3.1. Crystallized Polycarbonate Surface and Dust Properties
The solution treatment (30% concentration acetone) was used to crystallized polycarbonate surfaces via immersing technique.
Figure 1a,b show top views of crystallized surfaces. The crystallized surface had a texture, which consisted of globules (
Figure 1a) and fibers like whiskers (
Figure 1b) emanating from the surface of the globules. The formation of whiskers was related to the secondary branching from the initially formed crystal sites [
27]. The whiskers acted like nano-pins over the textured surface and contributed to the mobility of the liquid droplet, i.e., it created a Lotus effect on the surface. The size of the globules varies on the textured surface; however, they created a hierarchical texture morphology.
Figure 2a,b show the surface image (
Figure 2a) and texture profile (
Figure 2b) of the crystallized surface obtained from an atomic force microscope. The globules on the surface were apparent from the profile peaks. The average roughness of the surface 3.2 µm and the surface roughness parameter (ratio of area covered by globulus over the projected area) was about 0.56. The wetting state of the surface before and after the crystallization was evaluated using a goniometer) via measuring contact angle and hysteresis. The contact angle of water was measured as 132° ± 4° on the crystallized surface while the hysteresis was 38° ± 5°. Hence, the crystallized surface demonstrated hydrophobic behavior; however, droplets adhered on the surface due to high hysteresis. To evaluate the opaqueness of the textured samples, optical transmittance measurement was carried out.
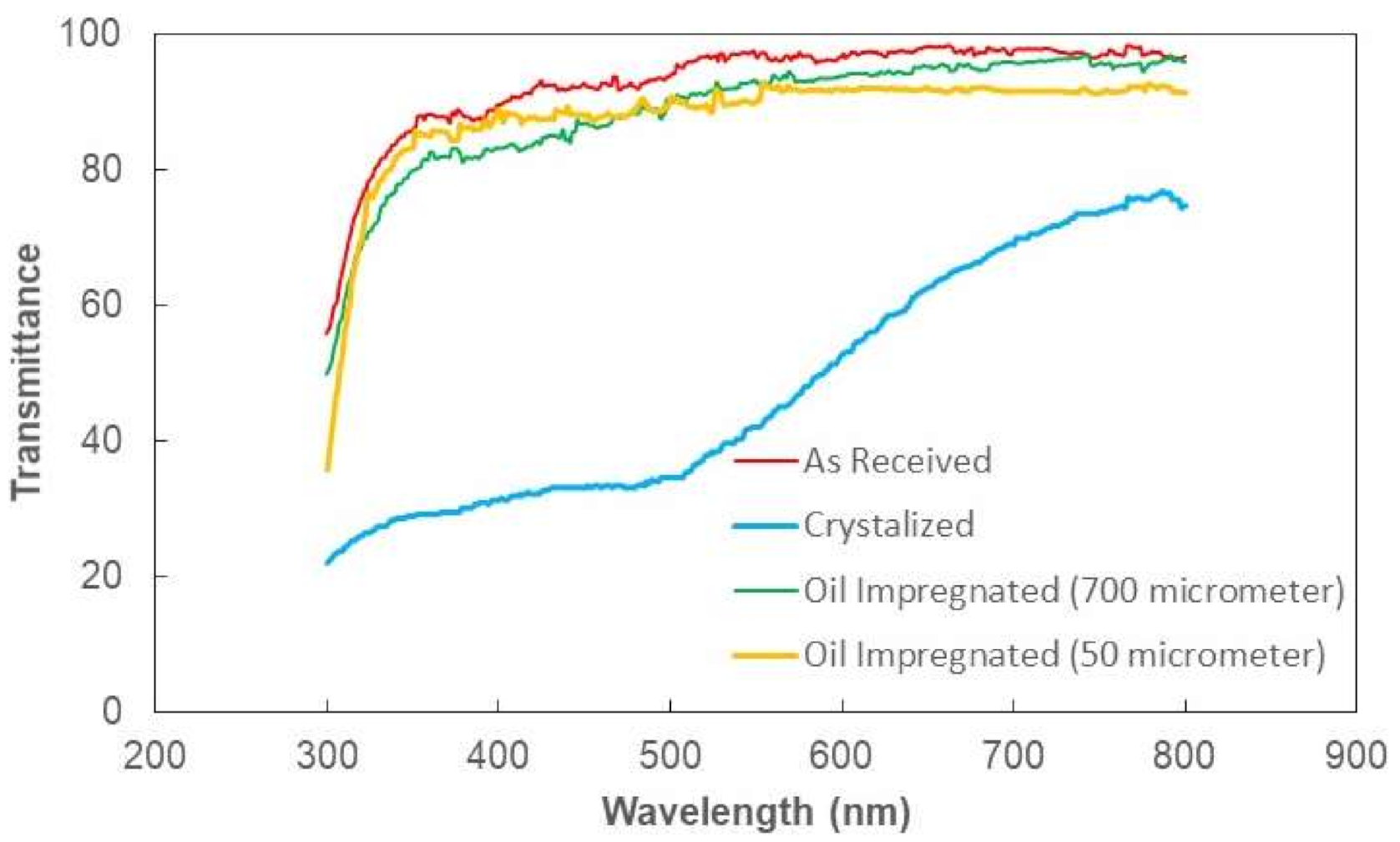
Figure 3 shows optical transmittance of the textured and as received polycarbonate surfaces. The transmittance of the crystallized sample reduced significantly because of the texture; in which case, incident electromagnetic radiation diffused and scattered at the crystallized surface. In this case, spherules and fibrils on the crystallized surface caused diffuse reflection and partial absorption of incident UV visible radiation while resulting in scattering of incident ration and lowering specular transmittance (wavelength-dependent transmittance). The increasing wavelength of incident optical radiation lowered diffuse reflection and absorption from the crystallized surface. To enhance optical transmittance, the surface was impregnated with silicon oil. To cover the surface with a complete oil film, spreading factor of silicon oil on crystallized polycarbonate surface (
) became important. The oil spreading is related to the surface tension of oil (
), interfacial-shear at the oil-crystallized surface interface (
), and surface free energy of crystallized surface (
). This yields [
11,
28]:
. The surface free energy of the crystallized sample was evaluated in the early work, which is
36.2 mJ/m
2 [
24] and the surface tension of silicon oil is 0.0187 N/m [
29]. The interfacial tension between oil and the crystalized surface could be evaluated through the Hemi–Wicking condition [
30], i.e., it became:
, here,
is the droplet contact angle on the crystallized sample. The interfacial tension (
) was estimated as 4.3 mN/m. Hence, the spreading factor (
) for silicon oil on the crystallized surface became 13.2 mN/m, which was greater than zero. This demonstrates that silicon oil spread over the crystallized surface. Hence, the silicon oil was spread over the crystallized surface under the controlled laboratory environment and, later, the oil film thickness was evaluated. Two extreme film thicknesses, 50 µm and 700 µm, were considered in the experiments. It is worth mentioning that as the oil film thickness reduced beyond 50 µm on the crystallized surface, the continuation of the oil film was not observed; oil formed islands like wet regions on the surface rather than developing a continuous film.
