Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean)
Abstract
1. Introduction
2. Results
2.1. General Biochemical Characteristics
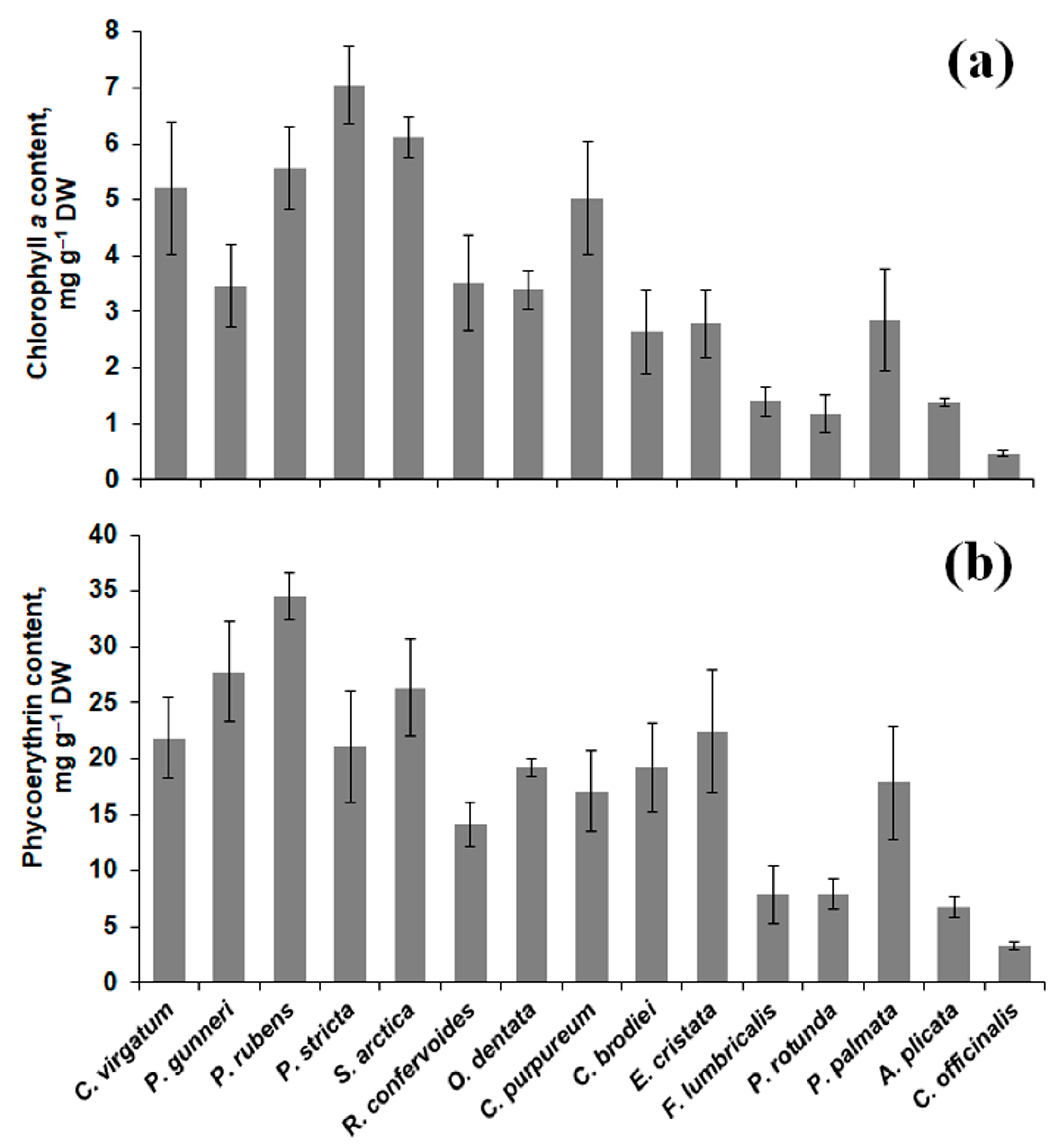
2.2. Pigment Content
2.3. Mineral Composition
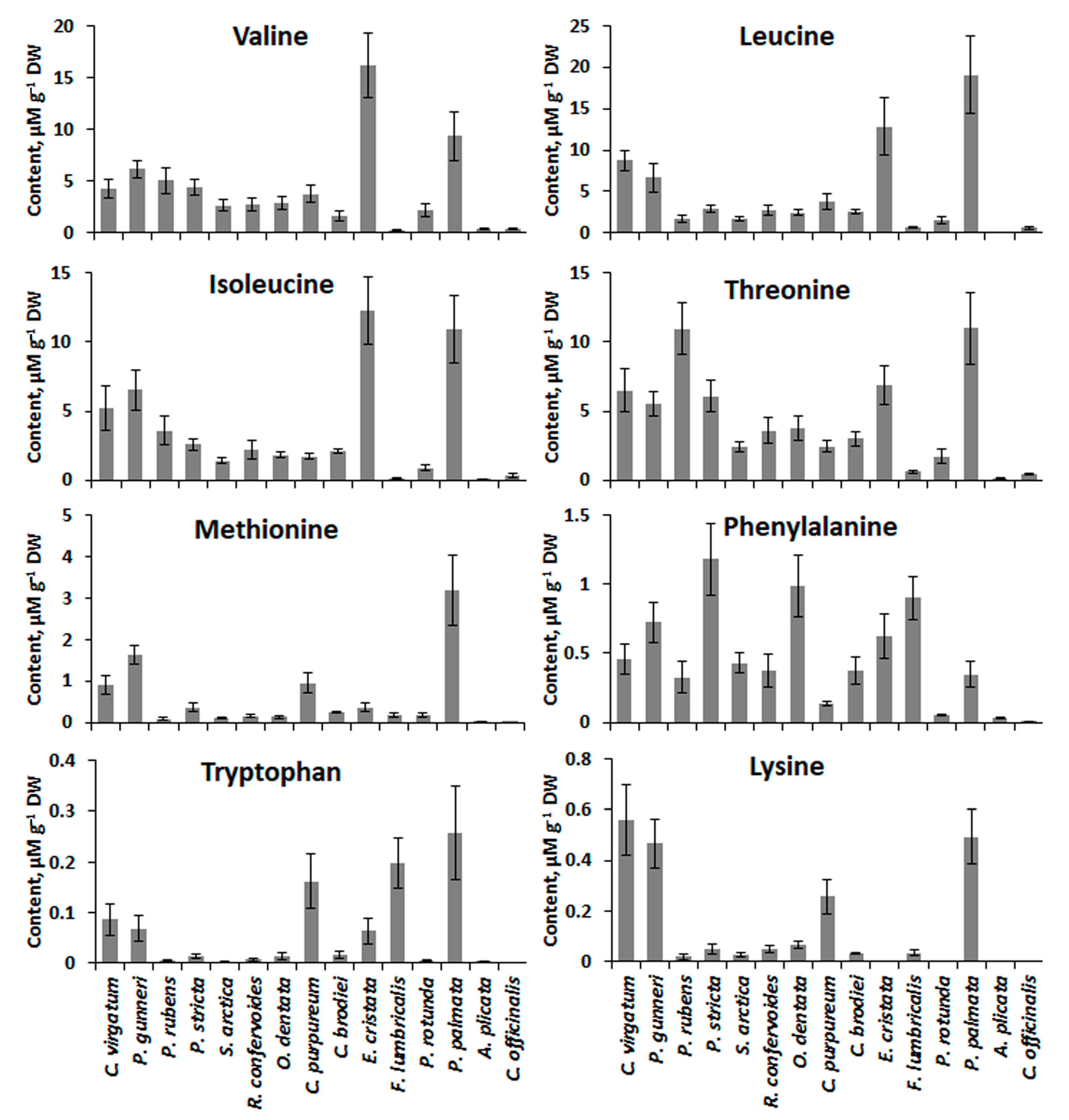
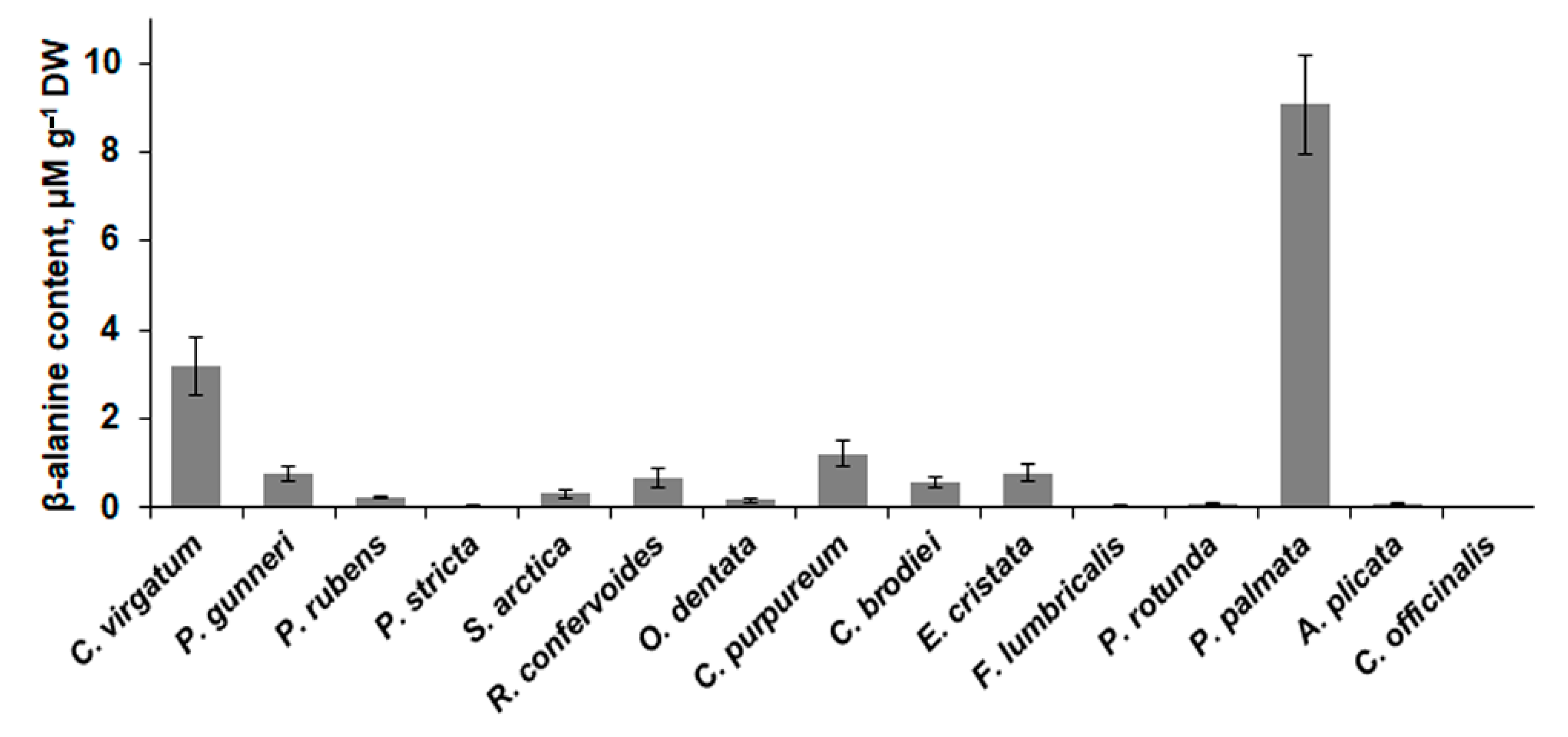
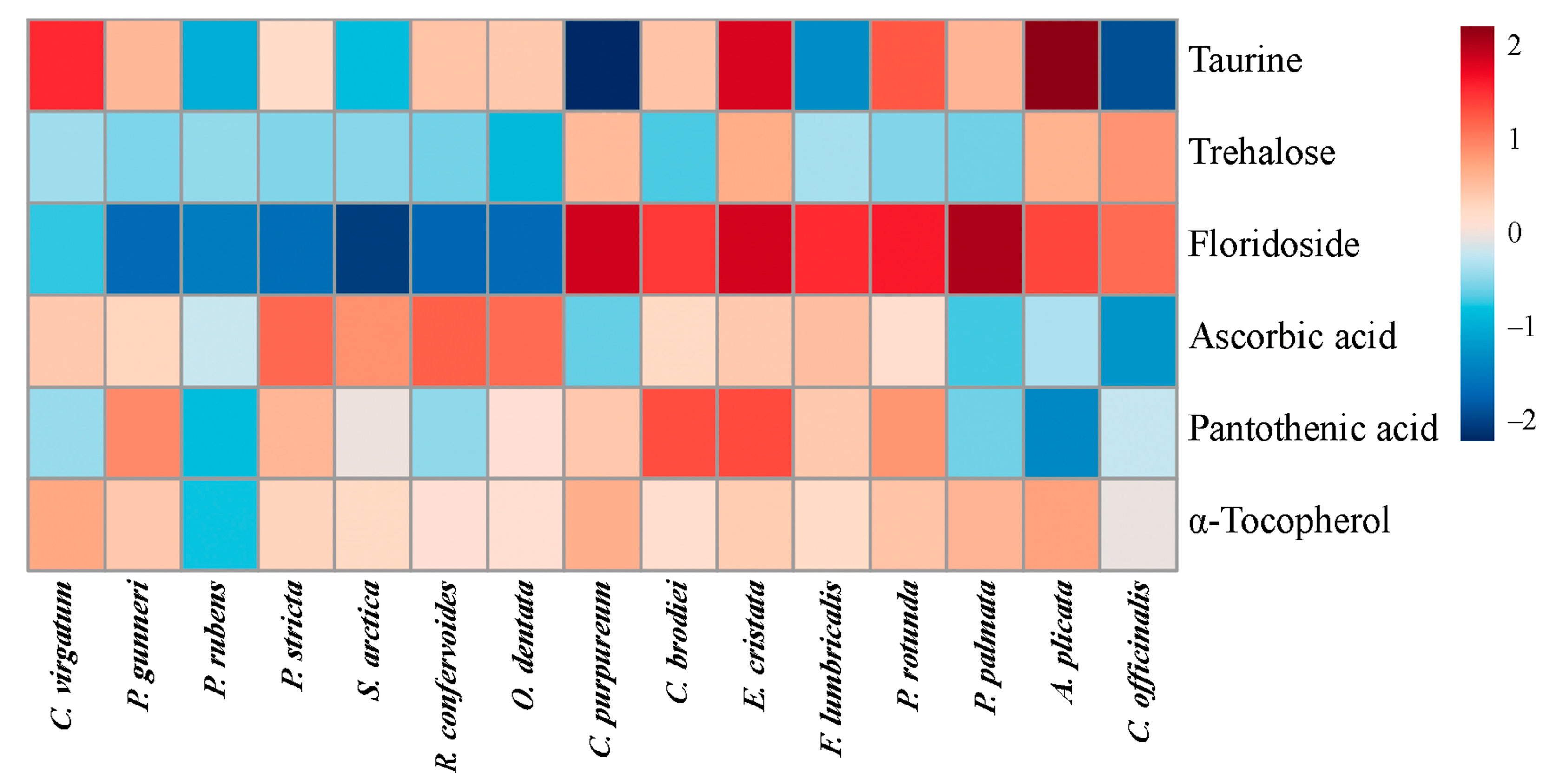
2.4. Low-Molecular-Weight Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection
4.2. Fresh Weight and Dry Weight Determination
4.3. Elemental Analysis
4.4. Carbohydrate Analysis
4.5. Protein Analysis
4.6. Pigment Analysis
4.7. Metabolite Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Lins, K.O.A.L.; Bezerra, D.P.; Alves, A.P.N.N.; Alencar, N.M.N.; Lima, M.W.; Torres, V.M.; Farias, W.R.L.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2008, 29, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Kasanah, N.; Hadisaputro, T.; Seto, D.S.; Amelia, W.; Isnansetyo, A. Antibacterial compounds from red seaweeds (Rhodophyta). Indones. J. Chem. 2015, 15, 201–209. [Google Scholar] [CrossRef]
- Kim, C.O.; Kim, Y.N.; Lee, D.C. Effects of Gelidium elegans on weight and fat mass reduction and obesity biomarkers in overweight or obese adults: A randomized double-blinded study. Nutrients 2019, 11, 1513. [Google Scholar] [CrossRef]
- Sørensen, L.E.; Jeppesen, P.B.; Christiansen, C.B.; Hermansen, K.; Gregersen, S. Nordic seaweed and diabetes prevention: Exploratory studies in KK-Ay mice. Nutrients 2019, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Biores. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Iqbal, N.; Mir, R.A.; Adeel, S.; Batool, F.; Khan, A.A.; Gul, S. Harnessing natural colorants from algal species for fabric dyeing: A sustainable eco-friendly approach for textile processing. J. Appl. Phycol. 2019, 31, 3941–3948. [Google Scholar] [CrossRef]
- Corey, P.; Kim, J.K.; Garbary, D.J.; Prithiviraj, B.; Duston, J. Bioremediation potential of Chondrus crispus (Basin Head) and Palmaria palmata: Effect of temperature and high nitrate on nutrient removal. J. Appl. Phycol. 2012, 24, 441–448. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S. Useful substances of marine red algae (Rhodophyta): Chemical structure and content. Izvestiya TINRO 2011, 165, 305–319. (In Russian) [Google Scholar]
- Lahaye, M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some “Sea vegetables”. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Seaweed proteins, peptides, and amino acids. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: London, UK, 2015; pp. 125–140. [Google Scholar] [CrossRef]
- Morgan, K.C.; Wright, J.L.C.; Simpson, F.J. Review of chemical constituents of the red alga Palmaria palmata (Dulse). Econ. Bot. 1980, 34, 27–50. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing, 7th ed.; Yada, R.Y., Ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 245–262. [Google Scholar] [CrossRef]
