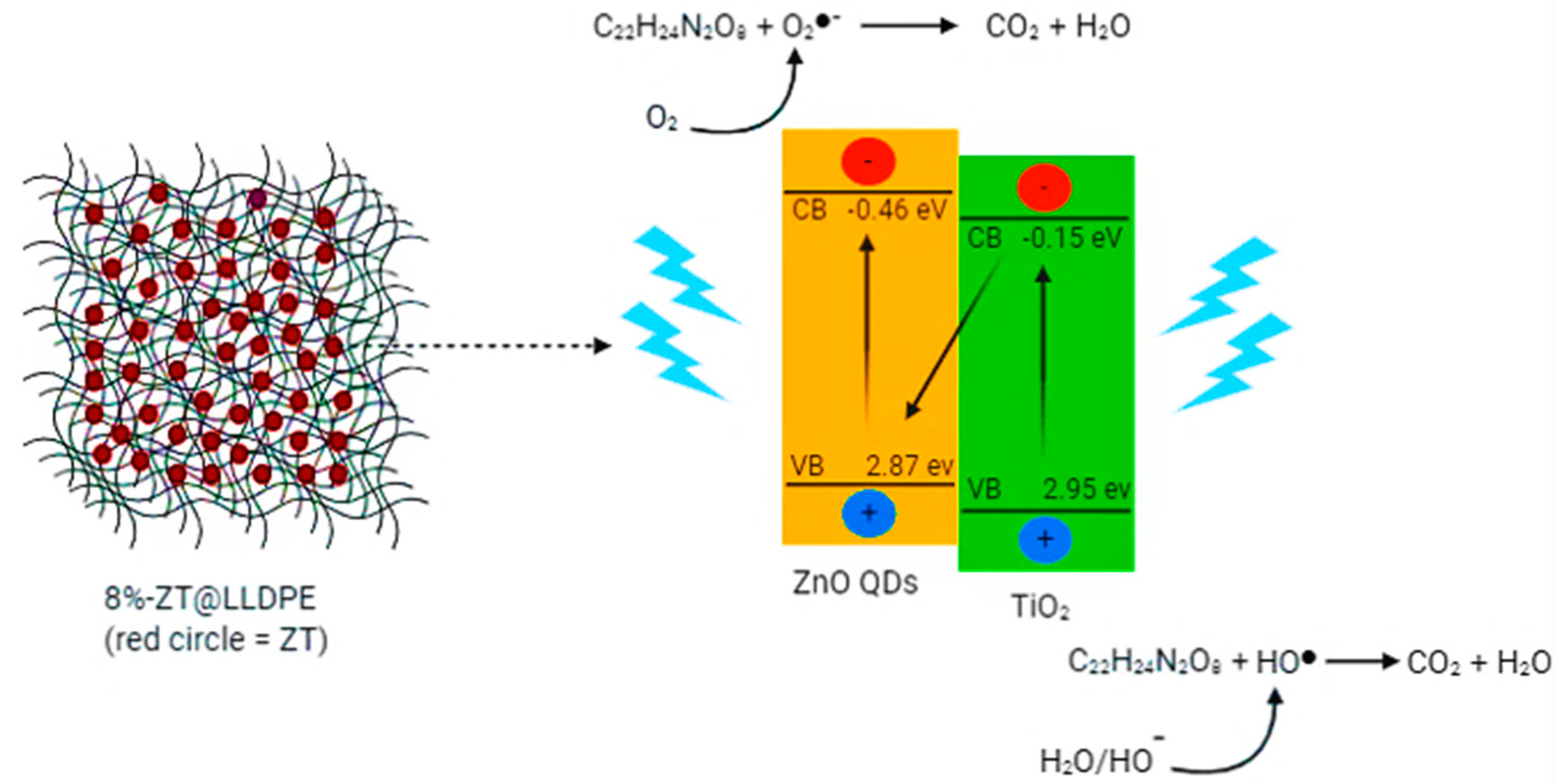
2.2.5. Mechanism of TC Degradation Using 8%-ZT@LLDPE
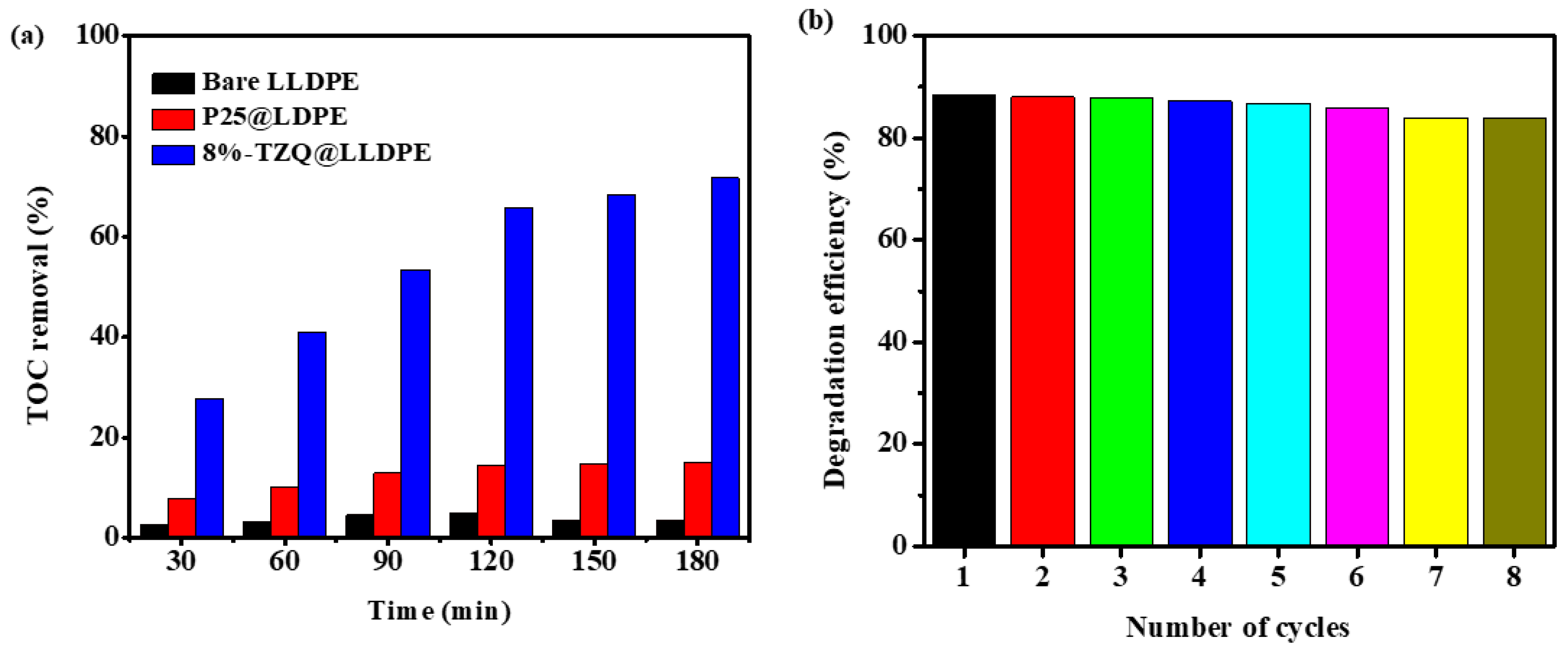
2.2.6. Mineralisation Efficiency
2.2.7. Reusability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZnO Quantum Dots (ZnO QDs)
3.3. Synthesis of ZnO QDs Modified TiO2
3.4. Synthesis of ZnO QDs Modified TiO2/LLDPE Floating Hybrid Polymer Film
3.5. Characterisation of the Hybrid Film Photocatalyst
3.6. Photocatalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterisations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Zhang, G.; Pang, T.; Zhang, X.; Cai, D.; Wu, Z. In situ degradation of antibiotic residues in medical intravenous infusion bottles using high energy electron beam irradiation. Sci. Rep. 2017, 7, 39928. [Google Scholar] [CrossRef] [PubMed]
- Safari, G.; Hoseini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 603–616. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Cui, W.; An, W.; Liu, L.; Hu, J.; Liang, Y. Novel Cu2O quantum dots coupled flower-like BiOBr for enhanced photocatalytic degradation of organic contaminant. J. Hazard. Mater. 2014, 280, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, J.; Zhao, Q.D.; Li, X.Y.; Zheng, N.; Xue, F.H. Synthesis of CdSe quantum-dots-sensitised TiO2 nanocomposites with visible-light photocatalytic activity. Adv. Mater. Res. 2014, 924, 3–9. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, J.; Jia, X.; Ma, Y.-H.; Zhang, X.; Wang, L.; Zou, J.-J. Highly efficient Z-scheme WO3−x quantum dots/TiO2 for photocatalytic hydrogen generation. Chin. J. Catal. 2017, 38, 253–259. [Google Scholar] [CrossRef]
- Chimmikuttanda, S.P.; Naik, A.; Akple, M.S.; Rajegowda, R.H. Hydrothermal synthesis of TiO2 hollow spheres adorned with SnO2 quantum dots and their efficiency in the production of methanol via photocatalysis. Environ. Sci. Pollut. Res. 2017, 24, 26436–26443. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Podzorski, M.; Bahnemann, D.; Subramanian, V.R. One-pot fabrication of high coverage PbS quantum dot nanocrystal-sensitised titania nanotubes for Photoelectrochemical Processes. J. Phys. Chem. C 2018, 122, 13659–13668. [Google Scholar] [CrossRef]
