Micro Salting-Out Assisted Matrix Solid-Phase Dispersion: A Simple and Fast Sample Preparation Method for the Analysis of Bisphenol Contaminants in Bee Pollen
Abstract
:1. Introduction
2. Results and Discussion
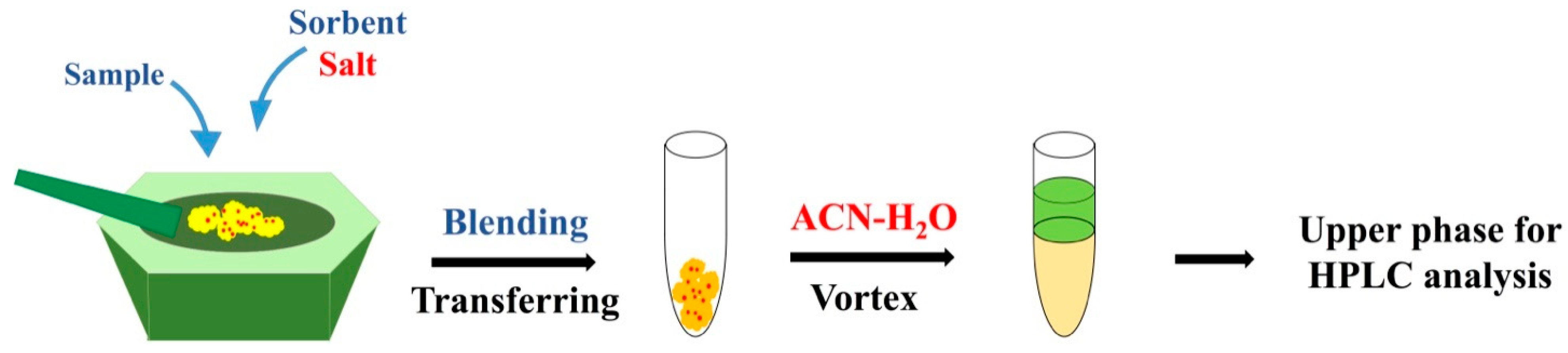
2.1. Micro Salting-Out Assisted MSPD
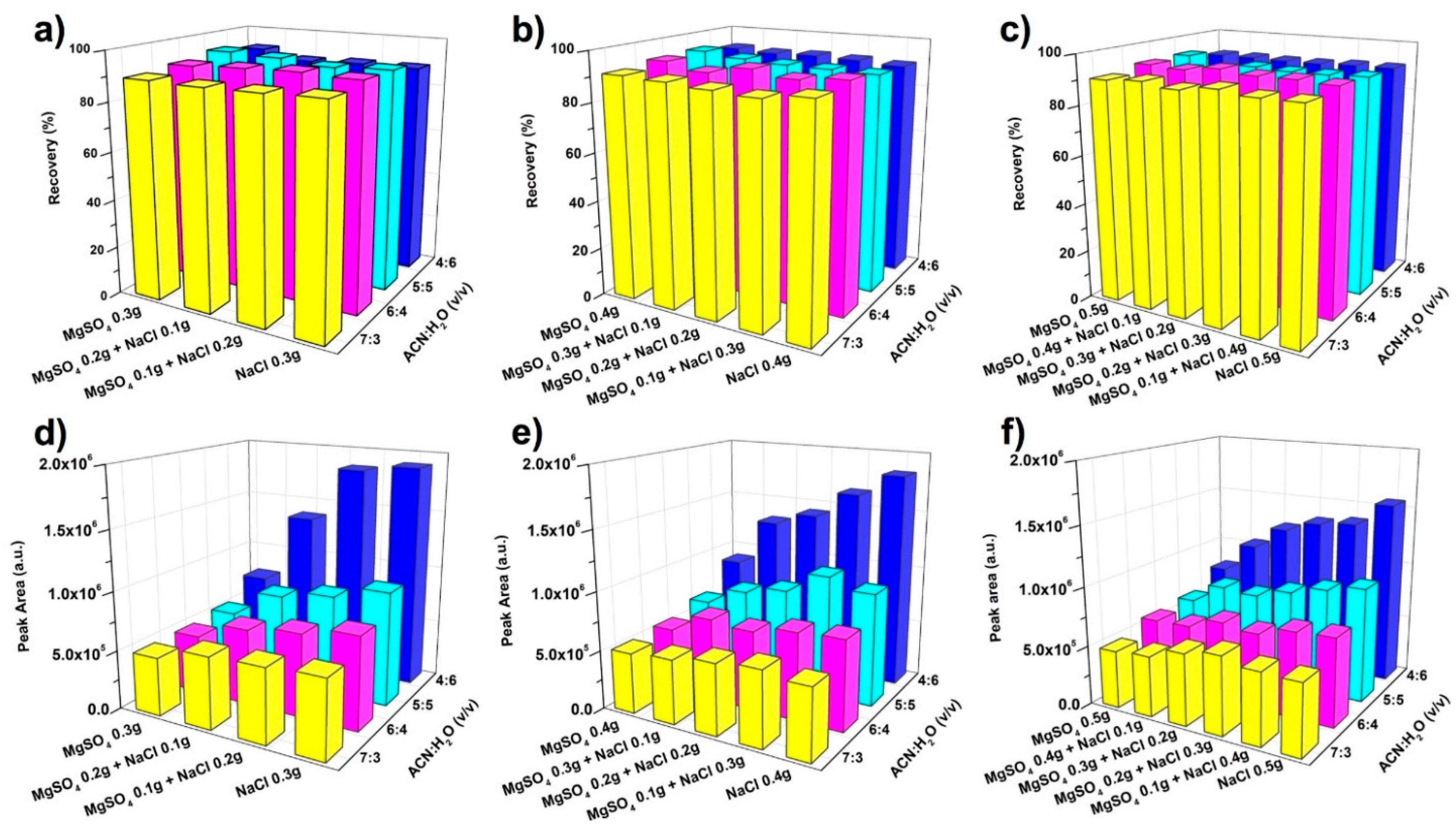
2.2. Salting-Out Parameters
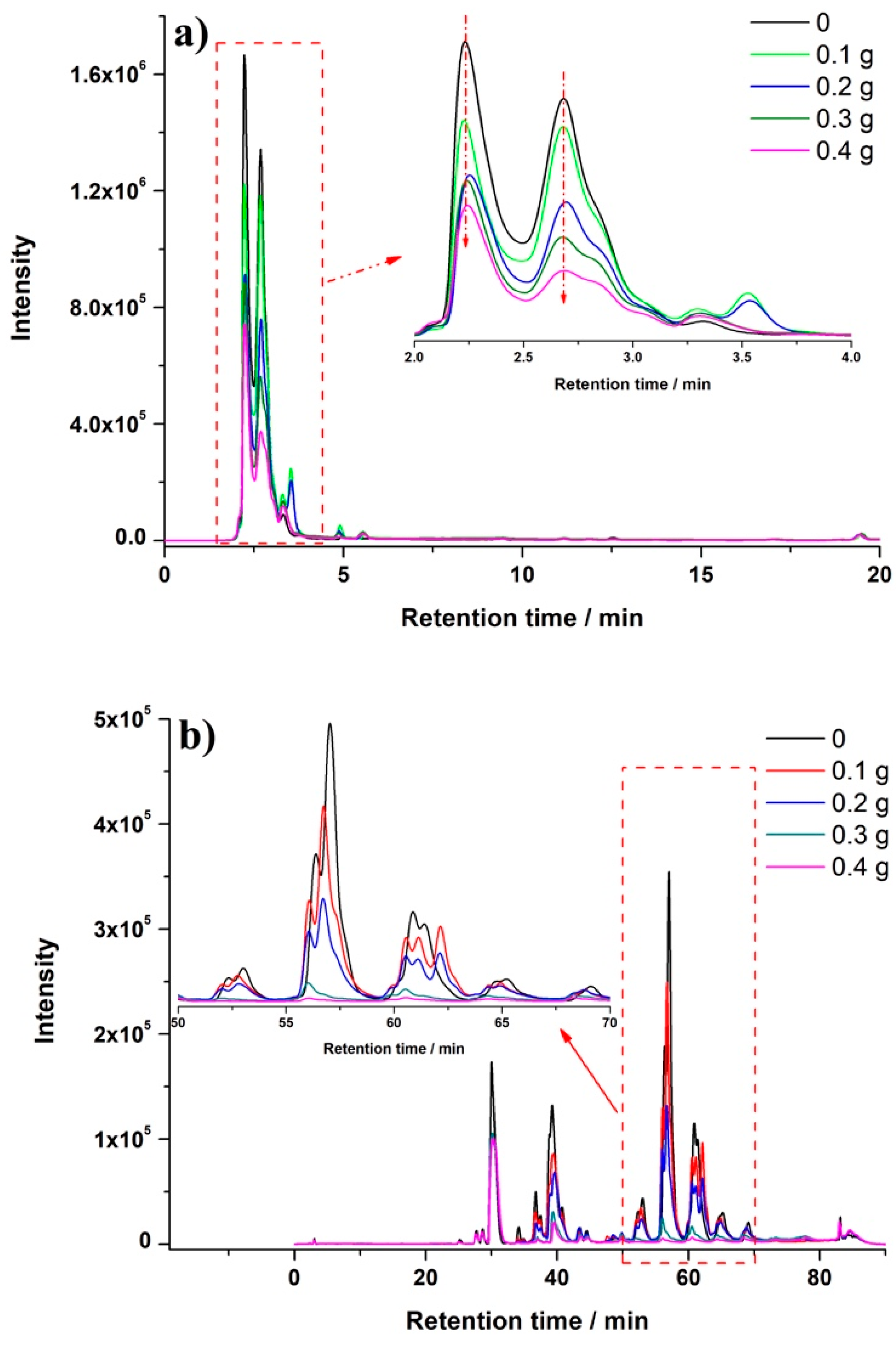
2.3. Clean-Up Performance
2.4. Method Validation
3. Materials and Methods
3.1. Materials
3.2. Optimal Micro Salting-Out Assisted MSPD
3.3. Optimization of Salting-Out Parameters
3.4. Clean-Up Effect of PSA in the Micro Salting-Out Assisted MSPD
3.5. HPLC Analysis
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Capriotti, A.L.; Cavaliere, C.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Recent developments in matrix solid-phase dispersion extraction. J. Chromatogr. A 2010, 1217, 2521–2532. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Laganà, A. Recent advances and developments in matrix solid-phase dispersion. Trends Anal. Chem. 2015, 71, 186–193. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. A Review on the Recent Progress in Matrix Solid Phase Dispersion. Molecules 2018, 23, 2767. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L. Use of new tailored and engineered materials for matrix solid-phase dispersion. Trends Anal. Chem. 2019, 118, 751–758. [Google Scholar] [CrossRef]
- Barker, S.A. Matrix solid-phase dispersion. J. Chromatogr. A 2000, 885, 115–127. [Google Scholar] [CrossRef]
- Barker, S.A. Matrix solid phase dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [Google Scholar] [CrossRef]
- Ramos, J.J.; Rial-Otero, R.; Ramos, L. Ultrasonic-assisted matrix solid-phase dispersion as an improved methodology for the determination of pesticides in fruits. J. Chromatogr. A 2008, 1212, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Aznar, R.; Albero, B.; Perez, R.A.; Sanchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Analysis of emerging organic contaminants in poultry manure by gas chromatography-tandem mass spectrometry. J. Sep. Sci. 2018, 41, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, G.I.; Soares, K.L.; Caldas, S.S.; Primel, E.G. Study of vortex-assisted MSPD and LC-MS/MS using alternative solid supports for pharmaceutical extraction from marketed fish. Anal. Bioanal. Chem. 2015, 407, 4793–4803. [Google Scholar] [CrossRef] [PubMed]
