Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Biosorption of Metals by Batch Technique
2.3. Biosorption Equilibirum
3. Results and Discussion
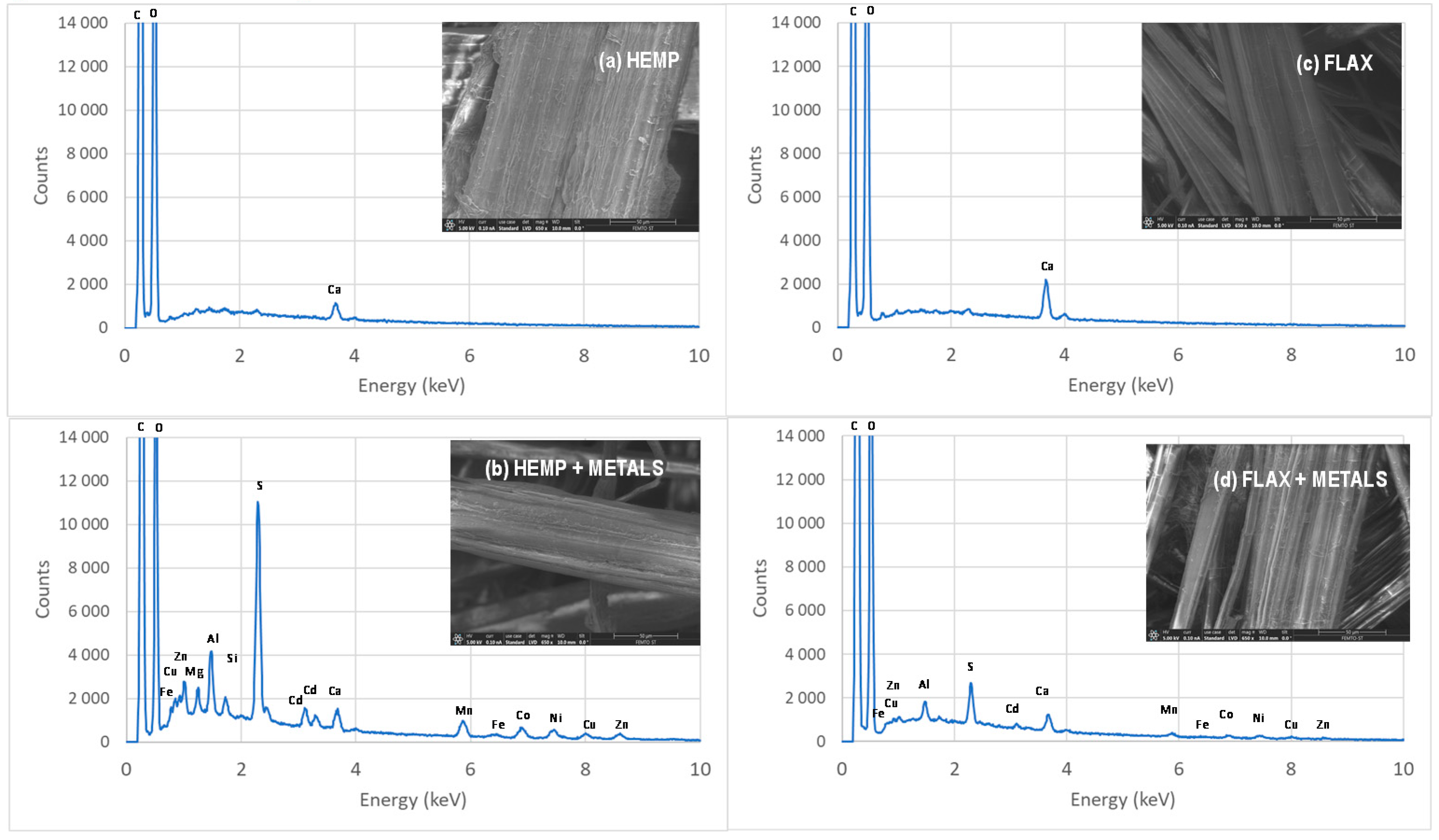
3.1. Characterization of Hemp- and Flax-Based Felts
3.2. Comparison between Hemp- and Flax-Based Biosorbents Regarding Metal Removal
3.3. Effect of Initial Metal Concentration
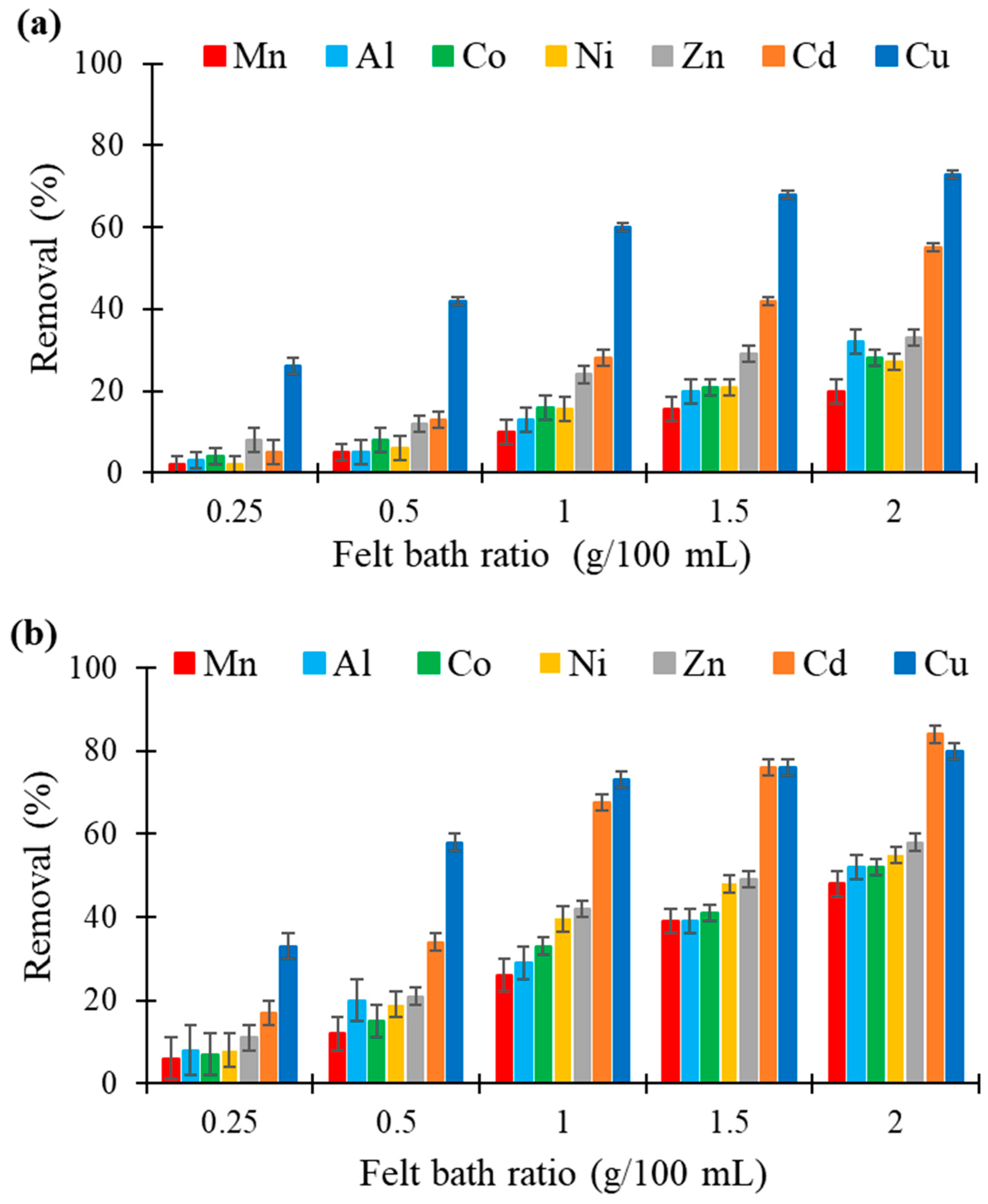
3.4. Effect of Adsorbent Dose
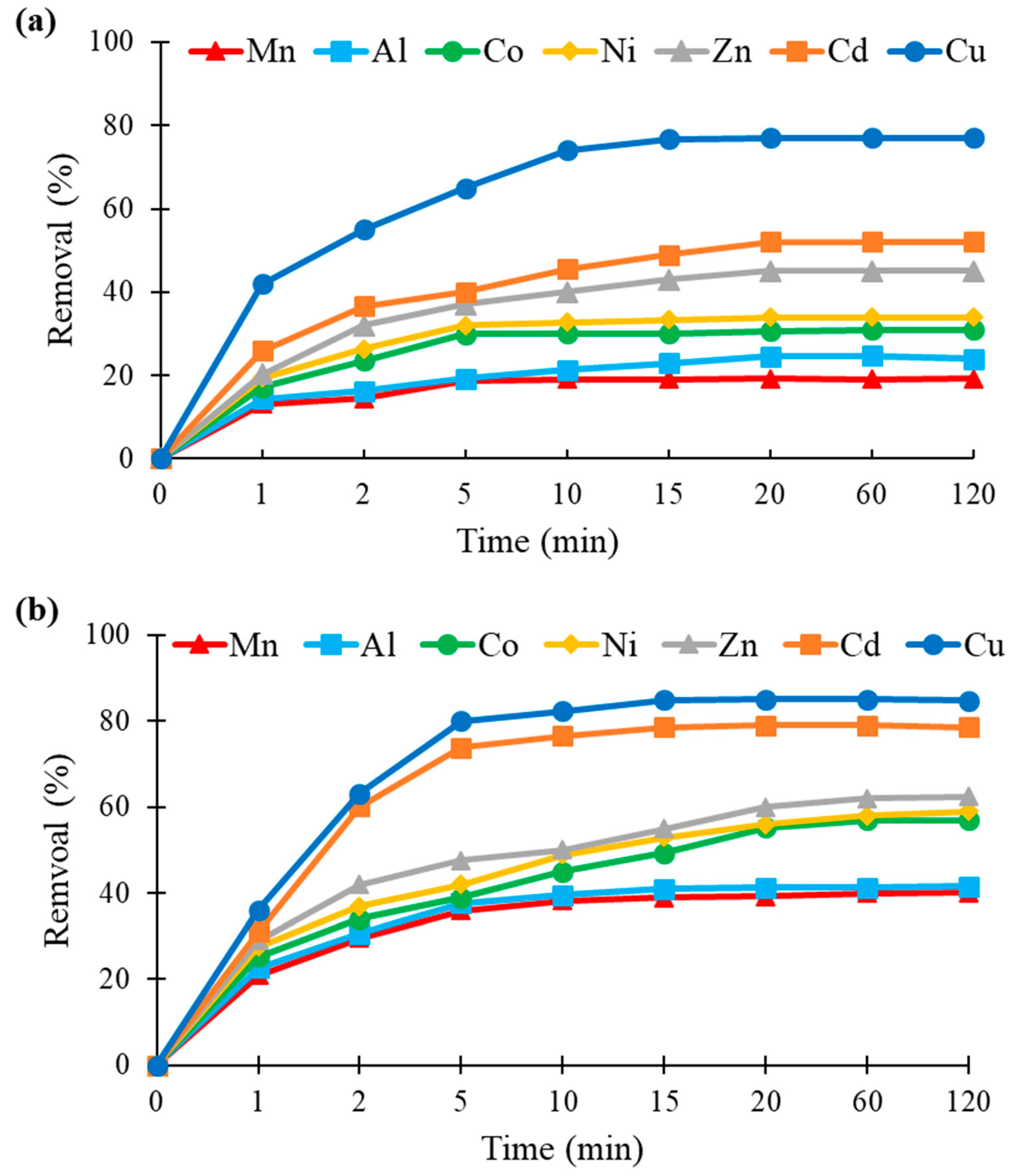
3.5. Effect of Contact Time
3.6. Effect of pH
3.7. Adsorption Isotherms
3.8. Tests with Polycontaminated Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barros, M.; Torres, M.; Bello, P.; Roca, E.; Casares, J. Integrated pollution prevention and control in the surface treatment industries in Galicia (NW Spain). Clean Technol. Environ. Policy 2008, 10, 175–188. [Google Scholar] [CrossRef]
- Druart, C.; Morin-Crini, N.; Euvrard, E.; Crini, G. Chemical and ecotoxicological monitoring of discharge water from a metal-finishing factory. Environ. Process. 2016, 3, 59–72. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Eaux Industrielles Contaminées, 1st ed.; Presses Universitaires de Franche-Comté: Besançon, France, 2017; pp. 57–102. (In French) [Google Scholar]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arabian J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A Critical Review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Charles, J.; Crini, G.; Morin-Crini, N.; Badot, P.M.; Trunfio, G.; Sancey, B.; de Carvalho, M.; Bradu, C.; Avramescu, S.; Winterton, P.; et al. Advanced oxidation (UV-ozone) and cyclodextrin sorption: Effects of individual and combined action on the Chemical abatement of organic pollutants in industrial effluents. J. Taiwan Inst. Chem. Eng. 2014, 45, 603–608. [Google Scholar] [CrossRef]
- Charles, J.; Bradu, C.; Morin-Crini, N.; Sancey, B.; Winterton, P.; Torri, G.; Badot, P.M.; Crini, G. Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: Chemical abatement. J. Saudi Chem. Soc. 2016, 20, 185–194. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Green Adsorbents for Pollutant Removal. Fundamentals and Design; Springer Nature: Cham, Switzerland, 2018; pp. 23–71. [Google Scholar]
