Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads
Abstract
1. Introduction
2. Results and Discussion
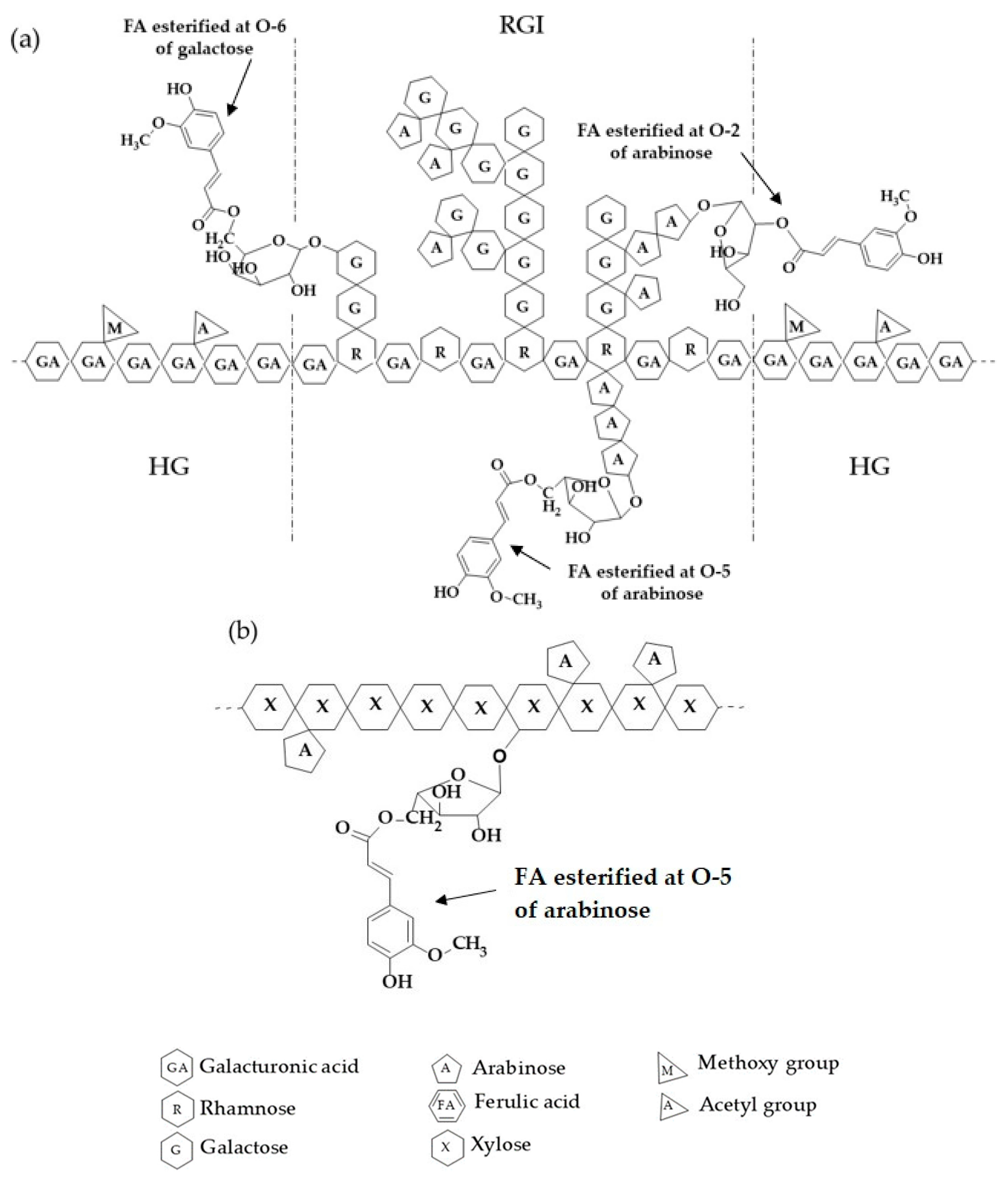
2.1. SBWP Characterization
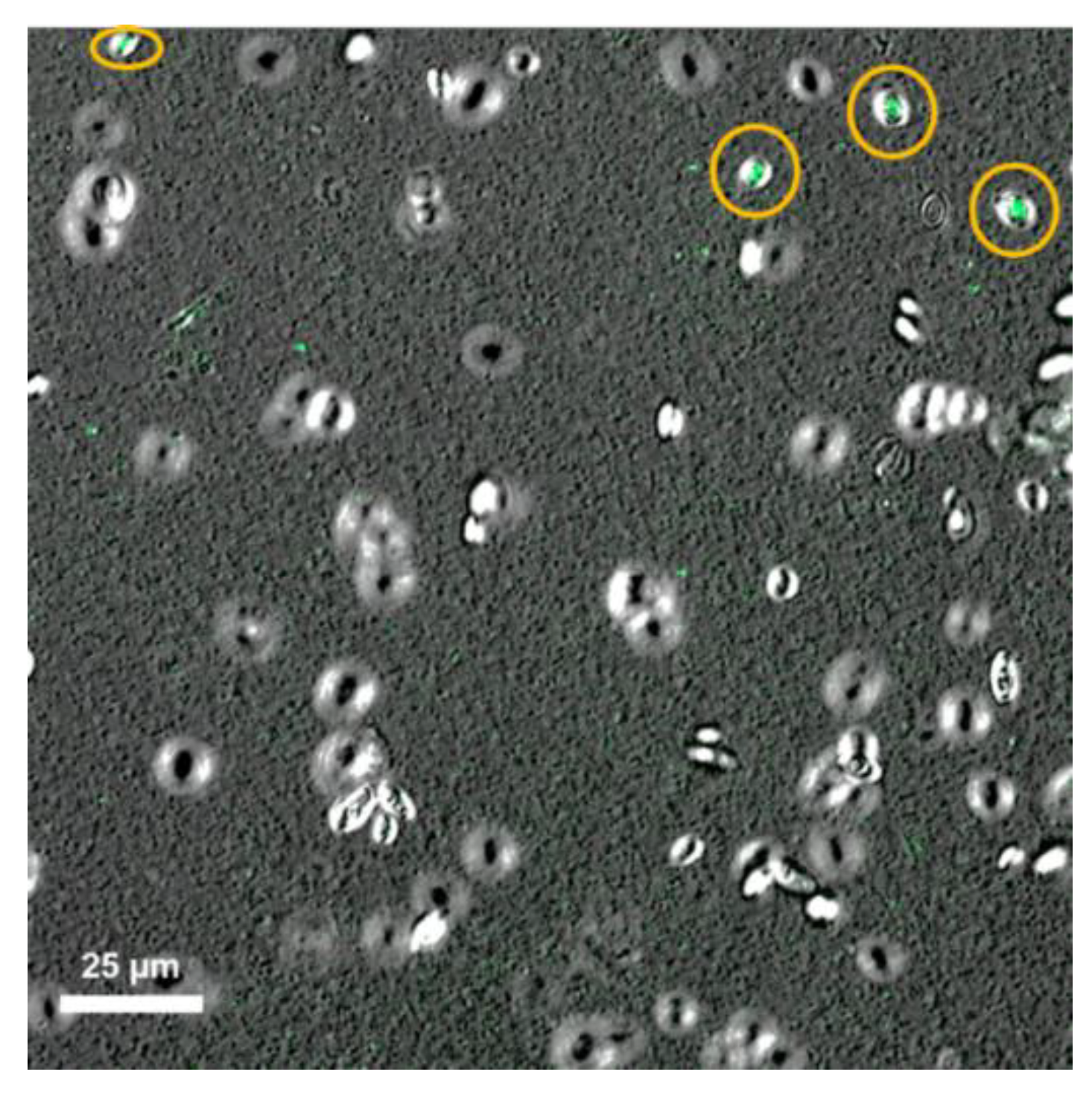
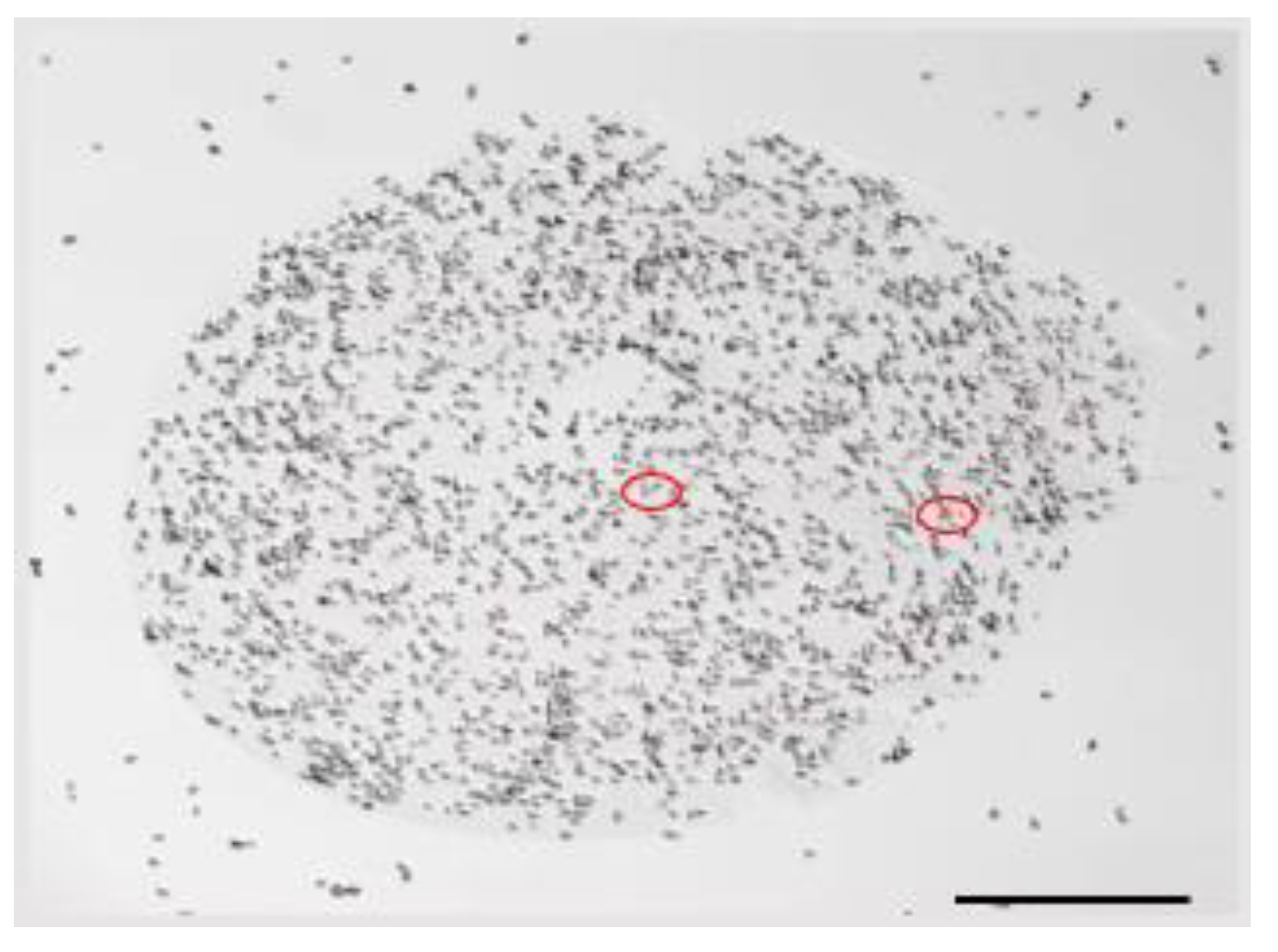
2.2. Biomass Production and Cells Viability
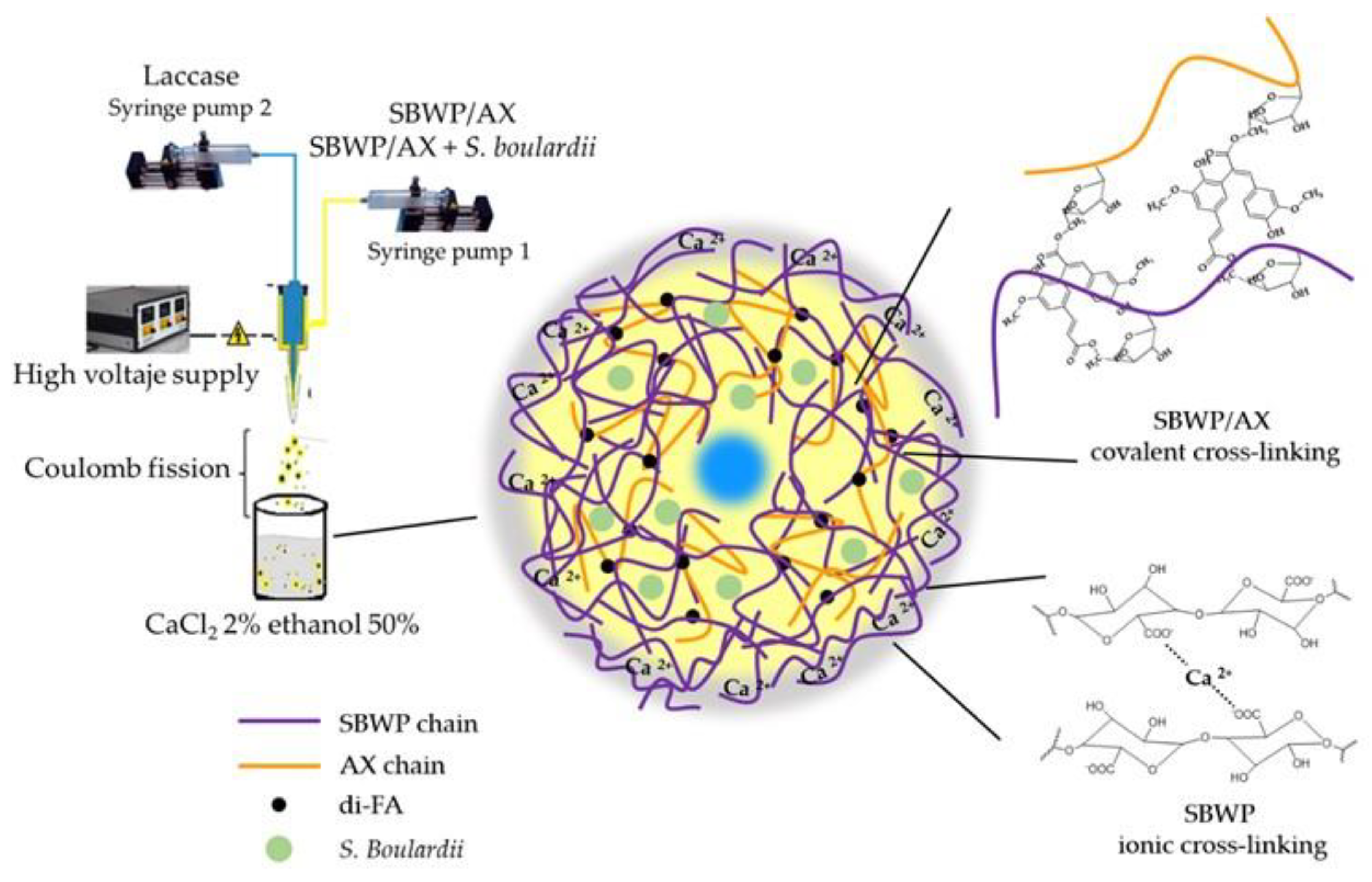
2.3. Polysaccharides Gelation and Microbeads Preparation
3. Materials and Methods
3.1. Materials
3.2. SBWP Extraction and Characterization
3.3. Biomass Production
3.4. Microbeads Preparation
3.5. S. boulardii Viability
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Douradinha, B.; Reis, V.C.B.; Rogers, M.B.; Torres, F.A.G.; Evans, J.D.; Marques, E.T.A. Novel insights in genetic transformation of the probiotic yeast Saccharomyces boulardii. Bioengineered 2013, 5, 21. [Google Scholar] [CrossRef]
- Kalkan, S.; Öztürk, D.; Selimoğlu, B.S. Determining some of the quality characteristics of probiotic yogurts manufactured by using microencapsulated Saccharomyces cerevisiae var. boulardii. Turk. J. Vet. Anim. Sci. 2018, 42, 617–623. [Google Scholar] [CrossRef]
- Tranquilino-Rodriguez, E.; Rodiles-Lopez, J.O.; Zamora-Vega, R.; Salgado-Garciglia, R.; Perez-Sanchez, R.E. Survival rate of Saccharomyces boulardii adapted to a functional freeze-dried yogurt: Experimental study related to processing, storage and digestion by Wistar rats. Funct. Foods Health Dis. 2017, 7, 98–114. [Google Scholar]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Argin, S. Microencapsulation of Probiotic Bacteria in Xanthan-Chitosan Polyelectrolyte Complex Gels; University of Maryland: College Park, MD, USA, 2002. [Google Scholar]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef]
