Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Assessment
2.2. Harvesting
2.3. Sample Preparation for Metabolomic Analysis
2.4. Derivatization
2.5. Data Evaluation
3. Results and Discussion
3.1. Growth of Chlorella Vulgaris
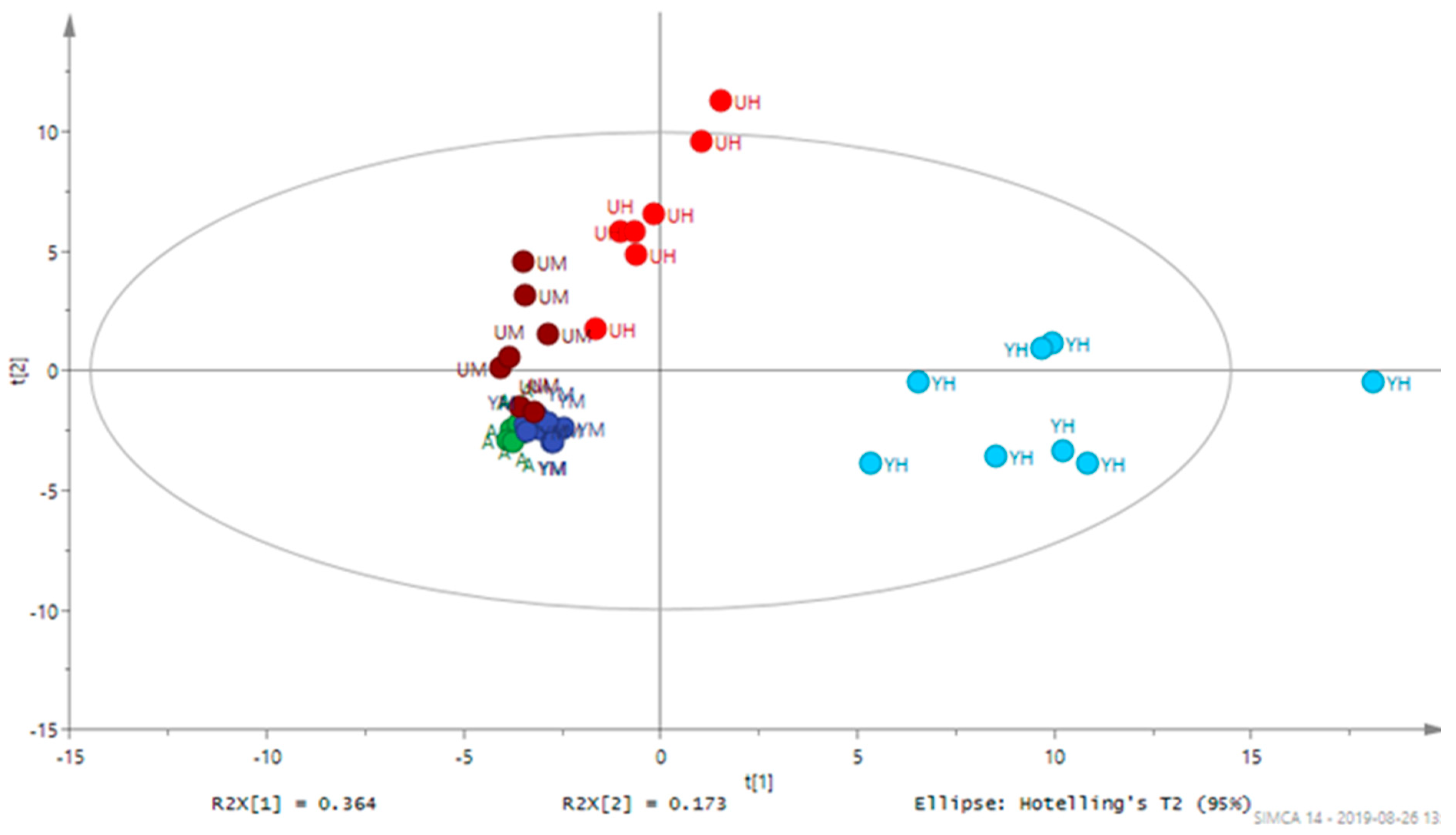
3.2. Metabolomic Analysis of Chlorella Alga Under Auto-, Mixo-, and Heterotrophic Conditions
3.3. Bioplastics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cent. 2019, 43, 1–4. [Google Scholar] [CrossRef]
- Zhang, C.; Show, P.-L.; Ho, S.-H. Progress and perspective on algal plastics—A critical review. Bioresour. Technol. 2019, 289, 121700. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Mudway, I.S.; Fussell, J.C. Air Pollution and Asthma: Critical Targets for Effective Action. Pulm. Ther. 2020, 1–16. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries: Volume 2: Products and Refin-ery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 2, pp. 61–131. [Google Scholar]
- Perez-Garcia, O.; De-Bashan, L.E.; Hernandez, J.-P.; Bashan, Y. Efficient of growth and nutrient uptake from wastewater by heterotrophic, autotrophic and mixotrophic cultivation of chlorella vul-garis immobilized with Azospirillum blasiliense. J. Phycol. 2010, 46, 800–812. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Eriksson, K.; Sellstedt, A. Mixotrophic and heterotrophic production of lipids and carbohydrates by a locally isolated microalga using wastewater as a growth medium. Bioresour. Technol. 