- Dhir, B. Potential of biological materials for removing heavy metals from wastewater. Environ. Sci. Pollut. Res. 2014, 21, 1614–1627. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green biosorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Aris, A.Z. A review on economically biosorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Bio/technol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is Biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental remediation of heavy metal ions from aqueous solution through hydrogel adsorption: A Critical Review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost biosorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost biosorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef] [Green Version]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based biosorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Beaugeard, V.; Muller, J.; Graillot, A.; Ding, X.Y.; Robin, J.J.; Monge, S. Acidic polymeric sorbents for the removal of metallic pollution in water: A review. React. Funct. Polym. 2020, 152, 104599. [Google Scholar] [CrossRef]
- Bouloc, P. Hemp: Industrial Production and Uses; CABI Publishing: Wallingford, UK, 2013; pp. 1–26. [Google Scholar]
- Bono, P.; le Duc, A.; Lozachmeur, M.; Day, A. Materials: New fields of research and development for the valorization of technical plant fibres (flax fiber and hemp). OCL Oilseeds Fats Crops Lipids 2015, 22, 1–11. [Google Scholar]
- Fike, J. Industrial hemp: Renewed opportunities for an Ancient crop. Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Bugnet, J.; Morin-Crini, N.; Chanet, G.; Cosentino, C.; Crini, G. Chapter XI. In Du Chanvre Pour Dépolluer des Eaux Polycontaminées en Métaux; Morin-Crini, G., Crini, G., Eds.; Presses Universitaires de Franche-Comté: Besançon, France, 2017; pp. 323–340. (In French) [Google Scholar]