- Lapsiri, W.; Bhandari, B.; Wanchaitanawong, P. Viability of Lactobacillus plantarum TISTR 2075 in Different Protectants during Spray Drying and Storage. Dry. Technol. 2012, 30, 1407–1412. [Google Scholar] [CrossRef]
- Chávez, B.E.; Ledeboer, A.M. Drying of probiotics: Optimization of formulation and process to enhance storage survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millan, E.; Pérez-López, E.; Hotchkiss, A.T.; González-Ríos, H.; Balandrán-Quintana, R.; Campa-Mada, A.C. Electrosprayed core-shell composite microbeads based on pectin-arabinoxylans for insulin carrying: Aggregation and size dispersion control. Polymers 2018, 10, 108. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Pawar, A.; Thakkar, S.; Misra, M. A bird’s eye view of nanoparticles prepared by electrospraying: Advancements in drug delivery field. J. Control. Release 2018, 286, 179–200. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Chasquibol Silva, N.; Arroyo Benites, E.; Morales Gomero, J.C. Extracción y caracterización de pectinas obtenidas a partir de frutos de la biodiversidad peruana. Ing. Ind. 2008, 26, 175–199. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.L.; Carvajal-Millán, E.; Ponce de León-Renova, N.E.; Márquez-Escalante, J.A.; Romo-Chacón, A. Pectin from low quality “Golden Delicious” apples: Composition and gelling capability. Food Chem. 2009, 116, 101–103. [Google Scholar] [CrossRef]