- León-González, M.E.; Rosales-Conrado, N. Determination of ibuprofen enantiomers in breast milk using vortex-assisted matrix solid-phase dispersion and direct chiral liquid chromatography. J. Chromatogr. A 2017, 1514, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Du, K.Z.; Li, J.; Chang, Y.X. A chitosan solution-based vortex-forced matrix solid phase dispersion method for the extraction and determination of four bioactive constituents from Ligustri Lucidi Fructus by high performance liquid chromatography. J. Chromatogr. A 2020, 1609, 460509. [Google Scholar] [CrossRef]
- Chung, W.H.; Lin, J.S.; Ding, W.H. Dual-vortex-assisted matrix solid-phase dispersion coupled with isotope-dilution ultrahigh-performance liquid chromatography-high resolution mass spectrometry for the rapid determination of parabens in indoor dust samples. J. Chromatogr. A 2019, 1605, 460367. [Google Scholar] [CrossRef]
- Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Madrid, Y. Determination of phenolic compounds in residual brewing yeast using matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef]
- Kemmerich, M.; Demarco, M.; Bernardi, G.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Balls-in-tube matrix solid phase dispersion (BiT-MSPD): An innovative and simplified technique for multiresidue determination of pesticides in fruit samples. J. Chromatogr. A 2020, 1612, 460640. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.G.; Anderson, J.L.; Stalikas, C.D. Matrix solid-phase dispersion based on magnetic ionic liquids: An alternative sample preparation approach for the extraction of pesticides from vegetables. J. Chromatogr. A 2018, 1581–1582, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically assisted matrix solid phase dispersion for extraction of parabens from breast milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef]
- Guerra, E.; Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1415, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, Y.; Tian, Y.; Wu, L.; Yang, L.; Hou, X.; Zheng, C. Single-Drop Solution Electrode Discharge-Induced Cold Vapor Generation Coupling to Matrix Solid-Phase Dispersion: A Robust Approach for Sensitive Quantification of Total Mercury Distribution in Fish. Anal. Chem. 2017, 89, 2093–2100. [Google Scholar] [CrossRef] [Green Version]