- Ito, K.; Hori, K. Seaweed: Chemical composition and potential food uses. Food Rev. Int. 1989, 5, 101–144. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Eggert, A.; Karsten, U. Low molecular weight carbohydrates in red algae—An ecophysiological and biochemical perspective. In Cellular Origins, Life in Extreme Habitats and Astrobiology. Red Algae in the Genomics Age; Seckbach, J., Chapman, D., Weber, A., Eds.; Springer: Berlin, Germany, 2010; pp. 445–456. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.-K., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 173–192. [Google Scholar] [CrossRef]
- Mikhaylova, T.A. Vegetation of the red algal belt of the White Sea (European Arctic, Russia). Nov. Sist. Nizshikh Rastenii 2019, 53, 39–65. [Google Scholar] [CrossRef]
- Mikhaylova, T.A. Checklist of Rhodophyta of the White Sea (the Arctic Ocean). Bot. Mar. 2017, 60, 55–65. [Google Scholar] [CrossRef]
- Garbary, D.J.; Tarakhovskaya, E.R. Marine macroalgae and associated flowering plants from the Keret Archipelago, White Sea, Russia. Algae 2013, 28, 267–280. [Google Scholar] [CrossRef]
- Mikhaylova, T.A.; Aristov, D.A.; Naumov, A.D.; Malavenda, S.S.; Savchenko, O.N.; Bijagov, K.L. Diversity and structure of epibenthic communities of the red algae zone in the White Sea. Polar Biol. 2019, 42, 953–968. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Morales, J.L. Antibacterial activity in marine algae from the coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Jeong Han, E.; Priyan Shanura Fernando, I.; Kim, E.-A.; Kim, J.; Jung, K.; Kim, S.-Y.; Cha, S.-H.; Kim, K.-N.; Heo, S.-J.; Ahn, G. 5-Bromo-3,4-dihydroxybenzaldehyde from Polysiphonia morrowii attenuate IgE/BSA-stimulated mast cell activation and passive cutaneous anaphylaxis in mice. Biochem. Pharmacol. 2020, 114087. [Google Scholar] [CrossRef]
- Dong, H.; Dong, S.; Erik Hansen, P.; Stagos, D.; Lin, X.; Liu, M. Progress of bromophenols in marine algae from 2011 to 2020: Structure, bioactivities, and applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef]
- Sampaio, A.H.; Rogers, D.J.; Barwell, C.J.; Saker-Sampaio, S.; Nascimento, K.S.; Nagano, C.S.; Farias, W.R.L. New affinity procedure for the isolation and further characterization of the blood group B specific lectin from the red marine alga Ptilota plumosa. J. Appl. Phycol. 2002, 14, 489–495. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Černá, M. Seaweed proteins and amino acids as nutraceuticals. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 297–312. [Google Scholar] [CrossRef]
- Macpherson, M.G.; Young, E.G. The chemical composition of marine algae. Can. J. Res. 1949, 27, 73–77. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.P.; Villaume, C.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Ramos, M.V.; Monteito, A.C.O.; Moreira, R.A.; Carvalho, A. Amino acid composition of some Brazilian seaweed species. J. Food Biochem. 2000, 24, 33–39. [Google Scholar] [CrossRef]