- Goutham, R.; Narayan, R.B.; Srikanth, B.; Gopinath, K. Supporting Materials for Immobilisation of Nano-photocatalysts. In Nanophotocatalysis and Environmental Applications. Environmental Chemistry for a Sustainable World; Sharma, G., Kumar, A., Lichtfouse, E., Asiri, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; Volume 29, Chapter 2; pp. 49–82. [Google Scholar]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A-Gen. 2013, 462, 178–195. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, L.; Wang, D.; Yin, R.; Li, X.; An, J.; Yang, X. Preparation, characterisation and visible-light photocatalytic performances of composite films prepared from polyvinyl chloride and SnO2 nanoparticles. J. Environ. Chem. Eng. 2015, 3, 622–629. [Google Scholar] [CrossRef]
- Kaur, T.; Sraw, A.; Wanchoo, R.; Toor, A.P. Solar assisted degradation of carbendazim in water using clay beads immobilised with TiO2 & Fe doped TiO2. Sol. Energy 2018, 162, 45–56. [Google Scholar]
- Erjavec, B.; Hudoklin, P.; Perc, K.; Tišler, T.; Dolenc, M.S.; Pintar, A. Glass fiber-supported TiO2 photocatalyst: Efficient mineralisation and removal of toxicity/estrogenicity of bisphenol A and its analogs. Appl. Catal. B 2016, 183, 149–158. [Google Scholar] [CrossRef]
- Veréb, G.; Ambrus, Z.; Pap, Z.; Mogyorósi, K.; Dombi, A.; Hernádi, K. Immobilization of crystallised photocatalysts on ceramic paper by titanium(IV) ethoxide and photocatalytic decomposition of phenol. React. Kinet. Mech. Catal. 2014, 113, 293–303. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Martins, P.M.; Ribeiro, J.M.; Teixeira, S.; Petrovykh, D.; Cuniberti, G.; Pereira, L.; Lanceros-Méndez, S. Photocatalytic microporous membrane against the increasing problem of water emerging pollutants. Materials 2019, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Vassalini, I.; Gjipalaji, J.; Crespi, S.; Gianoncelli, A.; Mella, M.; Ferroni, M.; Alessandri, I. Alginate-Derived Active Blend Enhances Adsorption and Photocatalytic Removal of Organic Pollutants in Water. Adv. Sustain. Syst. 2020, 1900112. [Google Scholar] [CrossRef]
- Vassalini, I.; Ribaudo, G.; Gianoncelli, A.; Casula, M.F.; Alessandri, I. Plasmonic hydrogels for capture, detection and removal of organic pollutants. Environ. Sci. Nano 2020, 7, 3888–3900. [Google Scholar]