- Morin-Crini, N.; Loiacono, S.; Placet, V.; Torri, G.; Bradu, C.; Kostić, M.; Cosentino, C.; Chanet, G.; Martel, B.; Lichtfouse, É.; et al. Hemp-based biosorbents for sequestration of Metals: A review. Environ. Chem. Lett. 2019, 17, 393–408. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Hemp Production and Applications; Springer Nature: Cham, Switzerland, 2020; pp. 38–87. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Pejić, B.M.; Vukčević, M.M.; Pajić-Lijaković, I.D.; Laušević, M.D.; Kostić, M.M. Mathematical Modeling of Heavy Metal Ions (Cd2+, Zn2+ and Pb2+) Biosorption by chemically modified short hemp fibres. Chem. Eng. J. 2011, 172, 35–360. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Păduraru, C.; Wenkert, R. Cobalt (II) removal from aqueous solutions by natural hemp fibers: Batch and fixed-bed columns studies. Appl. Surf. Sci. 2013, 285, 33–39. [Google Scholar] [CrossRef]
- Tofan, L.; Păduraru, C.; Teodosiu, C.; Toma, O. Fixed Bed columns study on the removal of chromium (III) ions from aqueous solutions by hemp fibers with improved sorption performance. Cellul. Chem. Technol. 2015, 49, 219–229. [Google Scholar]
- Tofan, L.; Păduraru, C.; Toma, O. Zinc remediation of aqueous solutions by natural hemp fibres: Batch desorption/regeneration study. Desalin. Water Treat. 2016, 57, 12644–12652. [Google Scholar] [CrossRef]
- Balintova, M.; Holub, M.; Stevulova, N.; Cigasova, J.; Tesarcikova, M. Sorption in acidic environment-Biosorbents in comparison with commercial biosorbents. Chem. Eng. Trans. 2014, 39, 625–630. [Google Scholar]
- Vukčević, M.; Pejić, B.; Laušević, M.; Pajić-Lijaković, I.; Kostić, M. Influence of chemically modified short hemp fiber structure on biosorption process of Zn2+ ions from waste water. Fibres Polym. 2014, 15, 687–697. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Terzopoulou, Z.; Nikolaidis, V.; Alexopoulou, E.; Bikiaris, D.N. Low-cost hemp biomaterials for nickel ions removal from aqueous solutions. J. Mol. Liq. 2015, 209, 209–218. [Google Scholar] [CrossRef]
- Bugnet, J.; Morin-Crini, N.; Cosentino, C.; Chanet, G.; Winterton, P.; Crini, G. Hemp decontamination of poly-metallic aqueous solutions. Environ. Eng. Manage. J. 2017, 16, 535–542. [Google Scholar]
