A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type
Abstract
1. Introduction
2. NMR
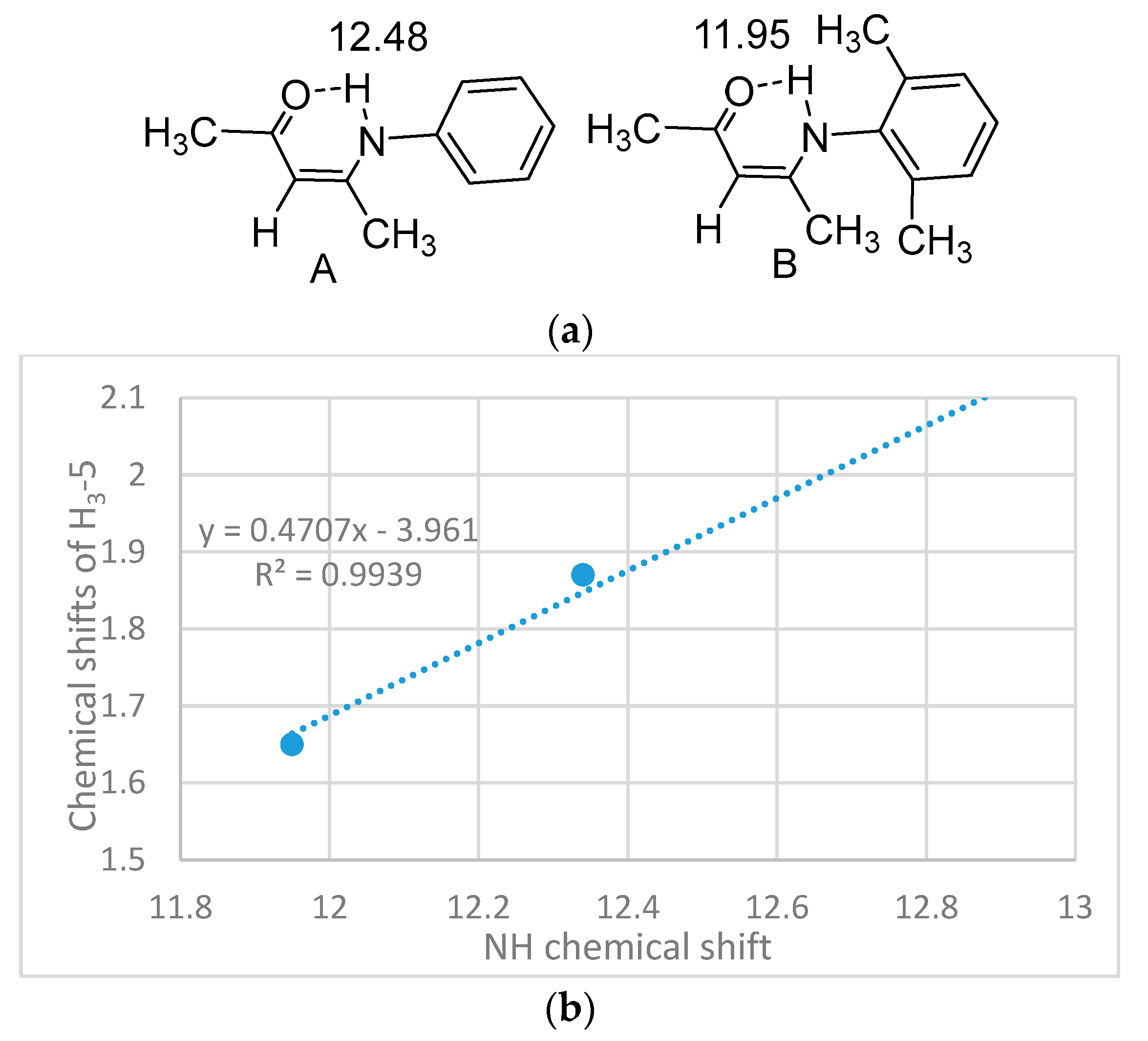
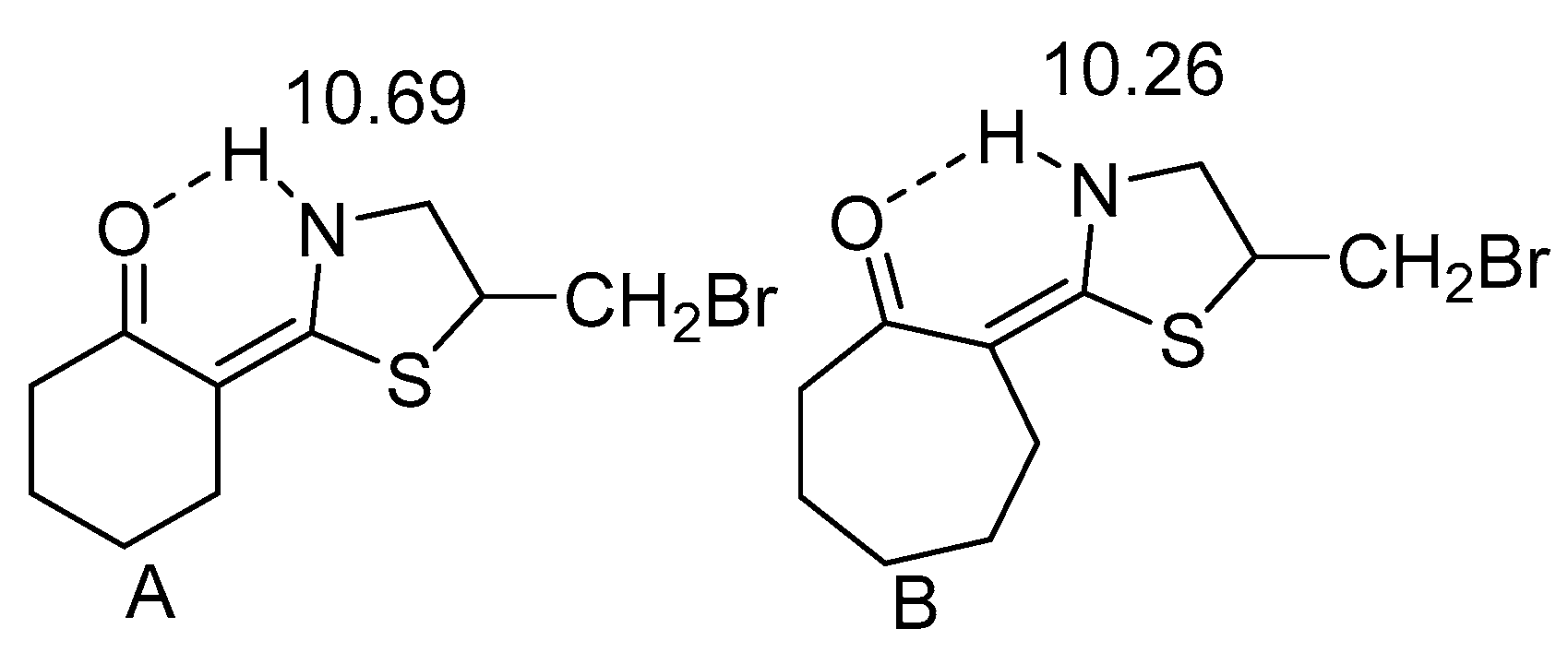
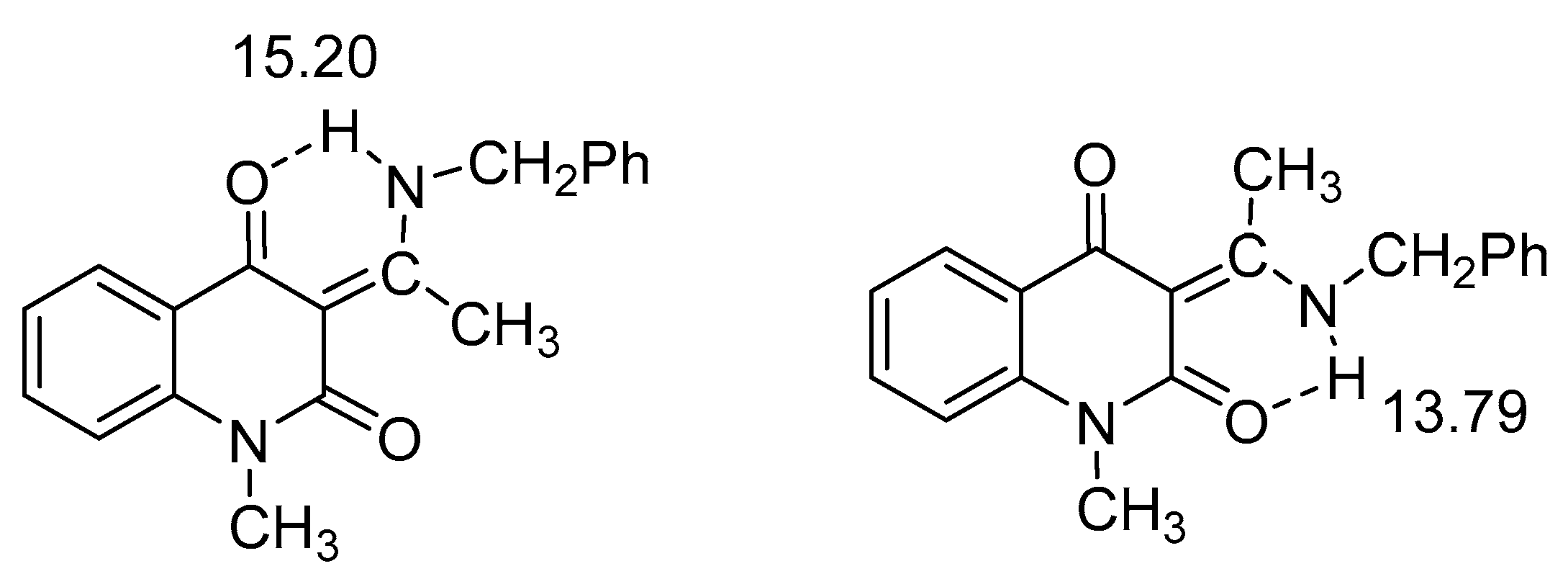
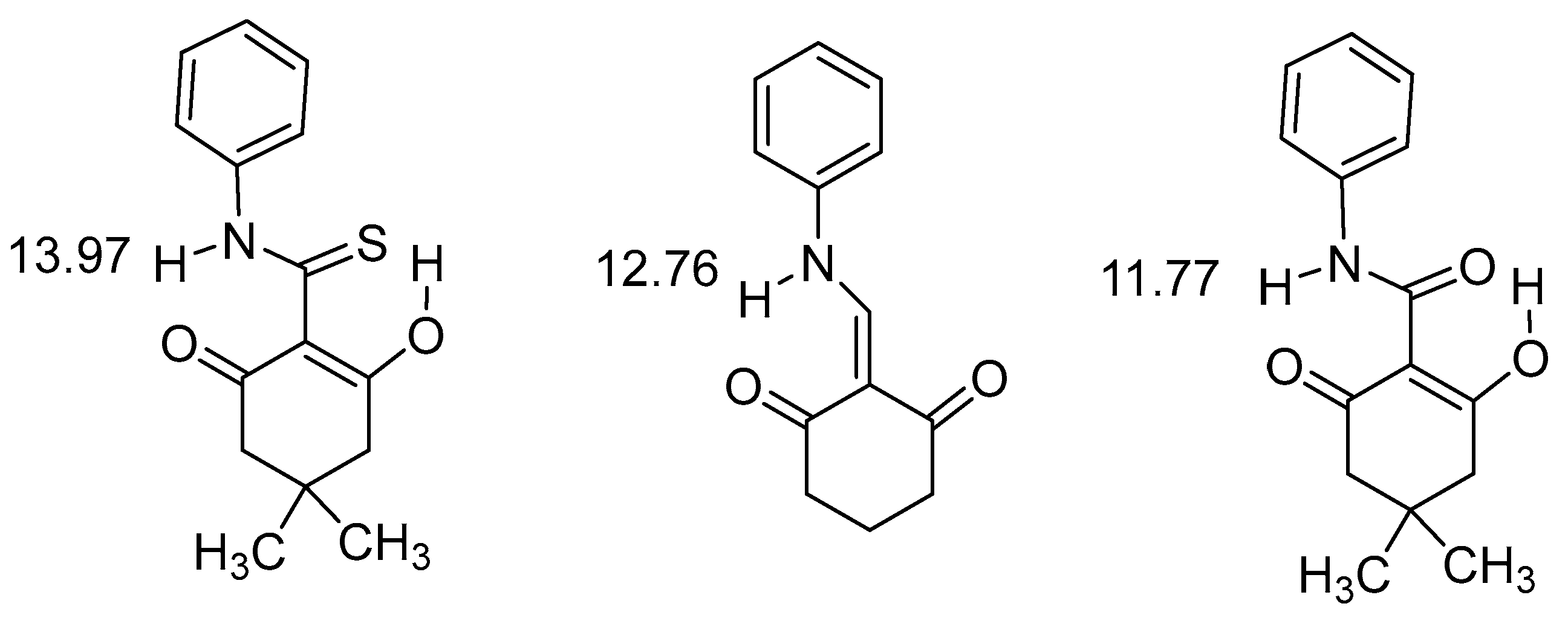
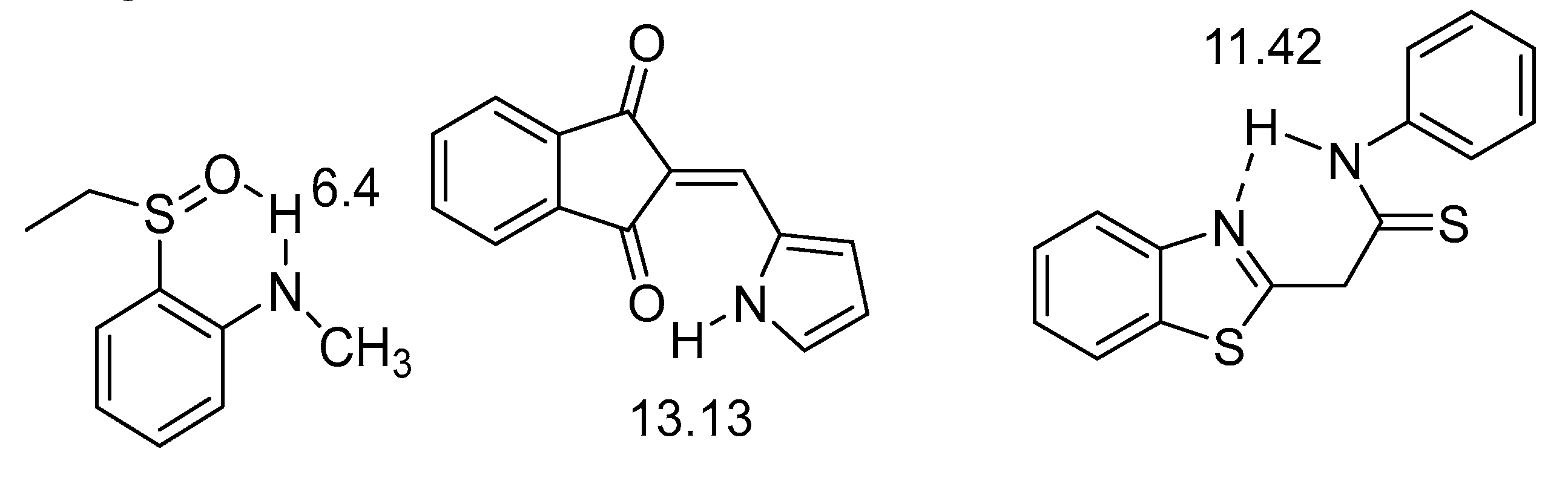
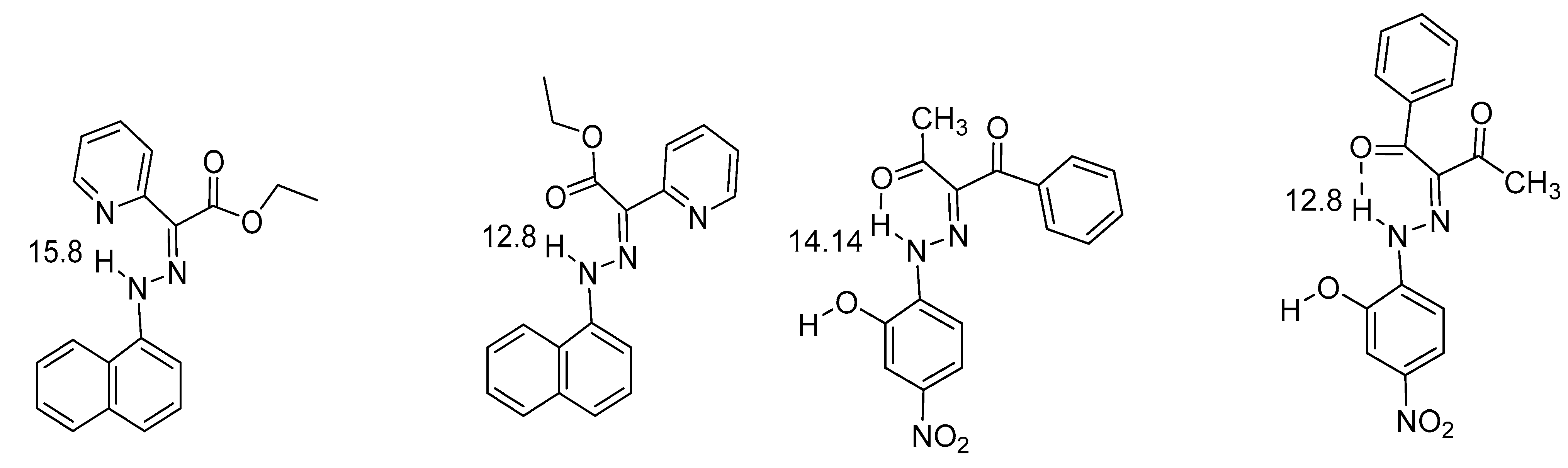
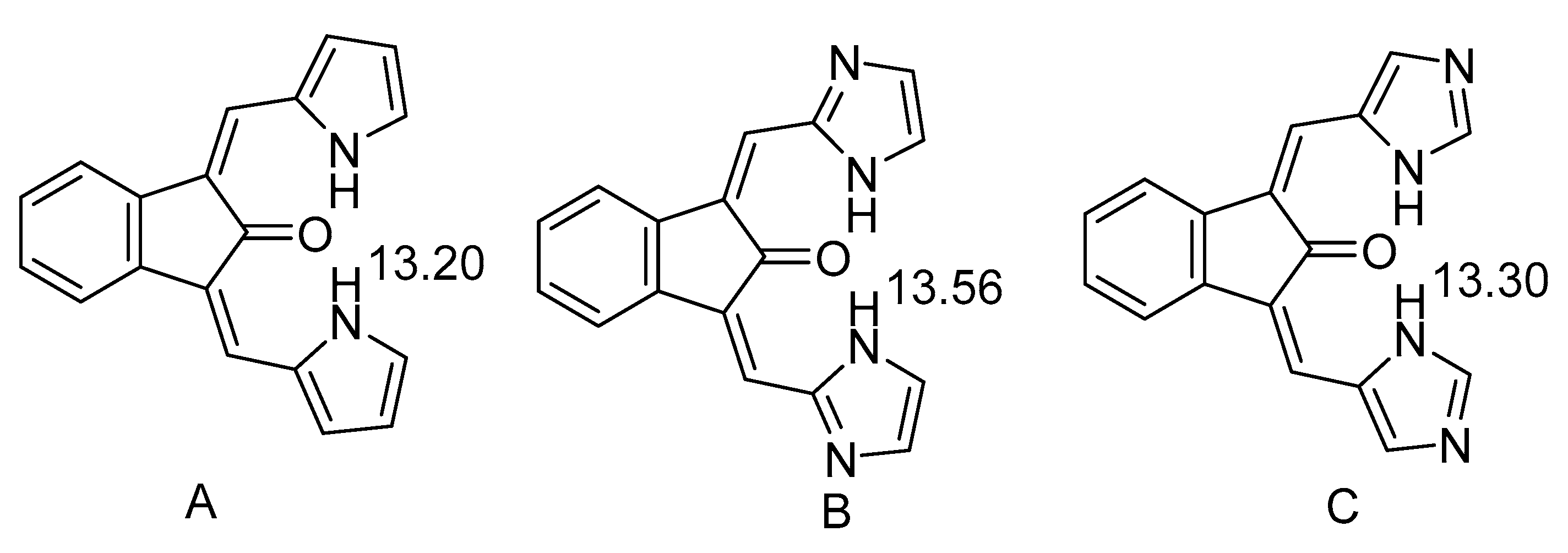
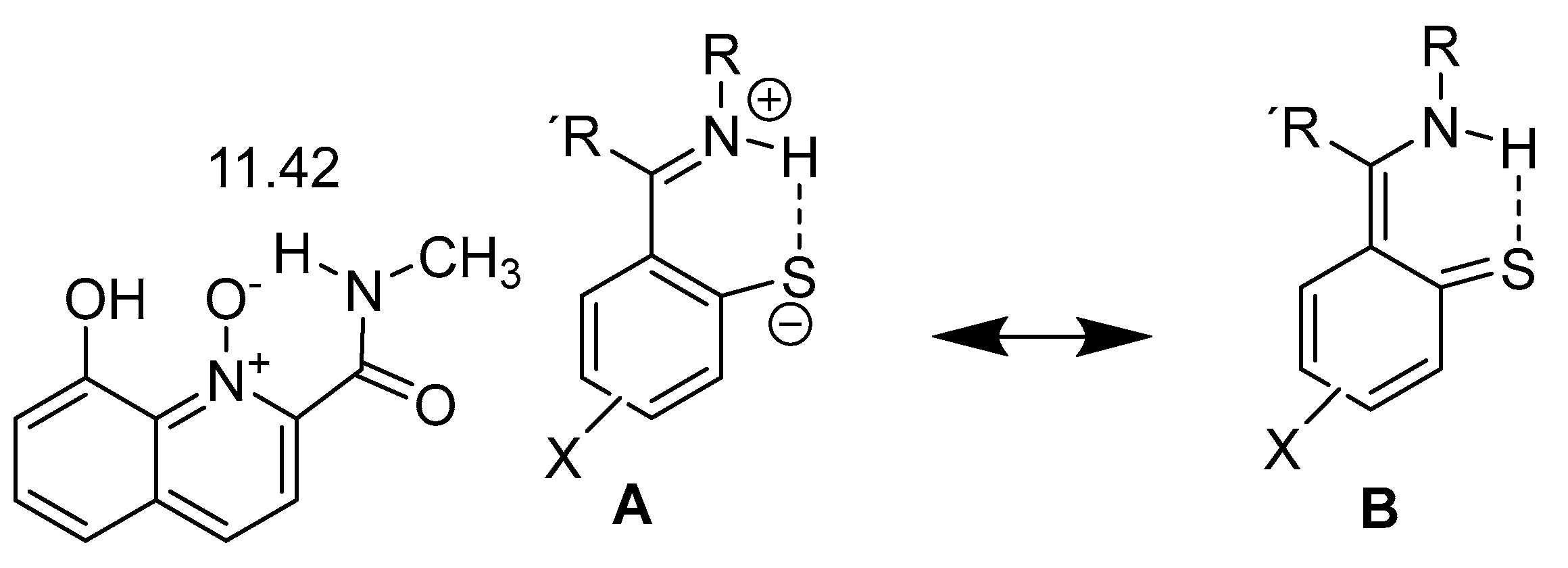
2.1. HN Chemical Shifts
2.2. Coupling Constants
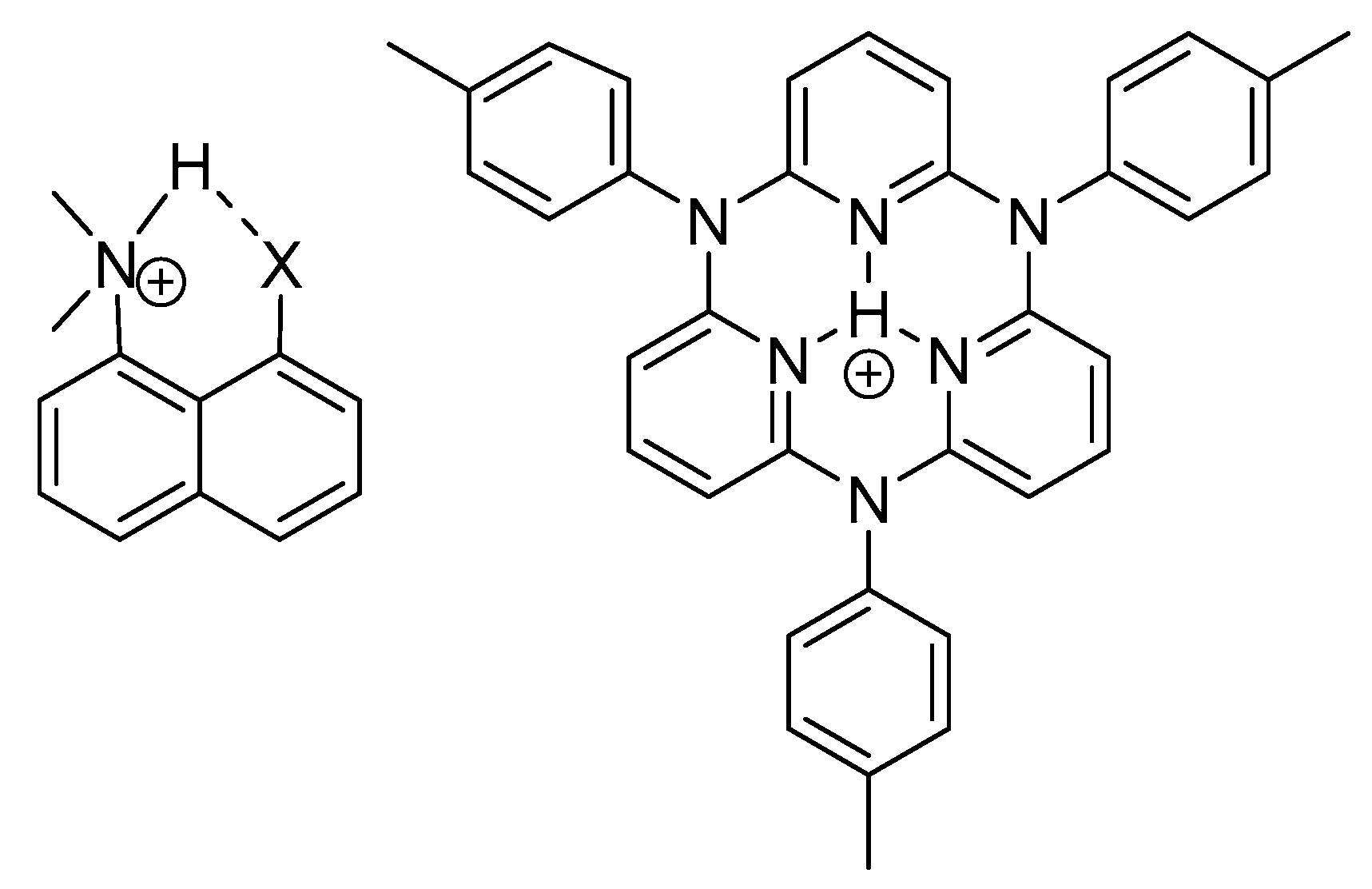
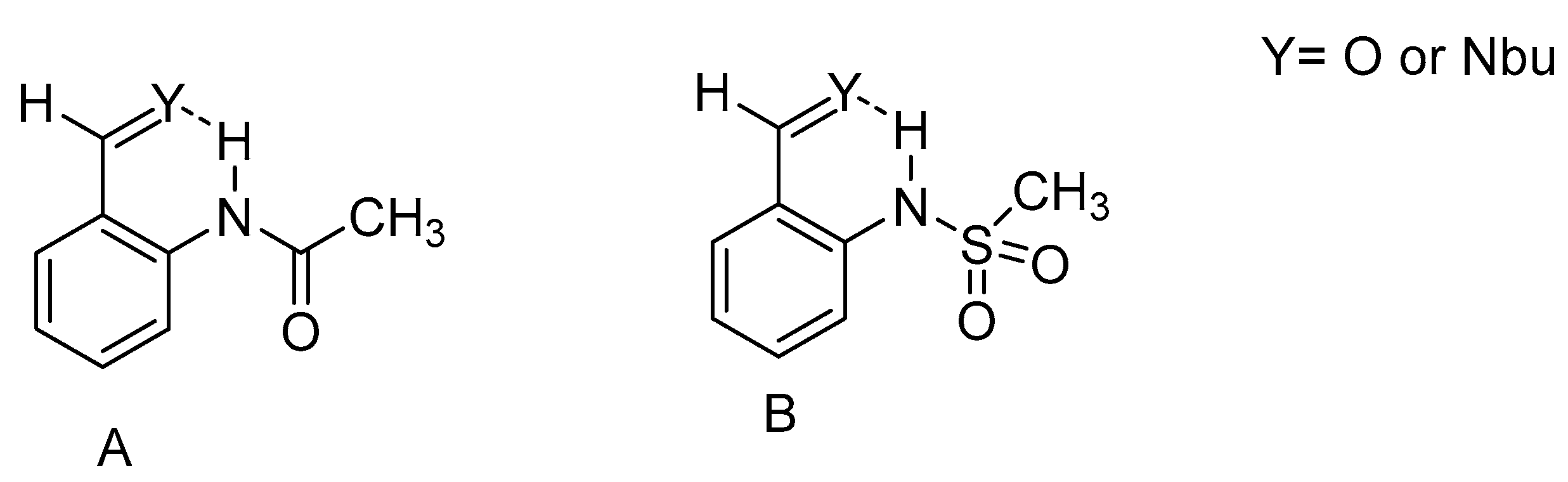
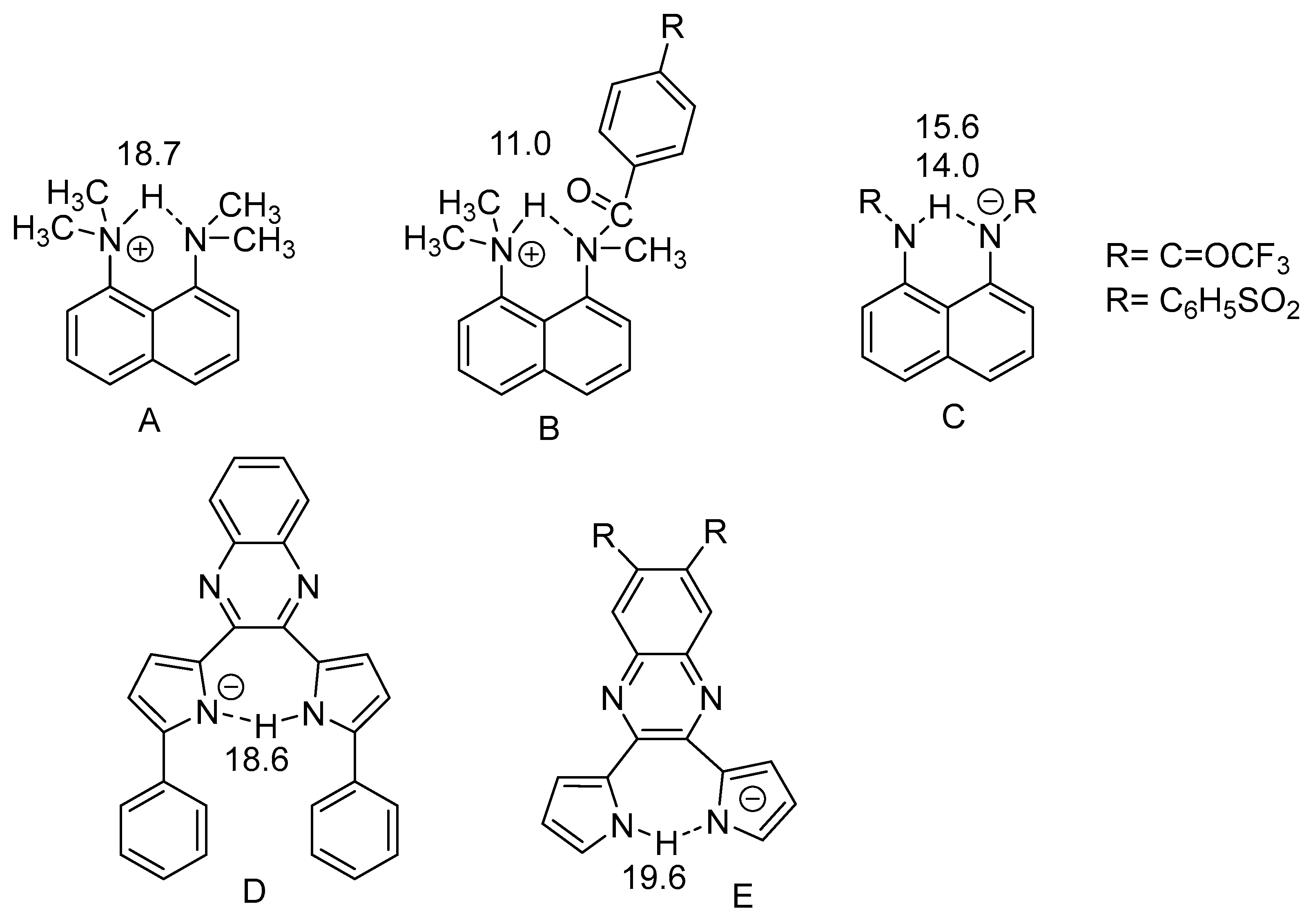
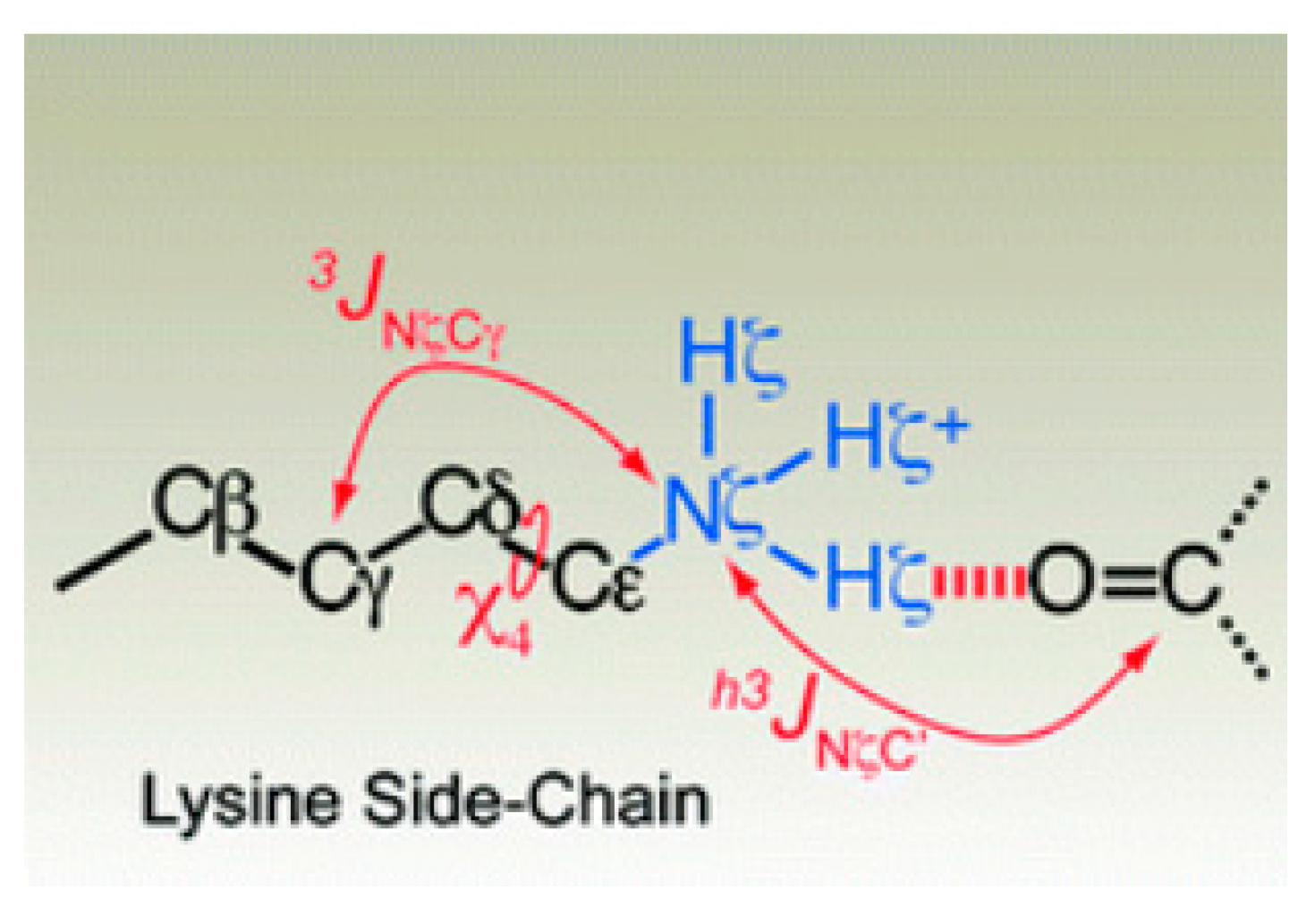
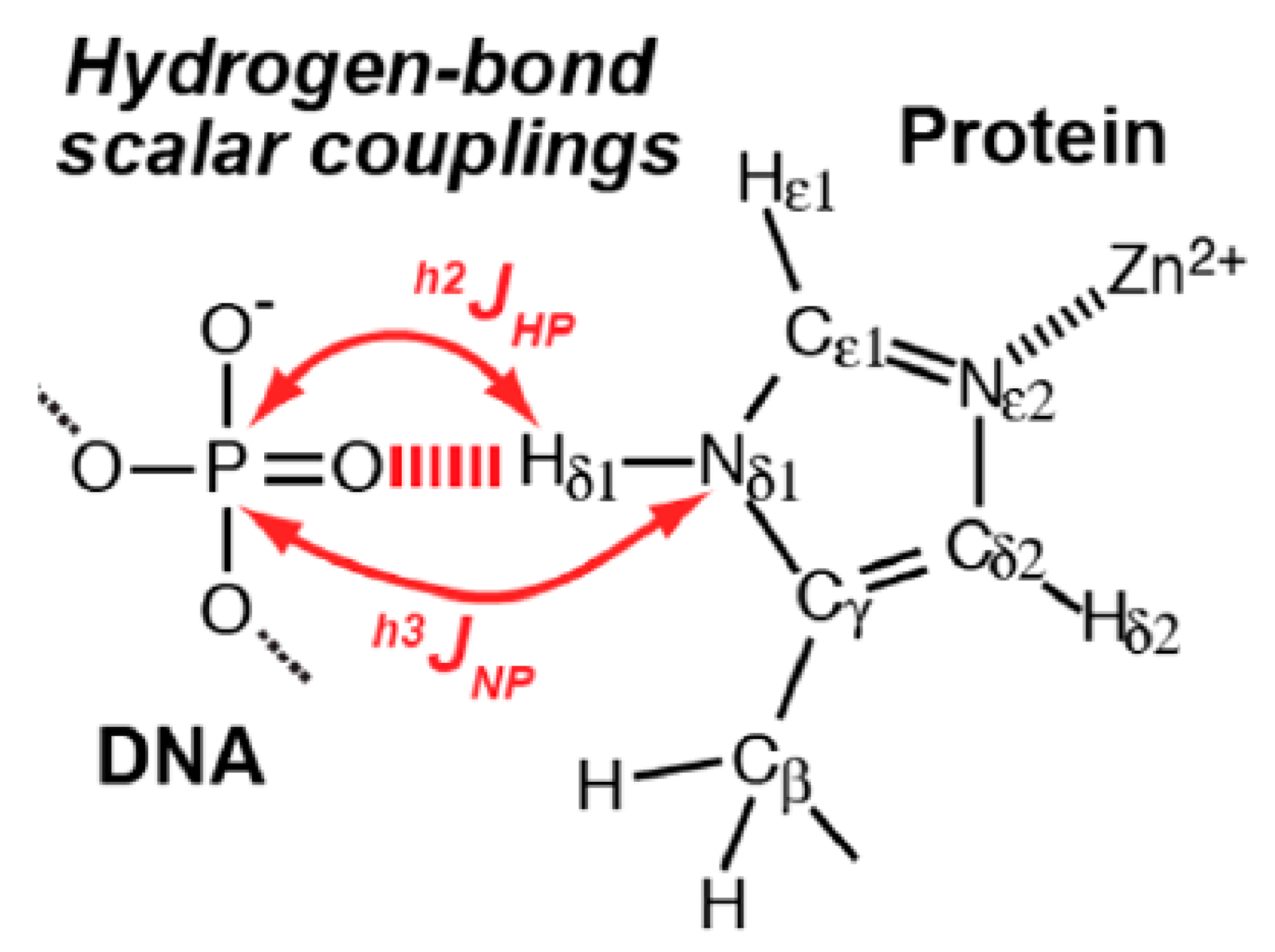
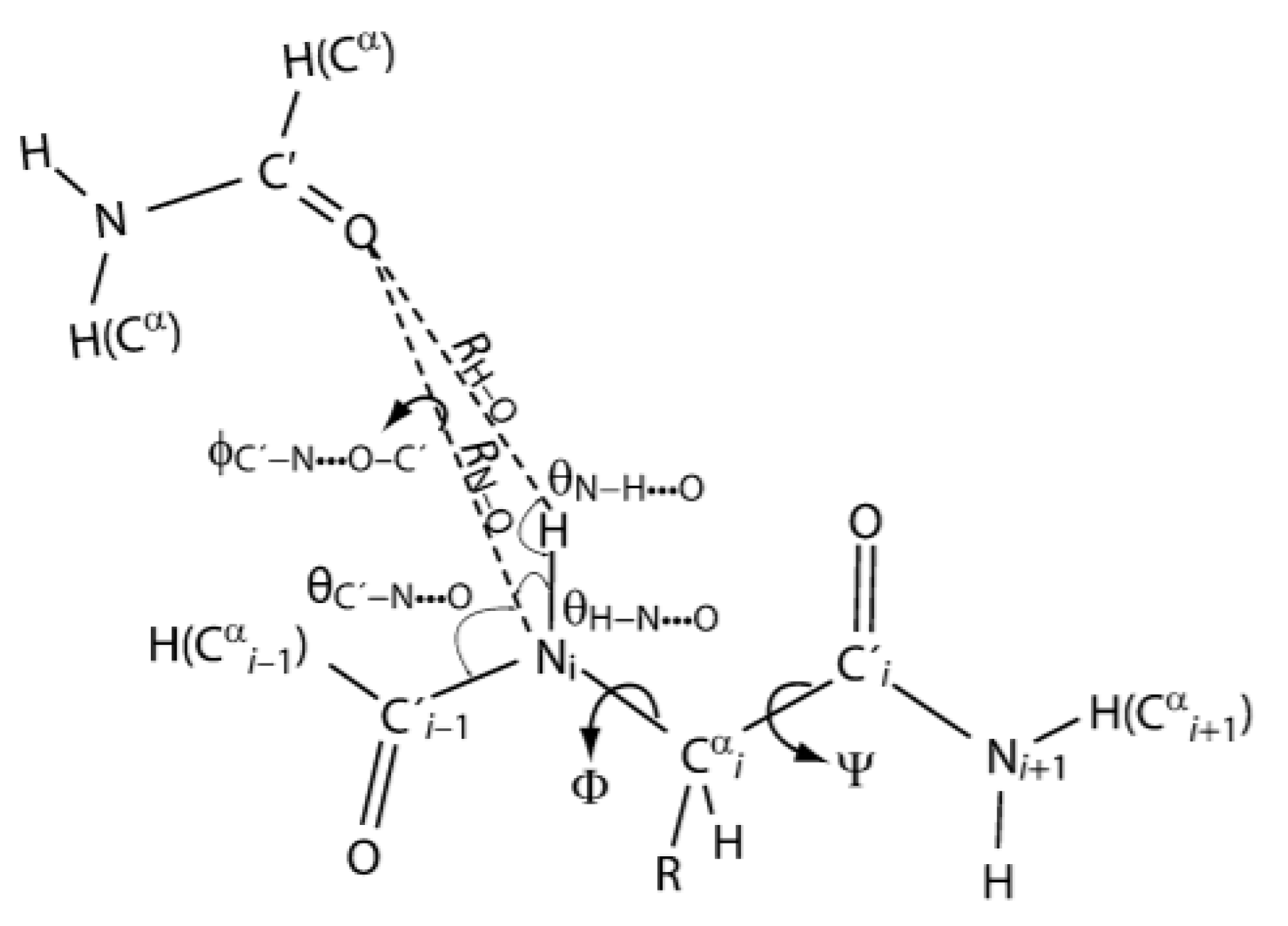
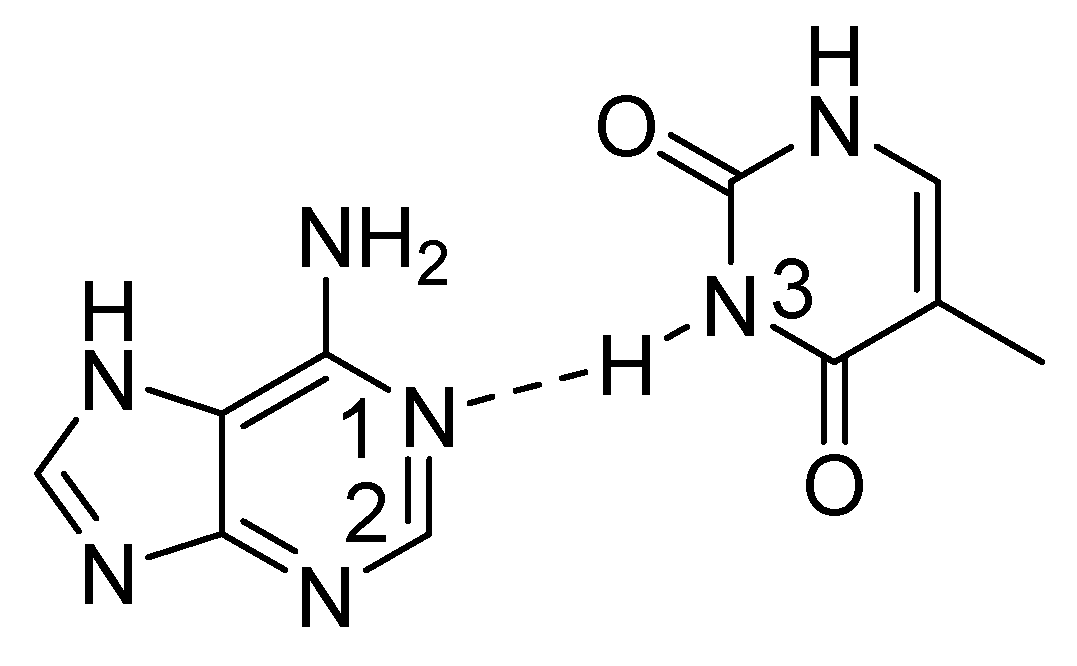
2.3. Non-RAHB Cases. Couplings across Hydrogen Bonds
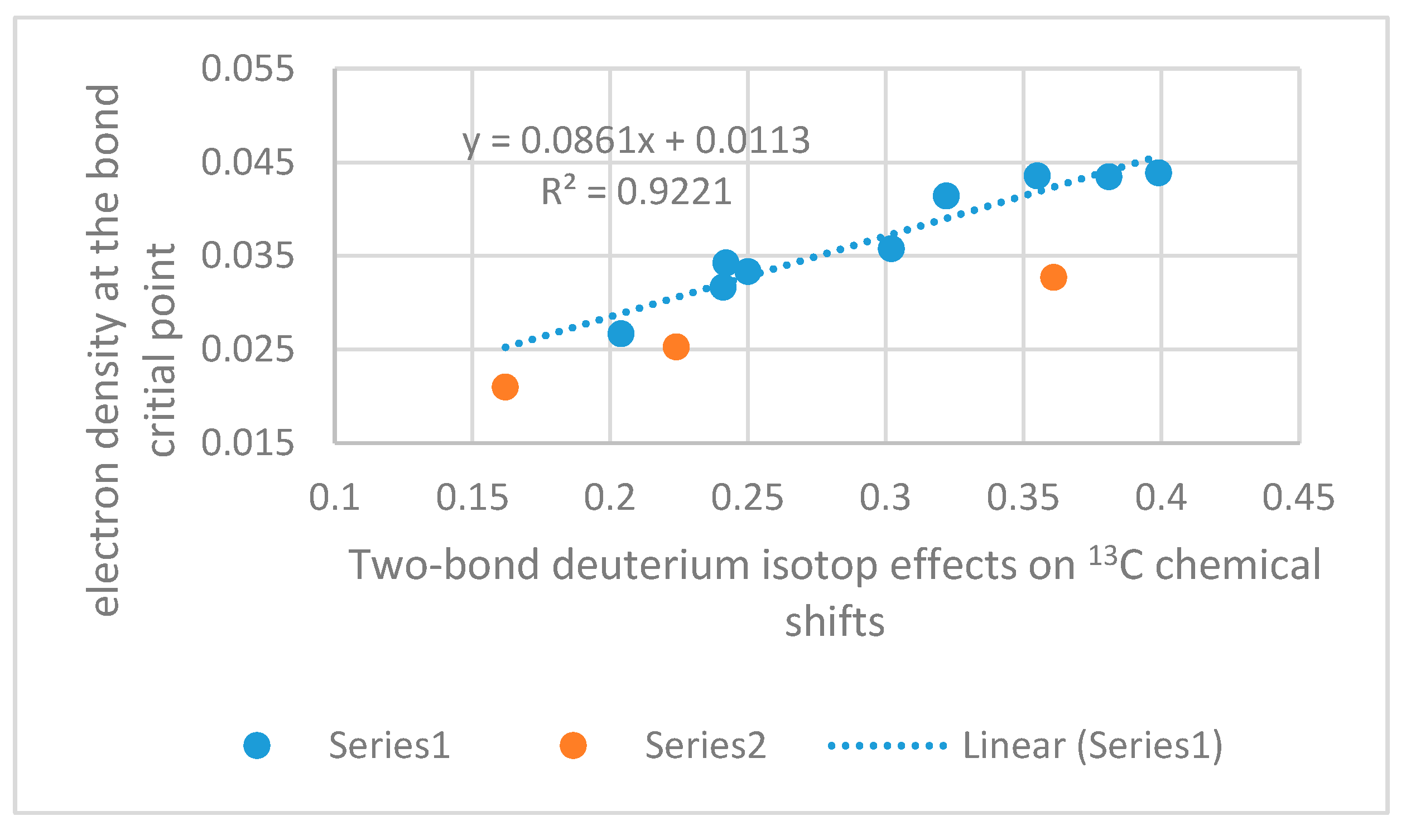
2.4. Isotope Effects on Chemical Shifts
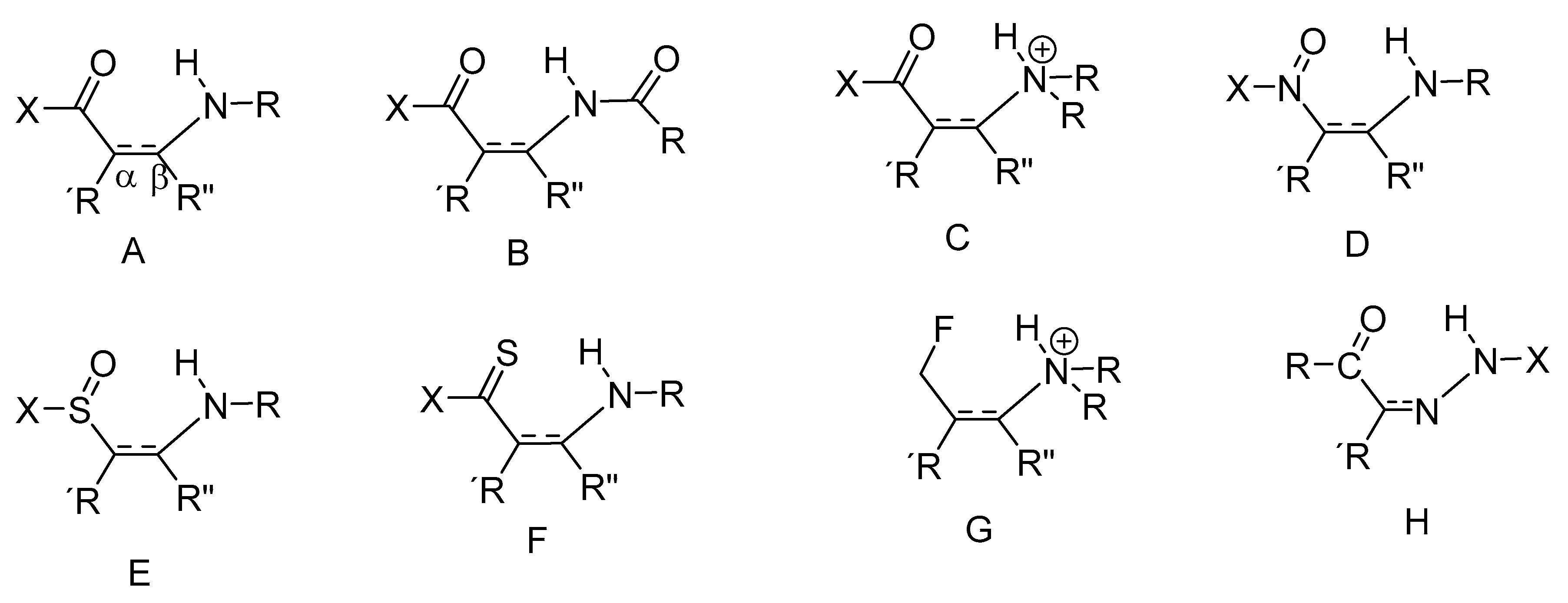
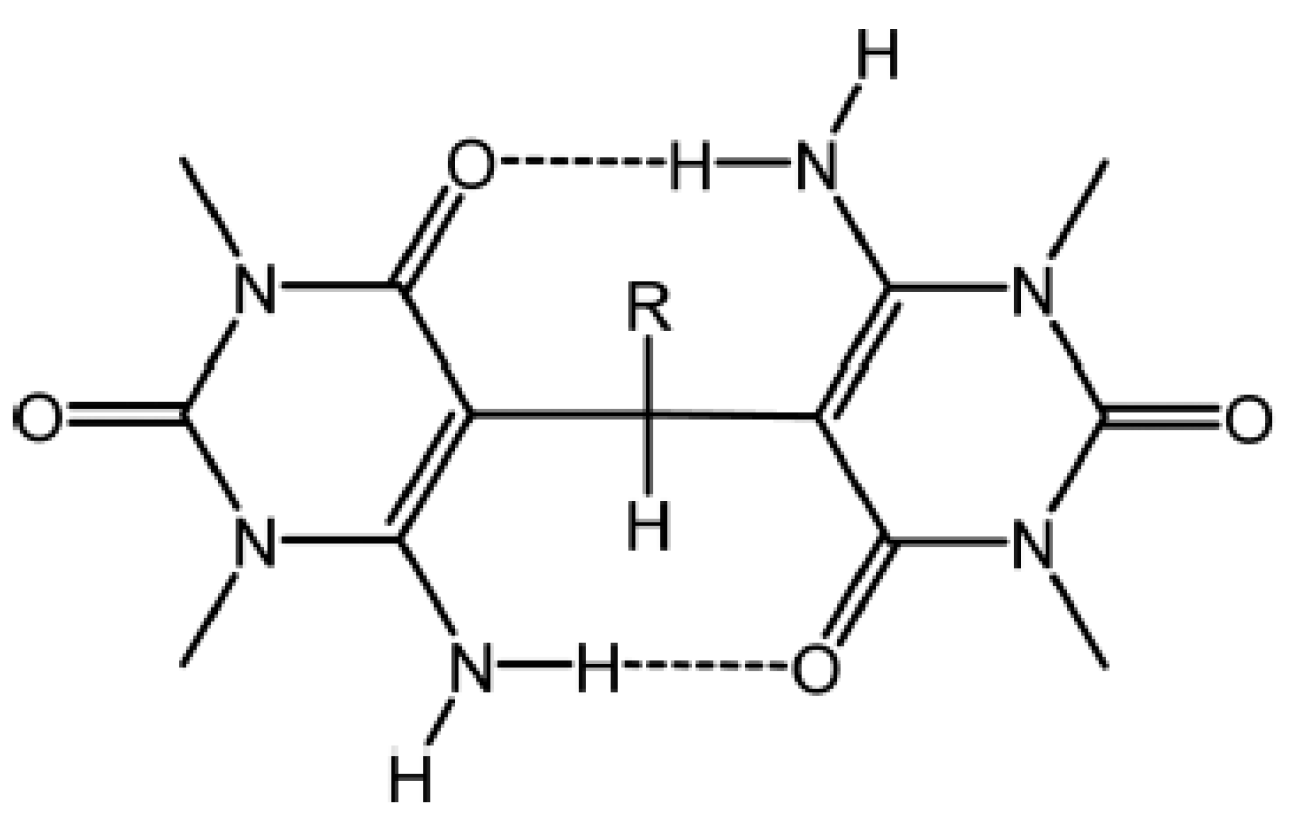
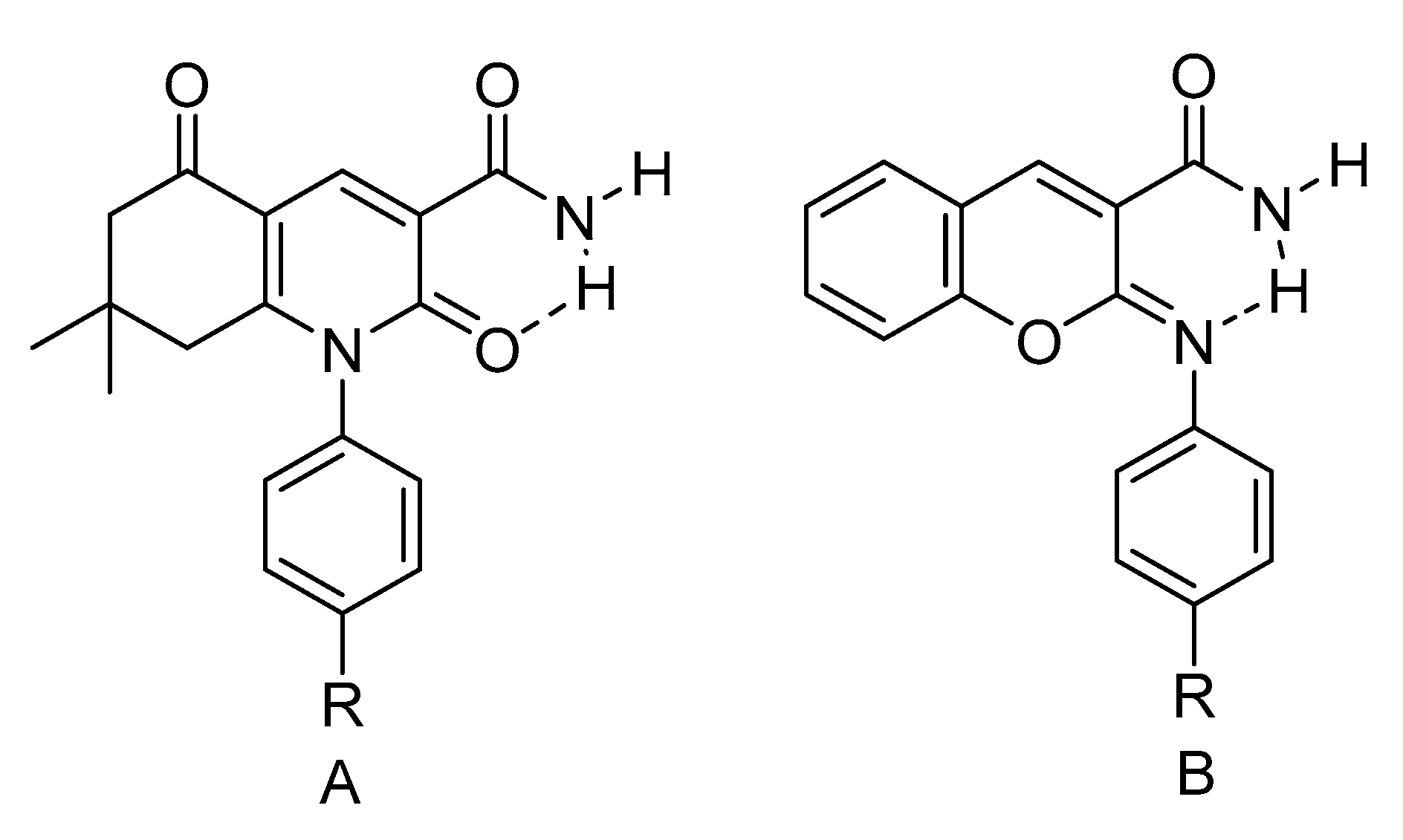
2.4.1. RAHB Cases (a “Double Bond” Connecting Cα and Cβ)
2.4.2. Non-RAHB Cases
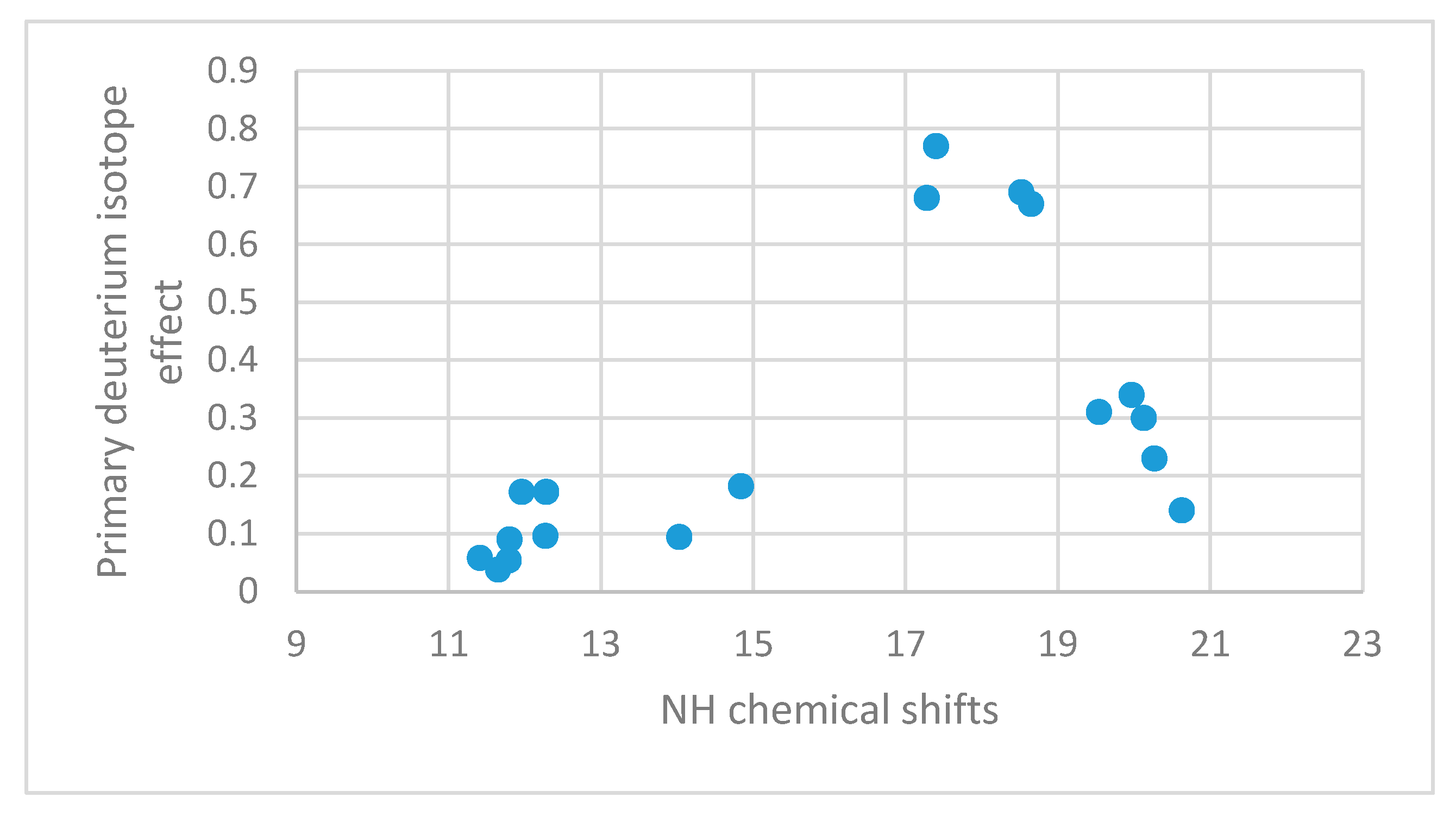
2.4.3. Primary Isotope Effects
3. Energy
4. Hydrogen Bond Strength
5. Assignments
5.1. CD Stretching Frequencies
5.2. Assignments
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinéz, R.F.; Matamoros, E.; Cintas, P.; Palacios, J.C. Imine or Enamine? Insights and Predictive Guidelines from the electronic Effect of Substituents in H-Bonded Salicylimines. J. Org. Chem. 2020, 85, 5838–5862. [Google Scholar] [CrossRef]
- Jezierska, A.; Tolstoy, P.M.; Panek, J.J.; Filarowski, A. Intramolecular Hydrogen Bonds in Selected Aromatic Compounds: Recent Developments. Catalysts 2019, 9, 909. [Google Scholar] [CrossRef]
- Hansen, P.E. Methods to distinguish tautomeric cases from static ones. In Tautomerism: Ideas, Compounds, Applications; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Hansen, P.E.; Spanget-Larsen, J. NMR and IR investigations of strong intramolecular hydrogen bonds. Molecules 2017, 22, 552. [Google Scholar] [CrossRef]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; Filarowski, A. Some Brief Notes on Theoretical and Experimental Investigations of Intramolecular Hydrogen Bonding. Molecules 2016, 21, 1657. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for intramolecular N-H…O Resonance–Assisted Hydrogen Bonding in β-Enaminones and Related Heterodienes. A Combined Crystal-Structural, IR and NMR Spectroscopic, and Quantum-Mechanical Investigation. J. Am. Chem. Soc. 2000, 122, 10405–10417. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Ozeryanskii, V.A.; Filatova, E.A.; Dyablo, O.V.; Pogosa, O.G.; Borodkin, G.S.; Filarowski, A.; Steglenko, D.V. Neutral Pyrrole nitrogen Atom as a π- and Mixed N,π-Donor in Hydrogen Bonding. J. Org. Chem. 2019, 84, 726–737. [Google Scholar] [CrossRef]
- Mikshiev, V.Y.; Pozharskii, A.F.; Filarowski, A.; Novikov, A.S.; Antonov, A.S.; Tolstoy, P.M.; Vovk, M.A.; Khoroshilova, O.V. How Strong is Hydrogen Bonding to Amide Nitrogen? ChemPhysChem 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, L. Quantum effects and 1H NMR chemical shifts of a bifurcated short hydrogen bond. J. Chem. Phys. 2020, 153, 114301. [Google Scholar] [CrossRef]