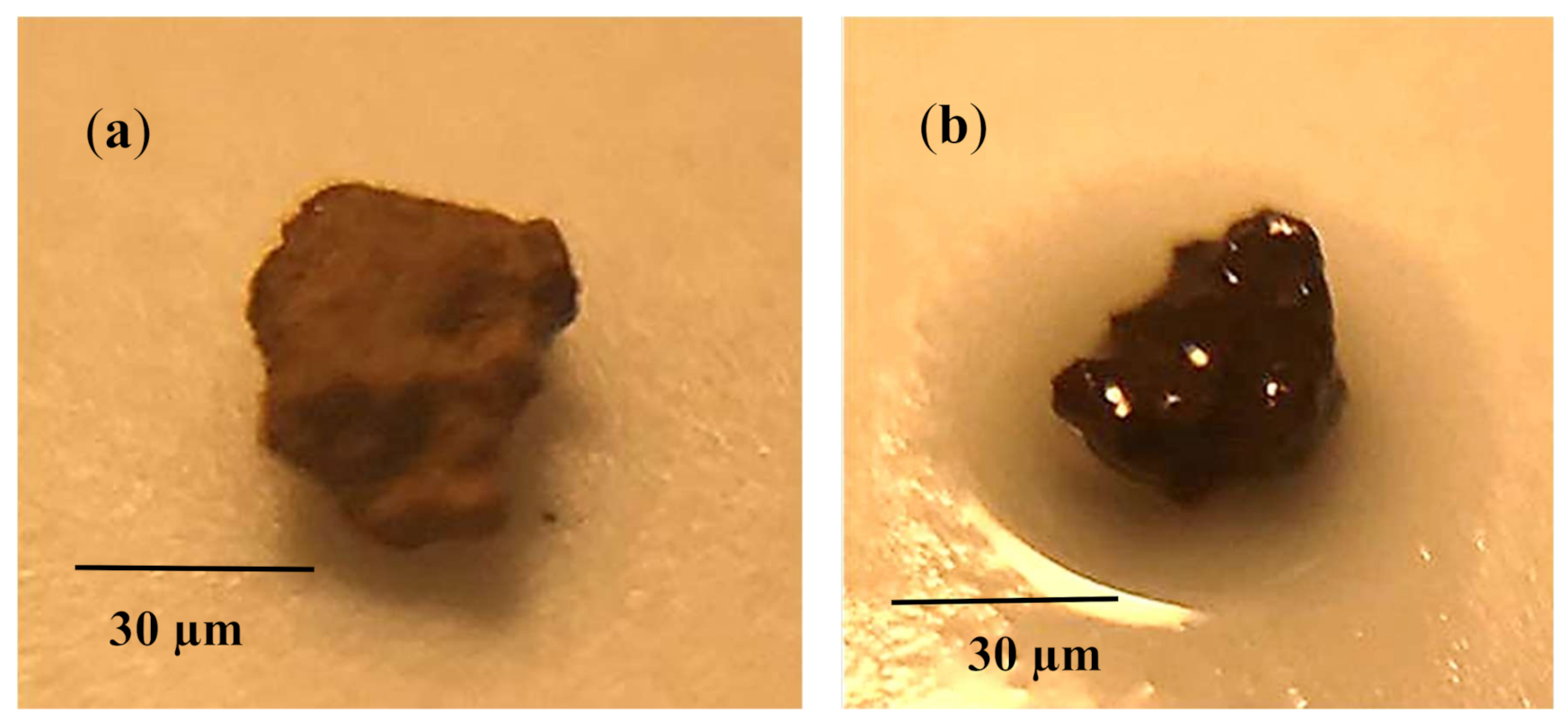
The dust particles have varying shapes and sizes, and the average dust particle was assessed via particle analyzer (Malvern Panalytical, Mastersizer 3000, Worcestershire, UK), which was about 1.2 µm.
Figure 4 shows dust particles, which were composed of small and large sizes. Small particles adhered to the large particle surface, which was because of the non-stoichiometric composition for the compounds (NaCl and KCl) formed in the dust. This is noted from
Table 2, in which elemental constitutes of dust is given. The dust particles were composed of inorganic compounds. For small dust particles (≤2.5 µm), the stoichiometric ratio of elements for NaCl and KCl did not satisfy, which in turn created charges on the particles while contributing to the attachment of small particles to the large ones (
Figure 4). The small size dust particles could adhere on the surface the charges and extra efforts were need for mitigating the dust from the dry crystallized sample surface. Therefore, the use of silicon oil may reduce the dust particle adhesion over the crystallized surface and sliding droplet can be able to pick up the dust from the oil film surface. The optical transmittance of the oil-impregnated surface was reassessed and findings are shown in
Figure 3. The optical transmittance of the crystallized surface enhanced notably after oil spreading; however, the influence of the oil film thickness (700 µm and 50 µm) was not significant on the optical transmittance, i.e., optical transmittance remains high for both impregnated surfaces.