- Kostić, M.; Pejić, B.; Vukčević, M. Waste Hemp (Cannabis sativa) Fibers as a biosorbent and a precursor for biocarbon sorbents: Influence of their chemical composition on Pb(II) removal. In Chemistry of Lignocellulosics: Current Trends; Stevanovic, T., Ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2018; pp. 3–21. [Google Scholar]
- Abbar, B.; Alem, A.; Marcotte, S.; Pantet, A.; Ahfir, N.D.; Bizet, D.; Duriatti, D. Experimental investigation from aqueous solution by flax fibres. Process Saf. Environ. Prot. 2017, 109, 369–647. [Google Scholar] [CrossRef]
- Abbar, B.; Alem, A.; Pantet, A.; Marcotte, S.; Ahfir, N.D.; Duriatti, D. Removal of dissolved and particulate contaminants from aqueous solution using natural flax fibres. Academic J. Civ. Eng. 2017, 35, 656–661. [Google Scholar]
- Melia, P.M.; Busquets, R.; Ray, S.; Cundy, A.B. Agricultural wastes from wheat, barley, flax and grape for the efficient removal of Cd from contaminated Water. RSC Adv. 2018, 8, 40378. [Google Scholar] [CrossRef] [Green Version]
- Jasim, N.A.; Hussein, T.K. Removal of cadmium ions from aqueous solutions using flax seeds as an adsorbent. Diyala J. Eng. Sci. 2019, 12, 1–8. [Google Scholar]
- Abutaleb, A.; Tayeb, A.M.; Mahmoud, M.A.; Daher, A.M.; Desouky, O.A.; Bakather, O.Y.; Farouq, R. Removal and recovery of U(VI) from aqueous effluents by flax fiber: Adsorption, desorption and batch adsorber proposal. J. Adv. Res. 2020, 22, 153–162. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Dehabadi, L.; Alabi, W.; Simonson, C.J.; Wilson, L.D. Hydratation and sorption properties of raw and milled flax fibers. ACS Omega 2020, 5, 6113–6121. [Google Scholar] [CrossRef]
- Loiacono, S.; Morin-Crini, N.; Cosentino, C.; Torri, G.; Chanet, G.; Winterton, P.; Crini, G. Simultaneous removal of Cd, Co, Cu, Mn, Ni and Zn from synthetic solutions on a hemp-based felt: Experimental design. J. Polym. Appl. Sci. 2017, 134, 44422. [Google Scholar] [CrossRef]
