Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome
Abstract
1. Introduction
2. Results
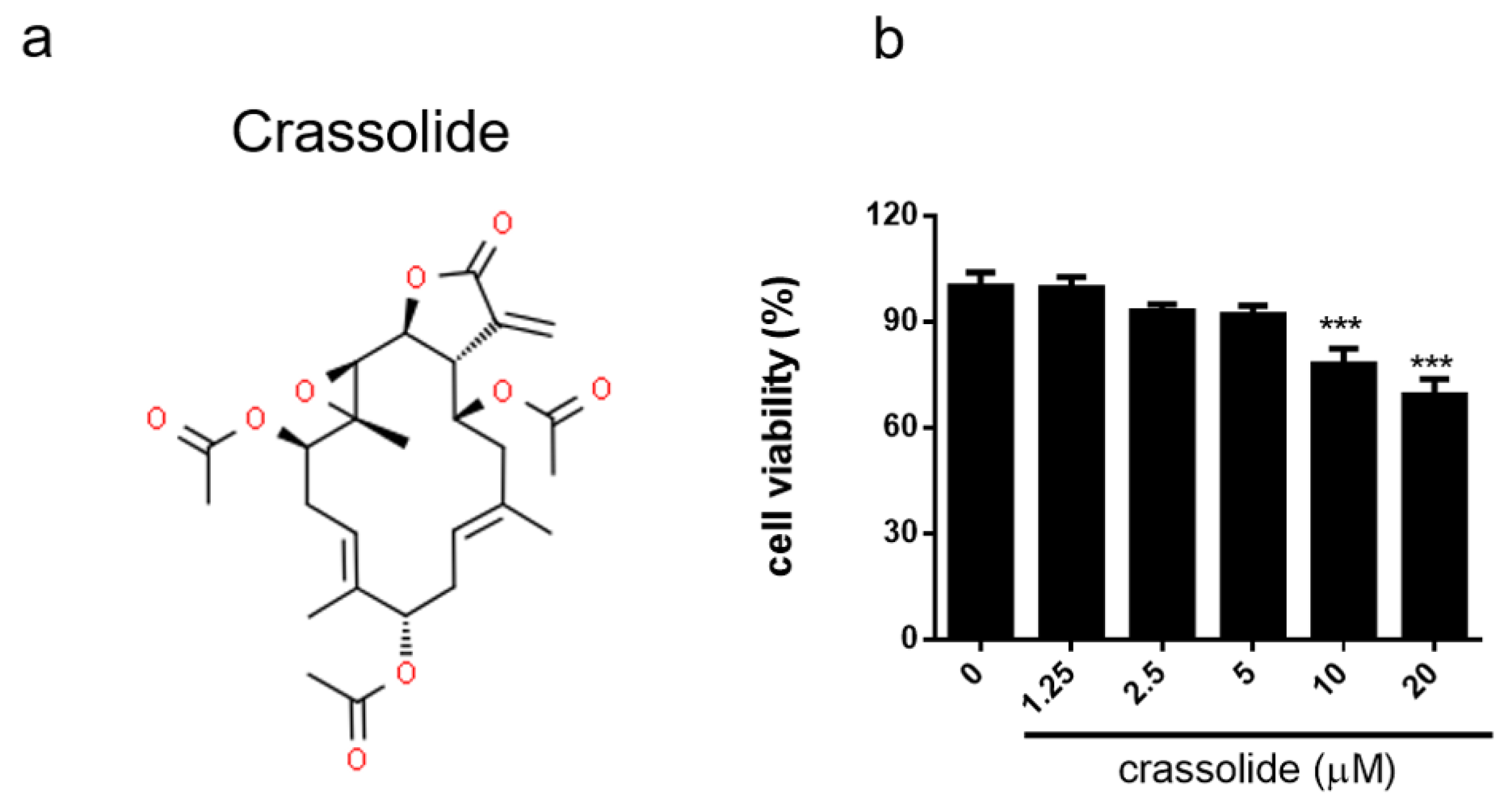
2.1. No Discernible Toxicity of Crassolide
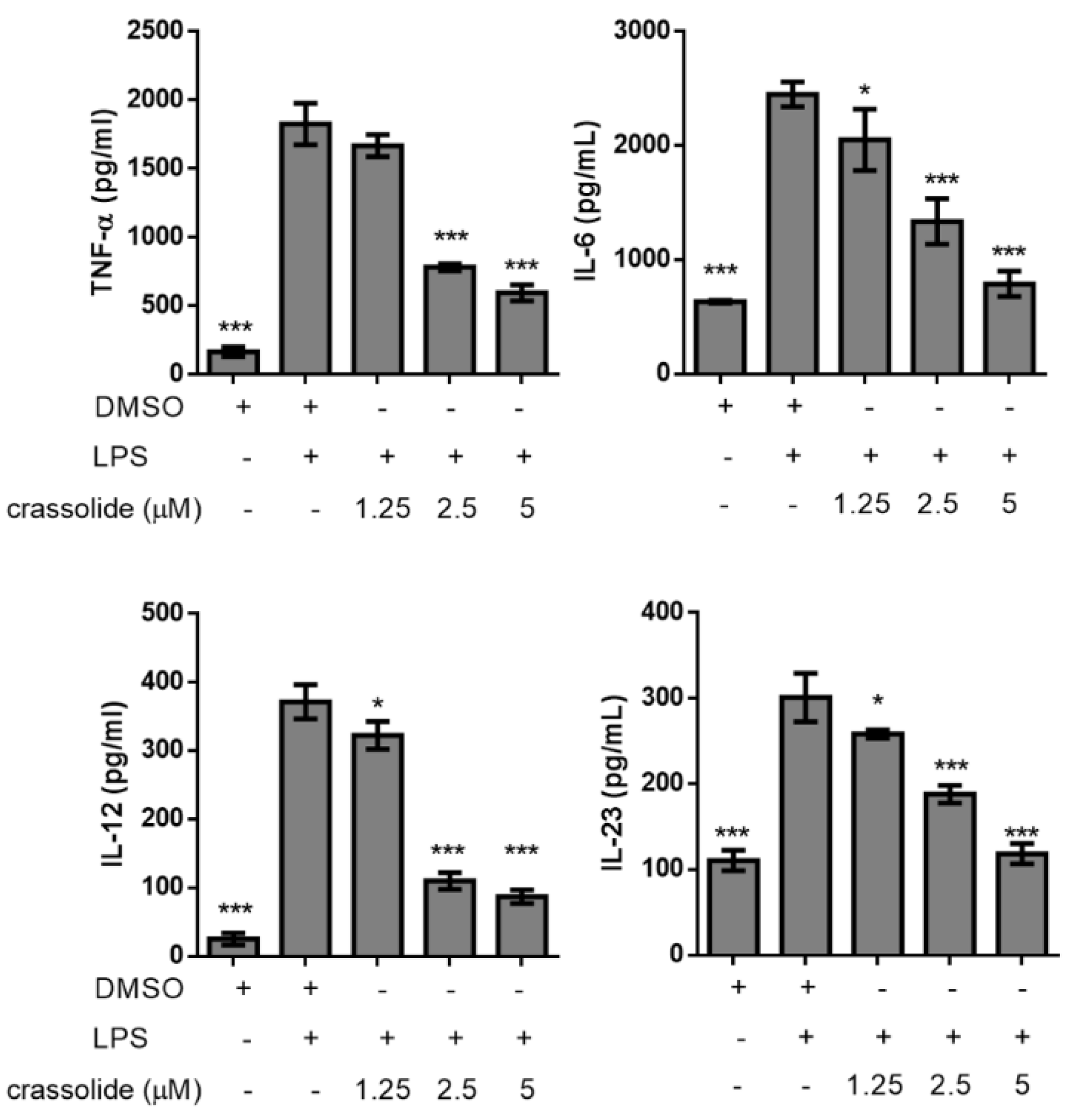
2.2. Crassolide Lowered LPS-Induced Expressions of Co-Stimulatory Molecules and Secretions of Pro-Inflammatory Cytokines in BMDCs
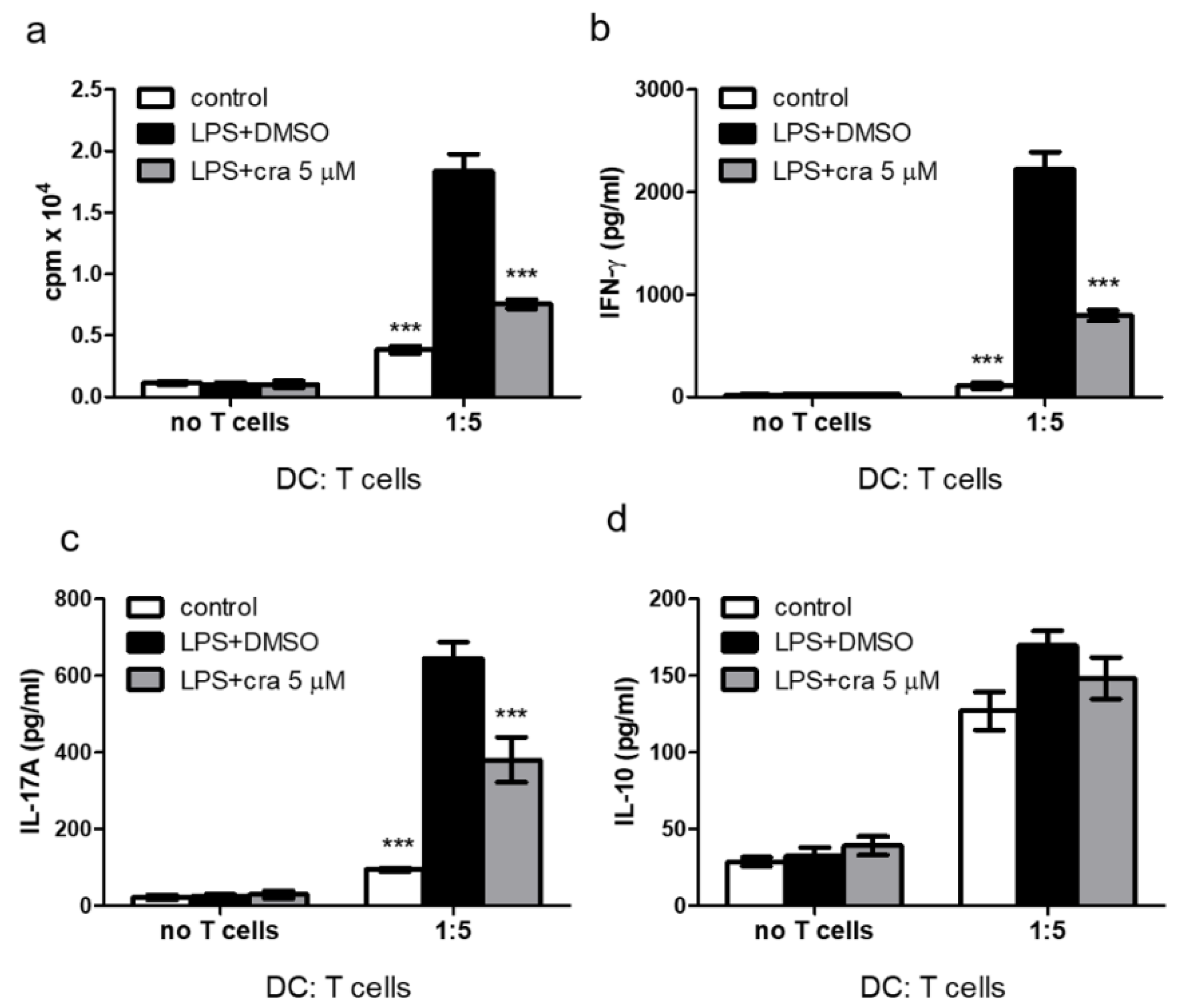
2.3. Crassolide Suppressed LPS-Induced Antigen-Specific T Cell Proliferation and Cytokines Production in DCs
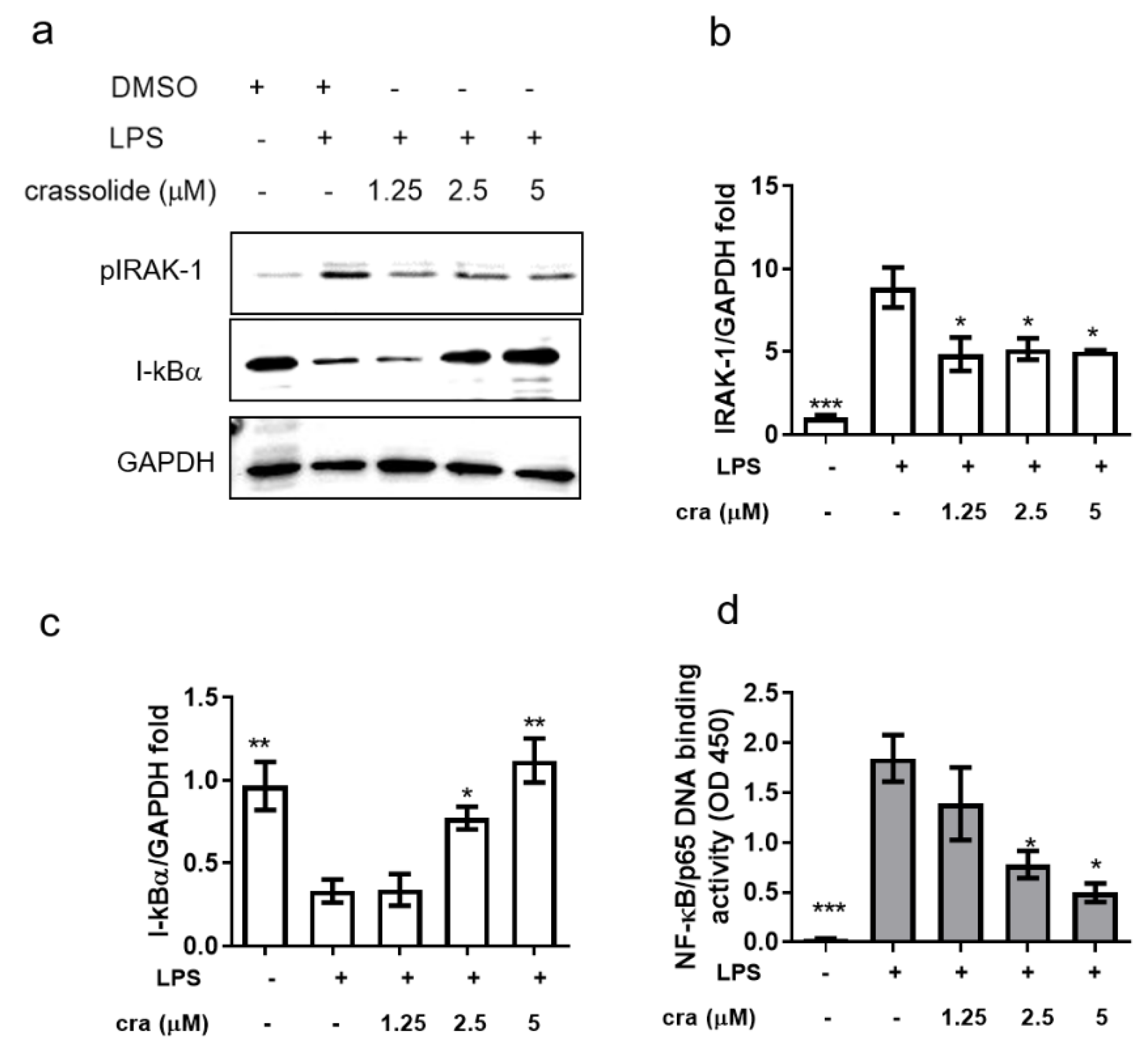
2.4. Crassolide Inhibited IRAK-1 Phosphorylation and NF-κB Activation in BMDCs
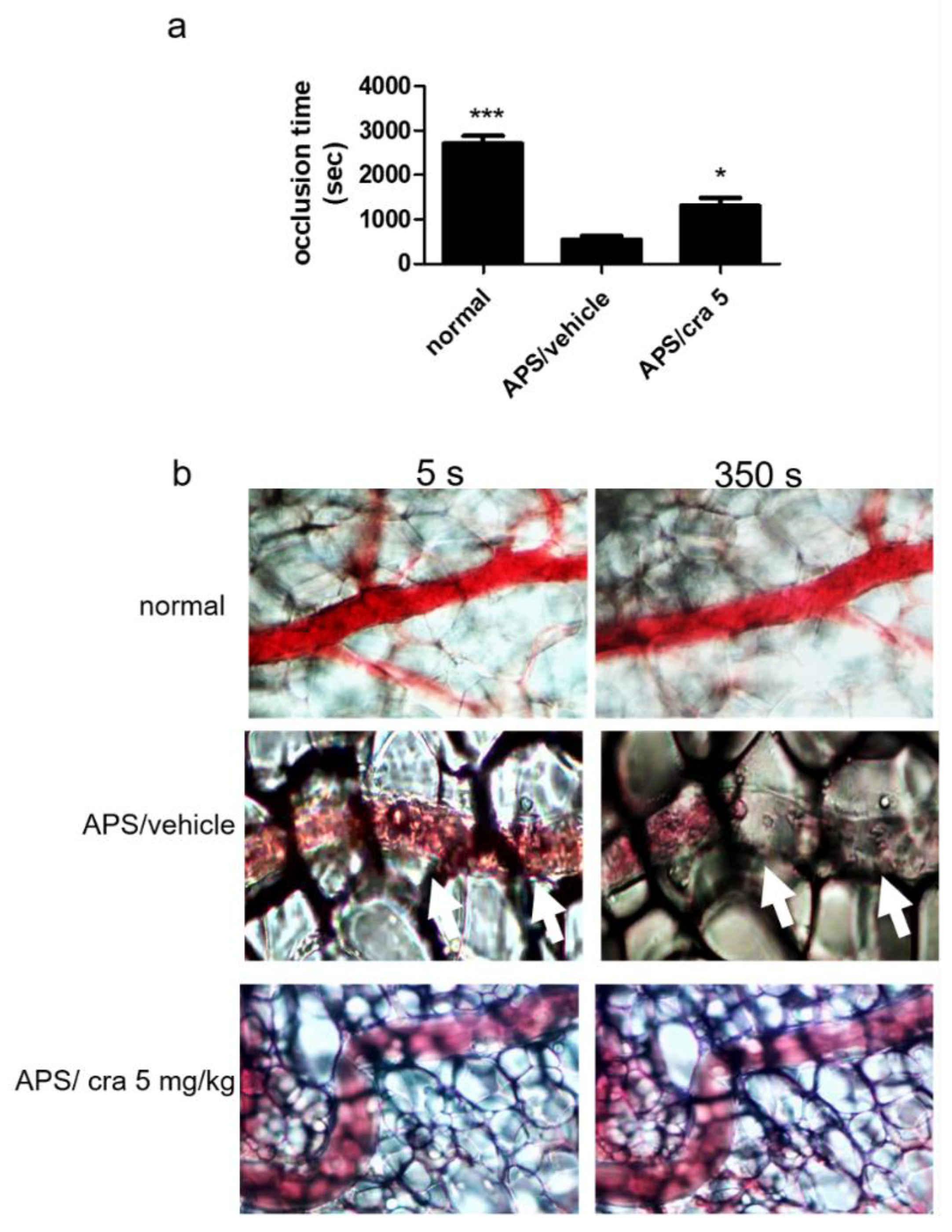
2.5. Therapeutic Effects of Crassolide on APS Manifestations
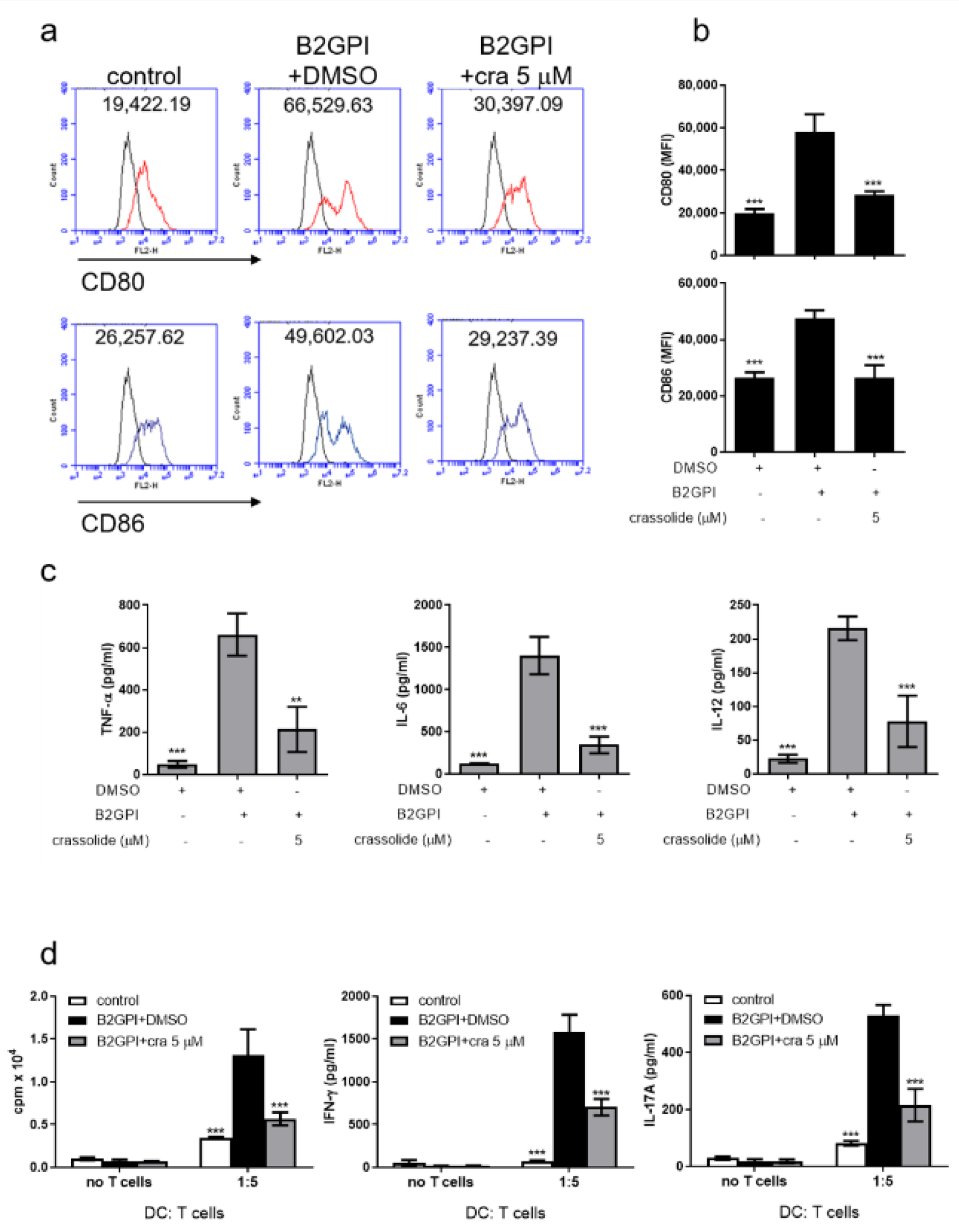
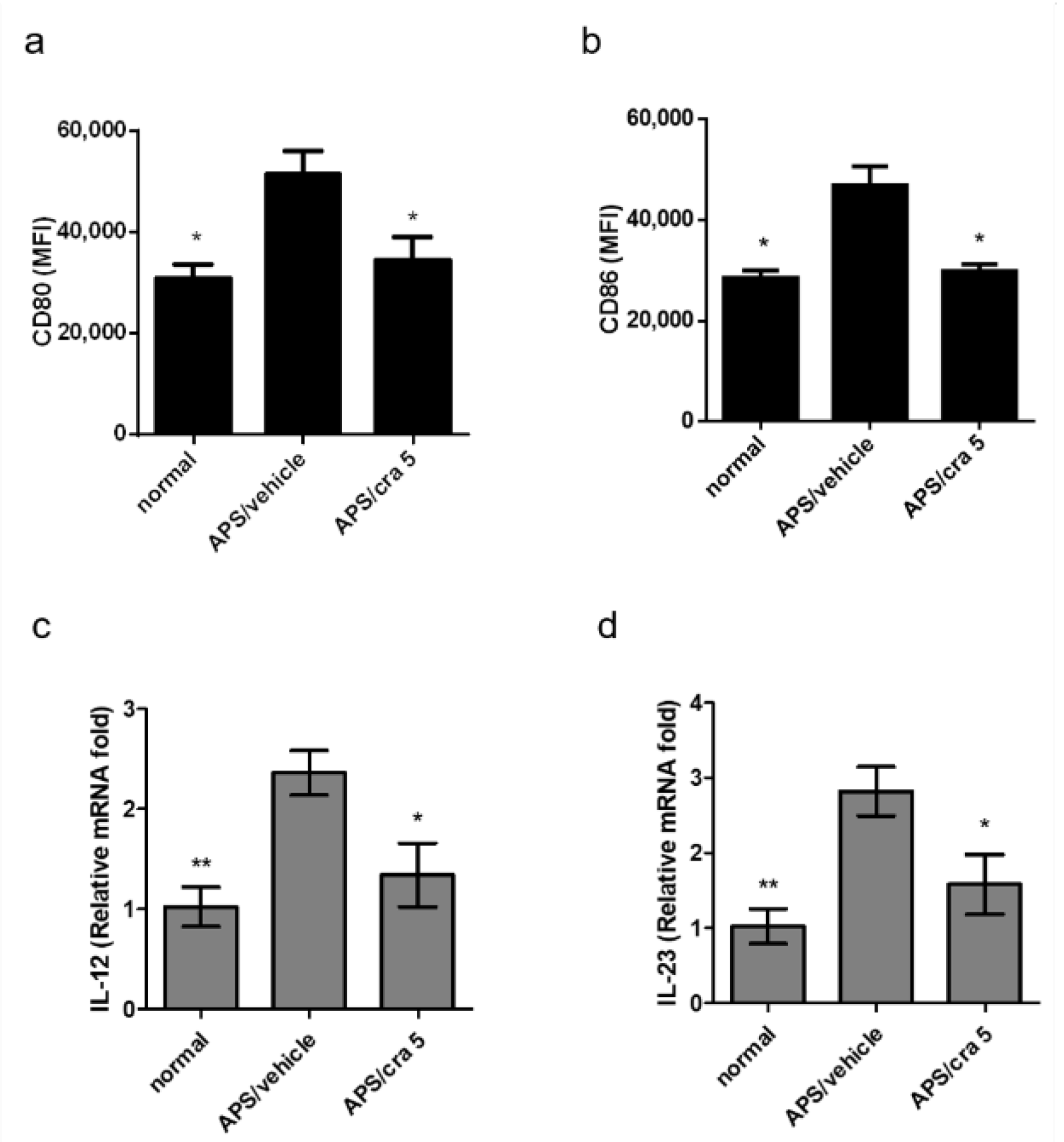
2.6. Crassolide Suppressed Splenic DC Maturation and Cytokines Production in APS Mice
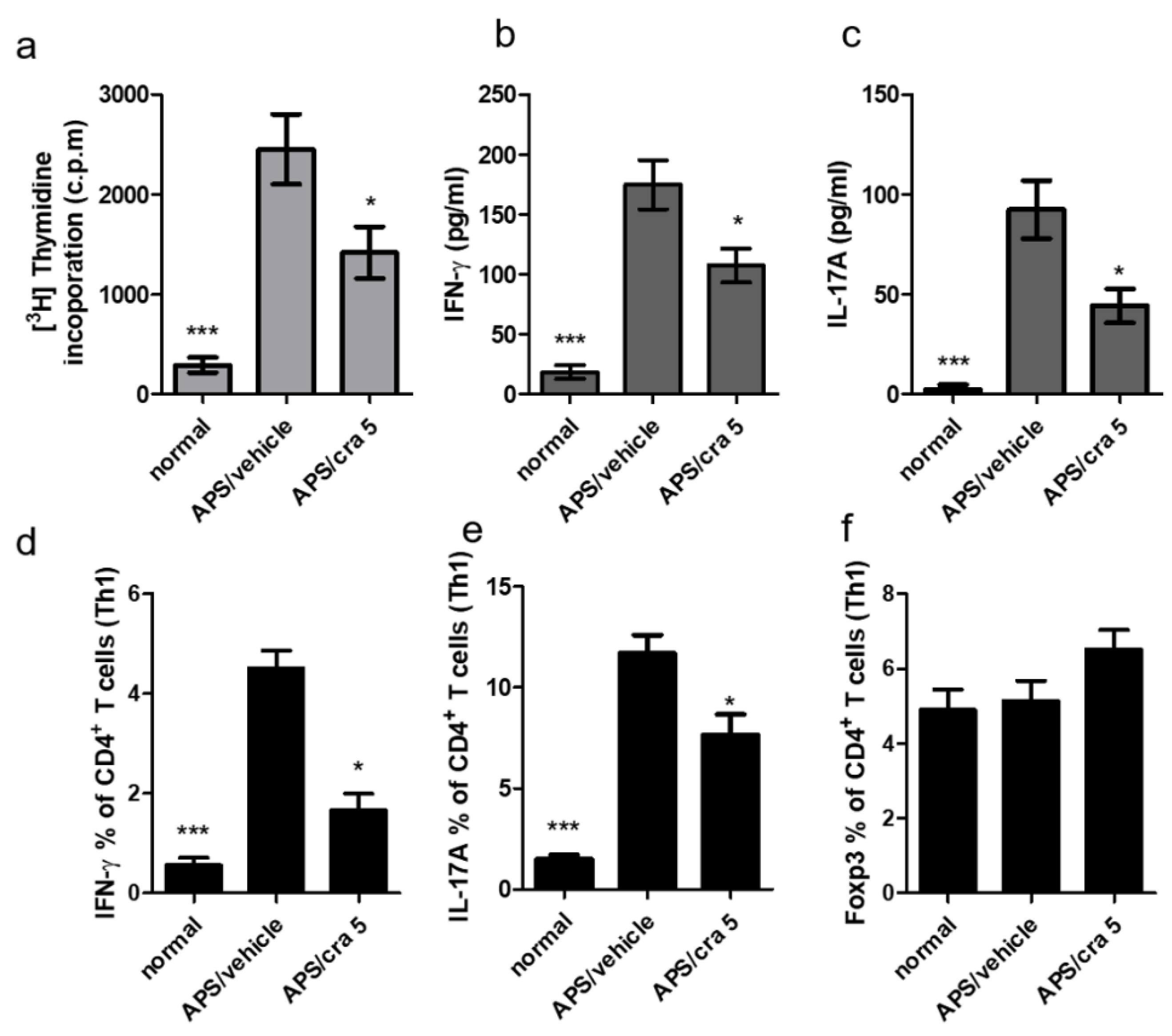
2.7. Crassolide Suppressed Β2GPI-Specific Splenic Th1 and Th17 Responses in APS Mice
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Preparation of Bone Marrow-Derived Dendritic Cells (BMDCs)
4.3. Chemicals
4.4. Cell Viability Assay
4.5. Flow Cytometric Analyses of Surface Makers on Both BMDCs In Vitro and Splenic DCs Ex Vivo
4.6. Analyses of Cytokine Expressions
4.7. Analyses of OT-II T Cell Activation
4.8. Western Blotting
4.9. Preparation of Nuclear Extracts and Measurement of NF-κB Activity