2018, 257, 260–265. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel pro-duction. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhou, W.; Noppol, L.; Liu, T. Mechanism and enhancement of lipid accumu-lation in filamentous oleaginous microalgae Tribonema minus under heterotrophic condition. Biotechnol. Biofuels 2018, 1, 328. [Google Scholar] [CrossRef]
- Zienkiewicz, K.; Du, Z.-Y.; Ma, W.; Vollheyde, K.; Benning, C. Stress-induced neutral lipid bio-synthesis in microalgae—Molecular, cellular and physiological insights. Biochim. Biophys. Acta 2016, 186, 1269–1281. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.Z.; Martinez-Rodriguez, O.A.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World J. Microbiol. Biotechnol. 2015, 31, 1–9. [Google Scholar] [CrossRef]
- Renberg, L.; Johansson, A.I.; Shutova, T.; Stenlund, H.; Aksmann, A.; Raven, J.A.; Gardeström, P.; Moritz, T.; Samuelsson, G. A Metabolomic Approach to Study Major Metabolite Changes during Acclimation to Limiting CO2 in Chlamydomonas reinhardtii. Plant Physiol. 2010, 154, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Hao, W.; Zou, Y.; Shi, M.; Jiang, Y.; Xiao, P.; Lei, A.; Hu, Z.; Zhang, W.; Zhao, L.; et al. Fatty acid and metabolomic profiling approaches differentiate heterotrophic and mixo-trophic culture conditions in a microalgal food supplement ‘Euglena’. BMC Biotechnol. 