- Marsham, S.; Scot, G.W.; Tobin, M.L. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef]
- Dere, S.; Dalkiran, N.; Karacaoglu, D.; Yildiz, G.; Dere, E. The determination of total protein, total soluble carbohydrate and pigment contents of some macroalgae collected from Gemlik-Karacaali (Bursa) and Erdek-Ormanli (Balikesir) in the Sea of Marmara. Turk. Oceanol. 2003, 45, 453–471. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional properties of plant lectins. Toxicon 2004, 44, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Birkemeyer, C.; Lemesheva, V.; Billig, S.; Tarakhovskaya, E. Composition of intracellular and cell wall-bound phlorotannin fractions in fucoid algae indicates specific functions of these metabolites dependent on the chemical structure. Metabolites 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Hori, K. Marine algal lectins: New developments. Hydrobiologia 1993, 260, 589–593. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Mangiafico, S.; Oriente, G.; Piattelli, M.; Sciuto, S. Amino acids and low-molecular-weight carbohydrates of some marine red algae. Phytochemistry 1975, 14, 1549–1557. [Google Scholar] [CrossRef]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic assessment of induced and activated chemical defense in the invasive red alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, e29359. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber a Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- Mai, K.; Mercer, J.P.; Donlon, J. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus Hannai Ino. II. Amino acid composition of abalone and six species of macroalgae with an assessment of their nutritional value. Aquaculture 1994, 128, 115–130. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100–109. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Pons, L.; Luçon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef]
- Martínez, B.; Rico, J.M. Seasonal variation of P content and major N pools in Palmaria palmata (Rhodophyta). J. Phycol. 2002, 38, 1082–1089. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.; Hurrell, R.F. Bioavailability of minerals and trace elements. Nutr. Res. Rev. 1996, 9, 295–324. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R.; Whittaker, N.F. Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. J. Agric. Food Chem. 1986, 34, 603–616. [Google Scholar] [CrossRef]
- Perucka, I.; Olszówka, K.; Chilczuk, B. Changes in the chlorophyll content in stored lettuce Lactuca sativa L. after pre-harvest foliar application of CaCl2. Acta Agrobot. 2013, 66, 137–142. [Google Scholar] [CrossRef]