4.10. Animal Models of Obstetric APS
4.11. Animal Models of Vascular APS
4.12. Evaluation of Side Effects Relevant to Carssolide Treatment in Mice
4.13. Treatments
4.14. Determination of Platelet Count and aPTT
4.15. Analyses of Blood Titer of Anti-β2GPI IgG Antibody
4.16. Proliferative Response and Cytokine Production of Murine Spleen Cells after β2GPI Stimulation
4.17. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.18. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannakopoulos, B.; Krilis, S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013, 368, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M. Beta2-glycoprotein I: Antiphospholipid syndrome and T-cell reactivity. Thromb. Res. 2004, 114, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, C.B.; Raimondo, M.G.; Meroni, P.L. Management of Thrombotic Antiphospholipid Syndrome. Semin. Thromb. Hemost. 2018, 44, 419–426. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, G.R.; Rodrigues, G.; de Jesus, N.R.; Levy, R.A. Pregnancy morbidity in antiphospholipid syndrome: What is the impact of treatment? Curr. Rheumatol. Rep. 2014, 16, 403. [Google Scholar] [CrossRef]
- Uthman, I.; Noureldine, M.H.A.; Ruiz-Irastorza, G.; Khamashta, M. Management of antiphospholipid syndrome. Ann. Rheum. Dis. 2019, 78, 155–161. [Google Scholar] [CrossRef]
- Giannakopoulos, B.; Krilis, S.A. How I treat the antiphospholipid syndrome. Blood 2009, 114, 2020–2030. [Google Scholar] [CrossRef]