3.2. Dust Removal from Oil Surface and Droplet Dynamics
Mitigating dust from the oil film by a sliding water droplet is challenging because of the oil and water infusions (cloaking) on the dust particle. It is worth mentioning that a water droplet can cloak over the dust particle surface during sliding transition over the oil surface. In order to determine the liquid (water and oil) infusion velocity over the dust particles, many experimental tests are conducted and temporal progression of water and oil height over the dust particle surface is recorded via the high speed facility.
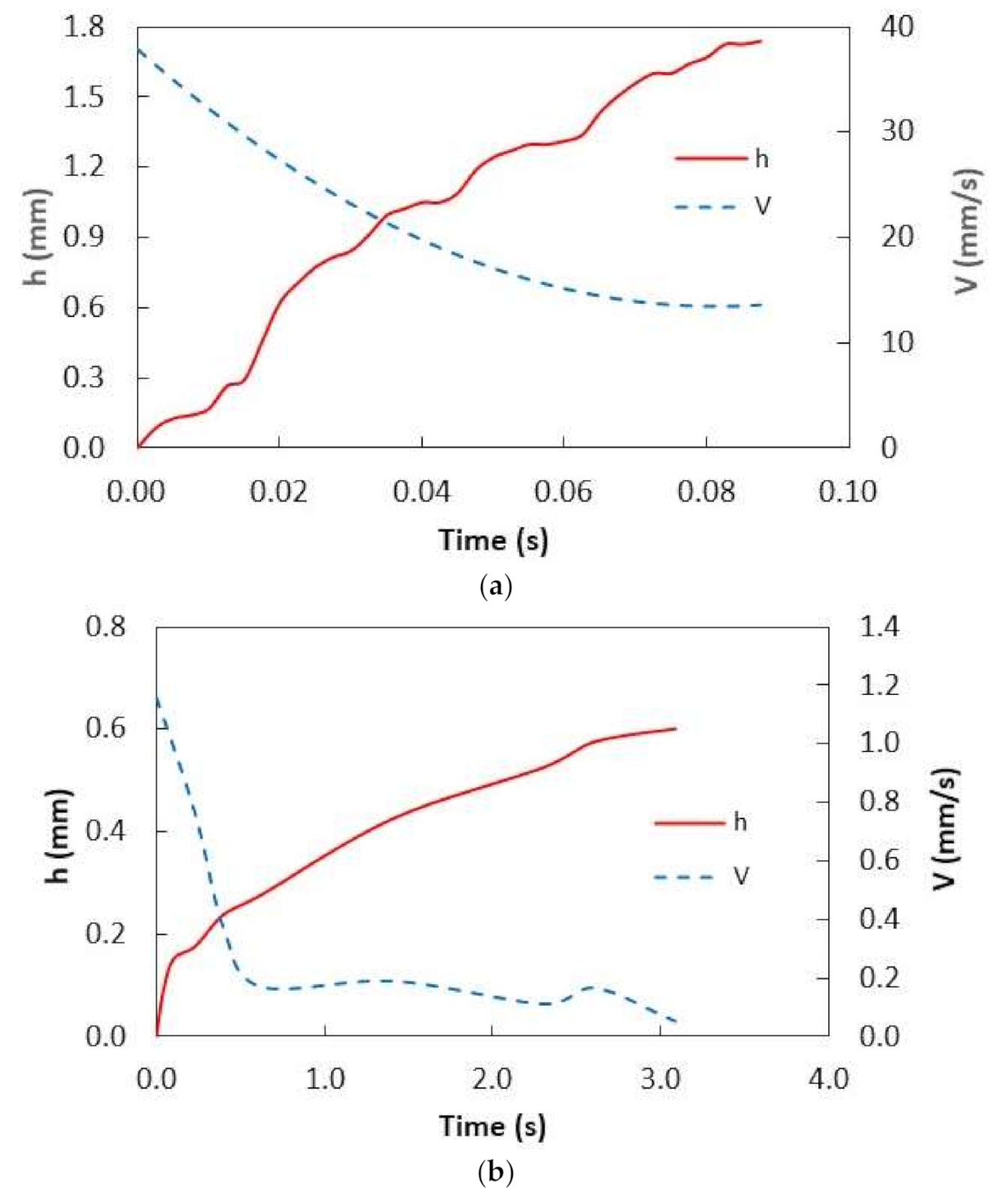
Figure 5a,b show the optical images of the stationary dust particle prior to and after oil infusion with time, respectively, while
Figure 6 shows the infusion (cloaking) velocity of water and silicon oil on the stationary dust particle. It is worth mentioning that the tracker program was used to obtain infusion velocity from the high-speed data. Infusion height of the water over the particle (~30 µm) was larger than that of silicon oil infusion height in the early period, i.e., water infusion took place at a faster rate than that of silicon oil. This resulted in larger water infusion velocity over the dust surface than that corresponding to silicon oil. As time progressed, the water and oil infusion velocities reduced and they became almost the same (silicon oil and water). Moreover, the decay of the infusion velocity was associated with the time, i.e.,
, here Vinf is the infusion velocity of the liquid and it takes the value of about 1/4 [
31]. Although silicon oil and water could spread over the dust particle surface, because of positive spreading coefficient (S), energy dissipation, due to viscous effect on the particle surface and gravitational pull over the particle height, slows down the liquid infusion on the surface [
31]. Liquid infusion over dust surface was associated with the Joos’ law [
32]. The liquid energy used during the infusion was related to the Ohnesorge number (
), where a represents the particle size [
33]. The Ohnesorge number took different values for water and silicon oil because of density and viscosity of the fluids, i.e., density of water was 1000 kg/m
3, viscosity was 0.7644 × 10
−3 Pa.s, and surface tension was 72.3 mN/m while the density of silicon oil was 935 kg/m
3, viscosity was 0.92 × 10
−2 Pa, and surface tension was 35 mN/m. Hence, the Ohnesorge number took the values of 0.082 and 0.016 for water infusion on the dust particle sizes of 1.2 µm and 30 µm, respectively while it took values of 1.47 and 2.94 for silicon oil for the same sizes dust particles. Hence, energy dissipated by the silicon oil became larger than water during the complete infusion (cloaking) over the dust particle surface. This became more evident for the large size dust particle. Hence, the temporal behavior of the infusion height of the silicon oil over the dust particle surface became gradual as compared to that of water.