2016, 16, 49. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalgaChlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate—Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, J.; Jonsson, P.; Nordstrom, A.; Sjostrom, M.; Moritz, T. Design of experiments: An effi-cient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal. Biochem. 2004, 331, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Jiye, A.; Trygg, J.; Gullberg, J.; Johansson, A.I.; Jonsson, P.; Antti, H.; Marklund, S.L.; Moritz, T. Ex-traction and GC/MS Analysis of the Human Blood Plasma Metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar]
- Mohan, S.V.; Rohit, M.V.; Chiranjeevi, P.; Chandra, R.; Beeramganti, N. Heterotrophic Mi-croalgae Cultivation to Synergize Biodiesel Production with Waste Remediation: Progress and Per-spectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Y.; Zhan, J.; He, C.; Wang, Q. Comparative metabolic profiling of the li-pid-producing green microalga Chlorella reveals that nitrogen and carbon metabolic pathways con-tribute to lipid metabolism. Biotechnol. Biofuels 2017, 10, 153. [Google Scholar] [CrossRef]
- Yang, Z.-K.; Niu, Y.-F.; Ma, Y.-H.; Xue, J.; Zhang, M.-H.; Yang, W.; Liu, J.-S.; Lu, S.; Guan, Y.; Li, H.-Y. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following ni-trogen deprivation. Biotechnol. Biofuels 2013, 6, 67. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.G.; Khalil, H.; Bressler, D.C. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