- Martinelli, I.; Abbattista, M.; Bucciarelli, P.; Tripodi, A.; Artoni, A.; Gianniello, F.; Novembrino, C.; Peyvandi, F. Recurrent thrombosis in patients with antiphospholipid antibodies treated with vitamin K antagonists or rivaroxaban. Haematologica 2018, 103, e315–e317. [Google Scholar] [CrossRef]
- Schreiber, K.; Radin, M.; Sciascia, S. Current insights in obstetric antiphospholipid syndrome. Curr. Opin. Obstet. Gynecol. 2017, 29, 397–403. [Google Scholar] [CrossRef]
- Crowther, M.A.; Ginsberg, J.S.; Julian, J.; Denburg, J.; Hirsh, J.; Douketis, J.; Laskin, C.; Fortin, P.; Anderson, D.; Kearon, C.; et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N. Engl. J. Med. 2003, 349, 1133–1138. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Hunt, B.J.; Khamashta, M.A. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007, 57, 1487–1495. [Google Scholar] [CrossRef]
- Skov, M.J.; Beck, J.C.; de Kater, A.W.; Shopp, G.M. Nonclinical safety of ziconotide: An intrathecal analgesic of a new pharmaceutical class. Int. J. Toxicol. 2007, 26, 411–421. [Google Scholar] [CrossRef]
- Lai, K.H.; You, W.J.; Lin, C.C.; El-Shazly, M.; Liao, Z.J.; Su, J.H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lu, M.C.; Su, J.H.; Chu, C.L.; Shiuan, D.; Weng, C.F.; Sung, P.J.; Huang, K.J. Immunomodulatory effect of marine cembrane-type diterpenoids on dendritic cells. Mar. Drugs 2013, 11, 1336–1350. [Google Scholar] [CrossRef]