Once the liquid droplet was created on the surface, droplet height reduced because of the bulging of droplet volume under gravity while causing droplet puddling on the surface. Once a water droplet was formed on the oil surface, a similar response was observed; however, silicon oil spread over the water droplet surface due to the lower surface tension of silicon oil as compared to water. It is worth mentioning that the interfacial resistance between oil and water was lower as compared to water surface tension. This caused oil infusion over the water droplet surface.
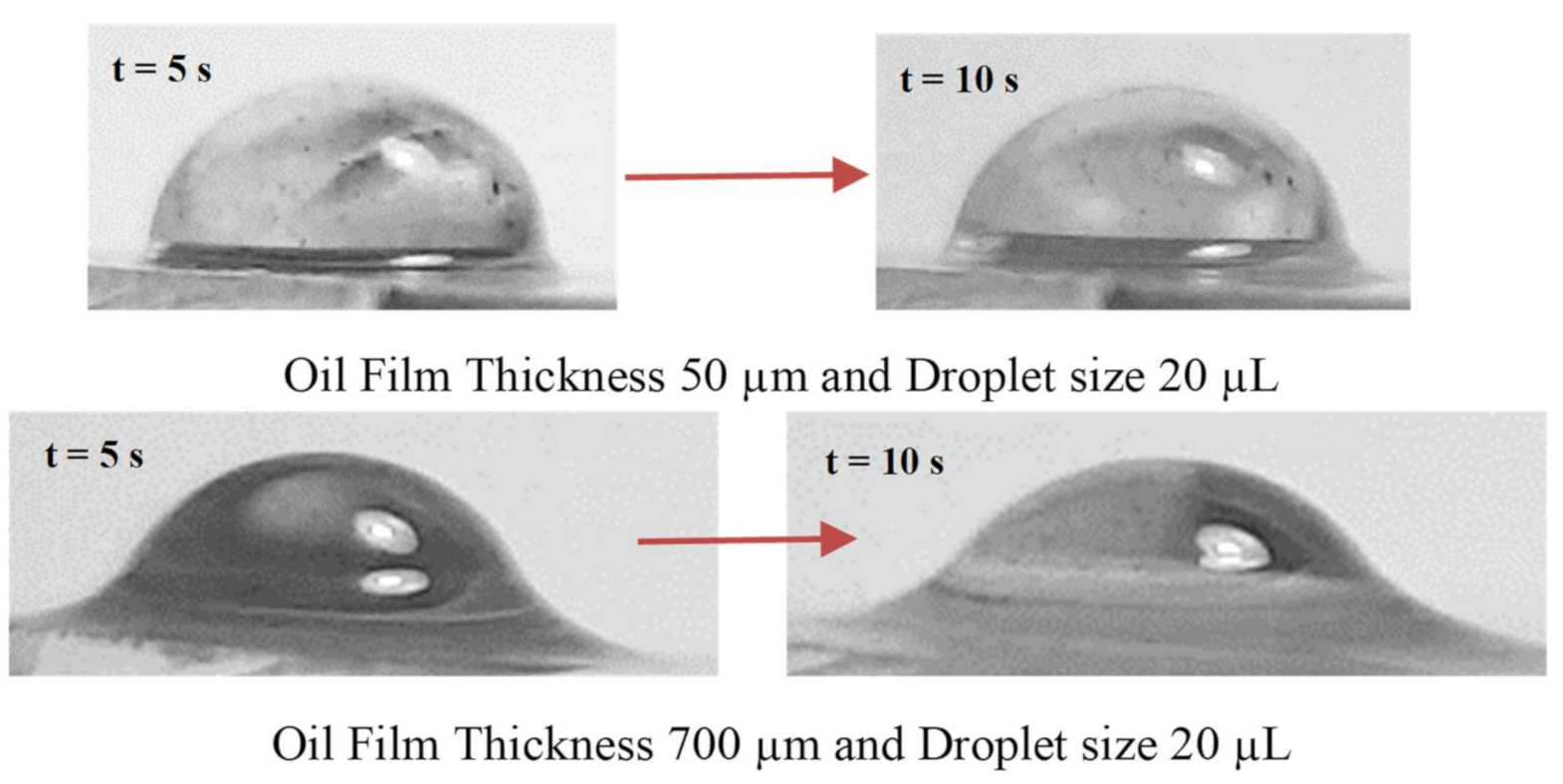
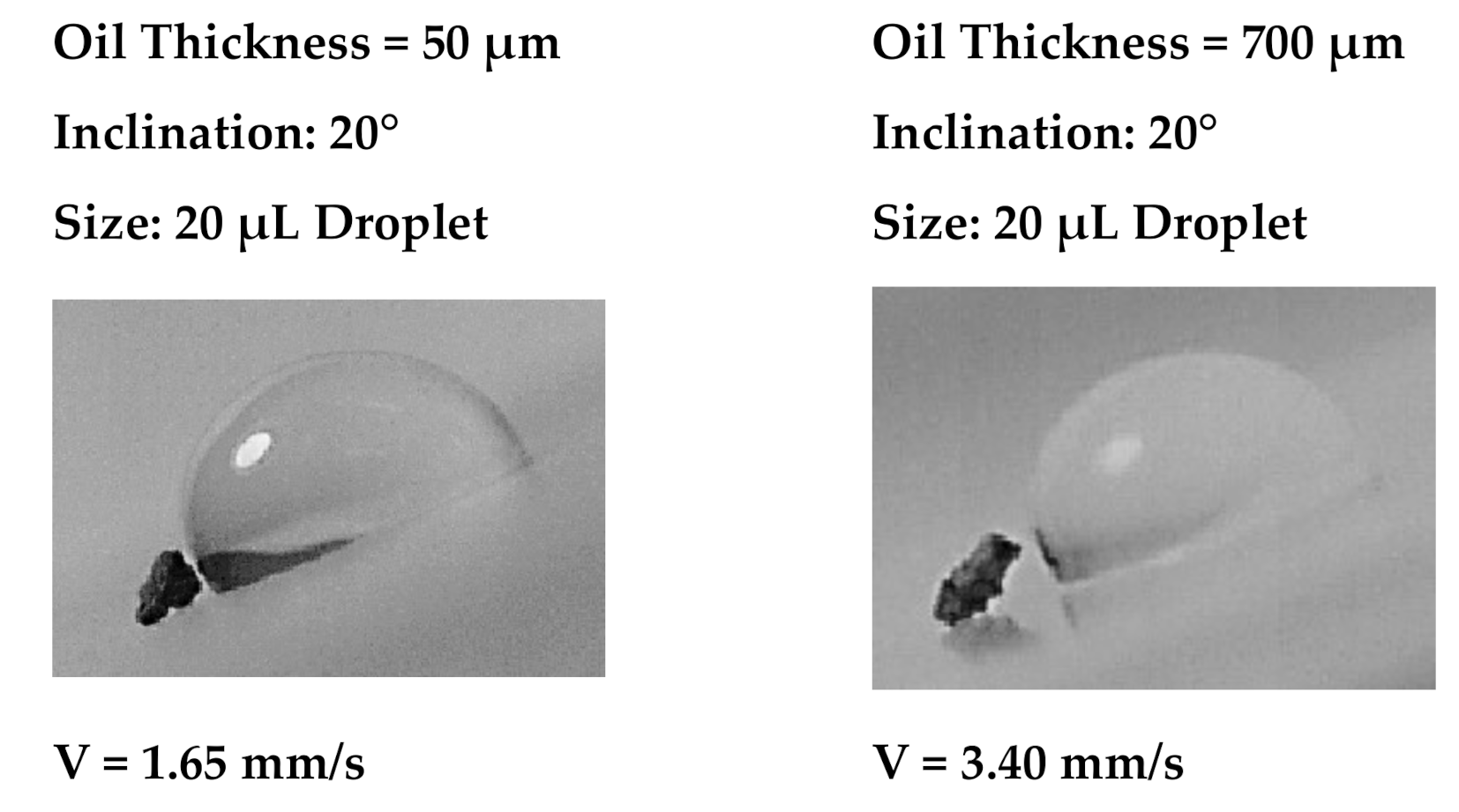
Figure 7 depicts images of the droplet over the thin (50 µm) and thick oil (700 µm) films at which the oil ridge was formed around the water droplet rim because of oil infusion over the water droplet surface, particularly for large oil film thickness (700 µm). The oil ridge height was about ~0.32 mm around the droplet rim (three-phase-contact-line) for 700 µm thick oil film; however, it became ~0.09 mm for 50 µm thick oil film. Hence, thinning the oil film thickness reduced the ridge height around the droplet. The water droplet free surface (exposed to air) could be approximated by a spherical cap with a radius of
, here ho is the height of the droplet cap in the air, r
o being the radius of the cap at the air-oil-water-contact-line, and rd is the droplet radius when droplet cap height was extended to form a circle [
26]. The forces could be encountered due to the droplet pinning under interfacial shear across the boundary of water and oil. The pinning forces are formulated in the early work [
26]; however, they are briskly described herein. The force equilibrium for the droplet, which is partially floating in the oil film, becomes:
, here
is the surface tension force,
being the buoyancy force, and
represents the weight of the water droplet. Moreover,
α being the water contact angle at the rim (air-water-oil-contact-line) and
is the filling angle of the rim about the vertical axis. Rearrangement of the vertical force balance yields;
, here
is water–silicon oil interface tension,
is silicon oil density, and
g is gravity. The experimental data demonstrate that
ho ≅ 0.92 mm for 20 µL droplet, which agrees with the value estimated from the vertical force balance (
ho ≅ 1.02 mm). Moreover, as the oil-impregnated surface is inclined, both the water droplet and the oil film flow in the direction of gravity. The state of the film velocity (
) on the tilted sample with the tilt angle of
δ ≤ π/2 is connected to the capillary number,
, where u is the velocity of the oil film and µS viscosity of oil [
34]. The liquid velocity of the film on the tilted surface is formulated in the early study [
34] and it takes the form:
, where
hf is the normalized film thickness (
, here
are the oil thickness and
hc being the oil in the upstream of the film—maximum thickness),
x represents the space along the inclined surface, and LD is the normalized parameter (
, here