| Growth Condition | |
|---|---|
| A | 1.25 ± 0.09 |
| Y M | 1.31 ± 0.10 |
| Y H | 0.52 ± 0.03 |
| U H | 1.29 ± 180 |
| U M | 0.70 ± 0.05 |
| Compounds | RI | C.C. | p Value |
|---|---|---|---|
| 1-myristoylglycerol | 2382 | FA | <0.0001 |
| 2-methylmalic acid | 1460 | CA | <0.0001 |
| 2-oxoisocaproic acid | 1207 | FA | 0.2423 |
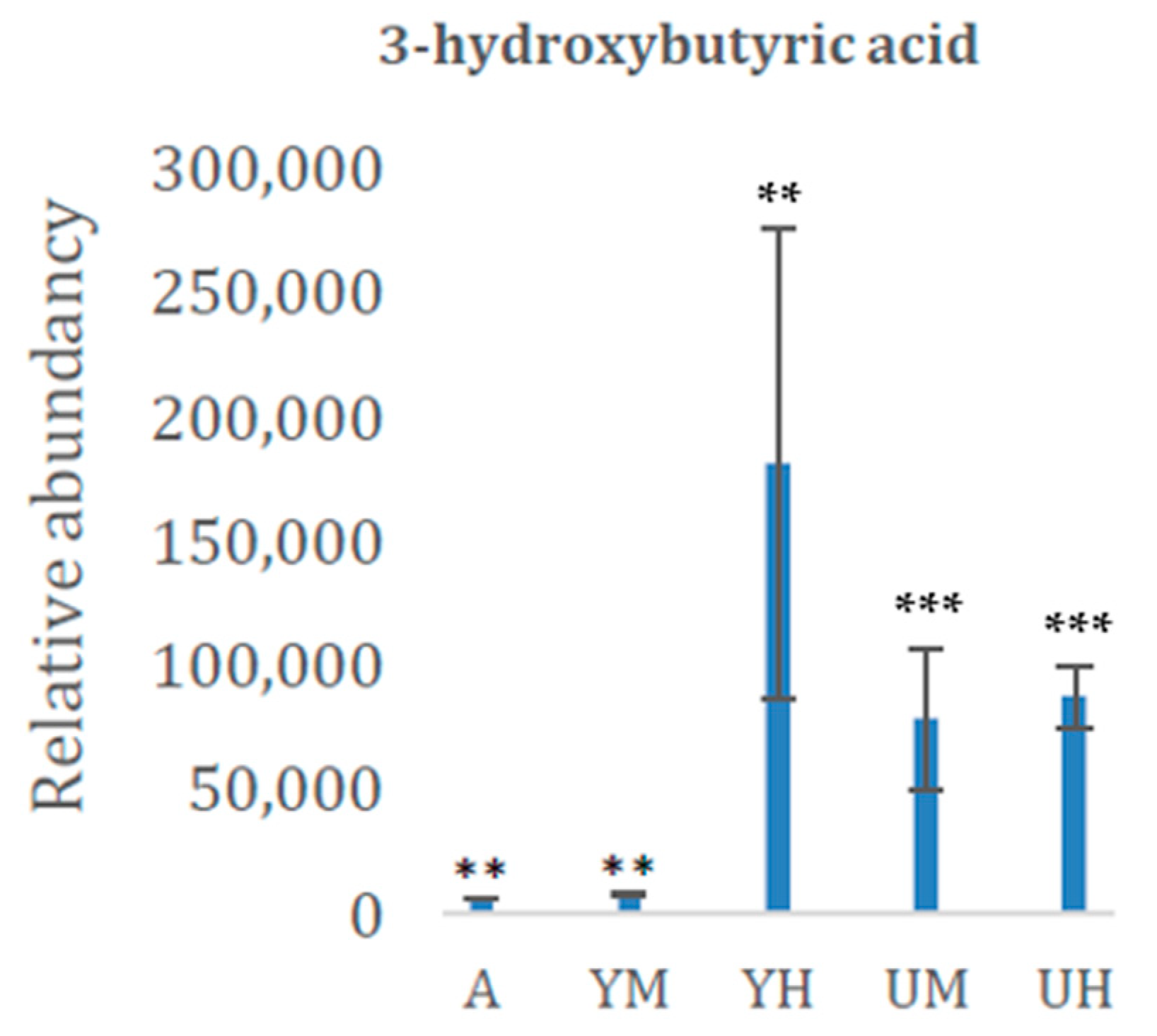
| 3-hydroxybutyric acid | 1156 | CA | 0.0377 |
| 3-hydroxyisobutyric acid | 1156 | CA | 0.0065 |
| 4-aminobutyric acid | 1522 | CA | 0.0860 |
| 4-hydroxyphenylacetic acid | 1636 | CA | 0.0064 |
| 5-hydroxypipecolic acid | 1588 | CA | 0.0139 |
| Adenosine-5-monophosphate | 3050 | EC | 0.0108 |
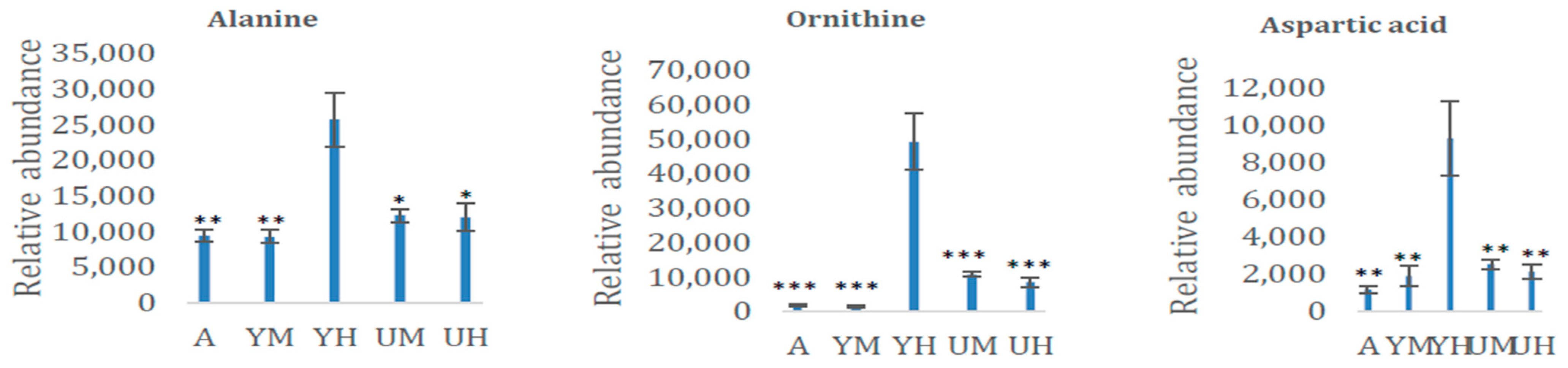
| Alanine | 1100 | AA | 0.0010 |
| Alpha-ketoglutaric acid | 1565 | CA | 0.0638 |
| Alpha-tocopherol | 3145 | V | 0.5274 |
| Aminomalonic acid | 1458 | ACA | 0.0425 |
| Arabinose/ribose/xylose | 1672 | CA | <0.0001 |
| Arabitol | 1697 | SA | 0.0002 |
| Asparagine | 1658 | AA | 0.0696 |
| Aspartic acid | 1507 | AA | 0.0011 |
| Benzoic acid | 1252 | P | 0.0420 |
| Beta-alanine | 1419 | AA | 0.0543 |
| Cadaverine | 1831 | AlA | <0.0001 |
| Citric acid | 1800 | CA | <0.0001 |
| Dehydroascorbic acid dimer | 1837 | V | 0.0002 |
| Disaccharide | 2515 | Carb | 0.0303 |