- Kaliaperumal, N.; Chennubhotla, V.; Kalimuthu, S.; Ramalingam, J.; Selvaraj, M.; Najmuddin, M. Chemical composition of seaweeds. Cent. Mar. Fish. Res. Inst. CMFRI Bull. 1987, 41, 31–99. [Google Scholar]
- Frikha, F.; Kammoun, M.; Hammami, N.; Mchirgui, R.A.; Belbahri, L.; Gargouri, Y.; Miled, N.; Ben-Rebah, F. Chemical composition and some biological activities of marine algae collected in Tunisia. Cienc. Mar. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Burdin, K.S.; Bird, K.T. Heavy metal accumulation by carrageenan and agar producing algae. Bot. Mar. 1994, 37, 467–470. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Idris, A.M.; Eltayeb, M.A.H.; El-Zahhar, A.A.; Ashraf, I.M. Bioaccumulation and health risk assessment of toxic metals in red algae in Sudanese Red Sea coast. Toxin Rev. 2019. [Google Scholar] [CrossRef]
- Strezov, A.; Nonova, T. Influence of macroalgal diversity on accumulation of radionuclides and heavy metals in Bulgarian Black Sea ecosystems. J. Environ. Radioact. 2009, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Boubonari, T.; Malea, P.; Koyro, H.W.; Kevrekidis, T. The red macroalga Gracilaria bursa-pastoris as a bioindicator of metals (Fe, Zn, Cu, Pb, Cd) in oligohaline coastal environments. Fresenius Environ. Bull. 2008, 17, 2207–2216. [Google Scholar]
- Ryan, S.; McLoughlin, P.; O’Donovan, O.A. comprehensive study of metal distribution in three main classes of seaweed. Environ. Pollut. 2012, 167, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Munda, I.M. Preliminary information on the ascorbic acid content in some Adriatic seaweeds. Hydrobiologia 1987, 151–152, 477–481. [Google Scholar] [CrossRef]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Jensen, A. Tocopherol content of seaweed and seaweed meal: 1.-Analytical methods and distribution of tocopherols in benthic algae. J. Sci. Food Agric. 1969, 20, 449–453. [Google Scholar] [CrossRef]
- Vinothkumar, R.; Murugesan, S.; Sivamurugan, V. Vitamin content of marine red alga Champia parvula. Asian J. Pharm. Clin. Res. 2019, 12, 207–209. [Google Scholar] [CrossRef]
- Teeri, A.E.; Bieber, R.E. B-Complex vitamins in certain brown and red algae. Science 1958, 127, 1500. [Google Scholar] [CrossRef]
- James, D.P. Nicotinic acid, pantothenic acid and biotin in fruits, vegetables and nuts. Br. J. Nutr. 1952, 6, 341–356. [Google Scholar] [CrossRef][Green Version]
- Craigie, J.S. Storage products. In Algal Physiology and Biochemistry; Stewart, W.D.P., Ed.; Blackwell Press: Oxford, UK, 1974; pp. 206–235. [Google Scholar]
- Reed, R.H. Osmoacclimation in Bangia atropurpurea (Rhodophyta, Bangiales): The osmotic role of floridoside. Br. Phycol. J. 1985, 20, 211–218. [Google Scholar] [CrossRef]