- Masmoudi, M.; Besbes, S.; Abbes, F.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Pectin Extraction from Lemon By-Product with Acidified Date Juice: Effect of Extraction Conditions on Chemical Composition of Pectins. Food Bioprocess Technol. 2012, 5, 687–695. [Google Scholar] [CrossRef]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Ralet, M.C.; Andre-Leroux, G.; Quemener, B.; Thibault, J.F. Sugar Beet (Beta vulgaris) Pectins are covalently Cross-linked through Diferulic Bridges in the Cell Wall. Phytochemistry 2005, 66, 2800–2814. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Oosterveld, A.; Grabber, J.H.; Beldman, G.; Ralph, J.; Voragen, A.G.J. Formation of Ferulic Acid Dehydrodimers through Oxidative Cross-Linking of Sugar Beet Pectin. Carbohydr. Res. 1997, 300, 179–181. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Yadav, M.P.; López-Franco, Y.L.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Brown-Bojorquez, F.; Silva-Campa, E.; Pedroza-Montero, M. Partial removal of protein associated with arabinoxylans: Impact on the viscoelasticity, crosslinking content, and microstructure of the gels formed. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Zaidel, D.N.A.; Chronakis, I.S.; Meyer, A.S. Enzyme catalyzed oxidative gelation of sugar beet pectin: Kinetics and rheology. Food Hydrocoll. 2012, 28, 130–140. [Google Scholar] [CrossRef]
- de Vries, J.A.; Rombouts, F.M.; Voragen, A.G.J.; Pilnik, W. Distribution of methoxyl groups in apple pectic substances. Carbohydr. Polym. 1983, 3, 245–258. [Google Scholar] [CrossRef]
- Saulnier, L.; Jean-Francó, T. Review Ferulic Acid and Diferulic Acids as Components of Sugar-Beet Pectins and Maize Bran Heteroxylans. J. Sci. Food Agric. 1999, 79, 396–402. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Guerrero, V.; Salas-Muñoz, E. Feruloylated arabinoxylans and arabinoxylan gels: Structure, sources and applications. Phytochem. Rev. 2010, 9, 111–120. [Google Scholar] [CrossRef]