- Kanbara, T.; Suzuki, Y.; Yamamoto, T. New proton-sponge-like macrocyclic compound: Synergistic hydrogen bonds of aminopyridine. Eur. J. Org. Chem. 2006, 3314–3316. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Pylaeva, S.A.; Tolstoy, P.M. Hydrogen Bonding in Bis(6-amino-1,3-dimehtyluracil-5-yl)-methane Derivatives: Dynamic NMR and DFT Evaluation. J. Phys. Chem. A 2016, 120, 2737–2748. [Google Scholar] [CrossRef]
- Zheglova, D.K.; Genov, D.G.; Bolvig, S.; Hansen, P.E. Deuterium Isotope Effects on 13C Chemical Shifts of Enaminones. Acta Chem. Scand. 1997, 51, 1016–1023. [Google Scholar] [CrossRef]
- Chen, X.; She, J.; Shang, Z.; Wu, J.; Wu, H.; Zhang, P. Synthesis of Pyrazoles, Diazepines, Enaminones, and Enamino Esters Using 12-Tungstenphosphoric Acid as a Reusable Catalyst in Water. Synthesis 2008, 21, 3478–3486. [Google Scholar]
- Kidwai, M.; Bardway, S.; Mishra, N.K.; Bansai, V.; Kumar, A.; Mozumdar, S. A novel method for the synthesis of β-enaminones using Cu-nanoparticles as catalyst. Catal. Commun. 2009, 10, 1514–1519. [Google Scholar] [CrossRef]
- Gholap, A.R.; Chakor, N.S.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. A remarkable rapid regioselective synthesis of β-enaminones using silica chloride in a heterogenouts as well as an ionic liquid in a homogenenous medium at room temperature. J. Mol. Catal. A Chem. 2006, 245, 37–46. [Google Scholar] [CrossRef]
- Lasić, V.; Jurković, M.; Jednacak, T.; Hrenar, T.; Vuković, J.P.; Novak, P. Intra-and intermolecular hydrogen bonding in acetylacetone and benzoylaceton derived enaminone derivatives. J. Mol. Struct. 2015, 1079, 243–249. [Google Scholar] [CrossRef]
- Rodriguez, M.; Santillan, R.; López, Y.; Farrán, N.; Barba, V.; Nakatani, K.; Garcia-Baez, E.V.; Padilla-Martinéz, J.J. N-H…O Assisted Structural Changes Induced on Ketoenamine Systems. Supramol. Chem. 2007, 19, 641–653. [Google Scholar] [CrossRef]
- Hansen, P.E.; Rozwadowski, Z.; Dziembowska, T. NMR Studies of Hydroxy Schiff bases. Curr. Org. Chem. 2009, 13, 194–215. [Google Scholar] [CrossRef]
- Sauer, M.; Yeung, C.; Chong, J.H.; Patrick, B.O.; MacLachalan, M.J. N-Salicylideneanilines: Tautomers for Formation of Hydrogen-Bonded Capsules, Clefts and Chains. J. Org. Chem. 2006, 71, 775–788. [Google Scholar] [CrossRef]
- Hansen, P.E.; Filarowski, A. Characterisation of the PT-form of o-Hydroxy Acylaromatic Schiff bases by NMR Spectroscopy and DFT Calculations. J. Mol. Struct. 2004, 707, 69–75. [Google Scholar] [CrossRef]
- Kwocz, A.; Panek, J.J.; Jezierska, A.; Hetmańczyk, Ł.; Pawlukojć, A.; Kochel, A.; Lipkowski, P.; Filarowski, A. A molecular roundabout: Triple cyclically arranged hydrogen bonds in light of experiment and theory. New J. Chem. 2018, 42, 19467–19477. [Google Scholar] [CrossRef]
- Prbybylski, P.; Wojciechowski, G.; Schilf, W.; Brezezinski, B.; Bartl, F. Spectroscopic study and PM5 semiempirical calculations of tautomeric forms of gossypol Schiff base with n-butylamine in the solid state and in the solution. J. Mol. Struct. 2003, 646, 161–168. [Google Scholar] [CrossRef]
- Quang, T.T.; Phung, K.P.; Hansen, P.E. Schiff Bases of Gossypol. A NMR and DFT Study. Magn. Reson. Chem. 2005, 43, 302–308. [Google Scholar]