- Deng, T.; Wu, D.; Duan, C.; Yan, X.; Du, Y.; Zou, J.; Guan, Y. Spatial Profiling of Gibberellins in a Single Leaf Based on Microscale Matrix Solid-Phase Dispersion and Precolumn Derivatization Coupled with Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 9537–9543. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Yang, R.; Ye, L.H.; Cao, J.; Cao, W.; Hu, S.S.; Peng, L.Q. Application of ionic liquids for elution of bioactive flavonoid glycosides from lime fruit by miniaturized matrix solid-phase dispersion. Food Chem. 2016, 204, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Cao, J.; Peng, L.Q.; Cao, W.; Zhu, Q.Y.; Zhang, Q.Y. Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2016, 1436, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Peng, L.Q.; Xu, J.J.; Du, L.J.; Zhang, Q.D. Simultaneous microextraction of inorganic iodine and iodinated amino acids by miniaturized matrix solid-phase dispersion with molecular sieves and ionic liquids. J. Chromatogr. A 2016, 1477, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized matrix solid-phase dispersion followed by liquid chromatography-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef]
- Chu, C.; Jiang, L.; Mao, H.; Yan, J. A simple and environmentally-friendly method by pipette-tip matrix solid-phase dispersion microextraction coupled with high-performance liquid chromatography for the simultaneous determination of lignans and terpenes. Sustain. Chem. Pharm. 2021, 20, 100384. [Google Scholar] [CrossRef]
- Pena-Abaurrea, M.; Ramos, L. Miniaturization of analytical methods. In Challenges in Green Analytical Chemistry, 1st ed.; de la Guardia, M., Garrigues, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 107–143. [Google Scholar]
- Anthemidis, A.N.; Ioannou, K.-I.G. Recent developments in homogeneous and dispersive liquid-liquid extraction for inorganic elements determination. A review. Talanta 2009, 80, 413–421. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Apyari, V.V.; Gorbunova, M.V.; Tolmacheva, V.V.; Zolotov, Y.A. Homogeneous Liquid–Liquid Microextraction of Organic Compounds. J. Anal. Chem. 2020, 75, 1371–1383. [Google Scholar] [CrossRef]