- Romero Saez, M.; Jaramillo, L.; Saravanan, R.; Benito, N.; Pabón, E.; Mosquera, E.; Gracia Caroca, F. Notable photocatalytic activity of TiO2-polyethylene nanocomposites for visible light degradation of organic pollutants. eXPRESS Polym. Lett. 2017, 11, 899–909. [Google Scholar]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.-J. Synthesis of M@ TiO2 (M= Au, Pd, Pt) core–shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Han, Z.; Weng, Q.; Lin, C.; Yi, J.; Kang, J. Development of CdSe–ZnO Flower-Rod Core-Shell Structure Based Photoelectrochemical Biosensor for Detection of Norovirus RNA. Sensors 2018, 18, 2980. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-Y.; Ren, T.-Z.; Vantomme, A.; Su, B.-L. Facile and Generalised Preparation of Hierarchically Mesoporous-Macroporous Binary Metal Oxide Materials. Chem. Mater. 2004, 16, 5096–5106. [Google Scholar] [CrossRef]
- Shafiq, M.; Yasin, T.; Saeed, S. Synthesis and characterisation of linear low-density polyethylene/sepiolite nanocomposites. J. Appl. Polym. Sci. 2012, 123, 1718–1723. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 Nanoparticles via Sol-Gel Method. Adv. Mat. Res. 2011, 173, 184–189. [Google Scholar]
- Mahshid, S.; Askari, M.; Sasani Ghamsari, M. Synthesis of TiO2 nanoparticles, by hydrolysis and peptisation of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Ambrosio, R.; Carrillo, A.; Mota, M.L.; de la Torre, K.; Torrealba, R.; Moreno, M.; Vazquez, H.; Flores, J.; Vivaldo, I. Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics. Polymers 2018, 10, 1370. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.; Jaafar, J.; Ismail, A.; Mutalib, M.A.; Sani, N.; Asri, S.; Ong, C. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Saharudin, K.; Sreekantan, S.; Basiron, N.; Khor, Y.; Harun, N.; Mydin, R.S.M.N.; Md Akil, H.; Seeni, A.; Vignesh, K. Bacteriostatic Activity of LLDPE nanocomposite embedded with sol–gel synthesised TiO2/ZnO coupled oxides at various ratios. Polymers 2018, 10, 878. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- El Mragui, A.; Daou, I.; Zegaoui, O. Influence of the preparation method and ZnO/(ZnO + TiO2) weight ratio on the physicochemical and photocatalytic properties of ZnO-TiO2 nanomaterials. Catal. Today 2019, 321, 41–51. [Google Scholar] [CrossRef]