- Dobosz, R.; Mućko, J.; Gawinecki, R. Using Chou´s 5-step rule to Evaluate the Stability of Tautomers: Suseptibility of 2-[Phenylimino)-methyl]-cyclohexane-1,3-dione to Tautomerization Based on the Calculated Gibbs Free Energy. Energies 2020, 13, 183. [Google Scholar] [CrossRef]
- Durgarao, N.; Hilemlan, B.; Cox, J.D.; Scott, H.; Hoang, P.; Robbins, A.; Bowers, K.; Tsebaot, L.; Mioa, K.; Cataneda, M.; et al. Acceleration of the Eschenmoser coupling reaction by sonication: Efficient synthes of enaminones. RSC Adv. 2013, 3, 181–188. [Google Scholar]
- Chen, J.-X.; Zhang, C.-F.; Gao, W.-X.; Jin, H.-L.; Dinga, J.-C.; Wu, H.-Y. B2O3/Al2O3 as a New, Highly Efficient and Reusable Heterogeneous Catalyst for the Selective Synthesis of β-Enamino Ketones and Esters under Solvent-Free Conditions. J. Braz. Chem. Soc. 2010, 21, S1–S28. [Google Scholar] [CrossRef][Green Version]
- Jebari, M.; Pasturaud, K.; Picard, B.; Maddaluno, J.; Rezgui, F.; Chataignera, I.; Legros, J. “On water” reaction of deactivated anilines with 4-methoxy-3-buten-2-one, an effective butynone surrogate. Org. Biomol. Chem. 2016, 14, 11085–11087. [Google Scholar] [CrossRef]
- Hansen, P.E.; Duus, F.; Neumann, R.; Jagodzinski, T.S. Deuterium Isotope Effects of 0-Hydroxythioamides, Thiazolines and 5-Acyl-2-thiobarbituric Acids. Pol. J. Chem. 2000, 74, 409–420. [Google Scholar]
- Bai, J.; Wang, P.; Cao, W.; Chen, X. Tautomer-selective derivatives of enolate, ketone and enaminone by addition reaction of picolyl-type anions with nitriles. J. Mol. Struct. 2017, 1128, 645–652. [Google Scholar] [CrossRef]
- Hansen, P.E.; Bolvig, S.; Kappe, T. Intramolecular Hydrogen Bonding and Tautomerism of Acyl Pyran-2,4-diones, 2,4,6-Triones and Pyridiones and Benzannelated Derivatives. Deuterium Isotope Effects on 13C NMR Chemical Shifts. J. Chem. Soc. Perkin Trans. 1995, 2, 1901–1907. [Google Scholar] [CrossRef]
- Hansen, P.E.; Duus, F.; Bolvig, S.; Jagodzinski, T.S. Intra-molecular Hydrogen Bonding of the Enol forms of β-Ketoamides and β-Ketothioamides. Deuterium Isotope Effects on 13C Chemical Shifts. J. Mol. Struct. 1996, 378, 45–59. [Google Scholar] [CrossRef]
- Kozerski, L.; Kawecki, R.; Hansen, P.E. Data for Characterization of the Geometrical Isomers of β -Sulfinylenamines. Magn. Reson. Chem. 1994, 32, 517–524. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef] [PubMed]
- Landge, S.M.; Thatchouuk, E.; Benitez, D.; Lanfranchi, D.A.; Elhabari, M.; Goodard, W.A., III; Aprahamian, I. Isomerization Mechanism in Hydrazone-Based Rotary Swithches: Lateral Shift, Rotation, or Tautomerization? J. Am. Chem. Soc. 2011, 133, 9812–9823. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.H.; Lampkin, B.J.; Venkateh, A.; Ellern, A.; VanVeller, B. Open-Resonance-assisted Hydrogen Bonds and Competing quasiaromaticity. J. Org. Chem. 2018, 83, 9850–9857. [Google Scholar] [CrossRef]
- Hristova, S.; Kamounah, F.S.; Molla, N.; Hansen, P.E.; Nedeltcheva, D.; Antonov, L. The possible tautomerism of the rotary switch 2-(2-(2-Hydroxy-4-nitrophenyl)hydrazono)-1-phenylbutane-1,3dione. Dyes Pigments 2017, 144, 249–261. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Switching Around Two Axles: Controlling the Configuration and Conformation of a Hydrazone-Based Switch. Org. Lett. 2011, 13, 30–33. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.; Strashnikova, N.; Sterkhova, I. Moleclular Structure and Photoinduced Hydrogen Bonding in 2-pyrrolemethylidene Cyclohexanones. J. Org. Chem. 2015, 80, 10521–10535. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Takeda, Y.; Haketa, Y.; Morimoto, Y.; Yasuda, N. Ion-pairing Assemblies of p-Electronic Anions formed by Intramolecular Hydrogen bonding. Chem. Eur. J. 2018, 24, 8910–8916. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P. Molecular Structure, Intramolecular Hydrogen Bonding, Solvent-Induced Isomerization, and Tautomerism in Azoylmethylidene Derivatives of 2-Indanone. Eur. J. Org. Chem. 2017, 1353–1364. [Google Scholar] [CrossRef]
- Ladarevic, J.; Bozic, B.; Natovic, L.; Nedeljkovic, B.B.; Minj, D. Role of the bifurcated intramolecular hydrogen bond on the physico-chemical profile of the novel azo pyridone dyes. Dyes Pigments 2019, 162, 562–572. [Google Scholar] [CrossRef]
- Diembowska, T.; Rozwadowski, A.; Hansen, P.E. Intramolecular Hydrogen bonding of 8- hydroxyquinoline N-oxides, Quinaldinic Acid N-oxides and Quinaldinamide N-oxide. Deuterium Isotope Effects on 13C Chemical Shifts. J. Mol. Struct. 1997, 436–437, 189–199. [Google Scholar] [CrossRef]
- Saeed, B.A.; Elias, R.S.; Kamounah, F.S.; Hansen, P.E. A NMR, MP2 and DFT Study of Thiophenoxyketenimines (o-ThioSchiff bases). Magn. Reson. Chem. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, J.L.; Arnold, J.; Bergman, R.G. Platinum Group Thiophenoxyimine Complexes, Syntheses and Crystallographic/Computational Studies. Organometalics 2007, 26, 897–909. [Google Scholar] [CrossRef]
- Corrigan, M.F.; Rae, I.D.; West, B.O. The Constitution of N-Substituted 2-(Iminomethyl) benzenethiols (o-mercaptobenzaldimines). Aust. J. Chem. 1978, 31, 587–594. [Google Scholar] [CrossRef]
- Feng, Z.; Jia, S.; Chen, H.; You, L. Modulation of imine chemistry with intramolecular hydrogen bonding. Effects from ortho-OH to NH. Tetrahedron 2020, 76, 131128. [Google Scholar] [CrossRef]
- Froimowicz, P.; Zhang, K.; Ishida, H. Intramolecular Hydrogen Bonding in Benzoxazines: When Structural design becomes functional. Chem. Eur. J. 2016, 22, 2691–2707. [Google Scholar] [CrossRef]
- Arya, N.; Mishra, S.K.; Suryaprakash, M. Intramolecular hydrogen bond directed distribution of conformational populations in the derivatives of N´-benzylidenebenzohydrazide. New. J. Chem. 2019, 43, 13134–13142. [Google Scholar] [CrossRef]
- Ren, Y.; Kravchenko, O.; Ramström, O. Configurational and Constitutional Dynamics of Enamine Molecular Swithches. Chem. Eur. J. 2020, 26, 15654–15663. [Google Scholar] [CrossRef]
- Petrova, M.; Mahamadejev, R.; Vigante, B.; Duburs, G.; Liepinsh, E. Intramolecular hydrogen bonds in 1,4-dihydropyridine derivatives. R. Soc. Open. Sci. 2018, 5, 1800088. [Google Scholar]
- Piękoś, P.; Jezierska, A.; Panek, J.J.; Goremychkin, E.A.; Pozharskii, A.F.; Antonov, A.S.; Tolstoy, P.M.; Filarowski, A. Symmetry/Asymmetry of the NHN Hydrogen Bond in Protonated 1,8-Bis(dimethylamino)naphthalene. Symmetry 2020, 12, 1924. [Google Scholar] [CrossRef]
- Vlasenko, M.P.; Ozeryanski, V.A.; Pozharskii, A.F.; Khoroshilova, O.V. Positively and negatively charged NHN hydrogen bonds in one molecule: Synergistic strengthening effect superbasicity and acetonitrile capture. New J. Chem. 2019, 43, 7557–7561. [Google Scholar] [CrossRef]