δ is the tilt angle of the surface). The numerical solution of the equation for the film velocity yielded that the film velocity was 0.02 mm/s for the film thickness 700 µm and the tilt angle of 5°. The film velocity obtained by a tracker program using the high-speed recorded data was about 0.017 mm/s, which is closer to the numerical prediction. Moreover, since the crystallized surface, where the oil film was deposited, had a hydrophobic texture with 3.2 µm average roughness, slip condition could occur across the textured surface and the oil film. The slip velocity can be formulated through the slip length [
35] (
, here
being the droplet fluid viscosity,
ϕ represents the solid fraction for crystallized surface, and
ht is the film thickness) [
30]. The solid fraction of the crystalized surface was evaluated via AFM and SEM micrographs and it was estimated at about
ϕ = 0.35. Incorporating the viscosity of silicon oil (0.92 × 10
−2 Pa.s) and water (0.89 × 10
−3 Pa.s), film thickness (700 µm), and a solid fraction, the slip length yielded about
b = 44 µm. Moreover, the slip velocity can be written as;
, here
is the interfacial shear stress across the oil and the crystallized surface [
30], which can be further simplified after considering a Couette flow in clearance between the bottom of the droplet and oil. Hence, the slip velocity in terms of film velocity and the slip length yielded:
. Moreover, the velocities of the partially immersed droplet and the oil film were different, which created a shear resistance across the droplet and the oil. The frictional stress can be;
, here
represents the velocity of the droplet and
lm is the height between the droplet base and the droplet mass center [
36]. It is also known that shear stress in between the textured surface and the oil can be approximated as
. The shear stresses on the droplet and the oil at the interface had similar orders (stress continuity). This yields the relation between the slip and the droplet velocities, i.e.,
. The ratio of
b/
ht is about 0.057 and
, which yields that the droplet velocity becomes much larger than the film velocity (
>
uf). Moreover, energy dissipated by the sliding water droplet on the oil film via shear resistance was related to (i) shear rate created at the ridges due to oil infusion over the droplet surface (ii) the air drag acting on the droplet cap in the air, (iii) lateral component of the surface tension around the droplet rim. The dissipated energy is formulated in the early study [
30] and it takes the form ~Δ
L(
). Here, the term Δ
L is an incremental length scale along the oil film surface,
represents the interfacial shear effect on the immersed droplet base [
36],
is the shear effect due to ridge height around the droplet meniscus [
30],
is the force of drag force created over the immersed droplet by the oil [
37], where
ho is the droplet immersion height in oil, which is experimentally evaluated to be ~62 µm, and
is the air drag acting on the droplet cap, where
Cd is the drag coefficient, and
Ac is the cross-sectional area of the droplet cap in the air. In addition, work was done by the sliding droplet towards overcoming the droplet pinning, which could account for the droplet’s additional energy dissipation. The work required overcoming the pinning was about
[
26]. The potential and kinetic energy change along the incremental length scale were
and is
, respectively. Here,
is the inclination angle of the surface and