- Nasseh, N.; Panahi, A.H.; Esmati, M.; Daglioglu, N.; Asadi, A.; Rajati, H.; Khodadoost, F. Enhanced photocatalytic degradation of tetracycline from aqueous solution by a novel magnetically separable FeNi3/SiO2/ZnO nano-composite under simulated sunlight: Efficiency, stability, and kinetic studies. J. Mol. Liq. 2020, 112434. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, L.; Liu, X.; Zhou, M.; Zhu, Z.; Lu, Z.; Ma, C.; Huo, P. Synthesis and characterisation of ZnS nanocrystals and its photocatalytic activity on antibiotics. Fresen Environ. Bull. 2015, 24, 2280–2288. [Google Scholar]
- He, J.; Zhang, Y.; Guo, Y.; Rhodes, G.; Yeom, J.; Li, H.; Zhang, W. Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: Kinetics, influencing factors, and mechanisms. Environ. Int. 2019, 132, 105105. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, S.; Shan, G.; Wu, W.; Qiang, L.; Zhu, L. Novel MWNTs–Bi2WO6 composites with enhanced simulated solar photoactivity toward adsorbed and free tetracycline in water. Appl. Catal. B 2015, 176, 11–19. [Google Scholar] [CrossRef]
- Gómez-Pacheco, C.; Sánchez-Polo, M.; Rivera-Utrilla, J.; López-Peñalver, J. Tetracycline degradation in aqueous phase by ultraviolet radiation. Chem. Eng. J. 2012, 187, 89–95. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Biglari, H.; Mahvi, A.H. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. J. Chem. 2012, 9, 2453–2461. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef]
- Song, C.; Wang, X.; Zhang, J.; Chen, X.; Li, C. Enhanced performance of direct Z-scheme CuS-WO3 system towards photocatalytic decomposition of organic pollutants under visible light. Appl. Surf. Sci. 2017, 425, 788–795. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Mohseni-Bandpei, A.; Safari, A.A.; Asadi, A. N, S co-doped TiO2 nanoparticles and nanosheets in simulated solar light for photocatalytic degradation of non-steroidal anti-inflammatory drugs in water: A comparative study. J. Chem. Technol. Biotechnol. 2016, 91, 2693–2704. [Google Scholar] [CrossRef]
- Shams, M.; Goharshadi, E.K.; Ghalehaskari, S.; Nezhad, N.T.; Aziznezhad, M.; Nejad, Z.D.; Wilson, L.D. Parameter optimisation of tetracycline removal by vanadium oxide nano cuboids. Coll. Surf. A Phyiscochem. Eng. Asp. 2021, 619, 126460. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Feng, Y. Bimetallic Au/Ag decorated TiO2 nanocomposite membrane for enhanced photocatalytic degradation of tetracycline and bactericidal efficiency. Appl. Surf. Sci. 2019, 487, 1008–1017. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilised TiO2 systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2 (P25) immobilised in biopolymer (chitosan) under UV irradiation. Water Sci Technol. 2020, 82, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, T.; Li, G.; An, L.; Li, F.; Zhang, Z. Electrospun H4SiW12O40/cellulose acetate composite nanofibrous membrane for photocatalytic degradation of tetracycline and methyl orange with different mechanism. Carbohydr. Polym. 2017, 168, 153–162. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Duoerkun, G.; Cao, W.; Liu, T.; Zhang, L.; Liu, J.; Li, M.; Chen, Z. Fabrication of MoS2/BiOBr heterojunctions on carbon fibers as a weaveable photocatalyst for tetracycline hydrochloride degradation and Cr(VI) reduction under visible light. Environ. Sci. Nano 2020, 7, 2708–2722. [Google Scholar] [CrossRef]
- Zhang, F.; Song, N.; Zhang, S.; Zou, S.; Zhong, S. Synthesis of sponge-loaded Bi2WO6/ZnFe2O4 magnetic photocatalyst and application in continuous flow photocatalytic reactor. J. Mater. Sci. Mater. Electron. 2017, 28, 8197–8205. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Yu, K.; Hu, X. Immobilization of 2D/2D structured g-C3N4 nanosheet/reduced graphene oxide hybrids on 3D nickel foam and its photocatalytic performance. Mater. Res. Bull. 2018, 97, 306–313. [Google Scholar] [CrossRef]
- Mahjoub, M.A.; Monier, G.; Robert-Goumet, C.; Réveret, F.O.; Echabaane, M.; Chaudanson, D.; Petit, M.; Bideux, L.; Gruzza, B. Synthesis and study of stable and size-controlled ZnO–SiO2 quantum dots: Application as a humidity sensor. J. Phys. Chem. C 2016, 120, 11652–11662. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, X.; Yang, Y. Facile synthesis of monodisperse porous ZnO spheres by a soluble starch-assisted method and their photocatalytic activity. J. Phys. Chem. C 2011, 115, 7145–7152. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Kaus, N.H.M.; Jiang, Z.-T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef]
- Nezhad, N.T.; Shams, M.; Deghan, A.; Aziznezhad, M.; Goharshadi, E.K.; Mohammadhosseini, F.; Wilson, L.D. Vanadium dioxide nanoparticles as a promising sorbent for controlled removal of waterborne fluoroquinolone ciprofloxacin. Mater. Chem. Phys. 2021, 259, 123993. [Google Scholar] [CrossRef]