- Dega-Szafran, Z.; Nowak-Wydra, B.; Szafran, M. Reinvestigtion of 1H and 13C NMR Spectra of 1,8-Bis(dimethylamino)naphthalene Complexes with Mineral Acids. Magn. Reson. Chem. 1993, 31, 726–730. [Google Scholar] [CrossRef]
- Pietrzak, M.; Grech, E.; Nowicka-Scheibe, J.; Hansen, P.E. Deuterium isotope effects on 13C chemical shifts of negatively charged NH…N systems. Magn. Reson. Chem. 2013, 51, 683–688. [Google Scholar]
- Yamakoda, R.; Ishibashi, H.; Motoyoshi, Y.; Yasuda, N.; Maeda, H. Ion-pairing assemblies based on p-extended dipyrrolylquinoxalines. Chem. Commun. 2019, 55, 326–329. [Google Scholar] [CrossRef]
- Pietrzak, M.; Try, A.C.; Andriolette, B.; Sessler, J.L.; Anzenbacher, P., Jr.; Limbach, H.-H. The largest 15N-15N Coupling Constant across an NHN Hydrogen bond. Angew. Chem. Int. Ed. 2008, 47, 1123–1126. [Google Scholar] [CrossRef]
- Osmialowski, B. Conformational equilibrium and substituent effects in hydrogen bonded complexes. Curr. Org. Chem. 2018, 22. [Google Scholar] [CrossRef]
- Sosnicki, J.G.; Hansen, P.E. Temperature coefficients of NH chemical Shifts of Thioamides in Relation to Structure. Mol. Struct. 2004, 700, 91–103. [Google Scholar] [CrossRef]
- Desseyn, H.O.; Perlepes, S.P.; Clou, K.; Blaton, N.; van der Veken, B.J.; Dommisse, R.; Hansen, P.E. Theoretical, Structural, Vibribrational, NMR and Thermal evidence of the Inter versus Intramolecular Hydrogen Bonding in Oxamides and Thiooxamides. J. Phys. Chem. A 2004, 108, 175–182. [Google Scholar] [CrossRef]
- Scheiner, S. Assesment of the Presence and Strength of H-Bonds by Means of Corrected NMR. Molecules 2016, 21, 1426. [Google Scholar] [CrossRef] [PubMed]
- Dudek, G.; Dudek, E.P. Spectroscopic Studies of Keto-Enol Equilibria. Part XIII. 15N Substituted Imines. J. Chem. Soc. B 1971, 1356–1360. [Google Scholar] [CrossRef]
- Hansen, P.E.; Saeed, B.A.; Rutua, R.S.; Kupka, T. One-bond 1J(15N,H) coupling constants at sp2-hybridized nitrogen of Schiff bases, enaminones and similar compounds: A theoretical study. Magn. Reson. Chem. 2020, 58, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Dingley, A.J.; Grzesiek, S. Direct observation of hydrogen bonds in nucleic acid base pairs by internucleotide (2)J(NN) couplings. S. J. Am. Chem. Soc. 1998, 120, 8293–8297. [Google Scholar] [CrossRef]
- Dingley, A.J.; Masse, J.E.; Peterson, R.D.; Barfield, M.; Feigon, J.; Grzesiek, S. Internucleotide Scalar Couplings across Hydrogen Bonds in Watson-Crick and Hoogsteen Base Pairs of a DNA Triplex. J. Am. Chem. Soc. 1999, 121, 6019–6027. [Google Scholar] [CrossRef]
- Grzesiek, S.; Cordier, F.; Jaravine, V.; Barfield, M. Insights into biomolecular hydrogen bonds from hydrogen bond scalar couplings. Prog. Nuc. Magn. Reson. Spectrosc. 2004, 45, 275–300. [Google Scholar] [CrossRef]
- Cornilescu, G.; Hu, J.S.; Bax, A. Identification of the hydrogen bonding network in a protein by scalar couplings. J. Am. Chem. Soc. 1999, 121, 2949–2950. [Google Scholar] [CrossRef]