- Ivanovska, A.; Cerovic, D.; Maletic, S.; Jankovic, I.; Asanovic, K.; Kostić, M. Influence of the alkali treatment on the sorption and dielectric properties of woven jute fabric. Cellulose 2019, 26, 5133–5146. [Google Scholar] [CrossRef]
- Loiacono, S.; Crini, G.; Martel, B.; Chanet, G.; Cosentino, C.; Raschetti, M.; Placet, V.; Torri, G.; Morin-Crini, N. Simultaneous removal of Cd, Co, Cu, Mn, Ni and Zn from synthetic solutions on a hemp-based Felt. II. Chemical modification. J. Polym. Appl. Sci. 2017, 134, 45138. [Google Scholar] [CrossRef]
- Ivanovska, A.; Dojcinovic, B.; Maletic, S.; Pavun, K.; Kostić, M. Waste jute fabric as a biosorbent for heavy metal ions from aqueous solution. Fibers Polym. 2020, 21, 1992–2002. [Google Scholar] [CrossRef]
- Placet, V.; Meteau, J.; Froehly, L.; Salut, R.; Boubakar, L. Investigation of the internal structure of hemp fibres using optical coherence tomography and focused ion beam transverse cutting. J. Mater. Sci. 2014, 49, 8317–8327. [Google Scholar] [CrossRef] [Green Version]
- Richely, E.; Durand, S.; Melelli, A.; Kao, A.; Magueresse, A.; Dhakal, H.; Gorshkova, T.; Callebert, F.; Bourmaud, A.; Beaugrand, J.; et al. Novel insight into the intricate shape of flax fibre lumen. Fibers 2021, 9, 24. [Google Scholar] [CrossRef]
- Loiacono, S.; Crini, G.; Chanet, G.; Raschetti, M.; Placet, V.; Morin-Crini, N. Metals in aqueous solutions and real effluents: Biosorption behavior onto a hemp-based felt. J. Chem. Technol. Biotechnol. 2018, 93, 2592–2606. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Loiacono, S.; Placet, V.; Torri, G.; Bradu, C.; Kostić, M.; Cosentino, C.; Chanet, G.; Martel, B.; Lichtfouse, É.; et al. Hemp-based materials for metal removal. In Green Biosorbents for Pollutant Removal; Crini, G., Lichtfouse, E., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 154–196. [Google Scholar]