- Courtois, A.; Simon-Colin, C.; Boisset, C.; Berthou, C.; Deslandes, E.; Guézennec, J.; Bordron, A. Floridoside extracted from the red alga Mastocarpus stellatus is a potent activator of the classical complement pathway. Mar. Drugs 2008, 6, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses proinflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. The effects of marine carbohydrates and glycosylated compounds on human health. Int. J. Mol. Sci. 2015, 16, 6018–6056. [Google Scholar] [CrossRef]
- Karsten, U.; Görs, S.; Eggert, A.; West, J.A. Trehalose, digeneaside and floridoside in the Florideophyceae (Rhodophyta)—A re-evaluation of its chemotaxonomic value. Phycologia 2007, 46, 143–150. [Google Scholar] [CrossRef]
- Kirst, G.O. Low MW carbohydrates and ions in Rhodophyceae: Quantitative measurement of floridoside and digeneaside. Phytochemistry 1980, 19, 1107–1110. [Google Scholar] [CrossRef]
- Rødde, R.S.H.; Vårum, K.M.; Larsen, B.A.; Myklestad, S.M. Seasonal and geographical variation in the chemical composition of the red alga Palmaria palmata (L.) Kuntze. Bot. Mar. 2004, 47, 125–133. [Google Scholar] [CrossRef]
- Elbein, A.D. The metabolism of α,α-trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [CrossRef]
- Cao, Y.; Ashline, D.J.; Ficko-Blean, E.; Klein, A.S. Trehalose and (iso)floridoside production under desiccation stress in red alga Porphyra umbilicalis and the genes involved in their synthesis. J. Phycol. 2020, 56, 1468–1480. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Usov, A.I.; Mysyakina, I.S.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Microbiology 2014, 83, 184–194. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and role of β-alanine in plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Duhazé, C.; Gagneul, D.; Leport, L.; Larher, F.R.; Bouchereau, A. Uracil as one of the multiple sources of β-alanine in Limonium latifolium, a halotolerant β-alanine betaine accumulating Plumbaginaceae. Plant Physiol. Biochem. 2003, 41, 993–998. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.; Tkeshelashvili, B. Women dealing with hot flushes: The role of β-alanine. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5148–5154. [Google Scholar] [CrossRef]
- Kawasaki, A.; Ono, A.; Mizuta, S.; Kamiya, M.; Takenaga, T.; Murakami, S. The taurine content of Japanese seaweed. In Taurine 10. Advances in Experimental Medicine and Biology; Lee, D.H., Schaffer, S.W., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 1105–1113. [Google Scholar] [CrossRef]
- Mehdinia, A.; Rostami, S.; Dadkhah, S.; Fumani, N.S. Simultaneous screening of homotaurine and taurine in marine macro-algae using liquid chromatography–fluorescence detection. J. Iran. Chem. Soc. 2017, 14, 2135–2142. [Google Scholar] [CrossRef]
- Kadnikova, I.A.; Selivanova, O.N.; Shcherbakova, N.S. Chemical composition of the Palmariales algae (Rhodophyta) from the coast of Kamchatka. Izv. TINRO 2012, 169, 246–254. (In Russian) [Google Scholar]