- Turquois, T.; Rinaudo, M.; Taravel, F.R.; Heyraud, A. Extraction of highly gelling pectic substances from sugar beet pulp and potato pulp: Influence of extrinsic parameters on their gelling properties. Food Hydrocoll. 1999, 13, 255–262. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Noreen, A.; Nazli, Z.H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef]
- Pi, F.; Liu, Z.; Guo, X.; Guo, X.; Meng, H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocoll. 2019, 89, 792–801. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Abbasi, A.M.; Luo, Z.-G. Preparation of environment-friendly pectin from sugar beet pulp and assessment of its emulsifying capacity. Int. J. Food Sci. Technol. 2015, 50, 1324–1330. [Google Scholar] [CrossRef]
- Pereira, C.; Saraiva, L. Interference of aging media on the assessment of yeast chronological life span by propidium iodide staining. Folia Microbiol. (Praha) 2013, 58, 81–84. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Khalighi, S.; Berger, R.G.; Ersoy, F. Cross-linking of fibrex gel by fungal laccase: Gel rheological and structural characteristics. Processes 2020, 8, 16. [Google Scholar] [CrossRef]
- Paz-Samaniego, R.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Brown, F.; Rascón-Chu, A.; López-Franco, Y.; Lizardi-Mendoza, J. Maize Processing Waste Water Upcycling in Mexico: Recovery of Arabinoxylans for Probiotic Encapsulation. Sustainability 2016, 8, 1104. [Google Scholar] [CrossRef]