- Duh, C.Y.; Wang, S.K.; Chung, S.G.; Chou, G.C.; Dai, C.F. Cytotoxic cembrenolides and steroids from the formosan soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Pesando, D.; Graillet, C.; Braekman, J.C.; Dubreuil, A.; Girard, J.P.; Puiseux-Dao, S. The use of sea urchin eggs as a model to investigate the effects of crassolide, a diterpene isolated from a soft coral. Toxicol. In Vitro 1991, 5, 395–401. [Google Scholar] [CrossRef]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, H.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef]
- Radhika, P.; Rao, P.R.; Archana, J.; Nalamolu, K.R. Anti-inflammatory activity of a new sphingosine derivative and cembrenoid diterpene (lobohedleolide) isolated from marine soft corals of Sinularia crassa TIXIER-DURIVAULT and Lobophytum species of the Andaman and Nicobar Islands. Biol. Pharm. Bull. 2005, 28, 1311–1313. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Mattei, V.; Siracusano, A.; Ortona, E.; Margutti, P.; Salvati, B.; Sorice, M.; Rigano, R. Oxidized beta2-glycoprotein I induces human dendritic cell maturation and promotes a T helper type 1 response. Blood 2005, 106, 3880–3887. [Google Scholar] [CrossRef]
- Hurst, J.; Prinz, N.; Lorenz, M.; Bauer, S.; Chapman, J.; Lackner, K.J.; von Landenberg, P. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology 2009, 214, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Pierangeli, S.S.; Gertel, S.; Blank, M. Tolerogenic dendritic cells specific for beta2-glycoprotein-I Domain-I, attenuate experimental antiphospholipid syndrome. J. Autoimmun. 2014, 54, 72–80. [Google Scholar] [CrossRef]