- Klindukh, M.P.; Ryzhik, I.V.; Dobuchina, E.O. Composition and content of free amino acids of sublittoral red algae from Murmansk coast of the Barents Sea. Tr. Kolskogo Nauchnogo Cent. RAN 2020, 5, 92–101. (In Russian) [Google Scholar]
- Yamori, Y.; Taguchi, T.; Hamada, A.; Kunimasa, K.; Mori, H.; Mori, M. Taurine in health and diseases: Consistent evidence from experimental and epidemiological studies. J. Biomed. Sci. 2010, 17, 1–14. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Cobussen-Pool, E.M.; van Egmond, B.F.; van Hal, J.W. Determination of carbohydrate composition of macroalgae. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 199–210. [Google Scholar] [CrossRef]
- Hewitt, B.R. Spectrophotometric determination of total carbohydrate. Nature 1958, 182, 246–247. [Google Scholar] [CrossRef]
- Lowry, D.H.; Rosebrough, N.J.; Farr, A.; Randall, R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lees, M.B.; Paxman, S. Modification of the Lowry procedure for the analysis of proteolipid protein. Anal. Biochem. 1972, 47, 184–192. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lüder, U.H.; Wiencke, C.; Knoetzel, J. Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (Rhodophyta): A study to simulate Antarctic winter sea ice cover. J. Phycol. 2002, 38, 904–913. [Google Scholar] [CrossRef]
- Hutschenreuther, A.; Kiontke, A.; Birkenmeier, G.; Birkemeyer, C. Comparison of extraction conditions and normalization approaches for cellular metabolomics of adherent growing cells with GC-MS. Anal. Meth. 2012, 4, 1953–1963. [Google Scholar] [CrossRef]
- Kovats, V.E. Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. GMD@ CSB. DB: The Golm metabolome database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
| Species | Moisture, % | Protein, % DW | Total Carbohydrates, % DW | |
|---|---|---|---|---|
| Soluble Protein | Total Protein | |||
| Ceramium virgatum | 86.77 ± 3.13 | 7.79 ± 1.90 | 32.25 ± 2.18 | 32.33 ± 4.31 |
| Ptilota gunneri | 82.90 ± 3.05 | 6.69 ± 0.36 | 8.92 ± 0.55 | 40.26 ± 4.06 |
| Phycodrys rubens | 76.71 ± 2.66 | 7.16 ± 0.72 | 13.21 ± 1.32 | 40.83 ± 3.00 |
| Polysiphonia stricta | 86.38 ± 4.32 | 11.63 ± 1.92 | 18.58 ± 4.18 | 29.38 ± 2.25 |
| Savoiea arctica | 81.93 ± 2.16 | 14.28 ± 2.45 | 24.95 ± 4.71 | 32.50 ± 8.19 |
| Rhodomela confervoides | 80.87 ± 2.98 | 12.15 ± 0.78 | 24.26 ± 1.26 | 44.38 ± 3.18 |
| Odonthalia dentata | 79.64 ± 2.40 | 6.43 ± 0.44 | 14.78 ± 2.64 | 38.09 ± 4.78 |
| Cystoclonium purpureum | 89.42 ± 3.71 | 11.88 ± 1.24 | 27.10 ± 2.36 | 27.24 ± 7.36 |
| Coccotylus brodiei | 72.53 ± 3.18 | 6.96 ± 0.74 | 15.87 ± 1.38 | 33.27 ± 3.59 |
| Euthora cristata | 84.24 ± 2.56 | 6.52 ± 0.62 | 13.06 ± 2.47 | 41.59 ± 4.50 |
| Furcellaria lumbricalis | 77.20 ± 2.02 | 10.45 ± 1.66 | 15.98 ± 1.44 | 56.92 ± 4.49 |
| Polyides rotunda | 73.26 ± 4.01 | 3.46 ± 0.54 | 9.23 ± 1.53 | 33.02 ± 0.95 |
| Palmaria palmata | 88.21 ± 2.65 | 5.60 ± 0.57 | 19.94 ± 3.58 | 24.06 ± 4.17 |