- Morin-Crini, N.; Staelens, J.N.; Loiacono, S.; Martel, B.; Chanet, G.; Crini, G. Simultaneous removal of Cd, Co, Cu, Mn, Ni and Zn from synthetic solutions on a hemp-based felt. III. Real discharge waters. J. Polym. Appl. Sci. 2020, 137, 48823. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.M.; Seshaiah, K. Removal of Cd(II) and Cu(II) from aqueous solution by agro biomass: Equilibrium, kinetic and thermodynamic studies. Environ. Eng. Res. 2012, 17, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Cyclodextrin-based biosorbents for the removal of pollutants from wastewater: A review. Environ. Sci. Pollut. Res. 2020, 28, 1317–1340. [Google Scholar] [CrossRef] [PubMed]
- Neris, J.B.; Luzardo, F.H.M.; da Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Jamshaid, A.; Hamid, A.; Muhammad, N.; Naseer, A.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Shah, N.S. Cellulose-based materials for the removal of heavy metals from wastewater-An overview. ChemBioEng 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Chauhan, S. Use of cellulose and its derivatives for metal ion sorption. J. Chem. Pharm. Res. 2016, 8, 416–420. [Google Scholar]
- Hubbe, M.A.; Hasan, S.H.; Ducoste, J.J. Cellulosic substrates for removal of pollutants from aqueous systems: A review. 1. Metals. BioResources 2011, 6, 2161–2287. [Google Scholar] [CrossRef]






| HEMP | FLAX | |
|---|---|---|
| Thickness (mm) | ~5 | ~3 |
| Surface weight (g/m2) | 665 | 280 |
| Synthetic fiber content (%) | 14.68 | 23.29 |
| Lignocellulosic fiber content (%) | 85.32 | 76.71 |
| α-Cellulose * (%) | 67.02 ± 0.2 | 72.77 ± 0.72 |
| Hemicelluloses * (%) | 19.32 ± 1.46 | 13.71 ± 1.00 |
| Lignin * (%) | 5.95 ± 0.33 | 6.93 ± 0.90 |
| Pectins * (%) | 1.50 ± 0.27 | 0.70 ± 0.31 |
| Fats and waxes * (%) | 1.23 ± 1.17 | 0.76 ± 0.12 |
| Water solubles (%) | 4.98 ± 0.18 | 5.12 ± 1.29 |
| % C | 41.1 | 43.5 |
| % N | 0.28 | 0.35 |
| % S | 0.06 | 0.09 |
| Ion exchange capacity (meq/g) | 0.10 ± 0.09 | 0.87 ± 0.09 |
| Metal Ion | Langmuir | Freundlich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/g) | aL (L/mg) | χ2 | R2 | KF (L/g) | nF | χ2 | R2 | ||
| HEMP | Mn | 0.33 | 0.08 | 0.24 | 0.00567 | 0.9337 | 0.18 | 4.06 | 0.01258 | 0.6537 |
| Al | 0.51 | 0.38 | 0.75 | 0.00398 | 0.9806 | 0.19 | 3.13 | 0.01554 | 0.7665 | |