md is the mass of the droplet (
,
ro being the droplet radius when the droplet volume is spherical). Hence, the change of droplet potential energy along the incremental length scale overcame the frictional energy dissipation and the work required for pinning, and, in addition, the kinetic energy for creating the droplet motion on the oil surface. The potential energy change of the droplet per unit droplet mass along the length scale of 44 mm of 10° inclined surface with 700 µm thick oil was 0.075 J/kg while the kinetic energy was 3.125 × 10
−3 J/kg. Hence, a large amount of energy was dissipated in the course of droplet sliding, i.e., almost 96% of droplet potential energy was dissipated to overcome shear resistance and pinning of the droplet.
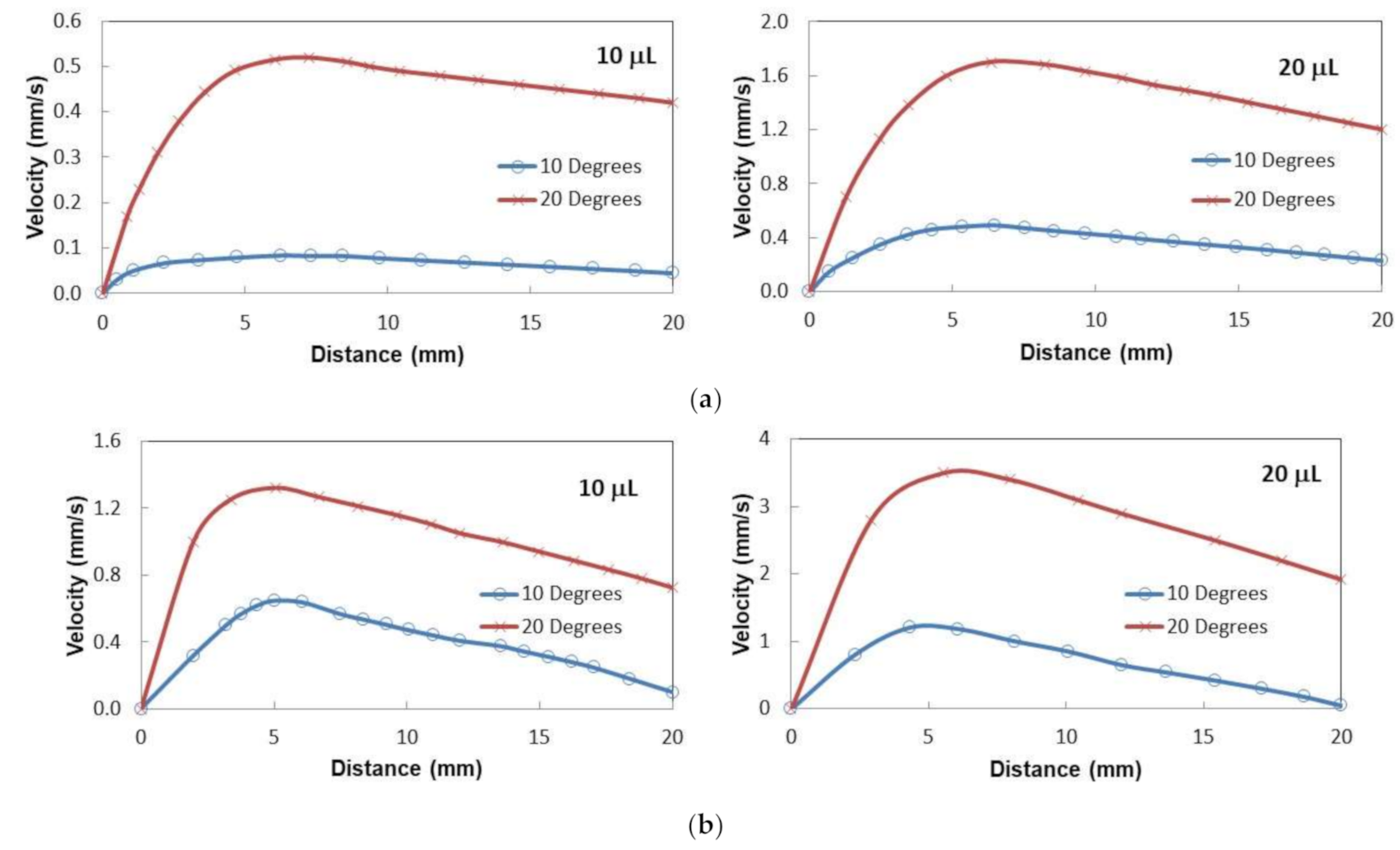
Figure 8a shows droplet velocity on the thin oil film (50 µm thick) with distance for two volumes and two tilt angles. In the early period of sliding, the velocity increased to attain maximum and as the droplet sliding continued along with the inclined film, the velocity decreased gradually. This behavior was observed for both inclination angles and the droplet volumes. This is mainly related to the oil ridge formation in the droplet neighborhood through oil infusion on the water droplet. Oil infusion in the early sliding period was small preventing the oil ridge formation. As the droplet sliding progressed, the oil ridge formed around the droplet because of the oil infusion during the droplet sliding. Hence, the interfacial fluid resistance between the oil and the droplet liquid increased along the droplet circumference, which caused slowing down the droplet motion along the inclined surface. Moreover, as the droplet size increased, interfacial resistance along the droplet ridge increased because of the increased droplet diameter with increasing droplet volume. However, increased droplet inertial force, because of the droplet mass increase, enhanced the droplet velocity despite the fact that interfacial resistance at oil-droplet along with the ridge increased. In the case of the thick silicon oil film (~700 µm) impregnated sample surface, the sliding velocity of the droplet increased to reach its maximum and, later, it decreased gradually along the inclined surface (
Figure 8b). This behavior was similar to the thin oil film case (
Figure 8a). The sliding velocity attains larger values for the thick oil film case than that of the thin film case. It is worth noting that the droplet immersion became more as the film thickness becomes larger (~700 µm). Hence, it was expected that the fluid resistance around the immersed area of the droplet would increase. However, the attainment of the large velocity on the thick oil film suggests that the droplet on the thin film (~50 µm) penetrated into the oil film and making physical contact on the crystallized surface. This increased the shear resistance at the droplet bottom. In addition, the droplet located on the thick oil film floated in the oil film and created slip condition at the droplet bottom, which in turn reduced the shear resistance across the film and the droplet. Therefore, the velocity of the droplet attained higher values for the large thickness oil-impregnated sample surface.