- Valente, I.M.; Rodrigues, J.A. Recent advances in salt-assisted LLE for analyzing biological samples. Bioanalysis 2015, 7, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tu, X.; Wu, D.; Gao, Z.; Wu, S.; Huang, S. Comparison of the Partition Efficiencies of Multiple Phenolic Compounds Contained in Propolis in Different Modes of Acetonitrile-Water-Based Homogenous Liquid-Liquid Extraction. Molecules 2019, 24, 442. [Google Scholar] [CrossRef] [Green Version]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Tu, X.; Wu, S.; Liu, W.; Gao, Z.; Huang, S.; Chen, W. Sugaring-Out Assisted Liquid-Liquid Extraction Combined with High-Performance Liquid Chromatography-Fluorescence Detection for the Determination of Bisphenol A and Bisphenol B in Royal Jelly. Food Anal. Methods 2019, 12, 705–711. [Google Scholar] [CrossRef]
- Inoue, K.; Murayama, S.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Contamination of xenoestrogens bisphenol A and F in honey: Safety assessment and analytical method of these compounds in honey. J. Food Compos. Anal. 2003, 16, 497–506. [Google Scholar] [CrossRef]
- Gałuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namiesnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Valente, I.M.; Goncalves, L.M.; Rodrigues, J.A. Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures. J. Chromatogr. A 2013, 1308, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Sun, F.; Wu, S.; Liu, W.; Gao, Z.; Huang, S.; Chen, W. Comparison of salting-out and sugaring-out liquid-liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their co-extracted protein content. J. Chromatogr. B 2018, 1073, 90–95. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. Miniaturized Salting-Out Assisted Liquid-Liquid Extraction Combined with Disposable Pipette Extraction for Fast Sample Preparation of Neonicotinoid Pesticides in Bee Pollen. Molecules 2020, 25, 5703. [Google Scholar] [CrossRef]
- Zhang, C.P.; Huang, S.; Wei, W.T.; Ping, S.; Shen, X.G.; Li, Y.J.; Hu, F.L. Development of high-performance liquid chromatographic for quality and authenticity control of Chinese propolis. J. Food Sci. 2014, 79, C1315–C1322. [Google Scholar]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]