| Co | 0.45 | 0.08 | 0.19 | 0.01860 | 0.8331 | 0.22 | 3.24 | 0.03517 | 0.7029 | |
| Ni | 0.53 | 0.15 | 0.28 | 0.01456 | 0.9207 | 0.22 | 3.10 | 0.03519 | 0.7284 | |
| Zn | 0.76 | 0.21 | 0.28 | 0.02939 | 0.8869 | 0.31 | 2.88 | 0.06693 | 0.7905 | |
| Cd | 1.02 | 0.57 | 0.56 | 0.02435 | 0.9563 | 0.38 | 2.88 | 0.07575 | 0.8237 | |
| Cu | 4.51 | 0.66 | 0.15 | 0.01485 | 0.9894 | 0.55 | 1.73 | 0.04286 | 0.9743 | |
| FLAX | Mn | 0.78 | 0.27 | 0.35 | 0.03427 | 0.9416 | 0.33 | 3.03 | 0.07986 | 0.7684 |
| Al | 0.90 | 0.38 | 0.42 | 0.03225 | 0.9564 | 0.45 | 3.82 | 0.07136 | 0.8545 | |
| Co | 1.45 | 13.24 | 9.13 | 0.01866 | 0.9738 | 0.43 | 2.6 | 0.07671 | 0.9020 | |
| Ni | 1.56 | 2.01 | 1.29 | 0.03575 | 0.9717 | 0.68 | 3.63 | 0.06871 | 0.9557 | |
| Zn | 2.05 | 2.09 | 1.02 | 0.03297 | 0.9914 | 0.72 | 3.33 | 0.04097 | 0.9764 | |
| Cd | 3.00 | 9.15 | 3.04 | 0.12359 | 0.9796 | 1.08 | 3.00 | 0.23426 | 0.9785 | |
| Cu | 5.53 | 1.75 | 0.32 | 0.02546 | 0.9906 | 1.40 | 2.70 | 0.18903 | 0.9802 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.R.L.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties. Molecules 2021, 26, 4199. https://doi.org/10.3390/molecules26144199
Mongioví C, Morin-Crini N, Lacalamita D, Bradu C, Raschetti M, Placet V, Ribeiro ARL, Ivanovska A, Kostić M, Crini G. Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties. Molecules. 2021; 26(14):4199. https://doi.org/10.3390/molecules26144199
Chicago/Turabian StyleMongioví, Chiara, Nadia Morin-Crini, Dario Lacalamita, Corina Bradu, Marina Raschetti, Vincent Placet, Ana Rita Lado Ribeiro, Aleksandra Ivanovska, Mirjana Kostić, and Grégorio Crini. 2021. "Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties" Molecules 26, no. 14: 4199. https://doi.org/10.3390/molecules26144199
APA StyleMongioví, C., Morin-Crini, N., Lacalamita, D., Bradu, C., Raschetti, M., Placet, V., Ribeiro, A. R. L., Ivanovska, A., Kostić, M., & Crini, G. (2021). Biosorbents from Plant Fibers of Hemp and Flax for Metal Removal: Comparison of Their Biosorption Properties. Molecules, 26(14), 4199. https://doi.org/10.3390/molecules26144199