- Barnden, M.J.; Allison, J.; Heath, W.R.; Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998, 76, 34–40. [Google Scholar] [CrossRef]
- Robertson, J.M.; Jensen, P.E.; Evavold, B.D. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J. Immunol. 2000, 164, 4706–4712. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, S.; Yuan, W.; Xi, Y.; Li, X.; Dong, J.; Huang, K.; Gustafson, K.R.; Yan, P. Cembranoids from a Chinese Collection of the Soft Coral Lobophytum crassum. Mar. Drugs 2016, 14, 111. [Google Scholar] [CrossRef]
- Li, W.; Zou, Y.H.; Ge, M.X.; Lou, L.L.; Xu, Y.S.; Ahmed, A.; Chen, Y.Y.; Zhang, J.S.; Tang, G.H.; Yin, S. Biscembranoids and Cembranoids from the Soft Coral Sarcophyton elegans. Mar. Drugs 2017, 15, 85. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Chen, Y.W.; Huang, C.Y.; Tseng, Y.J.; Lin, C.C.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Isolation and Structure Elucidation of Cembranoids from a Dongsha Atoll Soft Coral Sarcophyton stellatum. Mar. Drugs 2018, 16, 210. [Google Scholar] [CrossRef]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. New Casbane and Cembrane Diterpenoids from an Okinawan Soft Coral, Lobophytum sp. Molecules 2016, 21, 679. [Google Scholar] [CrossRef]