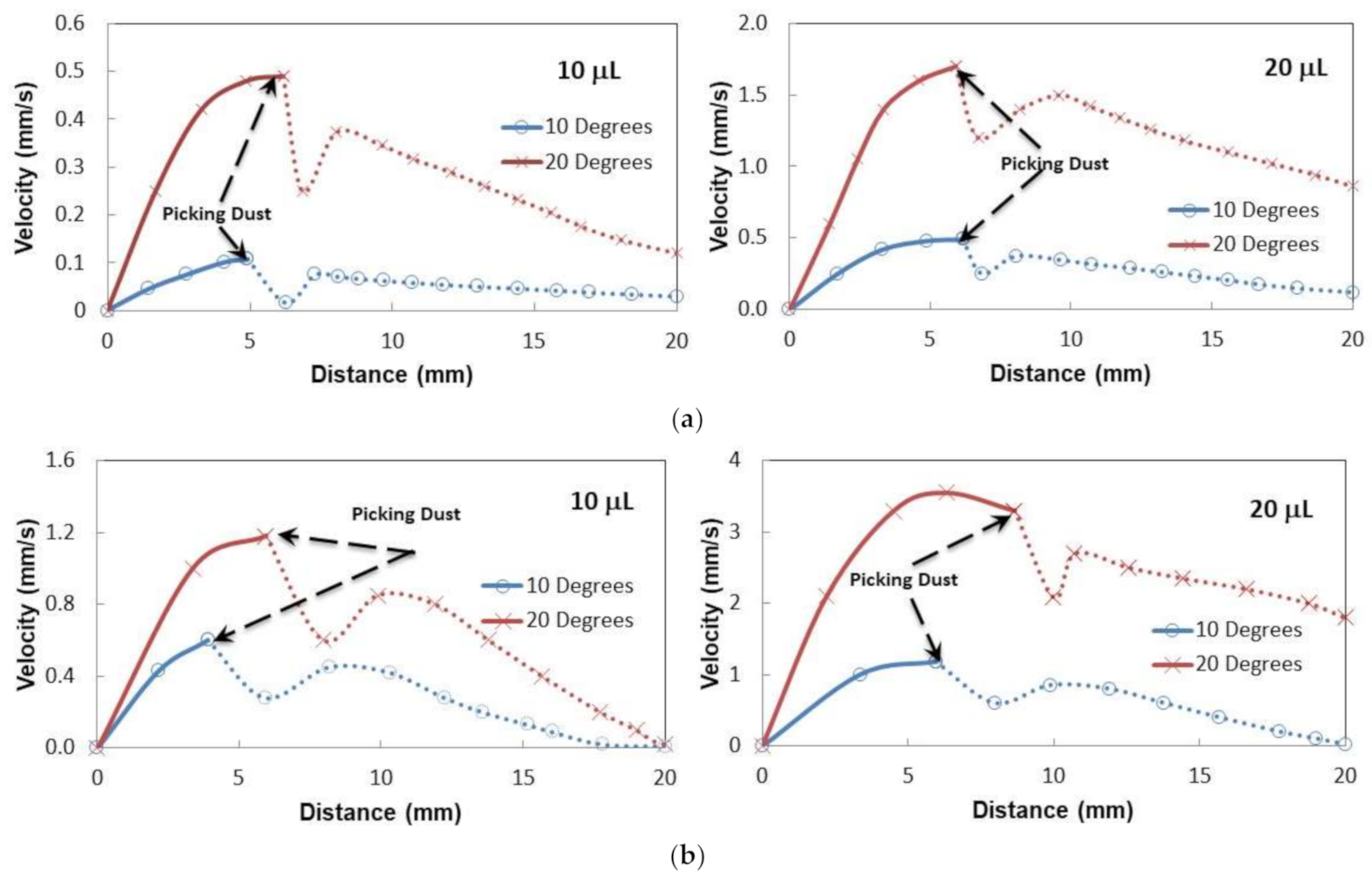
Figure 9a,b shows droplet sliding velocity on the inclined thin and thick oil films (~50 µm and 700 µm) with the presence of the dust particle over the oil surface for two droplet volumes and two tilting angles of the sample.