| Ahnfeltia plicata | 64.03 ± 2.01 | 3.02 ± 0.39 | 5.57 ± 1.14 | 41.27 ± 3.63 |
| Corallina officinalis | 31.28 ± 1.90 | 1.39 ± 0.23 | 3.37 ± 0.46 | 17.46 ± 3.76 |
| Species | Mineral Content, mg g−1 DW | |||||
|---|---|---|---|---|---|---|
| K | Ca | Mg | P | S | Fe | |
| Ceramium virgatum | 10.18 ± 0.15 | 5.78 ± 0.83 | 6.47 ± 0.44 | 2.52 ± 0.01 | 6.99 ± 0.09 | 0.32 ± 0.02 |
| Ptilota gunneri | 8.72 ± 0.21 | 6.63 ± 0.63 | 3.34 ± 0.28 | 2.42 ± 0.03 | 5.08 ± 0.25 | 0.43 ± 0.03 |
| Phycodrys rubens | 1.42 ± 0.26 | 6.66 ± 0.92 | 8.55 ± 0.63 | 2.68 ± 0.08 | 11.92 ± 0.75 | 0.25 ± 0.03 |
| Polysiphonia stricta | 10.33 ± 0.35 | 4.49 ± 0.62 | 3.50 ± 0.08 | 1.22 ± 0.03 | 10.00 ± 0.32 | 0.72 ± 0.16 |
| Savoiea arctica | 8.56 ± 0.24 | 4.19 ± 0.60 | 2.57 ± 0.10 | 1.12 ± 0.06 | 8.93 ± 0.67 | 1.84 ± 0.59 |
| Rhodomela confervoides | 14.32 ± 0.04 | 5.44 ± 0.05 | 5.03 ± 0.02 | 2.45 ± 0.01 | 12.81 ± 0.03 | 0.53 ± 0.11 |
| Odonthalia dentata | 10.70 ± 0.15 | 4.07 ± 0.33 | 2.86 ± 0.13 | 1.25 ± 0.03 | 9.25 ± 0.39 | 1.34 ± 0.07 |
| Cystoclonium purpureum | 14.99 ± 0.08 | 3.95 ± 0.20 | 4.61 ± 0.05 | 2.28 ± 0.08 | 6.96 ± 0.05 | 0.43 ± 0.06 |
| Coccotylus brodiei | 7.05 ± 0.35 | 4.50 ± 0.16 | 3.13 ± 0.12 | 1.38 ± 0.04 | 15.36 ± 0.89 | 0.39 ± 0.06 |
| Euthora cristata | 1.85 ± 0.22 | 6.73 ± 0.55 | 4.83 ± 0.30 | 2.37 ± 0.01 | 20.36 ± 0.26 | 0.39 ± 0.09 |
| Furcellaria lumbricalis | 6.09 ± 0.12 | 2.18 ± 0.10 | 5.55 ± 0.12 | 1.29 ± 0.02 | 13.44 ± 0.28 | 0.43 ± 0.02 |
| Polyides rotunda | 4.86 ± 0.24 | 3.69 ± 0.16 | 3.15 ± 0.10 | 0.99 ± 0.01 | 8.61 ± 0.19 | 0.29 ± 0.04 |
| Palmaria palmata | 19.99 ± 2.00 | 1.61 ± 0.13 | 0.92 ± 0.11 | 2.12 ± 0.05 | 1.73 ± 0.01 | 0.72 ± 0.08 |
| Ahnfeltia plicata | 3.47 ± 0.35 | 6.54 ± 0.40 | 2.05 ± 0.09 | 1.41 ± 0.02 | 4.37 ± 0.18 | 0.26 ± 0.03 |
| Corallina officinalis | 1.57 ± 0.21 | 309.7 ± 9.7 | 14.7 ± 0.71 | 0.56 ± 0.02 | 4.03 ± 0.21 | 0.28 ± 0.09 |
| Species | Mineral Content, μg g−1 DW | |||||
|---|---|---|---|---|---|---|
| Mn | Cu | Zn | Mo | Co | Ni | |
| Ceramium virgatum | 83.64 ± 5.16 | 6.79 ± 1.63 | 59.15 ± 5.52 | 1.41 ± 0.08 | <0.1 | 3.71 ± 0.25 |
| Ptilota gunneri | 210.4 ± 3.66 | 9.64 ± 0.94 | 75.66 ± 5.51 | 0.53 ± 0.07 | 1.09 ± 0.39 | 19.99 ± 4.02 |
| Phycodrys rubens | 43.10 ± 16.63 | 19.76 ± 2.31 | 100.3 ± 6.09 | 1.44 ± 0.23 | <0.1 | 9.36 ± 0.28 |
| Polysiphonia stricta | 279.1 ± 12.16 | 26.97 ± 2.64 | 86.33 ± 1.67 | 1.84 ± 0.10 | 1.35 ± 0.18 | 6.35 ± 0.39 |
| Savoiea arctica | 63.62 ± 8.81 | 42.54 ± 3.02 | 235.8 ± 8.38 | 1.23 ± 0.26 | 0.19 ± 0.03 | 10.57 ± 0.82 |
| Rhodomela confervoides | 4.72 ± 0.50 | 15.40 ± 0.73 | 286.7 ± 0.72 | 4.33 ± 0.19 | 0.08 ± 0.01 | 7.25 ± 0.81 |
| Odonthalia dentata | 1751.2 ± 56.2 | 30.30 ± 0.65 | 64.10 ± 2.96 | 0.70 ± 0.10 | 14.05 ± 0.30 | 14.82 ± 0.17 |
| Cystoclonium purpureum | 778.1 ± 31.28 | 7.39 ± 0.39 | 154.8 ± 8.67 | 2.00 ± 0.39 | 6.11 ± 0.32 | 9.78 ± 0.70 |