- González-Estrada, R.; Calderón-Santoyo, M.; Carvajal-Millan, E.; De Jesús Ascencio Valle, F.; Ragazzo-Sánchez, J.A.; Brown-Bojorquez, F.; Rascón-Chu, A. Covalently cross-linked arabinoxylans films for Debaryomyces hansenii entrapment. Molecules 2015, 20, 11373–11386. [Google Scholar] [CrossRef]
- Ekhart, P.F.; Van Der Saag, H.; Possemiers, S.; Van Den Abbeele, P.; Van De Wiele, T.; Neyrinck, A.M.; Nelly Delzenne, N.M.; Cani, P.C. Arabinoxylans for Modulating the Barrier Function of the Intestinal Surface. U.S. Patent 8465788 B2, 18 June 2013. [Google Scholar]
- Li, D.Q.; Du, G.M.; Jing, W.W.; Li, J.F.; Yan, J.Y.; Liu, Z.Y. Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohydr. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A novel pectin material: Extraction, characterization and gelling properties. Int. J. Mol. Sci. 2010, 11, 3686–3695. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Levigne, S.; Thomas, M.; Ralet, M.C.; Quéméner, B.; Thibault, J.F. Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocoll. 2002, 16, 547–550. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction and structure of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Ferrario, V.; Chernykh, A.; Fiorindo, F.; Kolomytseva, M.; Sinigoi, L.; Myasoedova, N.; Fattor, D.; Ebert, C.; Golovleva, L.; Gardossi, L. Investigating the Role of Conformational Effects on Laccase Stability and Hyperactivation under Stress Conditions. ChemBioChem 2015, 16, 2365–2372. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Component | Value |
|---|---|
| Galacturonic acid 1 | 52.2 ± 1.6 |
| Rhamnose 1 | 1.50 ± 0.02 |
| Arabinose 1 | 3.60 ± 0.04 |
| Xylose 1 | 1.20 ± 0.02 |