- Kao, C.Y.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Wang, W.H.; Chen, J.J.; Sheu, J.H.; Kuo, Y.H.; Weng, C.F.; Fang, L.S.; et al. Lobocrassins A-E: New cembrane-type diterpenoids from the soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar] [CrossRef]
- Lee, C.H.; Kao, C.Y.; Kao, S.Y.; Chang, C.H.; Su, J.H.; Hwang, T.L.; Kuo, Y.H.; Wen, Z.H.; Sung, P.J. Terpenoids from the octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea). Mar. Drugs 2012, 10, 427–438. [Google Scholar] [CrossRef]
- Peng, C.C.; Huang, C.Y.; Ahmed, A.F.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef]
- Wu, C.H.; Chao, C.H.; Huang, T.Z.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Cembranoid-Related Metabolites and Biological Activities from the Soft Coral Sinularia flexibilis. Mar. Drugs 2018, 16, 278. [Google Scholar] [CrossRef]
- Chung, T.W.; Li, Y.R.; Huang, W.Y.; Su, J.H.; Chan, H.L.; Lin, S.H.; Liu, C.S.; Lin, S.C.; Lin, C.C.; Lin, C.H. Sinulariolide suppresses LPS-induced phenotypic and functional maturation of dendritic cells. Mol. Med. Rep. 2017, 16, 6992–7000. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Bondanza, A.; Rovere-Querini, P.; Zimmermann, V.S.; Balestrieri, G.; Tincani, A.; Sabbadini, M.G.; Manfredi, A.A. Requirement for dendritic cells in the establishment of anti-phospholipid antibodies. Autoimmunity 2007, 40, 302–306. [Google Scholar] [CrossRef]