| Coccotylus brodiei | 597.0 ± 53.06 | 6.70 ± 0.08 | 67.85 ± 4.97 | 0.76 ± 0.03 | 3.69 ± 0.20 | 45.32 ± 3.62 |
| Euthora cristata | 132.9 ± 16.18 | 14.62 ± 0.26 | 75.70 ± 2.50 | 2.16 ± 0.55 | 0.76 ± 0.01 | 11.59 ± 0.75 |
| Furcellaria lumbricalis | 321.4 ± 14.35 | 7.14 ± 0.24 | 34.93 ± 2.17 | 0.52 ± 0.02 | 1.25 ± 0.16 | 5.14 ± 0.47 |
| Polyides rotunda | 157.2 ± 12.31 | 4.40 ± 0.88 | 34.80 ± 1.64 | 0.37 ± 0.03 | 1.02 ± 0.11 | 11.16 ± 0.45 |
| Palmaria palmata | 81.64 ± 10.19 | 11.06 ± 0.46 | 98.56 ± 9.97 | 0.52 ± 0.02 | 0.43 ± 0.02 | 11.17 ± 0.36 |
| Ahnfeltia plicata | 56.72 ± 3.27 | 4.63 ± 0.40 | 34.78 ± 1.29 | 0.56 ± 0.07 | 0.34 ± 0.01 | 5.79 ± 0.40 |
| Corallina officinalis | 171.1 ± 5.87 | 3.60 ± 0.21 | 31.05 ± 3.71 | 0.06 ± 0.01 | 0.30 ± 0.04 | 3.68 ± 0.18 |
| Order | Species | Typical Habitat |
|---|---|---|
| Ceramiales | Ceramium virgatum | Low intertidal–subtidal: exposed rocky shores |
| Ptilota gunneri | Subtidal: rocks, Saccharina holdfasts | |
| Phycodrys rubens | Subtidal: rocks, Saccharina stipes and holdfasts | |
| Polysiphonia stricta | Subtidal: rocks, Saccharina stipes and holdfasts | |
| Savoiea arctica | Subtidal: fucoid beds, Saccharina holdfasts | |
| Rhodomela confervoides | Low intertidal–subtidal: rock pools, fucoid beds, Saccharina holdfasts | |
| Odonthalia dentata | Subtidal: rocks, Saccharina stipes and holdfasts | |
| Gigartinales | Cystoclonium purpureum | Subtidal: rocky shores, frequently growing on Ahnfeltia thalli |
| Coccotylus brodiei | Subtidal: rocks, Saccharina stipes and holdfasts | |
| Euthora cristata | Subtidal: rocks, Saccharina stipes and holdfasts | |
| Furcellaria lumbricalis | Subtidal: rocks, Saccharina holdfasts | |
| Polyides rotunda | Low intertidal–subtidal: rock pools, sandy and rocky bottom | |
| Palmariales | Palmaria palmata | Mid-intertidal–subtidal: rock pools, Saccharina stipes |
| Ahnfeltiales | Ahnfeltia plicata | Subtidal: rocky shores |
| Corallinales | Corallina officinalis | Subtidal: rocks, fucoid beds, Saccharina stipes and holdfasts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. https://doi.org/10.3390/molecules26092489
Yanshin N, Kushnareva A, Lemesheva V, Birkemeyer C, Tarakhovskaya E. Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean). Molecules. 2021; 26(9):2489. https://doi.org/10.3390/molecules26092489
Chicago/Turabian StyleYanshin, Nikolay, Aleksandra Kushnareva, Valeriia Lemesheva, Claudia Birkemeyer, and Elena Tarakhovskaya. 2021. "Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean)" Molecules 26, no. 9: 2489. https://doi.org/10.3390/molecules26092489
APA StyleYanshin, N., Kushnareva, A., Lemesheva, V., Birkemeyer, C., & Tarakhovskaya, E. (2021). Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean). Molecules, 26(9), 2489. https://doi.org/10.3390/molecules26092489