- Cornilescu, G.; Ramirez, B.E.; Frank, M.K.; Clore, G.M.; Gronenborn, A.M.; Bax, A. Correlation between 3hJNC′ and Hydrogen Bond Length in Proteins. J. Am. Chem. Soc. 1999, 121, 6275–6279. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Jacob, J.; Cordier, F.; Wingfield, P.; Stahj, S.J.; Lee-Huang, S.; Torchia, D.; Grzesiek, S.; Bax, A. Measurement of 3hJNC′ connectivities across hydrogen bonds in a 30 kDa protein. J. Biomol. NMR 1999, 14, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Zandarashvili, L.; Li, D.-W.; Wang, T.; Brüschweiler, R.; Iwahara, J. Signature of Mobile Hydrogen Bonding of Lysine Side Chains from Long-Range 15N–13C Scalar J-Couplings and Computation. J. Am. Chem. Soc. 2011, 133, 9192–9195. [Google Scholar] [CrossRef]
- Manalo, M.N.; Kong, X.; LiWang, A. 1JNH Values show that N1...N3 Hydrogen Bonds are stronger in dsRNA A:U than dsDNA A:T Base pairs. J. Am. Chem. Soc. 2005, 127, 17974–17975. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Esadze, A.; Roy, S.; Iwahara, J. NMR Scalar Couplings across Intermolecular Hydrogen Bonds between Zinc-Finger Histidine Side Chains and DNA Phosphate Groups. J. Phys. Chem. B 2016, 120, 10679–10685. [Google Scholar] [CrossRef]
- Del Bene, J. Two-Bond NMR Spin–Spin Coupling Constants across Hydrogen Bonds. Encyclopedia of Theoretical Chemistry; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Sobczyk, L.; Obzrud, M.; Filarowski, A. H/D Isotope Effects in Hydrogen Bonded Systems. Molecules 2013, 18, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Kozerski, L.; von Philipsborn, W. N-15 NMR spectroscopy. 9. Solvent induced Deuterium isotope effects in C-13 NMR and N-15 NMR spectra of enaminones. Helv. Chim. Acta 1982, 65, 2077–2087. [Google Scholar] [CrossRef]
- Hansen, P.E. Isotope effects on chemical shift in the study of intramolecular hydrogen bonds. Molecules 2015, 20, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Kawecki, R.; Krowczynski, A.; Kozerski, L. Deuterium Isotope Effects on 13C and 15N Nuclear Shielding in Intramoleculary Hydrogen-bonded Compounds. II. Investigation of Enamine Derivatives. Acta Chem. Scand. 1990, 44, 826–832. [Google Scholar] [CrossRef]
- Tomlinson, J.H.; Ullah, S.; Hansen, P.E.; Williamson, M.P. Characterisation of salt bridges to lysines in the Protein G B1 domain. J. Am. Chem. Soc. 2009, 131, 4674–4684. [Google Scholar] [CrossRef]
- Williamson, M.P.; Hounslow, A.M.; Ford, J.; Fowler, K.; Hebditch, M.; Hansen, P.E. Detection of salt bridges to lysines in barnase in solution. Chem. Commun. 2013, 49, 9824–9826. [Google Scholar] [CrossRef]
- Ullah, S.; Ishimoto, T.; Williamson, M.P.; Hansen, P.E. Ab initio Calculations of Deuterium Isotope Effects on Chemical Shifts of Salt bridged lysines. J. Phys. Chem. B 2011, 115, 3208–3215. [Google Scholar] [CrossRef]
- Abildgaard, J.; LiWang, A.; Manalo, M.N.; Hansen, P.E. Peptide Deuterium Isotope Effects on Backbone 15N Chemical Shifts in Proteins. J. Biomol. NMR 2009, 44, 119–124. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.-I.; Manalo, M.N.; Peréz, L.M.; LiWang, A. Computation and empirical trans-hydrogen bond deuterium isotope shifts suggest that n1-N3 A.U hydrogen bonds of RNA are shorter than those of A:T bonds of DNA. J. Biomol. NMR 2006, 34, 229–236. [Google Scholar] [CrossRef]
- Manalo, M.N.; Pérez, L.M.; LiWang, A. Hydrogen-Bonding and π-π Base-stacking Interactions are Coupled in DNA as Suggested by Calculated and Experimental Trans-Hbond Deuterium Isotope Shifts. J. Am. Chem. Soc. 2007, 129, 11298–11299. [Google Scholar] [CrossRef]