- Kuwana, M.; Matsuura, E.; Kobayashi, K.; Okazaki, Y.; Kaburaki, J.; Ikeda, Y.; Kawakami, Y. Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood 2005, 105, 1552–1557. [Google Scholar] [CrossRef]
- Torres-Aguilar, H.; Blank, M.; Kivity, S.; Misgav, M.; Luboshitz, J.; Pierangeli, S.S.; Shoenfeld, Y. Tolerogenic dendritic cells inhibit antiphospholipid syndrome derived effector/memory CD4(+) T cell response to beta2GPI. Ann. Rheum. Dis. 2012, 71, 120–128. [Google Scholar] [CrossRef]
- Karakantza, M.; Theodorou, G.L.; Meimaris, N.; Mouzaki, A.; John, E.; Andonopoulos, A.P.; Maniatis, A. Type 1 and type 2 cytokine-producing CD4+ and CD8+ T cells in primary antiphospholipid syndrome. Ann. Hematol. 2004, 83, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Popovic-Kuzmanovic, D.; Novakovic, I.; Stojanovich, L.; Aksentijevich, I.; Zogovic, N.; Tovilovic, G.; Trajkovic, V. Increased activity of interleukin-23/interleukin-17 cytokine axis in primary antiphospholipid syndrome. Immunobiology 2013, 218, 186–191. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, F.; Liu, X.; Xiong, J. Th1/Th2/Th17/Treg expression in cultured PBMCs with antiphospholipid antibodies. Mol. Med. Rep. 2012, 6, 1035–1039. [Google Scholar] [CrossRef]
- Chao, Y.H.; Chen, D.Y.; Lan, J.L.; Tang, K.T.; Lin, C.C. Tolerogenic beta2-glycoprotein I DNA vaccine and FK506 as an adjuvant attenuates experimental obstetric antiphospholipid syndrome. PLoS ONE 2018, 13, e0198821. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chen, F.Y.; Hsu, J.F.; Fu, R.H.; Chang, C.M.; Chang, C.T.; Liu, C.H.; Wu, J.R.; Lee, A.S.; Chan, H.C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef] [PubMed]