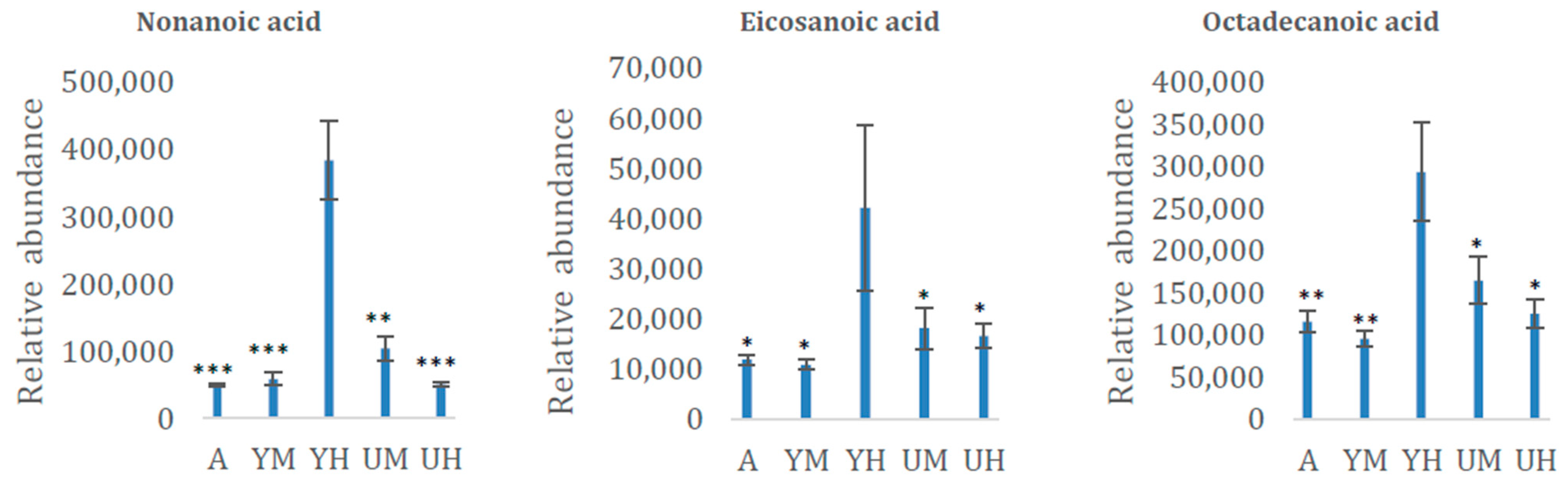
| Eicosanoic acid | 2435 | FA | 0.0388 |
| Ethanolamine | 1260 | AA | 0.0003 |
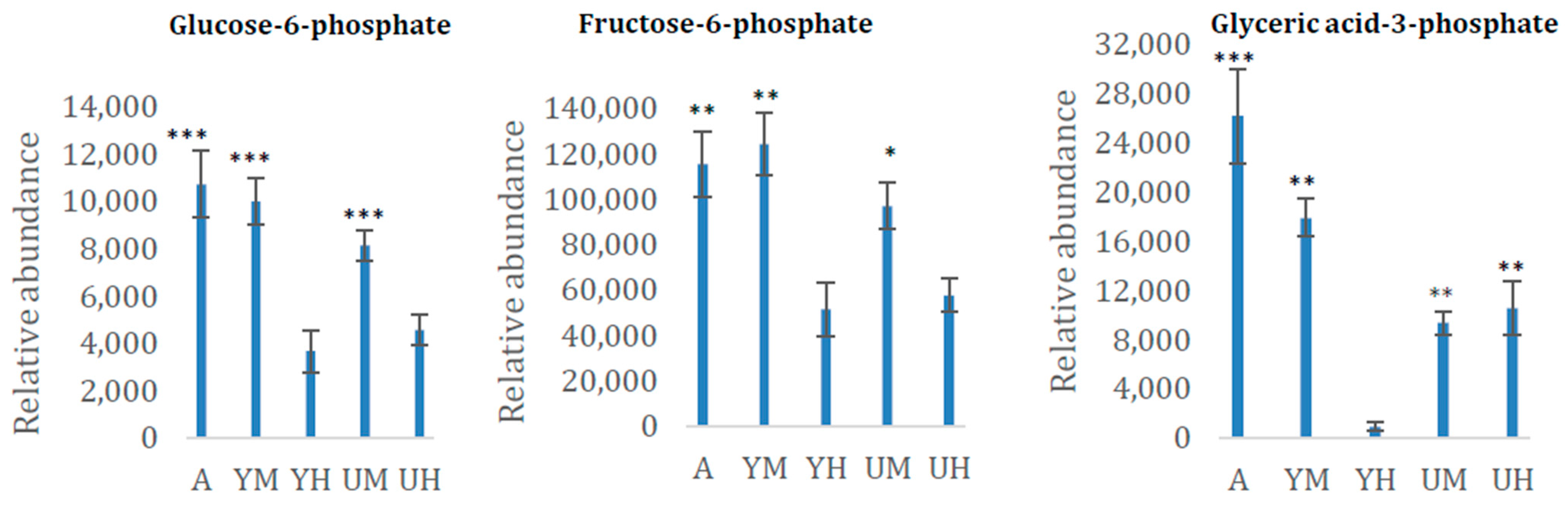
| Fructose-6-phosphate | 2280 | SP | 0.0041 |
| Fumaric acid | 1342 | CA | 0.0112 |
| Glucose | 1876 | S | 0.0393 |
| Glucose-6-phosphate | 2316/2327 | SP | 0.0008 |
| Glutamic acid | 1607 | AA | 0.0485 |
| Glutamine | 1763 | AA | 0.0438 |
| Glyceric acid | 1318 | CA | <0.0001 |
| Glyceric acid-3-phosphate | 1787 | CA | <0.0001 |
| Glycerol | 1267 | SA | <0.0001 |
| Glycerol-2-phosphate | 1704 | SP | 0.0001 |
| Glycerol-3-phosphate | 1743 | SP | <0.0001 |
| Glycine | 1120 | AA | 0.8101 |
| Glycolic acid | 1074 | CA | 0.0004 |
| Heptadecanoic acid | 2139 | L | 0.0052 |
| Hexadecanoic acid | 2042 | L | 0.0616 |
| Inositol, myo- | 2074 | V | 0.0151 |
| Inositol, scyllo- | 2008 | V | <0.0001 |
| Inositol-phosphate | 2396 | V | 0.3207 |
| Isoerythritol | 1479 | S | 0.0336 |
| Lactic acid | 1057 | CA | 0.1729 |
| Lysine | 1848 | AA | 0.0232 |
| Lyxose | 1633 | S | 0.0059 |
| Malic acid | 1474 | CA | 0.6389 |
| Maltose/turanose | 2727 | S | <0.0001 |
| Methylsuccinate | 1320 | CA | <0.0001 |
| Myristic acid 50 | 1844 | FA | 0.0027 |
| Nicotinamide | 1844 | V | 0.0002 |
| Nonanoic acid | 1357 | FA | <0.0001 |
| Octadecatrienoic acid, 6,9,12-(z,z,z)- | 2191 | FA | 0.061 |
| Octadecatrienoic acid, 9,12,15-(z,z,z)- | 2218 | FA | 0.0370 |