- Sosnicki, J.G.; Langaard, M.; Hansen, P.E. Long-range Deuterium Isotope Effects on 13C chemical Shifts of intramolecularly hydrogen bonded N-substituted-3-(cycloamino) thiopropionamides or amides: A case of electric field effects. J. Org. Chem. 2007, 72, 4108–4116. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E.; Morimoto, H.; Wemmer, D.; Williams, P. Primary Tritium and Deuterium Isotope Effects on Chemical Shifts of Compounds having an Intramolecular Hydrogen bond. Magn. Reson. Chem. 2000, 38, 525–535. [Google Scholar] [CrossRef]
- Chmielewski, P.; Ozeryanski, V.A.; Sobczyk, L.; Pozharskii, A.F. primary 1H/2H isotope effect in the NMR chemical shift of HClO4 salts of 1,8-bis(dimethylamino)naphthalene derivatives. J. Phys. Org. Chem. 2007, 20, 643–648. [Google Scholar] [CrossRef]
- Pozharski, A.F.; Ryabtsova, O.V.; Ozeryanski, V.A.; Degtyarev, A.V.; Kazheva, O.N.; Alexandrov, G.G.; Dyanchenko, O.A. Organometallic synthesis, molecular structure, and coloration of 2,7-disubstituted 1,8-bis(dimethylamino)naphthalenes. How significant is the influence of “buttressing effect” on their basicity? J. Org. Chem. 2003, 69, 10109–10122. [Google Scholar] [CrossRef] [PubMed]
- Reuben, J. Isotopic Multiplets in the Carbon-13 NMR Spectra of Aniline Derivatives and Nucleosides with Partially Deuterated Amino Groups: Effects of Intra- and Intermolecular Hydrogen Bonding. J. Am. Chem. Soc. 1987, 109, 316–321. [Google Scholar] [CrossRef]
- Schaefer, T. A Relationship between Hydroxy Proton Chemical Shifts and Torsional Frequencies in Some ortho-Substituted Phenol Derivatives. J. Phys. Chem. 1975, 79, 1888–1890. [Google Scholar] [CrossRef]
- Chiara, J.L.; Gomez-Sanchez, A.; Bellanato, J. Spectral properties and isomerism of nitroenamines. Part 4. β-Amino-α-nitro-α,β-unsaturated ketones. J. Chem. Soc. Perkin Trans. 1998, 2, 1797–1806. [Google Scholar] [CrossRef][Green Version]
- Tupikina, E.Y.; Sigalov, M.; Shenderovich, I.G.; Mulloyarova, V.V.; Denisov, G.S.; Tolstoy, P.M. Correlations of NHN hydrogen bond energy with geometry and 1H NMR chemical shift difference of NH protons for aniline complexes. J. Chem. Phys. 2019, 150, 114305. [Google Scholar] [CrossRef] [PubMed]
- Cuma, M.; Scheiner, S.; Kar, T. Competition between rotamerization and proton transfer in o-hydroxybenzaldehyde. J. Am. Chem. Soc. 1998, 120, 10497–10503. [Google Scholar] [CrossRef]
- Jablonski, M.; Kaczmarek, A.; Sadlej, S.J. Estimates of the Energy of Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef]
- Seyedkatouli, S.; Vakili, M.; Tayyari, S.F.; Hansen, P.E.; Kamounah, F. SMolecular structure, vibrational analysis, and intramolecular hydrogen bond strength (IHBs) of 3-methyl-4-amino-3-penten-2-one and the effect of N-methyl and N-phenyl substitutions on the IHBs. An Experimental and Theoretical approach. J. Mol. Struct. 2019, 1184, 233–245. [Google Scholar] [CrossRef]
- Gholipur, A.; Neyband, R.S.; Farhadi, S. NMR investigation of substituent effects on strength the intramolecular hydrogen bonding interaction in X-phenylhydrazones switches: A theoretical study. Chem. Phys. Lett. 2017, 676, 6–11. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of Ylides Containing N, O, and C Atoms as Hydrogen Bond Acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. [Google Scholar] [CrossRef]