| Catalyst | Efficiency (%) | k (min−1) | R2 |
|---|---|---|---|
| Bare LLDPE | 4 | 0.0002 | 0.99724 |
| P25@LLDPE | 20.5 | 0.00151 | 0.96392 |
| TiO2@LLDPE | 42.7 | 0.00334 | 0.99503 |
| ZnO@LLDPE | 53.4 | 0.00480 | 0.99690 |
| 8%-ZT@LLDPE | 85.4 | 0.01077 | 0.99897 |
| Photocatalyst | TC Conc. | Light Source | Results | Ref. |
|---|---|---|---|---|
| Au/Ag/TiO2 (cellulose acetate) | 5 mg/L | Xe lamp (λ > 420 nm) | 77.4% after 120 min (k = 0.01276 min−1). | [44] |
| TiO2 (alumina pellets) | 35 mg/L | UV light | 60% after 120 min (k = 0.0085 min−1) | [45] |
| TiO2 (chitosan) | 40 mg/L | UV light | 44% after 60 min (k = 0.0009 min−1) Reusability = 4 cycles | [46] |
| H4SiW12O40 (cellulose acetate) | 10 mg/L | 300W Hg lamp | 63.8% after 120 min Reusability = 3 cycles | [47] |
| MoS2/BiOBr (carbon fibres) | 20 mg/L | 300 W Xe lamp | 92.4% after 120 min Reusability = 4 cycles | [48] |
| Bi2WO6/ZnFe2O4 (polyurethane) | 50 mg/L | 500 W Xe lamp | 91.59% after 90 min Reusability = 4 cycles | [49] |
| g-C3N4/rGO (3D Ni foam) | 20 mg/L | 300W Xe lamp | 90% after 120 min Reusability = 4 cycles | [50] |
| 8%-ZT@LLDPE | 40 mg/L | 48W fluorescent lamp | 89.45% after 180 min (k = 0.01312 min−1) Reusability = 8 cycles | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Saidu, U.; Adam, F.; Sreekantan, S.; Yahaya, N.; Ahmad, M.N.; Ramalingam, R.J.; Wilson, L.D. Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation. Molecules 2021, 26, 2509. https://doi.org/10.3390/molecules26092509
Iqbal A, Saidu U, Adam F, Sreekantan S, Yahaya N, Ahmad MN, Ramalingam RJ, Wilson LD. Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation. Molecules. 2021; 26(9):2509. https://doi.org/10.3390/molecules26092509
Chicago/Turabian StyleIqbal, Anwar, Usman Saidu, Farook Adam, Srimala Sreekantan, Noorfatimah Yahaya, Mohammad Norazmi Ahmad, Rajabathar Jothi Ramalingam, and Lee D. Wilson. 2021. "Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation" Molecules 26, no. 9: 2509. https://doi.org/10.3390/molecules26092509
APA StyleIqbal, A., Saidu, U., Adam, F., Sreekantan, S., Yahaya, N., Ahmad, M. N., Ramalingam, R. J., & Wilson, L. D. (2021). Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation. Molecules, 26(9), 2509. https://doi.org/10.3390/molecules26092509