| Analytes | Spiked Levels (μg/kg) | Intra-Day | Inter-Day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||||||
| Recovery (Mean ± SD, %, n = 6) | RSD (%, n = 6) | Recovery (Mean ± SD, %, n = 6) | RSD (%, n = 6) | Recovery (Mean ± SD, %, n = 6) | RSD (%, n = 6) | Recovery (Mean ± SD, %, n = 18) | RSD (%, n = 18) | ||
| BPA | 60 | 92.70 ± 4.13 | 4.46 | 90.19 ± 4.41 | 4.89 | 94.64 ± 3.59 | 3.79 | 92.51 ± 4.24 | 4.59 |
| 300 | 89.13 ± 1.57 | 1.76 | 88.44 ± 2.75 | 3.11 | 87.70 ± 4.57 | 5.21 | 88.43 ± 3.07 | 3.47 | |
| 600 | 89.04 ± 3.08 | 3.46 | 90.16 ± 2.92 | 3.24 | 88.30 ± 2.25 | 2.55 | 89.16 ± 2.81 | 3.15 | |
| BPB | 80 | 85.14 ± 2.31 | 2.71 | 89.59 ± 4.46 | 4.98 | 85.25 ± 4.65 | 5.45 | 86.66 ± 4.28 | 4.94 |
| 400 | 84.29 ± 2.26 | 2.68 | 83.18 ± 2.20 | 2.64 | 83.46 ± 2.50 | 3.00 | 83.64 ± 2.24 | 2.68 | |
| 800 | 83.03 ± 1.72 | 2.07 | 83.26 ± 1.69 | 2.03 | 83.68 ± 2.61 | 3.12 | 83.32 ± 1.99 | 2.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yu, F.; Tao, Y.; Du, C.; Yang, W.; Chen, W.; Tu, X. Micro Salting-Out Assisted Matrix Solid-Phase Dispersion: A Simple and Fast Sample Preparation Method for the Analysis of Bisphenol Contaminants in Bee Pollen. Molecules 2021, 26, 2350. https://doi.org/10.3390/molecules26082350
Zhang J, Yu F, Tao Y, Du C, Yang W, Chen W, Tu X. Micro Salting-Out Assisted Matrix Solid-Phase Dispersion: A Simple and Fast Sample Preparation Method for the Analysis of Bisphenol Contaminants in Bee Pollen. Molecules. 2021; 26(8):2350. https://doi.org/10.3390/molecules26082350
Chicago/Turabian StyleZhang, Jianing, Fengjie Yu, Yunmin Tao, Chunping Du, Wenchao Yang, Wenbin Chen, and Xijuan Tu. 2021. "Micro Salting-Out Assisted Matrix Solid-Phase Dispersion: A Simple and Fast Sample Preparation Method for the Analysis of Bisphenol Contaminants in Bee Pollen" Molecules 26, no. 8: 2350. https://doi.org/10.3390/molecules26082350
APA StyleZhang, J., Yu, F., Tao, Y., Du, C., Yang, W., Chen, W., & Tu, X. (2021). Micro Salting-Out Assisted Matrix Solid-Phase Dispersion: A Simple and Fast Sample Preparation Method for the Analysis of Bisphenol Contaminants in Bee Pollen. Molecules, 26(8), 2350. https://doi.org/10.3390/molecules26082350