| Mannose 1 | 5.00 ± 0.04 |
| Galactose 1 | 20.7 ± 0.4 |
| Glucose 1 | 12.3 ± 0.2 |
| Ferulic acid 2 | 2.1 ± 0.1 |
| Component | Value |
|---|---|
| Molecular weight (kDa) | 468 ± 8 |
| Degree of esterification (%) | 30 ± 2 |
| Degree of acetylation (%) | 13 ± 2 |
| Time | FA | di-FA | tri-FA |
|---|---|---|---|
| (min) | (mg/g polysaccharides) | ||
| 0 | 3.58 ± 0.04 | 0.27 ± 0.04 | nd |
| 120 | 1.64 ± 0.02 | 1.01 ± 0.20 | 0.14 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohlmaier-Delgadillo, F.; Carvajal-Millan, E.; López-Franco, Y.L.; Islas-Osuna, M.A.; Micard, V.; Antoine-Assor, C.; Rascón-Chu, A. Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads. Molecules 2021, 26, 2478. https://doi.org/10.3390/molecules26092478
Ohlmaier-Delgadillo F, Carvajal-Millan E, López-Franco YL, Islas-Osuna MA, Micard V, Antoine-Assor C, Rascón-Chu A. Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads. Molecules. 2021; 26(9):2478. https://doi.org/10.3390/molecules26092478
Chicago/Turabian StyleOhlmaier-Delgadillo, Federico, Elizabeth Carvajal-Millan, Yolanda L. López-Franco, María A. Islas-Osuna, Valérie Micard, Carole Antoine-Assor, and Agustín Rascón-Chu. 2021. "Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads" Molecules 26, no. 9: 2478. https://doi.org/10.3390/molecules26092478
APA StyleOhlmaier-Delgadillo, F., Carvajal-Millan, E., López-Franco, Y. L., Islas-Osuna, M. A., Micard, V., Antoine-Assor, C., & Rascón-Chu, A. (2021). Ferulated Pectins and Ferulated Arabinoxylans Mixed Gel for Saccharomyces boulardii Entrapment in Electrosprayed Microbeads. Molecules, 26(9), 2478. https://doi.org/10.3390/molecules26092478