| O-phosphoetanolamine | 1771 | AM | <0.0001 |
| Ornithine | 1453 | AA | <0.0001 |
| Pantothenic acid | 1980 | V | 0.0043 |
| Phenylalanine | 1680 | A | 0.0036 |
| Putrescine | 1732 | AM | 0.0768 |
| Pyroglutamic acid | 1518 | AA | 0.0315 |
| Pyruvic acid | 1049 | CA | <0.0001 |
| Raffinose | 3373 | S | 0.0062 |
| Salicin | 2488 | S | <0.0001 |
| Succinic acid | 1308 | S | <0.0001 |
| Sucrose | 2616 | S | 0.2146 |
| Threonine/allo-threonine | 1372 | AA | 0.027205 |
| Trehalose/maltose | 2721 | S | <0.0001 |
| Tyrosine | 1933 | AA | 0.6952 |
| Valine | 1208 | AA | 0.0455 |
| Xylitol | 1684 | S | 0.0050 |
| Xylulose | 1654 | S | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzayisenga, J.C.; Sellstedt, A. Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden. Molecules 2021, 26, 2410. https://doi.org/10.3390/molecules26092410
Nzayisenga JC, Sellstedt A. Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden. Molecules. 2021; 26(9):2410. https://doi.org/10.3390/molecules26092410
Chicago/Turabian StyleNzayisenga, Jean Claude, and Anita Sellstedt. 2021. "Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden" Molecules 26, no. 9: 2410. https://doi.org/10.3390/molecules26092410
APA StyleNzayisenga, J. C., & Sellstedt, A. (2021). Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden. Molecules, 26(9), 2410. https://doi.org/10.3390/molecules26092410