| Normal Mice | Vehicle-Treated APS Mice | Crassolide 5 mg/kg-Treated APS Mice | |
|---|---|---|---|
| aPTT (s) | 19.3 ± 7.2 *** | 91.3 ± 21.4 | 43.3 ± 23.6 * |
| Platelet count (1 × 103 c3lls) | 673 ± 199 *** | 312 ± 183 | 495 ± 136 * |
| Anti-β2GPI (O.D. 450 nm) | 0.11 ± 0.21 *** | 1.96 ± 0.71 | 0.65 ± 0.42 ** |
| Fetal loss (%) | 14 ± 6 * | 46 ± 8 | 23 ± 4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-C.; Chang, Y.-K.; Lin, S.-C.; Su, J.-H.; Chao, Y.-H.; Tang, K.-T. Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome. Molecules 2021, 26, 2492. https://doi.org/10.3390/molecules26092492
Lin C-C, Chang Y-K, Lin S-C, Su J-H, Chao Y-H, Tang K-T. Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome. Molecules. 2021; 26(9):2492. https://doi.org/10.3390/molecules26092492
Chicago/Turabian StyleLin, Chi-Chien, Yu-Kang Chang, Shih-Chao Lin, Jui-Hsin Su, Ya-Hsuan Chao, and Kuo-Tung Tang. 2021. "Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome" Molecules 26, no. 9: 2492. https://doi.org/10.3390/molecules26092492
APA StyleLin, C.-C., Chang, Y.-K., Lin, S.-C., Su, J.-H., Chao, Y.-H., & Tang, K.-T. (2021). Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome. Molecules, 26(9), 2492. https://doi.org/10.3390/molecules26092492