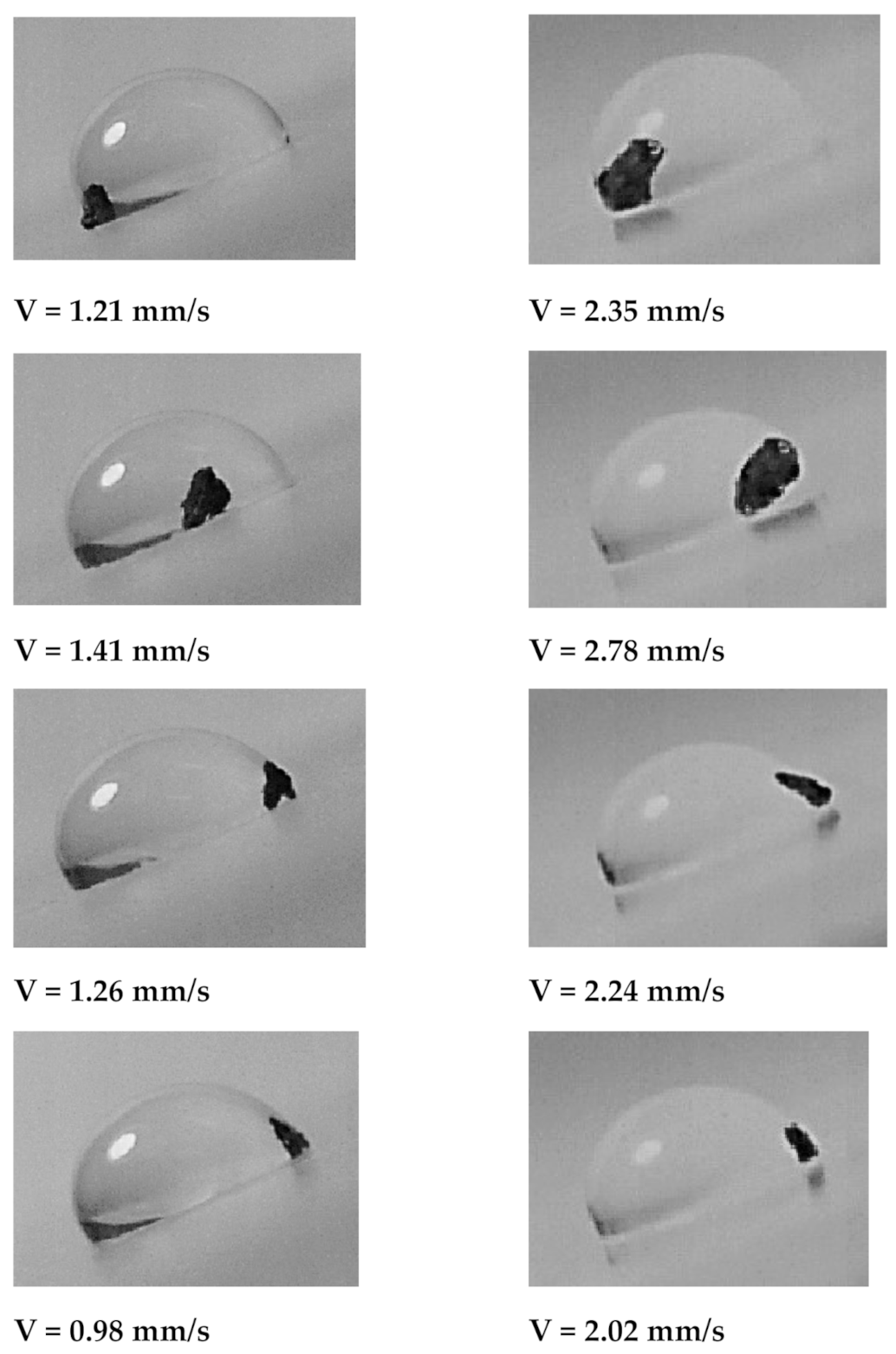
Figure 10 depicts the optical images of different stages of the dust particle mitigated by the sliding water droplet on the inclined oil film. It is worth mentioning that the dust particle size selected was large enough to be recorded by a high speed facility; hence, the selected dust particle was about 75 µm. As the sliding droplet approached the vicinity of the dust particle, the oil ridge around the droplet first contacted the dust particle surface. However, the dust particle height was larger than the oil ridge height, the infusion of oil from the oil ridge towards the dust particle surface was expected to be not considerable. In addition, the contact duration between the oil ridge and the dust particle was short, which reduced the amount of oil-infused over to the dust particle surface. Moreover, once the droplet fluid wetted the dust particle on the oil surface, droplet sliding velocity reduced, and the part of the dust particle surface, which was not infused by the oil, was infused by the fluid of the droplet. In the course of fluid infusion over the particle surface, the sliding velocity of the droplet reduced further. Once the droplet liquid cloaked over the dust, the particle was picked up by the droplet fluid and the particle was reoriented inside the sliding droplet, which can be observed from
Figure 10. During the dust particle reorientation, the sliding droplet mass increased slightly and the inertia force increases while causing droplet sliding velocity to increase along the inclined surface. However, as the dust particle was oriented at the droplet trailing edge, which was opposing to the droplet front edge in the sliding direction, the droplet velocity reduced considerably. This situation can be observed in
Figure 10. Moreover, it became true for all volumes of the droplet, the tilting angle of the sample, and oil film thickness. The possible explanation of this behavior is that the dust particle in the droplet trailing edge immersed into the oil and it was anchored by the oil film. This created the pinning influence on the sliding droplet, which gradually ceased sliding on the oil film, i.e., sliding motion terminated and the sliding velocity became zero on the oil film. The inclination angle had a significant influence on the droplet sliding velocity. In this case, reducing the inclination angle from 20° to 10° lowered the sliding speed from 0.98 mm/s to 2.02 mm/s. For a small inclination angle (10°), the dust particle position in the sliding droplet had a considerable effect on the sliding velocity. In this case, droplet sliding velocity attained high values as the dust particle was located in the central region of the droplet. As the dust particles moved towards the trailing edge of the droplet, the sliding velocity reduced considerably. Hence, the dust particles partially became immersed in the oil film at the droplet edge while creating the anchoring effect on the droplet sliding velocity.