- Hansen, P.E.; Tüchsen, E. Deuterium Isotope Effects on Carbonyl Chemical Shifts of BPTI. Hydrogen-bonding and Structure Determination in Proteins. Acta Chem. Scand. 1989, 43, 710–712. [Google Scholar] [CrossRef]
- Tüchsen, E.; Hansen, P.E. Hydrogen bonding monitored by deuterium isotope effects on carbonyl 13C chemical shift in BPTI: Intra-residue hydrogen bonds in antiparallel β-sheet. Int. J. Biol. Macromol. 1991, 13, 2–8. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Saeed, B.A.; MacLachlan, M.J.; Spanget-Larsen, J. Intramolecular Hydrogen Bonds in Normal and Sterically Compressed o-Hydroxy Aromatic Aldehydes. Isotope Effects on Chemical Shifts and Hydrogen Bond Strength. Molecules 2019, 24, 4533. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Bader, R.W.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Kieth, T.A. AIMAll (Version 16.10.31); TK Gristmill Software: Overland Park, KS, USA, 2016. [Google Scholar]
- Hansen, P.E.; Bolvig, S.; Duus, F.; Petrova, M.V.; Kawecki, R.; Kozerski, L. Deuterium Isotope Effects on 13C Chemical Shifts of Intramolecularly Hydrogen-bonded Olefins. Magn. Reson. Chem. 1995, 33, 621–631. [Google Scholar] [CrossRef]
- Chiara, J.L.; Gomez-Sanchez, A.; Sanchez Marcos, E. Spectral Properties and Isomerism of Nitro Enamines. Part 2. 3-Amino-2-nitrocrotronic Esters. J. Chem. Soc. Perkin Trans. 1990, 2, 385–392. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting Hydrogen-Bond Strength from Acid-Base Molecular Properties. The pKa Slide Rule: Toward the Solution of a Long-Lasting Problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Gorobets, N.Y.; Yermolayev, S.A.; Gurley, T.; Gurinov, A.A.; Tolstoy, P.M.; Shenderovich, I.G.; Leadbeater, N.E. Difference between 1H NMR signals of primary amide protons as a simple spectral index of the amide intramolecular hydrogen bond strength. J. Phys. Org. Chem. 2012, 25, 287–295. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Martyniak, A.; Majerz, I.; Filarowski, A. Pecularities of quasi-aromatic hydrogen bonding. RSC Adv. 2012, 2, 8135–8144. [Google Scholar] [CrossRef]
- Adhikary, R.; Zimmermann, J.; Liu, J.; Forrest, R.P.; Janicki, T.D.; Dawson, P.E.; Corcelli, S.A.; Romesberg, F.E. Evidence of an unusual N-H…N Hydrogen Bond in Proteins. J. Am. Chem. Soc. 2014, 136, 13474–13477. [Google Scholar] [CrossRef]
- Novak, A. Hydrogen Bonding in Solids. Correlation of Spectroscopic and Crystallographic Data. Struct. Bond. 1974, 18, 177–216. [Google Scholar]
- Grech, E.; Malarski, Z.; Sobczyk, L. Isotope Effects in NH.N Hydrogen bonds. Chem. Phys. Lett. 1986, 128, 259–263. [Google Scholar] [CrossRef]




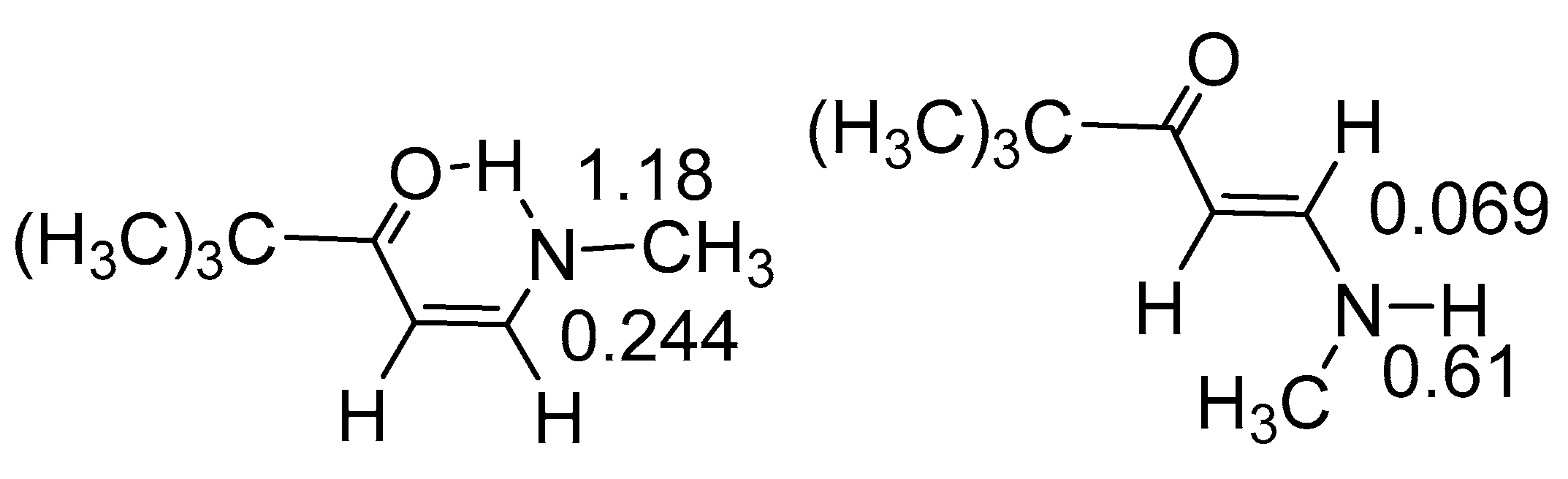
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, P.E. A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type. Molecules 2021, 26, 2409. https://doi.org/10.3390/molecules26092409
Hansen PE. A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type. Molecules. 2021; 26(9):2409. https://doi.org/10.3390/molecules26092409
Chicago/Turabian StyleHansen, Poul Erik. 2021. "A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type" Molecules 26, no. 9: 2409. https://doi.org/10.3390/molecules26092409
APA StyleHansen, P. E. (2021). A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type. Molecules, 26(9), 2409. https://doi.org/10.3390/molecules26092409